Abstract
SARS-CoV-2 remains a medical and economic challenge, due to the lack of a suitable drug or vaccine. The glycans in some proteins play a pivotal role in protein folding, oligomerization, quality control, sorting, and transport so the hindering of N-linked glycosylation of glycoproteins will prevent assembly of the virion. Tunicamycin an anticancer drug inhibit the N- linked glycans. Our study aimed to find out the mechanism action of tunicamycin on the viral glycoproteins. The growth of coronavirus in the presence inhibitor tunicamycin resulted in the production of spikeless, non-infectious virions which were devoid of S protein. We concluded that tunicamycin inhibits E2, S, and M glycoproteins of coronaviruses. Tunicamycin is also diminished glycosylation of PTMs such as HE, and 8 ab of SARS-CoV. Finally, we recommend using this drug to treat the SARS-CoV-2.
Keywords: Tunicamycin, Glycosylation, Virion, Coronavirus, PTMs
1. Introduction
Since last December, a new coronavirus has been a challenge for all of the world. The reason for the elevation number of infections and fatality that the researchers failed to find a suitable drug or vaccine to stem the outbreaks [1]. A few studies focused on the effect of N and O-glycosylation through the process of virion assembly. N-linked glycosylation is the attachment of an oligosaccharide (glycan), to a nitrogen atom an asparagine (Asn) residue of a protein. This linkage is important for the structure and function of some eukaryotic proteins. SARS–CoV is one of the viruses contain N-linked glycoproteins which are glycosylated by the transfer of core oligosaccharides from a dolichol pyrophosphate carrier to asparagine residues on the polypeptide [2]. O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom on serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesized. It occurs either in the endoplasmic reticulum or Golgi apparatus and it influences the stability and regulation of protein [3]. The main function of O-glycan is allowing the recognition of foreign material and controlling cell metabolism. The changes in O-glycosylation are important in many diseases including cancer, diabetes Alzheimer's as well as some viral infections [4]. Because glycosylation and transport of viral proteins depend upon cellular processes, prevention of this process depends on the type of the viral protein and the activity of the substrate to diminish it. Coronavirus glycoproteins may be glycosylated by a different mechanism. These glycoproteins serve as a useful model for the study of N and O-linked glycoproteins [5].
1.1. The role of tunicamycin (Tm)
Tunicamycin is an antibiotic was produced by Streptomyces clavuligerus and Streptomyces lysosuperficus bacteria along with several other species. and a proposed biosynthetic pathway was characterized. Tunicamycin is a white crystalline powder that is soluble in alkaline water, pyridine, and hot methanol, slightly soluble in ethanol and n-butanol and insoluble in acetone, ethyl acetate, chloroform, benzene, and acidic water [6]. Until now, there is no available information about the tunicamycin in pharmacodynamics, absorption, protein binding, metabolism, toxicity, affected organisms, and human clinical trials.
