Highlights
-
•
Recognizing sleep health as a modifiable risk factor is vital to CV health.
-
•
There is emerging evidence linking CSA with AF.
-
•
CSA may be a marker of abnormal autonomic function and cardiac function.
-
•
Invasive and expensive therapies may be used in moderate to severe CSA.
-
•
PNS is a promising new therapy for CSA to restore normal breathing patterns.
Keywords: Central sleep apnea, Atrial fibrillation, Pathophysiological mechanisms, Therapeutic options, Phrenic nerve stimulation
Abstract
Precipitating factors and chronic diseases associated with atrial fibrillation (AF) are detailed in the literature. Emerging evidence over the last several decades suggests a potential causal relationship between central sleep apnea (CSA) and AF. Mechanisms including apnea-induced hypoxia with intermittent arousal, fluctuating levels of carbon dioxide, enhanced sympathetic/neurohormonal activation and oxidative stress causing inflammation have been implicated as etiologic causes of AF within this subpopulation. CSA affects the efficacy of pharmacologic and catheter-based antiarrhythmic treatments, which is why treating CSA prior to these interventions may lead to lower rates of AF. Subsequently, a reduction in the AF burden with transvenous phrenic nerve stimulation (TPNS) has become a topic of interest. The present review describes the relationship between these conditions, pathophysiologic mechanisms implicating the role of CSA in development of AF, and emerging therapeutic interventions.
1. Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia [1]. It is associated with increased morbidity and mortality due to stroke, thromboembolic events, and heart failure (HF) [1]. By the year 2050, it is projected to affect 15.9 million people, significantly influencing healthcare expenditures [2].
Further contributing to the epidemic, sleep apnea has been associated with higher rates of AF recurrence following cardioversion and ablation [3], [4], [5], [6]. However, much of this data is based on studies analyzing the association of obstructive sleep apnea (OSA) and AF [3], [4], [5], [6]. While the link between central sleep apnea (CSA) and AF is not as well studied in the literature, evidence has been emerging on the topic over the last decade [7], [8]. The association of CSA and AF suggests CSA may be a marker of abnormal autonomic function, respiratory chemoreflex sensitivity, and cardiac function [9]. It has been hypothesized that CSA may have an acute beneficial effect, but the pathophysiology discussed below makes it unlikely that this is beneficial chronically [10]. Recognizing sleep health as a potential modifiable risk factor and coordinating early treatment strategies is fundamental to cardiovascular (CV) health.
This review details the association between CSA and AF, the pathophysiologic mechanisms behind AF occurrence in patients with CSA and provides an update of therapeutic interventions for patients with CSA and AF.
2. Central sleep apnea: Definition and diagnosis
CSA is characterized by diminished or absent respiratory effort during sleep, resulting in repetitive periods of insufficient ventilation and compromised gas exchange that cause individuals to experience oxygen desaturation and daytime somnolence [9], [11], [12]. Compared to OSA where hypoventilation occurs due to physical obstruction in the upper airways, CSA occurs because of a problem in the brain stem where signals are not transmitted effectively to the muscles of respiration, including the diaphragm [3], [13], [14], [15]. In some cases of CSA, individuals may experience recurrent central apneas, lasting greater than 10 s, with a crescendo-decrescendo breathing pattern during sleep [9], [11], [12], [16]. It is important to recognize that the two disorders can co-exist and patients may exhibit characteristics of both central and obstructive sleep apnea also known as complex or mixed sleep apnea [17]. The diagnostic criteria for CSA varies according to the type of CSA: primary (idiopathic) CSA, Cheyne-Stokes respiration (CSR), CSA due to high altitude periodic breathing or hypoventilation-related CSA due to a medical condition, drug or substance. Generally, the diagnosis requires evidence of recurrent central apneas via the gold standard diagnostic test, polysomnography, symptoms or signs of disrupted sleep, and exclusion of alternative diagnoses [18] (Table 1).
Table 1.
Diagnostic criteria for central sleep Apnea.
| Primary Central Sleep Apnea: |
|---|
Criteria A-D must be met
|
|
CSA with Cheyne-Stokes breathing: A + B + (C or D) satisfy the criteria
|
|
CSA due to high altitude periodic breathing: Criteria A-D must be met:
|
|
CSA due to a medication or substance: Criteria A-D must be met:
|
3. Central sleep apnea as a risk factor for atrial fibrillation
Several studies have confirmed the increased incidence of AF in CSA patients (Table 2) [7], [8], [19], [20], [21], [22], [23], [24], [25]. Javaheri et al. were one of the first to observe the increased prevalence of AF in patients with HF and sleep apnea [19]. These findings prompted more in depth studies analyzing the different sleep apnea subtypes including CSA. Although additional studies are needed, CSA was shown to be an independent risk factor for AF in the Sleep Heart Health Study [20]. In an analysis conducted by Tung et al., CSA demonstrated double the risk for AF (OR 2.06, 95% CI 1.23–3.44, p = 0.0057) in an unselected population without clinical signs of sleep-disordered breathing (SDB) [20]. Sin et al. analyzed risk factors for CSA and OSA in men and women with congestive heart failure (CHF) and demonstrated a strong association between CSA and AF in patients with CHF; however, Leung et al. revealed that the strong association between AF and CSA was not confined to patients with CHF, but also occurs in patients with idiopathic CSA [19], [22]. Mehra et al. evaluated nocturnal arrhythmias in older men with SDB and found that while OSA and hypoxia were strongly associated with complex ventricular ectopy (CVE), CSA was most strongly associated with AF [25]. Grimm et al. further confirmed a strong correlation between AF and severe CSA [7].
Table 2.
