Key Points
Question
Is the combination of a programmed cell death ligand 1 (PD-L1) inhibitor with etoposide-platinum chemotherapy associated with better tumor response among patients with extensive-stage small cell lung cancer compared with other first-line treatments?
Findings
In this systematic review and network meta-analysis of 3 phase 2 and 11 phase 3 randomized clinical trials, which included 4838 patients, the combination of a PD-L1 inhibitor (durvalumab or atezolizumab) with etoposide-platinum regimen was associated with better tumor response and safety than other regimens.
Meaning
The findings of this study suggest that the PD-L1 inhibitor plus etoposide-platinum regimen may be an optimal first-line treatment for patients with extensive-stage small cell lung cancer.
This systematic review and network meta-analysis clarifies which first-line treatment is associated with the best tumor response among patients with extensive-stage small cell lung cancer (SCLC).
Abstract
Importance
Combinations of chemotherapy with immunotherapy or bevacizumab in first-line treatments of extensive-stage small cell lung cancer (ES-SCLC) have been evaluated in various clinical trials. However, it remains unclear what the optimal combination regimen is.
Objective
To clarify which first-line combination regimen is associated with the best tumor response among patients with ES-SCLC.
Data Sources
Electronic databases (PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science) were systematically searched to extract eligible literature from database inception to December 2019.
Study Selection
Head-to-head randomized clinical trials on first-line treatments for patients with ES-SCLC were included with outcomes and toxic effects reported, including objective response rate (ORR, involving complete response and partial response), disease control rate (DCR, involving complete response, partial response, and stable disease), progression-free survival (PFS), overall survival (OS), and treatment related adverse events (TRAEs) of grades 3 to 5. Of 199 eligible articles, 14 were included.
Data Extraction and Synthesis
Data were independently extracted and collected by 2 reviewers based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. Data were pooled using a random-effects model.
Main Outcomes and Measures
Main outcomes were OS, PFS, DCR, ORR, and TRAEs of grades 3 to 5.
Results
A total of 3 phase 2 and 11 phase 3 randomized clinical trials involving 4838 patients were included. Programmed cell death ligand 1 (PD-L1) inhibitor (durvalumab and atezolizumab) plus etoposide-based chemotherapy, compared with etoposide-based chemotherapy alone, showed the most favorable OS (hazard ratio, 1.40; 95% CI, 1.09-1.80) and the best DCR (odds ratio [OR], 0.42; 95% CI, 0.21-0.81). Bevacizumab plus etoposide-based chemotherapy provided the best PFS compared with etoposide-based chemotherapy alone (hazard ratio, 1.54; 95% CI, 1.09-2.27), although this was not translated into OS benefit. The addition of PD-L1 inhibitors to etoposide-platinum chemotherapy caused no more toxic effects in general (compared with etoposide-based chemotherapy alone: OR, 1.14; 95% CI, 0.36-2.31), while bevacizumab plus etoposide-platinum regimen induced the most TRAEs grades 3 to 5 among all first-line treatments (eg, compared with irinotecan-platinum regimen: OR, 4.24; 95% CI, 1.26-14.57). Based on the surface under the cumulative ranking curve value, PD-L1 inhibitor plus etoposide-platinum had the highest probability of being ranked first for OS (0.87) and DCR (0.97).
Conclusions and Relevance
The findings of this systematic review and network meta-analysis suggest that the combination of a PD-L1 inhibitor (durvalumab and atezolizumab) and etoposide-based chemotherapy may be an optimal first-line treatment option for patients with ES-SCLC patients.
Introduction
Small cell lung cancer (SCLC), which is characterized by rapid growth and early development of metastasis, is an extremely aggressive type of lung cancer.1,2,3 Because most cases have metastasized to widespread sites at the time of diagnosis, 70% of patients present with extensive-stage SCLC (ES-SCLC).4 For several decades, the standard first-line chemotherapy for ES-SCLC has been etoposide combined with platinum (cisplatin or carboplatin).5,6,7 Despite its high response rate, nearly all patients experienced quick disease relapse, with a median progression-free survival (PFS) of as long as 3 months, and poor survival outcomes, with a median overall survival (OS) of approximately 10 months.8,9 Although some trials in Japan demonstrated that an irinotecan-based regimen as a first-line treatment for ES-SCLC had better PFS and OS, its OS benefit remained poor.10 Thus, improved first-line treatments are urgently needed.
