The continuously increasing prevalence of excessive bodyweight in the world’s population, combined with a sustained rise in life expectancy, has led nonalcoholic fatty liver disease (NAFLD) pathology to become the number one burden of liver disease. Indeed, an astonishing 20%–30% of the global adult population is affected by NAFLD, which frequently progresses to nonalcoholic steatohepatitis (NASH), in which excessive fat accumulation is accompanied by inflammation, hepatocyte ballooning, and even fibrosis.1 Because of the absence of approved anti-NAFLD pharmacotherapeutics, the clinical management of patients with NAFLD/NASH mainly relies on promoting significant lifestyle changes and/or bariatric surgery. However, these interventions are often insufficient for the treatment of the more advanced stages of liver fibrosis and for preventing further important complications. Therefore, the need for novel pharmaceuticals with metabolism-modifying, anti-inflammatory, or antifibrotic mechanisms is imperative. In the search for such novel drugs, various fibrosis-related mechanisms are proposed as potential beneficial targets, including cell stress/death, inflammation, the gut-liver axis, myofibroblastic activation, and metabolic pathways.2
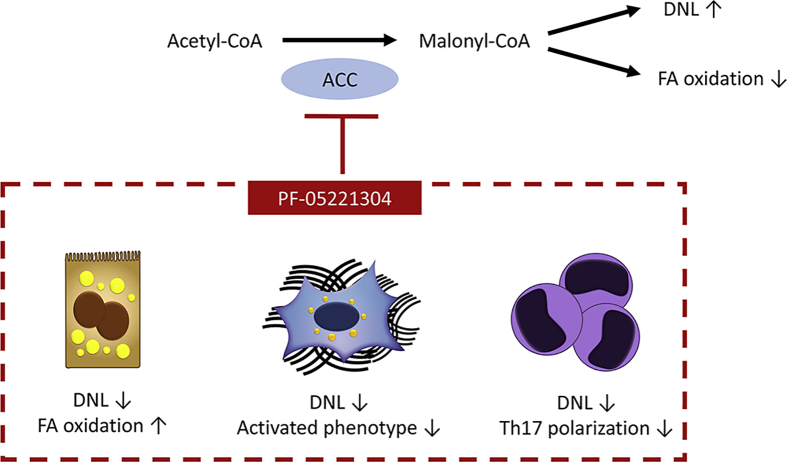
The metabolic perturbations that accompany the initiation and progression of NAFLD/NASH represent an interesting therapeutic target. One such promising strategy is the inhibition of acetyl-CoA carboxylase (ACC), a key enzyme in the regulation of lipid metabolism. ACC catalyzes the synthesis of malonyl-CoA from acetyl-CoA and carbonate, which is an important substrate for de novo lipogenesis, and which simultaneously inhibits fatty acid oxidation (Figure 1).3 Exploratory studies inhibiting ACC demonstrated its potential to reduce liver steatosis.4,5 However, whether inhibition of ACC has additional effects on key drivers of NASH (ie, fibrosis and inflammation), is unknown.
Figure 1.
PF-05221304, a dual acetyl-CoA carboxylase 1/2 (ACC1/2) inhibitor, is a promising therapeutic tool for NASH. ACC catalyzes acetyl-CoA and carbonate into malonyl-CoA, which promotes steatosis through stimulation of de novo lipogenesis (DNL) and inhibition of fatty acid (FA) oxidation. The inhibition of ACC through action of PF-05221304 was found to not only diminish steatosis, but also to hamper inflammation and stellate cell activation, promoting NASH amelioration in rodent models.