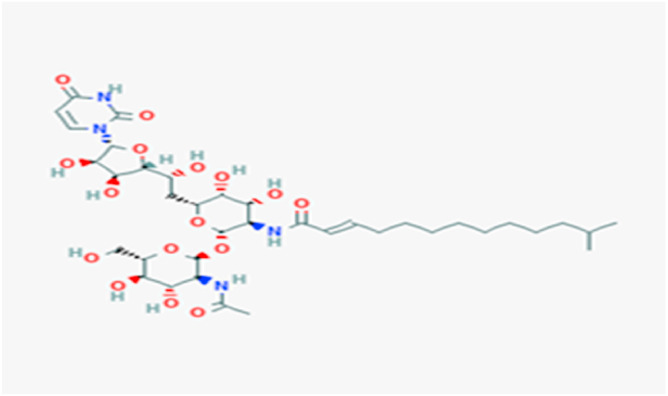
Tunicamycin Fig. 1 is an analog of UDP-N-acetylglucosamine, interfered with the formation of dolichol pyrophosphate-acetylglucosamine which acts as a carrier for N-glycosidic linkage of core oligosaccharides to asparagine (Asn) residues on glycoproteins. The bacteria utilize the enzymes in the tun gene cluster (TunA-N) to make tunicamycin. Because of many cellular glycoproteins such as fetuin, cornea1 proteoglycan, thyroglobulin, and immunoglobulins contain both asparagine-linked and serine or threonine linked oligosaccharides. Tunicamycin may interfere with the glycosylation of these glycoproteins. Tunicamycin induces ER stress in cells by inhibiting the first step in the biosynthesis of N-linked glycans in proteins resulting in many misfolded proteins [7]. When the antibiotic blocks glycosylation of N-glycans, the cell cycle arrests at the G1 phase in human cells. Previous studies suggested that tunicamycin was used as a therapeutic drug against human colon or prostate cancer cells via induced apoptosis [8]. The inhibited glycosylation of the structural glycoproteins was showed in alphaviruses, bunyaviruses, herpes viruses, myxoviruses, and all other viruses possess glycoprotein envelopes. Han et al proposed that inhibition biosynthesis of the N-glycosylation by tunicamycin may be an optimistic curative strategy to boost the sensibility of cancer cells to trastuzumab [9]. Some drugs and vaccines give interactions with tunicamycin illustrated in the Table 1 .
Fig. 1.
The structure of tunicamycin from DrugBank.
Table 1.
Drugs, vaccines, and antigens show interactions with tunicamycin.
| Drug | Interaction |
|---|---|
| Warfarin | The risk or severity of bleeding can be increased when Tunicamycin is combined with Warfarin. |
| Vibrio cholerae CVD Ag | The therapeutic efficacy of Vibrio cholerae CVD 103-HgR strain live antigen can be decreased when used in combination with Tunicamycin. |
| Typhoid vaccine | The therapeutic efficacy of the Typhoid vaccine can be decreased when used in combination with Tunicamycin. |
| Tioclomaro | The risk or severity of bleeding can be increased when Tunicamycin is combined with Tioclomarol |
| Picosulfuric acid | The therapeutic efficacy of Picosulfuric acid can be decreased when used in combination with Tunicamycin. |
| Phenprocoumon | The risk or severity of bleeding can be increased when Tunicamycin is combined with Phenprocoumon |
| Phenindione | The risk or severity of bleeding can be increased when Tunicamycin is combined with Phenindione. |
| Lactulose | The therapeutic efficacy of Lactulose can be decreased when used in combination with Tunicamycin. |
| Fluindione | The risk or severity of bleeding can be increased when Tunicamycin is combined with Fluindione. |
| Ethyl biscoumacetate | The risk or severity of bleeding can be increased when Tunicamycin is combined with Ethyl biscoumacetate. |
| BCG vaccine | The therapeutic efficacy of the BCG vaccine can be decreased when used in combination with Tunicamycin. |
| Dicoumarol | The risk or severity of bleeding can be increased when Tunicamycin is combined with Dicoumarol. |
1.2. Mechanism of action of tunicamycin
Tunicamycin inhibits the reverse reactions in the first step of the biosynthesis of N-linked oligosaccharides in cells. It prevents the formation of UDP-N-acetylglucosamine from N- acetylglucosaminylpyrophosryldolichol. The inhibition is increased by preincubating the enzyme with antibiotics for up to 5min before adding the substrate. The addition of phosphatidylcholine at the concentration up to 20 mM doesn't affect the inhibition regardless of whether it was added during the preincubation or at the same time at the substrate. Tunicamycin binds to the heat-denatured microsomal particles of the aorta as shown by the fact that preincubation of the antibiotic with these particles prevented the inhibition of the N-acetylglucosamin-1-phosphate transferase [10]. A previous study revealed that tunicamycin inhibits the aggressiveness of colon carcinoma cells by the promotion of apoptosis by downregulation of fibronectin, vimentin, and E‑cadherin expression levels. This study used Western blotting, immunohistochemistry, immunofluorescence, and apoptotic assays [11]. A study investigated the potential molecular mechanism of tunicamycin to inhibit the growth and aggressiveness of breast cancer cells. In vitro study revealed that tunicamycin treatment significantly inhibited tumor growth and significantly prolonged the survival of tumor-bearing mice, compared with the PBS-treated group. Mechanism analysis demonstrated that Tm inhibited the protein kinase B (Akt) and nuclear factor-κB (NF-κB) signaling pathways, whilst Akt overexpression significantly cancelled out the Tm inhibition growth and aggressiveness of breast cancer cells, as compared with control cells [12].