Summary of studies investigating risk of atrial fibrillation in central sleep apnea.
| Investigator | Population | Methods of diagnosis for CSA | Results |
|---|---|---|---|
| Sin et al. (1999) | N = 450 Patient with CHF |
PSG | Patients with CSA were older and had a higher prevalence of AF compared to those with OSA or no SDB (P < 0.05). AF is an independent risk factor for CSA but not for OSA. (OR 4.13; 95% CI 1.53 to 11.14) |
| Leung et al. (2005) | N = 67 Patients with idiopathic CSA |
PSG | The prevalence of AF in patients with idiopathic CSA (27%) was 16-fold higher than in the OSA group (1.7%) and 8-fold higher than in the no-SDB group (3.3%). (P < 0.001). |
| Oldenburg et al (2007) | N = 700 Stable patients with NYHA class ≥ II and impaired LVEF ≤ 40% |
Cardiorespiratory polygraphy (Embletta, Medcare, Island) | The severity of of SDB was significantly worse in CSA patients than OSA patients based on AHI (30.2/h vs. 18.5/h) The prevalence of AF was higher in CSA (35%) than in OSA (21%) and no SDB (14%). |
| Schulz et al. (2007) | N = 203 | 1). Stardust II system: for polygraphy 2) Pressure cannula3)Plethysmographic belt |
AF and lower PCO2 was more often seen in patients with CSR. |
| Mehra et al. (2009) | N = 2911 Older men |
PSG | Increasing SDB severity defined by RDI quartile in unadjusted analyses noted an increasing percentage of AF. CSA was more strongly associated with AF (OR: 2.69; 95%CI, 1.61–4.47) |
| Grimm et al. (2015) | N = 267 Patient with LVEF ≤ 50% |
PSG | Multivariate analysis revealed a significant association between AF and severe CSA (odds ratio [OR]: 5.21; 95% confidence interval [CI]: 1.67–16.27, P = 0.01) |
| May et al. (2016) | N = 843 Older men without prevalent AF |
PSG | Central sleep apnea (odds ratio [OR], 2.58; 95%CI, 1.18–5.66) and CSR with CSA (OR, 2.27; 95% CI, 1.13–4.56), but not OSA or hypoxemia, predicted Incidence of AF. |
| Tung et al. (2017) | N = 2912 Patients without a history of AF in the SHHS |
PSG | CSA was a predictor of incident AF in all adjusted models and associated with a 2- to 3-fold increased odds of developing AF (central apnea index ≥ 5 odds ratio [OR], 3.00, 1.40–6.44; Cheyne–Stokes respiration OR, 1.83, 0.95–3.54; CSA or Cheyne–Stokes respiration OR, 2.00, 1.16–3.44). |
CHF = congestive heart failure; PSG = polysomnography; CSA = central sleep apnea; OSA = obstructive sleep apnea; AF = atrial fibrillation; SDB = sleep-disordered breathing; OR = odds ratio; CI = confidence interval; NYHA = New York Heart Association; LVEF = left ventricular ejection fraction; AHI: Apnea hypopnea index; CSR = Cheyne-Stokes respiration; RDI = Respiratory disturbance index; SHHS = Sleep Heart Health Study.
4. Pathophysiological mechanisms implicating central sleep apnea in atrial fibrillation occurrence
Previous studies have documented the high prevalence of AF in patients with OSA; however, few studies have delved into the arrhythmogenic effects of CSA. Below we review the pathophysiology of the disease that result in similar fluctuations seen in OSA.
4.1. CSA as a marker for cardiac dysfunction
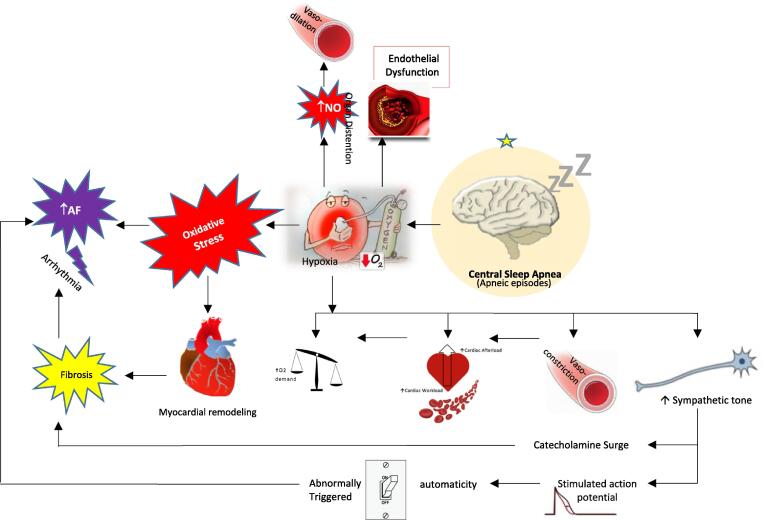
CSA adversely affects CV function by causing tissue hypoxia, sleep arousal, and activation of the sympathetic nervous system. There is emerging evidence implicating that CSA may be a marker of underlying cardiac dysfunction and even an independent pathologic factor, increasing the risk of death [21], [23], [26], [27]. The prevalence of CSA in patients with AF is less well defined in comparison to OSA; however, it has been identified more frequently in patients with HFrEF [28]. Potential mechanisms of AF occurrence in CSA patients are summarized in Fig. 1.
Fig. 1.
Potential pathophysiological mechanisms implicating atrial fibrillation in central sleep apnea patients. The black solid arrows represent the key components related to unstable breathing and central apnea/hypopnea during sleep.
4.2. Changes in blood and sympathovagal imbalance
Intermittent fluctuations in PaCO2 levels and periodic arousals, typically lasting 30–60 s, may be greater in CSA than OSA, predisposing patients to arrhythmogenic structural and electrical remodeling resulting from sympathetic overactivation [21], [29]. There are two forms of CSA: (1) hypercapnic genotype, where PaCO2 levels are elevated and (2) hypocapnic genotype, where PaCO2 levels are low to normal [30]. PaCO2 levels are elevated in CSA secondary to hypoventilation syndrome when there is a reduction in respiratory drive from chemoreceptor insensitivity. Respiratory drive is further suppressed at the onset of sleep with chemosensitivity depression resulting in increased PaCO2 levels and PaCO2 threshold for apnea [30], [31]. PaCO2 levels are low to normal in CSA secondary to medical conditions that increase respiratory drive (i.e. CHF) or idiopathic CSA, which is associated with increased chemosensitivity [32], [33]. Arousal events in patients with HF may precipitate an abrupt increase in respiratory drive that forces PaCO2 to drop and trigger central apneas through a vagally mediated mechanism of hypersensitivity to PaCO2 levels [30], [34]. These changes in CO2 levels and chemoreceptor sensitivity have been linked to autonomic nervous system dysregulation and concomitant electrical remodeling predisposing to AF [35], [36], [37].
Paroxysms of AF occurring after isolated apneic events may result from transient tachycardia-induced LV dysfunction and diastolic dysfunction that stems from a reduced cardiac output and increased pulmonary vascular pressure. These changes can result in ventilatory instability as seen in CSA [38]. Repeated pulmonary vagal stimulation during sympathetic activation increases the risk of AF by shortening the atrial refractory period and decreasing the threshold of fibrillation, predisposing individuals to focal atrial firing [39], [40].