In this issue of Cellular and Molecular Gastroenterology and Hepatology, Ross et al6 explore the effects of PF-05221304, a dual ACC1/2-inhibitor, in rodent models of NASH, and in vitro studies using primary human liver cells. In line with previous observations made through use of other ACC inhibitors,4,5 PF-05221304 diminished de novo lipogenesis, both in isolated primary human cells and in Western diet–fed rats.6 However, in contrast with previous studies that had reported an elevation in circulating triglyceride levels,4,5 no significant changes in body weight, fasting glucose, triglyceride, and cholesterol levels were observed. Interestingly, although dose-dependent reductions in hepatic triglyceride levels were identified in the PF-05221304-treated Western diet model, no effects on the extent of hepatic steatosis were observed when administrating the drug to diethylnitrosamine (DEN) and choline deficient and high-fat diet (CDAHFD) rat models.6
Because of the known association of increasing levels of infiltrating inflammatory cells during the NASH pathology, and the recent association of an elevated ratio of Th17-inflammatory over Treg-cells to NASH progression,7 Ross et al6 decided to also investigate the effect of PF-05221304 on the inflammatory outcome. Interestingly, they found that the drug suppresses the polarization of T cells toward the proinflammatory Th17 T cells, but not the anti-inflammatory Treg cells. Additionally, the administration of PF-05221304 to the DEN and CDAHFD rodent models led to an overall reduction in hepatic inflammation.6
Previous reports concerning the effects of ACC inhibitors on fibrogenesis were inconsistent. Although most studies did not describe fibrosis-alternating effects, or even observed negative results,5 a recent study reported direct inhibition of hepatic stellate cell (HSC)-activation and fibrosis outcome in a DEN and CDAHFD rat model using the ACC inhibitor firsocostat.8 In line with these observations, Ross et al6 also find, using the same rodent models of liver disease, reduced expression of the fibrogenic markers αSMA and Col1a1 following treatment with PF-05221304.6 Both studies suggest such antifibrotic effects to be caused by direct (inhibition of de novo lipogenesis in the HSC) and indirect (less lipotoxicity) mechanisms.
An aspect that is not addressed by the current study, but worth further exploration, is to test whether ACC1/2 inhibition impacts hepatocarcinogenesis. ACC and de novo lipogenesis have been linked to the development of hepatocellular carcinoma (HCC), and pharmacologic ACC inhibition reduced HCC progression in rat models.9 Such a tumor-suppressing activity of ACC inhibitors could provide particular value for patients with NASH that are at risk for HCC.
The perspective of blocking NAFLD pathogenesis via ACC inhibition at different levels (hepatocyte defattening, diminishing inflammation, stellate cell deactivation, and potentially also limiting tumorigenesis) is intriguing. Although the current work highlights the prospects of PF-05221304 in the battle against NAFLD/NASH, the preclinical data do not allow immediate translation into clinical scenarios. Clinical data using the ACC1/2 inhibitor firsocostat raised important safety concerns. In a randomized controlled phase 2 trial with 126 participants, asymptomatic grade 3 or 4 triglyceride elevations (>500 mg/dL) were observed in 16 patients receiving firsocostat 20 mg (n = 7) or 5 mg (n = 9).5 Same as for the PF-05221304 compound,6 this side effect was not seen in the rodent model.8 In addition, increasing fatty oxidation by ACC2 inhibition may result in increased accumulation of mitochondrial acetyl-CoA and activation of pyruvate carboxylase, which could promote increased hepatic gluconeogenesis.10 Although ACC-induced hypertriglyceridemia could be mitigated by fish oil or fibrates, the immediate consequences for glucose metabolism and the long-term cardiovascular effects require proper investigations.
The study by Ross et al6 provides encouraging effects of PF-05221304 administration, through diminishment of steatosis, hampering of inflammatory outcome, and reduction of HSC-activation in rodent models (Figure 1). This work supports the future clinical exploration of PF-05221304, which is already suggested to be safe, well-tolerated, and with preliminary beneficial effects for patients with NAFLD in clinical trials.11,12 Nonetheless, safety signals from competing ACC inhibitors in clinical development favor the concept of integrating ACC inhibition in a combinatorial therapeutic approach to mitigate risks and preserve benefits in the treatment of NASH.
Footnotes
Conflicts of interest Frank Tacke’s laboratory has received research funding from Allergan, Bristol-Myers Squibb, Galapagos, and Inventiva; and he is a consultant for Allergan, Bayer, Boehringer Ingelheim, Galapagos, Galmed, Intercept, Inventiva, Novartis, and Pfizer. Joeri Lambrecht discloses no conflicts.
Funding Joeri Lambrecht is supported by the Federal Ministry of Education and Research (BMBF, ImmuneAvatar). Frank Tacke is supported by the German Research Foundation (DFG SFB/TRR57, CRC1382, Ta434/3-1, Ta434/5-1).