1.3. The glycosylation of E1 and E2 glycoproteins of coronavirus
The transmembrane glycoprotein El composed of three domains: A glycosylated domain projects from the envelope. The second domain lies within the membrane, and the third domain interacts with the nucleocapsid inside the viral envelope. El may be an O-linked glycoprotein [13]. Glycosylation of El appears posttranslational event. The glycoprotein E2 forms the large petal-shaped peplomers characteristic of the coronavirus envelope. E2 is a 180 kDa which can be cleaved by trypsin to yield two 90 kDa components. It has been recorded that tunicamycin inhibits the formation of the E2 glycoprotein but does not prevent synthesis or glycosylation of the glycoprotein El through the formation or releasing of the virion from the infected cell. Moreover, the shift from the nonglycosylated 20 kDa form to the glycosylated 23 kDa form doesn't inhibit by tunicamycin [14]. This provides indirect evidence that E1 is not an N-linked glycoprotein but maybe an O-linked glycoprotein. Although El glycoproteins are more negatively charged than those of E2, these glycoproteins differ in carbohydrate composition, electrophoretic patterns of glycopeptides, and response to the antibiotic [15]. These data suggested that E1 is an O-linked glycoprotein while E2 is N-linked glycosylation. In contrast, the effects of tunicamycin on the synthesis and glycosylation of the two SARS-CoV-E glycoproteins have permitted tentative assignment of functions to these proteins [16].
1.4. The glycosylation of coronavirus S protein
Since the 1980s, the N-linked glycosylation S protein of coronavirus was defined for hepatitis virus MHV. S protein in the rough ER was found to acquire high mannose oligosaccharides. It has been found that the Golgi transport blocker (monensin) inhibited the transport of S protein from the trans-Golgi network to the cell surface [17]. Other studies revealed that MHV S protein also modified the N-linked glycosylation of bovine coronavirus BCoV, alpha-coronavirus TGEV, and gamma-coronavirus IBV. The high mannose oligosaccharides of SARS-CoV S protein was trimerized as early as 30 min post-entry into ER, before the acquisition of complex glycans in the Golgi apparatus using pulse-chase experiments coupled with fractionation. Then, the maturation status of S protein can be monitored by its sensitivity to endoglycosidase H. This enzyme hydrolyzes the high mannose glycans [18]. After that, the structure of the N-linked glycosylation of S protein was determined using mass spectrometry. This link was enriched with high mannose, hybrid and complex glycans with or without bisecting N-acetyl-galactosamine (GalNAc) and core fucose. A 12 out of 23 putative glycosylation sites detected of SARS-CoV S protein were glycosylated [19].