4.3. Cardiac remodeling
Electrical, contractile and structural remodeling play a crucial role in the pathogenesis of AF [41]. Specifically, atrial mechanical and electrical features of CSA may be responsible for the incidence of AF given a significantly lower left atrial reservoir, conduit, and contractile phasic function observed primarily in HFrEF patients with CSA compared to OSA [41]. As mentioned previously, fluctuating levels of CO2 and intermittent arousal are suggested risk factors for electrical and structural remodeling. Hyperventilation-induced hypocapnia has been associated with a shorter effective refractory period, but not conduction time, making more patients prone to fibrillation [41].
CSA patients have been noted to have increased concentrations of plasma and urinary norepinephrine and epinephrine which has been associated with LV dysfunction [29]. Irwin et al. analyzed the effect of sleep disturbances on catecholamine levels in humans and found that partial night sleep deprivation and nocturnal arousal was associated with significant increases in catecholamine levels [42]. Subsequently, enhancing sympathoadrenal activity increases the risk for CV disorders through a combination of transient hemodynamic, vasoconstrictive, and prothrombotic processes that are thought to increase the risk of plaque disruption, thrombosis and LV dysfunction [43], [44].
Abnormal myocardial mechanics evolve during disease progression in the HF continuum from Stage A to D generating a proarrhythmic state [45], [46]. Bitter et al., investigated the prevalence and type of SDB in patients with AF and normal systolic LV function. Patients with AF had a high prevalence of OSA (43%) and CSA/CSR (31%). Furthermore, patients with CSA/CSR had a higher pulmonary artery pressure, higher apnea-hypopnea index (AHI), a greater LA diameter, and a lower capillary blood pCO2 than patients with OSA [47]. As CSR is highly prevalent amid individuals with CHF, treatment should focus on underlying mechanisms by which CHF increases loop gain and promotes unstable breathing [48]. Floras and colleagues have recently speculated a mechanism whereby increased cardiac output triggered by atrial overdrive pacing result in reductions in lung to chemoreceptor circulation time and LV filling pressure. This would improve upper airway patency and stabilize breathing by reducing the loop gain and preventing hyperventilation that initiates CSA [49].
4.4. Heart failure and CSA
CSA is present in 25–40% of patients with chronic heart failure (HF) [27]. Arrhythmias in SDB have a higher prevalence in patients with HF, and are associated with increased morbidity and mortality and deterioration in quality of life [27], [50], [51]. Furthermore, several studies have reported a higher incidence of AF and reduced systolic LV-function in CSA patients compared to OSA patients [23]. CSA leads to marked activation of the sympathetic system, promoting adverse CV effects and further impairment of cardiac function [30]. Whether CSA is a reflection of HF severity or an independent pathological effect in HF is unclear; however, it is clear that CSA is concomitantly found in patients with advanced systolic dysfunction [16], [23], [29]. The mechanism behind LV dysfunction is mentioned above; however right ventricular (RV) function may also be affected in CSA. One of the major factors of RV systolic function is pulmonary artery pressure [52]. Severe pulmonary arterial hypertension is often seen in patients with sleep apnea and nocturnal desaturation which can result in further impairment of RV function [50]. This is important because RV dysfunction is a strong predictor for developing AF in patients with acutely decompensated systolic HF and may lead to worse outcomes when combined with LV dysfunction [53].
5. Oxidative stress, inflammation, and neurohumoral activation
Inflammation in patients with chronic HF is associated with adverse outcomes [54], [55], [56]. High-sensitivity C-reactive protein (hs-CRP) is an established risk factor for coronary artery disease and has been used as a biomarker of inflammation given its effect on vascular endothelium [57]. Currently, there is limited evidence regarding potential inflammatory response to CSA; however, some studies have investigated the relationship [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]. While the exact pathophysiological mechanism is poorly understood, similar to OSA, CSA presents with ventilation arrests resulting in intermittent episodes of hypoxia and insufficient sleep. These mechanisms may link CSA to elevated inflammatory markers by triggering CRP biosynthesis and hepatic CRP release via two main pathways: (1) elevated catecholamine levels caused by sympathetic overactivity and/or (2) elevated serum levels of inflammatory cytokines (principally IL-6) caused by reactive oxygen metabolites due to oxidative stress [60], [62], [63], [64], [65], [66], [67], [68], [69]. Oxidative stress has been involved with structural and electrical remodeling of the heart likely contributing to initiation and perpetuation of AF [3], [70], [71]. Schmalgemeier et al. conducted one of the largest studies to date demonstrating a significant correlation between levels of CRP as a marker of inflammation and LVEF reflecting severity of HF, and between CRP and AF [54], [61], [62], [63].
In addition, there are other mechanisms that may contribute to the negative prognostic impact of CSA, such as a neurohumoral activation [22]. Atrial oxidative stress may drive the sympathetic system to activate the renin-angiotensin aldosterone system (RAAS) which is involved in myocardial fibrosis in hypertensive heart disease, HF, myocardial injury, and cardiomyopathy. Angiotensin II stimulates collagen synthesis, while mitogen-activated protein kinases (MAPK) are essential mediators of Angiotensin II effects on tissue structure. Ultimately, activation of MAPK and excessive collagen deposition may promote arrhythmogenic atrial structural remodeling [3], [72], [73], [74].
6. Therapeutic options
Initial management of CSA should begin with assessing, screening and identifying comorbidities such as hypertension, heart failure and diabetes and target modifiable risk factors such as HF, rate or rhythm control of AF or elimination of medications. As discussed earlier, underlying CSA in HF results in low cardiac output, elevated sympathetic activation, and pulmonary congestion. Sympathetic overactivity in the setting of pulmonary congestion leads to hyperventilation that result in a decline in PaCO2 below apneic threshold. Patients will develop an episodic breathing pattern from the low cardiac output and delay of PaCO2 reaching the respiratory control center. Consequently, several investigators consider CSA to be a compensatory response to advanced HF [10], [75], [76]. Oldenburg and colleagues agree that CSA could initially have short-term beneficial compensatory outcomes; however, long-term effects are detrimental and cause chronic insult to the CV system that result in progressive LV dysfunction [77].