Scholars have investigated the outcomes of a biologically synergistic combination of etoposide-based chemotherapy with bevacizumab, a humanized monoclonal anti–vascular endothelial growth factor (VEGF) antibody, as a first-line option to prolong survival. They observed that bevacizumab plus etoposide-based chemotherapy as the first-line treatment for patients with ES-SCLC resulted in positive signals, such as increased PFS, but not in OS.11,12 Additionally, immunotherapies targeting either programmed cell death ligand 1 (PD-L1) or cytotoxic T-cell lymphocyte antigen 4 (CTLA-4) have also been used as first-line treatments for ES-SCLC in recent years, including durvalumab and atezolizumab, 2 human monoclonal antibodies that inhibit PD-L1–PD-1 signaling to enhance the T-cell immunity, and ipilimumab, a fully humanized immunoglobin G1 monoclonal antibody that blocks CTLA-4 binding to its ligands (CD80 and CD86).13,14,15 Previous studies indicated that first-line immune checkpoint inhibitor (ICI) plus etoposide-platinum chemotherapy might improve survival among patients with ES-SCLC.16,17,18,19 Furthermore, a PD-L1 inhibitor with chemotherapy has been included in National Comprehensive Cancer Network guideline as a first-line treatment option for patients with ES-SCLC. However, with advancements in first-line treatments for patients with ES-SCLC, the outcomes and relative safety profiles of these treatment regimens have not been fully compared.
The existing randomized clinical trials only provided a model to directly compare the outcomes and safety of etoposide-platinum chemotherapy with etoposide-platinum chemotherapy plus immunotherapies and monoclonal antibodies. Furthermore, previous meta-analyses have only partly compared different chemotherapy regimens for patients with ES-SCLC without including the recent randomized clinical trials that use the recommended addition of immunotherapy to chemotherapy as the first-line treatment of ES-SCLC.20,21,22 Therefore, we aimed to investigate the outcomes and safety profiles of chemotherapy-only regimens as well as chemotherapy plus either PD-L1 antibody, CTLA-4 antibody, or VEGF antibody as the first-line treatment23 for patients with ES-SCLC; to directly and indirectly compare the advantages of these treatments using network meta-analyses of randomized clinical trials; to identify the optimal treatment regimen in clinical practice; and to provide comprehensive evidence to help clinicians and patients select treatment.
Methods
Data Sources and Searches
Based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline24,25 (eTable 1 in the Supplement), we systematically searched the PubMed, Embase, and Web of Science databases as well as the Cochrane Central Register of Controlled Trials to find relevant studies published until December 2019. The main search terms and their combinations included extensive stage, SCLC, and randomized controlled trial. The detailed search strategy is presented in eTable 1 in the Supplement. Furthermore, we also reviewed relevant abstracts and presentations presented in major conference proceedings including the American Society of Clinical Oncology, the World Conference on Lung Cancer, and the European Society for Medical Oncology from 2010 to 2019. The manual search of reference lists of all available reviews was additionally performed to confirm the final selection. Three reviewers (T.Z., Z.Z., and Y.Z.) independently carried out the literature retrieval.
Study Selection
Studies were included if they (1) were randomized clinical head-to-head phase 2 or 3 trials; (2) enrolled patients with either histologically or cytologically confirmed ES-SCLC; (3) compared 2 or more first-line treatments for patients with ES-SCLC, including immunotherapy plus chemotherapy and an etoposide-platinum chemotherapy regimen; and (4) reported detailed outcomes and toxic effects including PFS, OS, objective response rate (ORR), disease control rate (DCR), and treatment-related adverse events (TRAEs) of grade 3 or higher. Studies failing to meet these criteria were excluded.