The SARS-CoV S protein has two domains S1 and S2. When the S1 domain of Bovine-CoV S protein was cloned and expressed in insect cells, the mature S protein was glycosylated and bound by neutralizing monoclonal antibodies. On the other hand, the infected cells with TGEV in the presence of tunicamycin, the antigenicity of both S and M protein was significantly reduced [20]. This may confirm that tunicamycin is an inhibitor factor of the glycosylation proteins. It has been demonstrated that the inhibition of N-linked glycosylation by tunicamycin or removal of N-linked glycans by PNGase F reduced TGEV-induced IFN- α production. Therefore, the N-linked glycans on SARS-CoV S protein may be a pathogen-associated molecular pattern recognized by host pattern recognition receptors. These receptors activate the downstream antiviral innate immune response. The growth of coronavirus in the presence of inhibitor tunicamycin resulted in the production of spikeless, non-infectious virions which were devoid of S protein [[21], [22]].The normal pathway of protein maturation starts in the ER and the protein modification from 2D to 3D structure happens after the protein transmits from the ER to the Golgi bodies, Fig. 2A. When the presence of tunicamycin, Tm trimmers the mannose in the protein for example SARS-CoV-2 spike protein. This may allow the endoglycosidase H to hydrolyzes mannose of spike protein resulting in the spikeless virion, Fig. 2B.
Fig. 2.
A: Normal pathway of protein modification starts from the lumen of the endoplasmic reticulum (ER) to the Golgi body (GB). B: In the presence of TM, Tunicamycin trimmers the mannose in the spike protein of SARS-CoV. Endoglycosidase H hydrolyzes mannose of S protein.
1.5. The glycosylation of coronavirus M protein
M protein of coronaviruses is the most abundant protein comprising of 220–260 amino acids. It plays a central role in the viral assembly. M protein is a multipass transmembrane protein with a short N-terminal ectodomain, three hydrophobic TM domains, and a large C-terminal endodomain. O-linked glycosylation of the mouse hepatitis virus M protein was first revealed in 1981. It was noted that in the presence of tunicamycin, M protein was still normally produced and glycosylated, resulting in the formation of none infectious virions containing normal amounts of N and M protein, but lacking S completely [[23], [24]].
Distinct from the O-linked glycosylation perceived in the M protein of bovine- coronavirus BCoV, human-coronavirus HCoV-OC43, alpha-coronavirus TGEV, gamma-coronavirus IBV, and turkey enteric coronavirus are all modified by N-linked glycosylation. This link of M protein is sensitive to endoglycosidase H and can be inhibited by tunicamycin. The N-linked glycosylation sites were mapped to N3 and N6 of IBV. M protein of beta coronaviruses in other lineages is also N-linked glycosylated [25]. For example, SARS-CoV M protein contains a single N-glycosylation site at N4. When transiently transfected as a C-terminally FLAG-tagged protein, SARS-CoV M protein was found to obtain high mannose N-glycans and was modified into complex N-glycans in the Golgi. Although the glycosylation of the coronavirus M protein is a strongly conserved feature, this glycosylation is not important for virus assembly or replication [[26], [27]].
1.6. The glycosylation of coronavirus nonstructural proteins nsp3 & nsp4
Some of the luminal domains of NSPs coronaviruses proteins undergo N-linked glycosylation in the ER. For instance, MHV nsp3 is inserted into ER co-transnationally and glycosylated at N1525. Glycosylation of nsp4 was detected in IBV at N48 residue while for the nsp4 of MHV, two glycosylation sites were predicted at N176 and N237 residues. Till now, no study supports that tunicamycin inhibits N-linked glycans either in nsp3 or nsp4 [[28], [29], [30]].
1.7. The glycosylation of coronavirus PTMs proteins
Coronavirus genome encodes various accessory proteins called apart from the structural and nonstructural proteins (PTMs), most of which share no homology to any known proteins. However some of the PTMs accessory proteins are incorporated in mature virions, others have been concerned in the modulation of host immune response and in vivo pathogenesis. One of the PTMs proteins is HE protein which is a part of Beta-coronaviruses S protein. The HE protein of Bovine CoV was also shown to be glycosylated when expressed using the human adenovirus vector. Furthermore, HE protein of MHV was found to be modified by N-linked glycosylation and was inhibited by tunicamycin but not monensin. The importance of N-linked glycosylation of coronavirus HE protein has not been fully characterized [[31], [32]].