Bitter and colleagues reported 29% of patients with drug refractory and symptomatic AF who had been referred for ablation procedures had CSA or moderate-to severe OSA documented [78]. Guidelines recognize SDB as a clinical risk factor for AF and better management of SBD should allow the effectiveness of antiarrhythmic treatment to be optimized [79]. Currently, the severity scale for CSA is the same as OSA: Normal AHI < 5 per hour; mild AHI 5–14 events per hour; moderate AHI 15–30 events per hour; severe AHI > 30 events per hour [47]. Symptomatic management is used for mild CSA, while more invasive and expensive therapies may be used in moderate to severe CSA (i.e. ASV and PNS). Fig. 2 is our proposed treatment scheme based on current options available for CSA. Furthermore, beneficial effects and outcomes of different therapies can be found in Table 3. It is important to note that low oxygen level is a risk factor for development of AF; therefore, primary treatment for AF in patients with CSA includes reversing the underlying cause.
Fig. 2.
Recommended management of central sleep apnea based on etiology.
Table 3.
Beneficial effects of different therapies for CSA on AF outcomes.
| Therapy | Beneficial Effect | Outcomes |
|---|---|---|
| CPAP | Diminish frequency of periodic arousals seen with apneic episodes resulting in:
|
|
| Supplemental Oxygen |
|
|
| Bi-PAP |
|
|
| ASV |
|
|
| Respiratory Stimulants |
|
|
| Phrenic nerve stimulation |
|
|
CSA = Central sleep apnea; AF = Atrial fibrillation; CPAP = Continuous positive airway pressure; RAAS = Renin angiotensin aldosterone system; OSA = Obstructive sleep apnea; Bi-PAP = Bilevel positive airway pressure; AHI = apnea-hypopnea index; ASV = Adaptive sero-ventilation; PNS = Phrenic Nerve stimulation
6.1. Continuous positive airway pressure (CPAP)
CPAP has traditionally been the first-line therapy for symptomatic patients with hyperventilation-related CSA seen in conditions such as HF. It may diminish the frequency of central apnea by preventing pharyngeal narrowing that can result in greater negative airway pressure triggering hyperpnea, hypocapnia and further episodes of central apnea [80]. CPAP therapy has been associated with improved sympathetic overactivity and down-regulation of RAAS caused by periodic arousals and fluctuating levels of PaCO2. Furthermore, there is evidence that CPAP therapy may reduce plasma norepinephrine levels along with 24-hour urinary catecholamine excretion consistent with a reduction in sympathetic nerve activity [3]. This can ultimately prevent or reverse structural changes associated with SDB that predispose patients to developing AF.
In patients with CSA-CSR, CPAP therapy has indicated improvement in cardiac function; yet its effect on mortality benefit is inconclusive. Currently, The Canadian Prospective Continuous Positive Airway Pressure (CANPAP) trial is the largest study evaluating CSA concomitant with HF, revealing CPAP’s association with improved nocturnal oxygenation, diminished norepinephrine levels, recovery of systolic function, and increased exercise tolerance with a six-minute walk test. However, patients had a mean AHI of 19 events per hour and no morbidity or mortality benefit with the use of CPAP [81], [82]. In a post hoc analysis by Arzt et al., HF patients treated early with CPAP and with significant reduction in AHI to < 15 events per hour, had improvement in LVEF and transplant-free survival [82].
A metaanalysis by Shukla et al. analyzed a large OSA population and determined that patients receiving CPAP had a 40% reduction of AF recurrence after catheter ablation or cardioversion [83]. Similar mechanisms are expected in CSA although data is lacking.
Increasingly, home medical devices such as CPAP machines provide long-term patient monitoring that have detected AF induced CSA in patients requiring a CPAP for previously diagnosed OSA [84]. Although studies are lacking on treatment options, many have identified a relationship between CSA and AF [7], [9], [20], [22], [25], [38], [57], [84]. Risk factor management has emerged as a critical component of AF treatment; however, gaining insight into the role of CSA in patients with AF is the key to development of AF prevention strategies.
6.2. Supplemental oxygen
Patients with hyperventilation-related CSA who experience hypoxemia during sleep may benefit from supplemental oxygen by preventing hyperventilation that may result in central apneas [81].
6.3. Bilevel positive airway pressure (Bi-PAP)
Bi-PAP is an alternative therapy option to consider in patients with hypercapnic CSA associated with hypoventilation and no response to CPAP or oxygen supplementation. It aims to normalize the AHI with a high inspiratory PAP to expiratory PAP difference increasing ventilation through tidal volume augmentation [81]. In addition to reinforcing the spontaneous breaths, a back-up respiratory rate is required when managing CSA because an increased tidal volume can lead to worsened hyperventilation, hypocapnia, and CSA [85], [86].
6.4. Adaptive servo-ventilation (ASV)
6.4.1. Patients with EF > 45%
Adaptive servo ventilation (ASV) is a form of positive airway pressure that remains a therapy option for patients with hyperventilation-related CSA and HF with preserved EF. Servo-controlled inspiratory pressure is delivered over expiratory positive airway pressure based on the detection of apneas during sleep [87]. Several small studies have demonstrated an improvement of AHI by a mean of 30 events per hour resulting in improved symptoms related to disrupted sleep, LVEF, exercise capacity, and arrhythmic events in patients with implanted cardioverter-defibrillator devices [81], [88], [89], [90]. Most recently, a substudy from the CAT-HF trial by Piccini et al. demonstrated proof of concept that ASV combined with optimized medical therapy (OMT) led to a reduction in AF, ventricular tachycardia and ventricular fibrillation events in comparison to OMT alone [91].
6.4.2. Patients with EF ≤ 45%
Further studies are needed to better assess management options in patients with CSA and HFrEF intolerant to CPAP. Despite improvement in LVEF and normalization of AHI in all patients, the use of ASV in the treatment of HFrEF associated CSA that is moderate to severe is discouraged based on an increased risk of cardiac mortality [85], [92].
Cowie et al. investigated the effects of ASV in patients with HFrEF and CSA. Despite effective control in SDB, patients had an increase in all-cause and CV mortality with the use of ASV. The authors postulated that CSA may be a compensatory mechanism in patients with HF; diminishing the compensatory adaptive response with ASV could lead to unfavorable outcomes in HF patients [87]. Interestingly, Kihara et al. wrote a correspondence piece, discussing their contradictory results found in the SAVIOUR-C (Study of the Effects of Adaptive Servo-ventilation Therapy on Cardiac Function and Remodeling in Patients with Chronic Heart Failure trial). Patients who received ASV had significant improvement in quality of life and New York Heart Association (NYHA) functional class III HF to class II HF [90]. The difference may lie in servo-ventillation settings. Kihara et al. maintained airway pressure at or below default levels while Cowie et al. manually increased inspiratory PAP and expiratory PAP to suppress sleep apnea [87], [90]. It is plausible that higher increases in positive airway pressure may reduce cardiac output, induce reflex sympathetic hyperactivity and as seen with Cowie et al. precipitate adverse events.