References
- 1.Estes C., Anstee Q.M., Arias-Loste M.T., Bantel H., Bellentani S., Caballeria J., Colombo M., Craxi A., Crespo J., Day C.P., Eguchi Y., Geier A., Kondili L.A., Kroy D.C., Lazarus J.V., Loomba R., Manns M.P., Marchesini G., Nakajima A., Negro F., Petta S., Ratziu V., Romero-Gomez M., Sanyal A., Schattenberg J.M., Tacke F., Tanaka J., Trautwein C., Wei L., Zeuzem S., Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 2.Lambrecht J., van Grunsven L.A., Tacke F. Current and emerging pharmacotherapeutic interventions for the treatment of liver fibrosis. Expert Opin Pharmacother. 2020:1–13. doi: 10.1080/14656566.2020.1774553. [DOI] [PubMed] [Google Scholar]
- 3.Harada N., Oda Z., Hara Y., Fujinami K., Okawa M., Ohbuchi K., Yonemoto M., Ikeda Y., Ohwaki K., Aragane K., Tamai Y., Kusunoki J. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol. 2007;27:1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X., Burgess S.C., Li C., Ruddy M., Chakravarthy M., Previs S., Milstein S., Fitzgerald K., Kelley D.E., Horton J.D. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26:576. doi: 10.1016/j.cmet.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R., Kayali Z., Noureddin M., Ruane P., Lawitz E.J., Bennett M., Wang L., Harting E., Tarrant J.M., McColgan B.J., Chung C., Ray A.S., Subramanian G.M., Myers R.P., Middleton M.S., Lai M., Charlton M., Harrison S.A. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155:1463–1473. doi: 10.1053/j.gastro.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross T.T., Crowley C., Kelly K.L., Rinaldi A., Beebe D.A., Lech M.P., Martinez R.V., Carvajal-Gonzalez S., Boucher M., Hirenallur-Shanthappa D., Morin J., Opsahl A.C., Vargas S.R., Bence K.K., Pfefferkorn J.A., Esler W.P. Acetyl-CoA carboxylase inhibition improves multiple dimensions of NASH pathogenesis in model systems. Cell Mol Gastroenterol Hepatol. 2020;10:829–851. doi: 10.1016/j.jcmgh.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C.N., Bahre H., Tschirner S.K., Gorinski N., Gohmert M., Mayer C.T., Huehn J., Ponimaskin E., Abraham W.R., Muller R., Lochner M., Sparwasser T. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 8.Bates J., Vijayakumar A., Ghoshal S., Marchand B., Yi S., Kornyeyev D., Zagorska A., Hollenback D., Walker K., Liu K., Pendem S., Newstrom D., Brockett R., Mikaelian I., Kusam S., Ramirez R., Lopez D., Li L., Fuchs B.C., Breckenridge D.G. Acetyl-CoA carboxylase inhibition disrupts metabolic reprogramming during hepatic stellate cell activation. J Hepatol. 2020 May 4;S0168-8278(20)30281-6 doi: 10.1016/j.jhep.2020.04.037. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Lally J.S.V., Ghoshal S., DePeralta D.K., Moaven O., Wei L., Masia R., Erstad D.J., Fujiwara N., Leong V., Houde V.P., Anagnostopoulos A.E., Wang A., Broadfield L.A., Ford R.J., Foster R.A., Bates J., Sun H., Wang T., Liu H., Ray A.S., Saha A.K., Greenwood J., Bhat S., Harriman G., Miao W., Rocnik J.L., Westlin W.F., Muti P., Tsakiridis T., Harwood H.J., Jr., Kapeller R., Hoshida Y., Tanabe K.K., Steinberg G.R., Fuchs B.C. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019;29:174–182. doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkhouri N., Lawitz E., Noureddin M., DeFronzo R., Shulman G.I. GS-0976 (Firsocostat): an investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH) Expert Opin Investig Drugs. 2020;29:135–141. doi: 10.1080/13543784.2020.1668374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin N., Carvajal-Gonzalez S., Aggarwal N., Tuthill T., Inglot M., Bergman A., Esler W. Abstract 31: PF-05221304 (PF’1304), a liver-targeted acetyl-CoA carboxylase inhibitor (ACCI), in adults with nonalcoholic fatty liver disease (NAFLD) demonstrates robust reductions in liver fat and ALT: phase 2a, dose-ranging study. Hepatology. 2019;70:21. [Google Scholar]
- 12.Bergman A., Carvajal-Gonzalez S., Tarabar S., Saxena A.R., Esler W.P., Amin N.B. Safety, tolerability, pharmacokinetics, and pharmacodynamics of a liver-targeting acetyl-CoA carboxylase inhibitor (PF-05221304): a three-part randomized phase 1 study. Clin Pharmacol Drug Dev. 2020;9:514–526. doi: 10.1002/cpdd.782. [DOI] [PMC free article] [PubMed] [Google Scholar]