The O-linked glycosylation of SARS-CoV 3a protein and M share the same N-exo/C-endo membrane topology. Both proteins contain three TM domains. O-linked glycans of the SARS-CoV protein 3a are resistant to the treatment of PNGase F, and pulse-chase analysis suggested that the oligosaccharides were acquired post-translationally. Protein 3a has been implicated in modulating host immune response [33].
The sgRNA8 of SARS-CoV encodes a single protein 8 ab. A 29-nt deletion in the center split open reading frame ORF8 into two smaller frames, encoding proteins 8a and 8b respectively. The 8 ab protein is co-translationally smuggled into the ER and is N-linked glycosylated at N81. The 8b protein is synthesized in the cytosol and not modified. Both proteins 8b and 8 ab were shown to interact and modified by ubiquitination. The glycosylation at N81 stabilized 8 ab protein and protected it from proteasomal degradation. Protein 8b is unstable and undergoes rapid proteasomal degradation. The ubiquitinated 8b and 8 ab may mediate the rapid degradation of IRF3 and regulate host antiviral innate immunity. The inhibition N-linked glycosylation of SARS-CoV 8 ab protein by tunicamycin is not completely understood [34].
2. Conclusions
The transmembrane structural proteins S, E, M, nsp3, nsp4, and accessory proteins (HE, 3a, and 8 ab) of the most coronavirus family are modified by glycosylation. Although it is assumed that Beta-coronavirus M proteins are O-linked glycosylation, other coronavirus M proteins are N-linked glycosylation, the latter being sensitive to tunicamycin. It has been registered that glycosylation of coronavirus S protein is essentially N-linked. The folding and intracellular trafficking N-linked glycans of the coronavirus S protein also constitute a significant part of the confirmation of the mature protein. Tunicamycin inhibits the formation of the coronavirus E2 glycoprotein but does not prevent synthesis or glycosylation of the glycoprotein El. The antigenicity of TGEV S and M protein was significantly reduced in the presence of tunicamycin. M protein of the most Coronaviridae is sensitive to endoglycosidase H and can be inhibited by tunicamycin. Lack of information about the effect tunicamycin on SARS-CoV N-linked glycans ns3 and ns4. HE and 8 ab proteins of SARS-CoV glycosylation are inhibited by tunicamycin. Although tunicamycin inhibits N-linked glycosylation of coronaviruses glycoproteins, no drug available to inhibit O-linked glycosylation has been identified yet. So far, there are no experimental studies that show the effect of tunicamycin clinically, whether on tissues or viruses, but our report may open the way for researchers to use Tm in clinical trials.
We concluded that tunicamycin inhibits E2, S, M glycoproteins of coronaviruses. Tunicamycin is also diminished glycosylation od PTMs such as HE, and 8 ab of SARS-CoV. Since tunicamycin has long been used as an anti-cancer and can inhibit glycoproteins of coronaviruses, we recommend using this drug to treat the SARS-CoV-2.
Funding
We didn't receive any specific grant from any institution.
Declaration of competing interest
The author has declared that no competing interest exists.
Acknowledgments
The author thanks University of Mosul for documenting this work.