6.5. Pharmacological therapy
Respiratory stimulants such as acetazolamide or theophylline may benefit patients who did not tolerate or benefit from PAP or supplemental oxygen.
6.5.1. Acetazolamide
Acetazolamide stimulates respiration and decreases frequency of central apneas by provoking mild metabolic acidosis. This medication has been studied in patients with hyperventilation-related CSA and reduced AHI while improving sleep quality and daytime fatigue in short term studies [93], [94].
6.5.2. Theophylline
Theophylline, another respiratory stimulant studied in patients with Cheyne Stokes-CSA breathing, has reduced the number of central apnea and hypopnea episodes along with duration of oxygen desaturation during nocturnal sleep [94]. Patients with other types of hyperventilation-related CSA have not been evaluated using Theophylline. Neither Theophylline nor Acetazolamide have been studied in patients with hypoventilation-related CSA.
6.6. Phrenic nerve stimulation
Phrenic nerve stimulation (PNS) is a new FDA approved therapy for CSA that uses transvenous phrenic nerve stimulation (TPNS) to restore normal breathing patterns during sleep by contracting the diaphragm and stabilizing gas exchange. The remedē system (Respicardia Inc, Minnetonka, MN, USA) is a fully implantable system that results in bilateral diaphragmatic activation during episodes of central apnea. Studies have shown promising results leading to FDA approval in October of 2017 for moderate to severe CSA.
Zang et al. first demonstrated the safety and efficiency of phrenic nerve stimulation to treat CSA-CSR in patients with HF [95]. Oldenberg et al. proved a 55% reduction in AHI after 3 months of therapy in patients with CSA and HF (22.4 ± 13.6 episodes vs 49.5 ± 14.6). Efficacy was maintained at 6 months and 6% of serious adverse events were related to device, implantation procedure, or therapy. No lead dislodgements were reported [96]. Jagielski et al. evaluated long-term outcomes of CSA patients treated with chronic nocturnal PNS. Results were consistent with those observed in the pilot study with sustained improvement in AHI, sleep parameters and quality of life after 12 months [97]. Costanzo et al. found a significant reduction in severity of CSA with improvements in sleep parameters, oxygenation and quality of life by phrenic stimulation [98]. The most recent study published by Fox et al. evaluated sleep metrics and safety in patients from the remedē System Pivotal Trial at 24 months and 36 months. The results confirmed long-term safety and sustained improvement in sleep metrics from PNS in patients with moderate to severe CSA [99].
Currently, PNS is being used in patients with symptomatic CSA who fail or do not tolerate CPAP or other therapies. Adverse events related to the device or procedure are representative of early experience with the implantation technique, technology and tools available [100]. These studies demonstrate that CSA can be treated successfully with phrenic pacing. Augostini et al. demonstrated the safety of PNS and observed an improvement in sleep and quality of life in CSA patients with AF regardless of HF status following 6 months of PNS therapy [101]. By directly stimulating the phrenic nerve, improvements in cardiac symptoms, sympathetic surges, and patient reported outcomes can be achieved with a more natural breathing pattern.
7. Conclusion
CSA is known to cause a number of physiologic stressors, including hypoxia and sympathetic nervous system activation associated with poor CV outcomes. The association between CSA and atrial arrhythmias has been supported by several small studies of patients initially referred for evaluation of treatment of heart disease or sleep disorders. Recognizing sleep health as a potential modifiable CV risk factor, emphasizes the potential usefulness in screening patients with AF for CSA. While there is increasing evidence that CSA exists independent of symptomatic HF, the mechanisms triggering CSA are likely common to both AF and HF. Initial management should focus on optimization of all co-morbidities; nevertheless, effective prevention of central respiratory events stabilizes gas exchange, reduces sympathovagal activation and may even prevent or reverse structural changes associated with central breathing disturbances that predispose to AF. While this has been demonstrated in OSA, there is a clear lack of evidence on the efficacy of treating CSA on AF burden and therefore we anticipate an improvement. This is an area for future research and could be of particular interest in patients with or without HF, who have a high incidence of both CSA and AF.
Disclosure
Dr. Robin Germany currently serves as the Chief Medical Officer of Respicardia.
CRediT authorship contribution statement
Alexandra M. Sanchez: Writing - original draft, Project administration, Methodology. Robin Germany: Conceptualization, Writing - review & editing, Supervision. Matthew R. Lozier: Resources, Visualization, Writing - review & editing. Michael D. Schweitzer: Resources, Writing - review & editing. Semaan Kosseifi: Writing - review & editing, Supervision. Rishi Anand: Conceptualization, Writing - review & editing, Supervision.
Footnotes
Authorship statement: All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100527.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Martin R.R., Bates M.D. Management of atrial fibrillation and concomitant coronary artery disease. Continuing Cardiol. Edu.. 2017;3(2):47–55. [Google Scholar]
- 2.Miyasaka Y., Barnes M.E., Bernard J.G. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Goudis C.A., Ketikoglou D.G. Obstructive sleep and atrial fibrillation: Pathophysiological mechanisms and therapeutic implications. Int. J. Cardiol. 2017;230:293–300. doi: 10.1016/j.ijcard.2016.12.120. [DOI] [PubMed] [Google Scholar]
- 4.Fein A.S., Shvilkin A., Shah D. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J. Am. Coll. Cardiol. 2013;62:300–305. doi: 10.1016/j.jacc.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 5.Hoyer F.F., Lickfett L.M., Mittmann-Braun E. High prevalence of obstructive sleep apnea in patients with resistant paroxysmal atrial fibrillation after pulmonary vein isolation. J. Interv. Card. Electrophysiol. 2010;29:37–41. doi: 10.1007/s10840-010-9502-8. [DOI] [PubMed] [Google Scholar]
- 6.Kanagala R., Murali N.S., Friedman P.A. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 7.Grimm W., Sass J., Sibai E. Severe central sleep apnea is associated with atrial fibrillation in patients with left ventricular systolic dysfunction. Pacing Clin. Electrophysiol. 2015;38(6):706–712. doi: 10.1111/pace.12495. [DOI] [PubMed] [Google Scholar]
- 8.May A.M., Blackwell T., Stone P.H. Central Sleep-disordered breathing predicts incident atrial fibrillation in older men. Am. J. Respir. Crit. Care Med. 2016;193(7):783–791. doi: 10.1164/rccm.201508-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tung P., Levitzky Y.S., Wang R. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J. Am. Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.116.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naughton M.T. Cheyne-Stoke respiration: friend or foe? Thorax. 2012;67:357–360. doi: 10.1136/thoraxjnl-2011-200927. [DOI] [PubMed] [Google Scholar]
- 11.Tung P., Anter E. Atrial fibrillation and sleep apnea: considerations for a dual epidemic. J. Atr. Fibrillation. 2016;8(6):1283. doi: 10.4022/jafib.1283. Published 2016 Apr 30. doi:10.4022/jafib.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drager L.F., McEvoy R.D., Barbe F., Lorenzi-Filho G., Redline S. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. doi: 10.1161/CIRCULATIONAHA.117.029400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haaland C., Grigg-Damberger M. 1132 bradypnea and central sleep apnea due to ventrolateral medullary infarction. SLEEP Web. 2018;41(Suppl1):A419. [Google Scholar]
- 14.Kasai T., Floras J.S., Bradley T.D. Sleep apnea and cardiovascular diseases: a bidirectional relationship. Circulation. 2012;126:1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 15.Somers V.K., White D.P., Amin R. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing Council. J. Am. Coll. Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Bitter T., Westerheide N., Prinz C. Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur. Heart J. 2011;32(1):61–74. doi: 10.1093/eurheartj/ehq327. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Wang Y., Feng J., Chen B.Y., Cao J. Complex sleep apnea syndrome. Patient Prefer Adherence. 2013;7:633–641. doi: 10.2147/PPA.S46626. Published 2013 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014.