OS was defined as the time from randomization to death from any cause. PFS was defined as the time from randomization to the date of objective disease progression or death from any cause in the absence of progression. ORR was defined as the proportion of patients with a complete or partial response. DCR was defined as the proportion of patents with a complete response, partial response, or stable disease.
Data Extraction and Quality Assessment
The data on study identification, first author, year of publication, study phase, therapeutic regimen, number of patients, and clinical outcomes were retrieved and summarized separately by 2 authors (F.L. and T.L.) following Cochrane Collaboration guidelines. The preferred survival outcomes included PFS and OS assessed by independent review committees rather than the investigators to reduce potential assessment bias. TRAEs commonly reported in most of the included studies were retrieved. In the cases that these data were not available, all adverse events were used. The original tests, supplementary materials, data in conference proceedings, and information at ClinicalTrials.gov were evaluated to obtain the most extensive and updated data.
Two other investigators (T.Z. and Z.Z.) assessed the risk of bias of the included studies by using Cochrane risk of bias tool.26 All disagreements were resolved in discussion, and consensus was reached.
Statistical Analysis
The hazard ratio (HR) for survival outcomes (OS and PFS), the odds ratio (OR) for binary outcomes (ORR and TRAEs grade 3 or higher), and their 95% CIs were used to measure outcomes and safety. For a specific comparison, an agent with an HR less than 1 for OS or PFS or an OR greater than 1 for ORR was deemed preferable, while an OR greater than 1 for TRAEs grade 3 or higher indicated greater likelihood of toxic effects.
First, we performed Bayesian network meta-analysis with R version 3.5.1 (R Project for Statistical Computing; gemtc package)27 using a random-effects hierarchical model by assuming that different comparisons for each survival outcome (ie, PFS, OS) shared a common heterogeneity parameter.28,29 The 95% CIs of either the pooled HR excluding 1 or a 2-sided P < .05 was considered statistically significant. Second, we established a random-effects network within a Bayesian framework using Markov chain Monte Carlo methods in ADDIS version 1.15 (Drugis).30 Third, we established a network of binary clinical outcomes (ie, ORR, DCR, and TRAEs grade ≥3) within studies and specified the associations among ORs across studies to make comparisons of different treatments in immunotherapy regimens. Moreover, for each outcome, we estimated the probability of every agent at each possible rank and presented the distribution of probabilities of each therapeutic regimen ranked at each of the possible positions in rankograms. To be more intuitive, the surface under the cumulative ranking (SUCRA) curve was used to rank the safety and clinical outcomes of various immunotherapy regimens.31 The results were displayed using the rank-heat plot32 to provide a simple numerical summary for the relative ranking of the regimens. The SUCRA value would be 1 if the agent was certain to be the best and 0 if it was certain to be the worst.
We considered the distribution that might affect outcomes to be similar in all of the pairwise comparisons according to the transitivity assumption. Inconsistency standard deviation and random effects standard deviation were used to evaluate the inconsistency within the multiple treatment comparison. A 95% CI that includes 1 indicated a low risk of inconsistency. A P < .05 was considered significant inconsistency.
Results
Systematic Review and Characteristics of All Trials
We identified 199 eligible articles according to the primary search strategy and finally included 14 trials, with 4838 patients, of which 3 were phase 2 studies11,33,34 and 11 were phase 3 studies.10,12,16,17,18,35,36,37,38,39,40 eFigure 1 in the Supplement summarizes the flowchart of study selection. These patients received 8 different treatments, including etoposide-platinum chemotherapy (etoposide plus cisplatin or carboplatin), irinotecan-platinum chemotherapy (irinotecan plus cisplatin or carboplatin), an ICI with conventional therapy (durvalumab, atezolizumab, or ipilimumab plus etoposide-platinum chemotherapy), and bevacizumab with conventional therapy. Table 1 presents the characteristics of all trials.10,11,12,16,17,18,33,34,35,36,37,38,39,40
Table 1. Baseline Characteristics of Studies Included in the Network Meta-analysis of Patients With ES-SCLC.