References
- 1.Dawood A. Mutated COVID-19, may foretells mankind in a great risk in the future. N. Mic. N. Inf. 2020 doi: 10.1016/j.nmni.2020.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merlieg J.P., Sebbane R. Inhibition of glycosylation with tunicamycin blocks assembly of newly synthesized acetylcholine receptor subunits in muscle C. J. Biol. Chem. 1982;257(10):2694–2701. [PubMed] [Google Scholar]
- 3.Locker J.K., Griffiths G., Horzinek M.C., Rottier P.J. O-glycosylation of the coronavirus M protein. Differential localization of sialyltransferases in N- and O-linked glycosylation. J. Biol. Chem. 1992;267(20):14094–14101. doi: 10.1016/S0021-9258(19)49683-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapps W., Hogue B.G., Brian D.A. Deduced amino acid sequence and potential O-glycosylation sites for the bovine coronavirus matrix protein. Adv. Exp. Med. Biol. 1987;218:123–129. doi: 10.1007/978-1-4684-1280-2_14. [DOI] [PubMed] [Google Scholar]
- 5.Yamada Y.K., Yabe M., Ohtsuki T., Taguchi F. Unique N-linked glycosylation of murine coronavirus MHV-2 membrane protein at the conserved O-linked glycosylation site. Virus Res. 2000;66(2):149–154. doi: 10.1016/S0168-1702(99)00134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiraishi T., Yoshida T., Nakata S., Hironaka M., Wakanda M., Mizutani Y., Miki T., Sakai T. Tunicamycin enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human prostate cancer cells. Canc. Res. 2005;15–65(14):6364–6370. doi: 10.1158/0008-5472.CAN-05-0312. [DOI] [PubMed] [Google Scholar]
- 7.Takatsuki A., Tamura G. Inhibition of glycoconjugate biosynthesis by tunicamycin. Tunicamycin. Jap. Sci. Soc. Pre. 1982:35–70. [Google Scholar]
- 8.Hiss D., Gabriels G., Folb P. Combination of tunicamycin with anticancer drugs synergistically enhances their toxicity in multidrug-resistant human ovarian cystadenocarcinoma cells. Can Cel Int. 2007;7(5) doi: 10.1186/1475-2867-7-5. https://doi:10.1186/1475-2867-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.HeifetzRoy W. KeenanAlan, Elbein D. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichol-phosphate GlcNAc-1-phosphate transferase. Bio. 1979;18(11):2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- 10.Niemann H., Boschek B., Evans D., Rosing M., Tamura T., Klenk H.D. Post-translational glycosylation of coronavirus glycoprotein E1: inhibition by monensin. EMBO J. 1982;1(12):1499–1504. doi: 10.1002/j.1460-2075.1982.tb01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luytjes W., Sturman L.S., Bredenbeek P.J. Primary structure of the glycoprotein E2 of coronavirus MHV-A59 and identification of the trypsin cleavage site. Virology. 1987;161(2):479–487. doi: 10.1016/0042-6822(87)90142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corse E., Machamer C.E. Infectious bronchitis virus E protein is targeted to the Golgi complex and directs the release of virus-like particles. J. Virol. 2000;74(9):4319–4326. doi: 10.1128/jvi.74.9.4319-4326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tooze S.A., Tooze J., Warren G G. Site of the addition of N-acetyl-galactosamine to the E1 glycoprotein of mouse hepatitis virus-A59. J. Cell Biol. 1988;106(5):1475–1487. doi: 10.1083/jcb.106.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes K.V., Doller E.W., Sturman L.S. Tunicamycin resistant glycosylation of a coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981;11:334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada Y., Liu D.X. Proteolytic activation of the spike protein at a novel RRRR/S motif is implicated in the furin-dependent entry, syncytium formation, and infectivity of coronavirus infectious bronchitis virus in cultured cells. J. Virol. 2009;83(17):8744–8758. doi: 10.1128/JVI.00613-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavallette D., Barbouche R., Yao Y Y., et al. Significant redox insensitivity of the functions of the SARS-CoV spike glycoprotein: comparison with HIV envelope. J. Biol. Chem. 2006;281(14):9200–9204. doi: 10.1074/jbc.M512529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen S., Tan T., TanJ Y.