- 19.Javaheri S., Parker T.J., Liming J.D. Sleep apnea in 81ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 20.Tung P., Levitzky Y., Wang R. Obstructive and central sleep apnea and risk of atrial fibrillation. Abstract. Am. Heart Assoc. Sci. Sessions. 2014;17143:130. [Google Scholar]
- 21.Sin D.D., Fitzgerald F.S., Parker J.D., Newton G., Floras J.S., Bradley T.D. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am. J. Respir. Crit. Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 22.Leung R.S.T., Huber M.A., Rogge T., Maimon N., Chiu K.-L., Bradley T.D. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;28(12):1543–1546. doi: 10.1093/sleep/28.12.1543. [DOI] [PubMed] [Google Scholar]
- 23.Oldenburg O., Lamp B., Faber L., Teschler H., Horstkotte D., Töpfer V. Sleep disordered breathing in patients with symptomatic heart failure. Eur. J. Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Schulz R, Blau A, B¨orgel J, et al. Working group Kreislauf und Schlaf of the German Sleep Society (DGSM). Sleep apnoea in heart failure. Eur Respir J 2007; 29:1201–1205. [DOI] [PubMed]
- 25.Mehra R., Stone K.L., Varosy P.D. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS Sleep) Study. JAMA Int. Med. 2009;169(12):1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arzt M., Floras J.S., Logan A.G. CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 27.Bradley T.D., Logan A.G., Kimoff R.J. Continuous positive airway pressure for central sleep apnea and heart failure. New Engl. J. Med. 2005;353(19):2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 28.Lavergne F., Morin L., Armitstead J., Benjafield A., Richards G., Woehrle H. Atrial fibrillation and sleep-disordered breathing. J. Thoracic Disease. 2015;7(12) doi: 10.3978/j.issn.2072-1439.2015.12.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naughton M.T., Benard D.C., Rutherford R., Bradley T.D. Effect of continuous positive airway pressure on central sleep apnea and nocturnal PCO2 in heart failure. Am. J. Respir. Crit. Care Med. 1994;150:1598–1604. doi: 10.1164/ajrccm.150.6.7952621. [DOI] [PubMed] [Google Scholar]
- 30.Bradley T.D. Crossing the threshold: implications for central sleep apnea. Am. J. Respir. Crit. Care Med. 2002;165:1203–1204. doi: 10.1164/rccm.2203016. [DOI] [PubMed] [Google Scholar]
- 31.Bradley T.D., Phillipson E.A. Central sleep apnea. Clin. Chest Med. 1992;13:493–505. [PubMed] [Google Scholar]
- 32.Solin P., Roebuck T., Johns D.P., Walters E.H., Naughton M.T. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am. J. Respir. Crit. Care Med. 2000;162:2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 33.Xie A., Rutherford R., Rankin F., Wong B., Bradley T.D. Hypocapnia and increased ventilatory responsiveness in patients with idiopathic central sleep apnea. Am. J. Respir. Crit.Care Med. 1995;152:1950–1955. doi: 10.1164/ajrccm.152.6.8520761. [DOI] [PubMed] [Google Scholar]
- 34.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N. Engl. J. Med. 1999;341:949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 35.Rossi V.A., Stradling J.R., Kohler M. Effects of obstructive sleep apnoea on heart rhythm. Eur. Respir. J. 2013;41:1439–1451. doi: 10.1183/09031936.00128412. [DOI] [PubMed] [Google Scholar]
- 36.Javaheri S., Corbett W.S. Association of low PaCO2 with central sleep apnea and ventricular arrhythmias in ambulatory patients with stable heart failure. Ann. Intern. Med. 1998;128:204–207. doi: 10.7326/0003-4819-128-3-199802010-00006. [DOI] [PubMed] [Google Scholar]
- 37.Orban M., Bruce C.J., Pressman G.S. Dynamic changes of left ventricular performance and left atrial volume induced by the mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am. J. Cardiol. 2008;102:1557–1561. doi: 10.1016/j.amjcard.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rupprecht S., Hutschenreuther J., Brehm B., Figulla H.-R., Witte O.W., Schwab M. Causality in the relationship between central sleep apnea and paroxysmal atrial fibrillation. Sleep Med. 2008;9:462–464. doi: 10.1016/j.sleep.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Tse H.F., Lau C.P. Electrophysiological properties of the fibrillating atrium: implications for therapy. Clin. Exp. Pharmacol. Physiol. 1998;25:293–302. doi: 10.1111/j.1440-1681.1998.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 40.A. Bayé s de Luna, A. Bayé s Geńıs, J. Guindo, et al., Mechanisms favoring and triggering atrial fibrillation [in French], Arch Mal Coeur Vaiss 87 (1 Spec No) (1994) 19–2 [PubMed]
- 41.Floras J.S. Sleep apnea and cardiovascular disease: an enigmatic risk factor. Circ. Res. 2018;122:1741–1764. doi: 10.1161/CIRCRESAHA.118.310783. [DOI] [PubMed] [Google Scholar]
- 42.Thompson Irwin, Gillin Miller, Ziegler, Effects of Sleep and Sleep Deprivation on Catecholamine and Interleukin-2 Levels in Humans: Clinical Implications, J. Clin. Endocrinol. Metabolism 84.6 (1999) 1979–1985. Web. [DOI] [PubMed]
- 43.R.P. Van Diest, W. Appels, Sleep physiological characteristics of exhausted men, Psychosomatic Med. 56.1 (1994) 28–35. Web. [DOI] [PubMed]
- 44.D.L. Feng, G.H. Tofler, Diurnal physiologic processes and circadian variation of acute myocardial infarction, J. Cardiovas. Risk 2.6 (1995) 494–498. Web. [DOI] [PubMed]
- 45.D. Hoit, Left atrial size and function. Role in prognosis, J. Am. Coll. Cardiol. 63 (2014) 493–505. [DOI] [PubMed]
- 46.Christopher Bianco M., Peter Farjo D., Yasir Ghaffar A., Partho Sengupta P. Myocardial Mechanics in Patients with Normal LVEF and Diastolic Dysfunction. J. Am College Cardiol.: Cardiovasc. Imag. 2020;13:258–271. doi: 10.1016/j.jcmg.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 47.Thomas Bitter, Langer, et al. Sleep-disordered breathing in patients with atrial fibrillation and normal systolic left ventricular function, Dtsch Arztebl Int. 106(10) (2009) 164–170. [DOI] [PMC free article] [PubMed]