| Source | Phase | Treatment | Participants, No. | ORR, No./total No. (%) | DCR | PFS, median, m | HR (95% CI) | P value | OS, median, m | HR (95% CI) | P value | TRAE 3-5, No./total No. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paz-Ares et al,16 2019 | 3 | Durvalumab plus etoposide-platinum chemotherapy | 268 | 182/268 (67.9) | NR | 5.10 | 0.78 (0.65-0.94) | NR | 13.00 | 0.73 (0.59-0.91) | .005 | 163/265 (61.5) |
| Etoposide-platinum chemotherapy | 269 | 155/269 (57.6) | NR | 5.40 | 10.30 | 166/266 (62.4) | ||||||
| Kim et al,37 2019 | 3 | Irinotecan plus carboplatin | 173 | 108/173 (62.4) | 132/173 (76.3) | 6.50 | 0.846 (0.709-1.008) | .12 | 10.90 | 0.879 (0.73-1.05) | .12 | NR |
| Etoposide plus carboplatin | 189 | 91/189 (48.1) | 142/189 (75.1) | 5.80 | 10.30 | NR | ||||||
| Horn et al,17 2018 | 3 | Atezolizumab plus etoposide plus carboplatin | 201 | 121/201 (60.2) | 163/201 (81.1) | 5.20 | 0.77 (0.62-0.96) | .02 | 12.30 | 0.70 (0.54-0.91) | .007 | 115/198 (58.1) |
| Etoposide plus carboplatin | 202 | 130/202 (64.4) | 173/202 (85.6) | 4.30 | 10.30 | 113/196 (57.7) | ||||||
| Tiseo et al,12 2017 | 3 | Etoposide-platinum chemotherapy plus bevacizumab | 103 | 57/103 (55.3) | 73/103 (70.9) | 5.70 | 0.72 (0.54-0.97) | .03 | 8.90 | 0.78 (0.58-1.06) | .11 | 64/103 (62.1) |
| Etoposide plus platinum | 101 | 59/101 (58.4) | 68/101 (67.3) | 6.70 | 9.80 | 52/95 (54.7) | ||||||
| Reck et al,18 2016 | 3 | Ipilimumab plus etoposide-platinum chemotherapy | 566 | 297/478 (62.1) | 422/478 (88.3) | 4.60 | 0.85 (0.74-0.97) | .02 | 11.00 | 0.94 (0.81-1.09) | .38 | 231/478 (48.3) |
| Etoposide-platinum chemotherapy | 566 | 196/476 (41.2) | 422/476 (88.7) | 4.40 | 10.90 | 214/476 (45.0) | ||||||
| Shi et al,34 2015 | 2 | Irinotecan plus cisplatin | 30 | 21/30 (70.0) | 21/30 (70) | 6.00 | NR | NR | 18.10 | NR | NR | NR |
| Etoposide plus cisplatin | 32 | 21/32 (65.6) | 23/32 (71.9) | 6.00 | 15.10 | NR | ||||||
| Schmittel et al,39 2011 | 3 | Irinotecan plus carboplatin | 106 | 66/106 (62.3) | NR | 6.00 | 1.29 (0.96-1.73) | .07 | 10.00 | 1.34 (0.97-1.85) | .06 | NR |
| Etoposide plus carboplatin | 110 | 69/110 (62.7) | NR | 6.00 | 9.00 | NR | ||||||
| Spigel et al,11 2011 | 2 | Etoposide-platinum chemotherapy plus bevacizumab | 52 | 30/52 (57.7) | NR | 5.50 | 0.52 (0.32-0.83) | NR | 9.40 | 1.16 (0.66-2.04) | NR | 38/51 (74.5) |
| Etoposide-platinum chemotherapy | 50 | 24/50 (48.0) | NR | 4.40 | 10.50 | 28/47 (59.6) | ||||||