-J. Expression, glycosylation, and modification of the spike (S) glycoprotein of SARS CoV. Methods Mol. Biol. 2007;379:127–135. doi: 10.1007/978-1-59745-393-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng J., Yamada Y., Fung T.S., Huang M., Chia R., Liu D.X. Identification of N-linked glycosylation sites in the spike protein and their functional impact on the replication and infectivity of coronavirus infectious bronchitis virus in cell culture. Virology. 2018;513:65–74. doi: 10.1016/j.virol.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noda S. Fujieda, Seki M., Tanaka N., Sunaga H., Ohtsubo T., Tsuzuki H., Fan G.K., Saito H. Inhibition of N-linked glycosylation by tunicamycin enhances sensitivity to cisplatin in human head-and-neck carcinoma cells. Int. J. Canc. 1999;18;80(2):279–284. doi: 10.1002/(sici)1097-0215(19990118)80:2<279::aid-ijc18>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Charley B., Lavena L., Delmas B. Glycosylation is required for coronavirus TGEV to induce an efficient production of IFN alpha by blood mononuclear cells. Scand. J. Immunol. 1991;33(4):435–440. doi: 10.1111/j.1365-3083.1991.tb01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locker J.K., Rose J.K., Horzinek M.C., Rottier P.J. Membrane assembly of the triple-spanning coronavirus M protein. Individual transmembrane domains show a preferred orientation. J. Biol. Chem. 1992;267(30):21911–21918. doi: 10.1016/S0021-9258(19)36699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Haan C., de Wit M., Kuo L., Montalto-Morrison C., Haagmans B., Weiss S., Masters P., Rottier P. The glycosylation status of the murine hepatitis coronavirus M protein affects the interferometric capacity of the virus in vitro and its ability to replicate in the liver but not the brain. Virology. 2003;312:395–406. doi: 10.1016/S0042-6822(03)00235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogue B.G., Nayak D.P. Expression of the porcine transmissible gastroenteritis coronavirus M protein. Adv. Exp. Med. Biol. 1990;276:121–126. doi: 10.1007/978-1-4684-5823-7_18. [DOI] [PubMed] [Google Scholar]
- 24.Fung T., Liu D. Coronavirus infection, ER stress, apoptosis and Innate Immunity. Front.Microb. 2014;5:296. doi: 10.3389/fmicb.2014.00296. http://doi:10.3389/fmicb.2014.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J. Virol. 1990;64(11):5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White T.C., Yi Z., Hogue B.G. Identification of mouse hepatitis coronavirus A59 nucleocapsid protein phosphorylation sites. Virus Res. 2007;126(1–2):139–148. doi: 10.1016/j.virusres.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oostra M., Hagemeijer M.C., van Gent M., et al. Topology and membrane anchoring of the coronavirus replication complex: not all hydrophobic domains of nsp3 and nsp6 are membrane-spanning. J. Virol. 2008;82(24):12392–12405. doi: 10.1128/JVI.01219-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang C., Sue S.-C., Yu T., et al. Modular organization of SARS coronavirus nucleocapsid protein. J. Biomed. Sci. 2006;13(1):59–72. doi: 10.1007/s11373-005-9035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo P.C., Lau S.K., Laml C.S. Discovery of seven novel mammalian and avian coronaviruses in Deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012;86(7):3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes K.V., Doller E.W., Sturman L.S. Tunicamycin resistant glycosylation of coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981;115(2):334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritchie G., Harvey D.J., Feldmann F., et al. Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology. 2010;399(2):257–269. doi: 10.1016/j.virol.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio. 2013;4(4) doi: 10.1128/mBio.00524-13. e00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong H.H., Fung T.S., Fang S., Huang M., Le M.T., Liu D.X. Accessory proteins 8b and 8ab of severe acute respiratory syndrome coronavirus suppress the interferon signaling pathway by mediating ubiquitin-dependent rapid degradation of interferon regulatory factor 3. Virology. 2017;515:165–175. doi: 10.1016/j.virol.2017.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindner H.A., Lytvyn V., Qi H., Lachance P., Ziomek E., Menard R. Selectivity in ISG15 and ubiquitin recognition by the SARS coronavirus papain-like protease. Arch. Biochem. Biophys. 2007;466(1):8–14. doi: 10.1016/j.abb.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]