- 48.Sands S.A., Owens R.L. Congestive heart failure and central sleep apnea. Crit Care Clin. 2015;31(3):473–495. doi: 10.1016/j.ccc.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Floras J.S., Bradley T.D. Atrial overdrive pacing for sleep apnea. A door now closed? Am. J. Respir. 2005;172:1–2. doi: 10.1164/rccm.2503007. [DOI] [PubMed] [Google Scholar]
- 50.Javaheri S., Shukla R., Zeigler H., Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J. Am. Coll. Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 51.Fox H., Bitter T., Horstkotte D., Oldenburg O. Sleep-disordered breathing and arrhythmia in heart failure patients. Sleep Med. Clin. 2017;12(2):229–241. doi: 10.1016/j.jsmc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Ghio S., Gavazzi A., Campana C. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 53.Aziz, Kukin, Javed, et al., Right ventricular dysfunction is a strong predictor of developing atrial fibrillation in acutely decompensated heart failure patients, ACAP-HF Data Analysis, J. Cardiac Failure 16.10 (2010): 827–834. Web. [DOI] [PubMed]
- 54.Rauchhaus M., Doehner W., Francis D.P. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 55.Deswal A., Petersen N.J., Feldman A.M., Young J.B., White B.G., Mann D.L. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 56.Vasan R.S., Sullivan L.M., Roubenoff R. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 57.H. Schmalgemeier, Bitter, Fischbach, Horstkotte, Oldenburg, C-reactive protein is elevated in heart failure patients with central sleep apnea and cheyne-stokes respiration, Respiration 87.2 (2014) 113–120. Web. [DOI] [PubMed]
- 58.E.K. Larkin, C.L. Rosen, H.L. Kirchner, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep. [DOI] [PubMed]
- 59.Koyama T., Watanabe H., Kobukai Y. Beneficial effects of adaptive servo ventilation in patients with chronic heart failure. Circ. J. 2010;74:2118–2124. doi: 10.1253/circj.cj-10-0082. [DOI] [PubMed] [Google Scholar]
- 60.Naughton M.T., Benard D.C., Liu P.P., Rutherford R., Rankin F., Bradley T.D. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am. J. Respir. Crit. Care Med. 1995;152:473–479. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 61.Pye M., Rae A.P., Cobbe S.M. Study of serum Creactive protein concentration in cardiac failure. Br. Heart J. 1990;63(228–230):40. doi: 10.1136/hrt.63.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Y., Lip G.Y., Apostolakis S. Inflammation in atrial fibrillation. J. Am. Coll. Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 63.Anand I.S., Latini R., Florea V. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112:1428–1434. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- 64.Ryan S., Taylor C.T., McNicholas W.T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 65.Somers V.K., Dyken M.E., Clary M.P., Abboud F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conner E.M., Grisham M.B. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 67.Castell J.V., Gmez-Lechn M.J., David M., Fabra R., Trullenque R., Heinrich P.C. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- 68.Mills P.J., Natarajan L., von Knel R., Ancoli-Israel S., Dimsdale J.E. Diurnal variability of Creactive protein in obstructive sleep apnea. Sleep Breath. 2009;13:415–420. doi: 10.1007/s11325-009-0268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartmann G., Tschp M., Fischer R. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12:246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 70.Korantzopoulos P., Galaris K.D., Goudevenos J.A. The role of oxidative stress in the pathogenesis and perpetuation of atrial _brillation. Int. J. Cardiol. 2007:135–143. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 71.Youn J.Y., Zhang J., Zhang Y. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J. Mol. Cell Cardiol. 2013;62:72–79. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danish Li D., Shinagawa L., Pang K., Leung T.K., Cardin S., Wang Z., Nattel S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104(21):2608–2614. doi: 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 73.Reil J.C., Hohl M., Selejan S. Aldosterone promotes atrial fibrillation. Eur. Heart J. 2012;33:2098–2108. doi: 10.1093/eurheartj/ehr266. [DOI] [PubMed] [Google Scholar]
- 74.Tsai C.T., Chiang F.T., Tseng C.D. Increased expression of mineralocorticoid receptor in human atrial fibrillation and a cellular model of atrial fibrillation. J. Am. Coll. Cardiol. 2010;55:758–770. doi: 10.1016/j.jacc.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 75.Naughton Matthew T. phrenic nerve stimulation for central sleep apnea: wiping out apnea or whipping the muscles? JACC Heart Failure. 2015;3(5):370–372. doi: 10.1016/j.jchf.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 76.Naughton M., Bernard D., Tam A., Rutherford R., Bradley T.D. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am. Rev. Respir. Dis. 1993;148:330–338. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 77.Oldenburg Olaf, Coats Andrew. CSA is not beneficial long term in heart failure patients with reduced ejection fraction. Int. J. Cardiol. 2017;227:474–477. doi: 10.1016/j.ijcard.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Harms, Zeng, Smith, Vidruk, Dempsey, Negative Pressure-induced Deformation of the Upper Airway Causes Central Apnea in Awake and Sleeping Dogs, J. Appl. Physiol. (Bethesda, Md. : 1985) 80.5 (1996) 1528–1539. [DOI] [PubMed]