| Zatloukal et al,40 2010 | 3 | Irinotecan plus cisplatin | 202 | 79/202 (26.1) | 156/202 (77.2) | NR | NR | NR | 10.20 | 0.81 (0.65-1.01) | .06 | NR |
| Etoposide plus cisplatin | 203 | 94/203 (46.3) | 155/203 (76.4) | NR | 9.70 | NR | ||||||
| Lara et al,10 2009 | 3 | Irinotecan plus cisplatin | 324 | 194/324 (59.9) | NR | 5.80 | NR | .07 | 9.90 | NR | .71 | 213/317 (67.2) |
| Etoposide plus cisplatin | 327 | 186/327 (78.5) | NR | 5.20 | 9.10 | 274/324 (84.6) | ||||||
| Hermes et al,36 2008 | 3 | Irinotecan plus carboplatin | 105 | NR | NR | NR | NR | NR | 8.50 | 1.41 (1.06-1.87) | .02 | NR |
| Etoposide plus carboplatin | 104 | NR | NR | NR | 7.10 | NR | ||||||
| Hanna et al,35 2006 | 3 | Irinotecan plus cisplatin | 221 | 106/221 (48.0) | 115/221 (52) | 4.10 | NR | .37 | 9.30 | NR | .74 | NR |
| Etoposide plus cisplatin | 110 | 48/110 (43.6) | 56/110 (50.9) | 4.60 | 10.20 | NR | ||||||
| Schmittel et al,33 2006 | 2 | Irinotecan plus carboplatin | 35 | 22/35 (62.9) | 25/35 (71.4) | 9.00 | NR | .03 | NR | NR | NR | NR |
| Etoposide plus carboplatin | 35 | 20/35 (57.1) | 23/35 (65.7) | 6.00 | NR | NR | ||||||
| Noda et al,38 2002 | 3 | Irinotecan plus cisplatin | 77 | 65/77 (84.4) | NR NR |
6.90 | 0.61 (0.44-0.84) | .003 | 12.80 | NR | .002 | NR |
| Etoposide plus cisplatin | 77 | 52/77 (67.5) | 4.80 | 9.40 | NR |
Abbreviations: DCR, disease control rate; ES-SCLC, extensive-stage small cell lung cancer; HR, hazard ratio; NR, not reported; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TRAE 3-5, treatment-related adverse event, grades 3 to 5.
Risk of Bias in the Included Studies
Risk of bias assessment of the 14 included trials was performed by 2 investigators (T.Z. and Z.Z). The studies were considered adequate for performing random sequence generation and allocation concealment as well as having a low risk of detection and reporting bias. All studies required the masking of participants and personnel, and except for 6 trials10,33,34,35,36,40 with incomplete outcome data, 8 trials11,12,16,17,18,37,38,39 were considered to have a low risk of attrition bias (eFigure 2 in the Supplement).
Network Meta-analyses for Outcomes
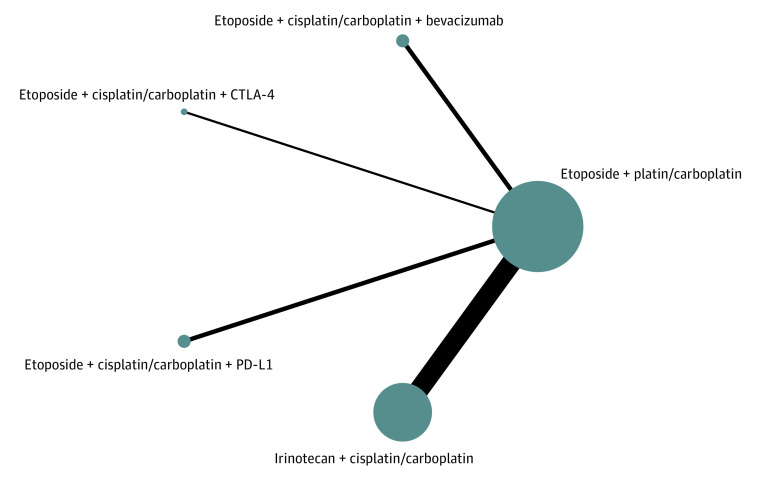
The network was designed to allow for multiple comparisons of different drugs added to chemotherapy and conventional therapy (Figure 1). It contained 8 studies10,12,17,18,35,37,38,39 for OS and PFS, and 10 studies10,17,18,33,34,35,36,37,38,39 for ORR and DCR.
Figure 1. Network Plot of Multiple Therapies in the First-Line Treatment of Extensive-Stage Small Cell Lung Cancer.
The size of each dot represents the number of patients receiving the corresponding intervention. The width of each line represents the number of corresponding comparison studies. CTLA-4 indicates T-cell lymphocyte antigen 4; and PD-L1, programmed cell death ligand 1.
In terms of ORR (Table 2), no significant outcome difference was found among etoposide-platinum chemotherapy, etoposide-platinum chemotherapy plus bevacizumab, etoposide-platinum chemotherapy with PD-L1 inhibitors, and irinotecan-platinum chemotherapy, while the addition of ipilimumab to an etoposide-platinum regimen showed a significant benefit in ORR compared with etoposide-platinum chemotherapy (OR, 0.43; 95% CI, 0.18-0.97). PD-L1 inhibitors plus etoposide-platinum chemotherapy showed a better DCR than etoposide-platinum chemotherapy (OR, 0.42; 95% CI, 0.21-0.81).
Table 2. Multiple Treatment Comparison of Clinical Outcomes Based on Network Consistency Model.
| Treatment | OR (95% CI)a | HR (95% CI)b | ||
|---|---|---|---|---|
| ORR | DCR | PFS | OS | |
| Etoposide plus cisplatin/carboplatinc | ||||
| Etoposide plus cisplatin/carboplatin plus bevacizumab | 0.91 (0.44-1.85) | 0.85 (0.40-1.83) | 1.54 (1.09-2.27) | 1.16 (0.81-1.59) |
| Etoposide plus cisplatin/carboplatin plus ipilimumab | 0.43 (0.18-0.97) | 1.04 (0.54-2.07) | 1.18 (0.79-1.77) | 1.06 (0.78-1.46) |
| Etoposide plus cisplatin/carboplatin plus PD-L1 | 0.86 (0.48-1.58) | 0.42 (0.21-0.81) | 1.29 (0.96-1.75) | 1.40 (1.09-1.80) |
| Irinotecan plus cisplatin/carboplatind | 0.82 (0.57-1.12) | 0.94 (0.65-1.33) | 1.30 (1.03-1.74) | 1.29 (1.11-1.56) |
| Etoposide plus cisplatin/carboplatin plus bevacizumab | ||||
| Etoposide plus cisplatin/carboplatin plus ipilimumab | 0.47 (0.15-1.37) | 1.20 (0.46-3.36) | 0.77 (0.44-1.27) | 0.92 (0.60-1.49) |
| Etoposide plus cisplatin/carboplatin plus PD-L1 | 0.94 (0.37-2.47) | 0.50 (0.17-1.31) | 0.84 (0.51-1.31) | 1.21 (0.81-1.88) |
| Irinotecan plus cisplatin/carboplatin | 0.90 (0.40-1.99) | 1.10 (0.46-2.58) | 0.85 (0.54-1.32) | 1.12 (0.79-1.69) |
| Etoposide plus cisplatin/carboplatin plus ipilimumabd | ||||
| Etoposide plus cisplatin/carboplatin plus PD-L1 | 2.02 (0.74-5.72) | 0.40 (0.16-1.01) | 1.10 (0.66-1.81) | 1.31 (0.88-1.97) |
| Irinotecan plus cisplatin/carboplatin | 1.93 (0.78-4.63) | 0.90 (0.42-1.88) | 1.11 (0.70-1.84) | 1.21 (0.87-1.77) |
| Etoposide plus cisplatin/carboplatin plus PD-L1d | ||||
| Irinotecan plus cisplatin/carboplatin | 0.95 (0.47-1.84) | 2.26 (1.06-4.75) | 1.01 (0.69-1.54) | 0.93 (0.69-1.28) |
Abbreviations: HR, hazard ratio; OR, odds ratio; PD-L1, programmed cell death ligand 1.