- 79.Monahan K., Brewster J., Wang L. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am. J. Cardiol. 2012;110:369–372. doi: 10.1016/j.amjcard.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bradley T.D., Logan A.G., Kimoff R.J. Continuous positive airway pressure for central sleep apnea and heart failure. N. Engl. J. Med. 2005;353(19):2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 81.Aurora J., Nisha R., Chowdhuri Susmita, Ramar Kannan, Bista Sabin R., Casey Kenneth R., Lamm Carin I., Kristo David A., Mallea Jorge M., Rowley James A., Zak Rochelle S., Tracy Sharon L. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arzt, Floras, Logan, Kimoff, Series, Morrison, Ferguson, Belenkie, Pfeifer, Fleetham, Hanly, Smilovitch, Ryan, Tomlinson, Bradley, Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the canadian continuous positive airway pressure for patients with Central Sleep Apnea and Heart Failure Trial (CANPAP), Circulation 115 (25) (2007) 3173–3180. [DOI] [PubMed]
- 83.Shukla A., Aizer A., Holmes D., Fowler, Park Fowler D.S., Bernstein S., Bernstein N., Chinitz L. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin. Electrophysiol. 2015;1:41–51. doi: 10.1016/j.jacep.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 84.Light M., Orr J.E., Malhotra A., Owens R.L. Continuous positive airway pressure device detects atrial fibrillation induced central sleep apnoea. Lancet. 2018;392(10142):160. doi: 10.1016/S0140-6736(18)31381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bordier Lataste, Hofmann Robert, Bourenane Nocturnal oxygen therapy in patients with chronic heart failure and sleep apnea: a systematic review. Sleep Med. 2016;17:149–157. doi: 10.1016/j.sleep.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 86.Johnson Karin G., Johnson Douglas C. Bilevel positive airway pressure worsens central apneas during sleep. Chest. 2005;128(4):2141–2150. doi: 10.1378/chest.128.4.2141. Web. [DOI] [PubMed] [Google Scholar]
- 87.Cowie, R. Martin, Holger Woehrle, Karl Wegscheider, ER AL. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. New Engl. J. Med. 373 (12) (2015) 1095–1105. [DOI] [PMC free article] [PubMed]
- 88.Bitter Thomas, Gutleben Klaus-Jürgen. treatment of cheyne-stokes respiration reduces arrhythmic events in chronic heart failure. J. Cardiovasc. Electrophysiol. 2013;24(10):1132–1140. doi: 10.1111/jce.12197. [DOI] [PubMed] [Google Scholar]
- 89.Sharma, Bakker, Mcsharry, Desai, Javaheri, and Malhotra, Adaptive servoventilation for treatment of sleep-disordered breathing in heart failure: a systematic review and meta-analysis, Chest 142 (5) (2012) 1211–1221. [DOI] [PMC free article] [PubMed]
- 90.Yasuki Kihara. Adaptive servo-ventilation for central sleep apnea in heart failure. New Engl. J. Med. 2016;374(7) doi: 10.1056/NEJMc1515007. pp. 687–91. Web. [DOI] [PubMed] [Google Scholar]
- 91.Piccini Jonathan P. Adaptive servo-ventilation reduces atrial fibrillation burden in patients with heart failure and sleep apnea. Heart Rhythm. 2019;16(1):91–97. doi: 10.1016/j.hrthm.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 92.Aurora R Nisha, Bista Sabin R, Casey Kenneth R. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses. J. Clin. Sleep Med.: JCSM: Off. Publ. Am. Acad. Sleep Med. 2016;12(5):757–761. doi: 10.5664/jcsm.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Javaheri Shahrokh. Acetazolamide Improves Central Sleep Apnea in Heart Failure: A Double-blind, Prospective Study. Am. J. Respir. Crit. Care Med. 2006;173(2):234–237. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 94.Hu K., Li Q., Yang J., Hu S., Chen X. The Effect of Theophylline on Sleep-disordered Breathing in Patients with Stable Chronic Congestive Heart Failure. Chin. Med. J. 2003;116(11):1711–1716. [PubMed] [Google Scholar]
- 95.Zhang Xi-Long, Ding Ning, Wang, et al. Transvenous Phrenic Nerve Stimulation in Patients With Cheyne-Stokes Respiration and Congestive Heart Failure: A Safety and Proof-of-Concept Study: A Safety and Proof-of-Concept Study, Chest 142 (4) (2012) 927–934. [DOI] [PubMed]
- 96.Oldenburg O., Augostini R., Krueger S. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail. 2015;3(5):360–369. doi: 10.1016/j.jchf.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 97.Jagielski D., Ponikowski P., Augostini R., Kolodziej A., Khayat R., Abraham W.T. Transvenous stimulation of the phrenic nerve for the treatment of central sleep apnea: 12 months experience with Remedē System. Eur. J. Heart Fail. 2016;18:1386–1393. doi: 10.1002/ejhf.593. [DOI] [PubMed] [Google Scholar]
- 98.Costanzo M.R., Ponikowski P., Javaheri S. Transvenous neurostimulation for central sleep apnoea: a randomized controlled trial. Lancet. 2016;388:974–982. doi: 10.1016/S0140-6736(16)30961-8. [DOI] [PubMed] [Google Scholar]
- 99.H. Fox, O. Oldenburg, S. Javaheri, et al., Remede System Pivotal Trial Study Group, Long-term Efficacy and Safety of Phrenic Nerve Stimulation for the Treatment of Central Sleep Apnea Outcomes of Phrenic Nerve Stimulation for Central Sleep Apnea, Sleep. doi.org/10.1093/sleep/zsz158. [DOI] [PMC free article] [PubMed]
- 100.Alonso C., Leclercq C., d’Allones F.R. Six year experience of transvenous left ventricular lead implantation for permanent biventricular pacing in patients with advanced heart failure: technical aspects. Heart. 2001;86:405–410. doi: 10.1136/heart.86.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Augostini R., Stellbrink C., Jagielski D., Beyerbach D., Gupta S., Gutleben K., Hayes J., Germany R., Costanzo M.R. Phrenic Nerve Stimulation is Safe and Improves Sleep and QOL in Patients with Central Sleep Apnea and Atrial Fibrillation Regardless of Heart Failure Status. J. Cardiac Fail. 2019;25(8):S83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.