An OR greater than 1 indicates that the treatment listed in the row header is worse than the treatment it is being compared with.
An HR greater than 1 indicates that the treatment listed in the row header is better than the treatment it is being compared with.
All comparisons with etoposide plus cisplatin/carboplatin were direct.
All comparisons with etoposide plus cisplatin/carboplatin and bevacizumab, etoposide plus cisplatin/carboplatin and ipilimumab, etoposide plus cisplatin/carboplatin with PD-L1, and irinotecan plus cisplatin/carboplatin are indirect.
In terms of PFS, bevacizumab plus chemotherapy showed a better PFS than etoposide-platinum chemotherapy (HR, 1.54; 95% CI, 1.09-2.27). Likewise, irinotecan-platinum chemotherapy also presented a longer PFS than an etoposide-platinum alone regimen (HR, 1.30; 95% CI, 1.03-1.74). Similar outcomes were found among etoposide-platinum chemotherapy plus ipilimumab, etoposide-platinum chemotherapy with PD-L1 inhibitors (durvalumab and atezolizumab), and etoposide-platinum chemotherapy (Table 2).
An OS benefit was observed in only 2 included trials16,17 (CASPIAN and IMpower-133); moreover, our results indicated that a statistically significant OS benefit was associated with the addition of PD-L1 inhibitors (durvalumab and atezolizumab) to etoposide-platinum chemotherapy compared with etoposide-platinum chemotherapy alone regimen (HR, 1.40; 95% CI, 1.09-1.80) (Table 2). A significant advantage was also observed in irinotecan-based chemotherapy compared with etoposide-based chemotherapy (HR, 1.29; 95% CI, 1.11-1.56). No significant difference was found among etoposide-platinum chemotherapy plus ipilimumab, etoposide-platinum chemotherapy with bevacizumab, and etoposide-platinum chemotherapy, with the HRs close to 1.
Network Meta-analyses for TRAEs of Grade 3 or Greater
Six studies10,11,12,16,17,18 were included in the network meta-analysis for TRAE. The safety profile analysis indicated that there were nonsignificant differences in the incidence of TRAEs of grade 3 or greater among any 2 of the following therapy regimens: etoposide-platinum chemotherapy plus PD-L1 inhibitors, etoposide-platinum chemotherapy with ipilimumab, and irinotecan-platinum chemotherapy. The addition of PD-L1 inhibitors to etoposide-platinum chemotherapy caused no more toxic effects in general (compared with etoposide-based chemotherapy alone: OR, 1.14; 95% CI, 0.36-2.31). Compared with irinotecan-platinum, both etoposide-platinum chemotherapy and bevacizumab plus etoposide-platinum chemotherapy were associated with higher incidence of TRAE of grade 3 or higher (compared with etoposide-platinum chemotherapy: OR, 2.71; 95% CI, 1.04-7.00; compared with bevacizumab plus etoposide-platinum chemotherapy: OR, 4.24, 95% CI: 1.26-14.57). The addition of an ICI to conversional chemotherapy, such as ipilimumab plus etoposide-platinum chemotherapy, durvalumab plus etoposide-platinum chemotherapy, or atezolizumab plus etoposide-platinum chemotherapy, had similar safety profiles as conventional chemotherapy alone (Table 3).
Table 3. Multiple Treatment Comparison for Tolerability Based on Network Consistency Model.
| Comparison | OR (95% CI)a | |||||||
|---|---|---|---|---|---|---|---|---|
| TRAE 3-5 | Leukopenia | Neutropenia | Anemia | Thrombocytopenia | Diarrhea | Vomiting | Nausea | |
| Etoposide plus cisplatin/carboplatinb | ||||||||
| Etoposide plus cisplatin/carboplatin plus bevacizumab | 0.64 (0.29-1.35) | 1.10 (0.14-8.83) | 1.12 (0.34-3.98) | NA | 0.51 (0.11-2.20) | 0.00 (0.00-0.37) | 0.63 (0.06-5.54) | 0.14 (0.01-1.82) |
| Etoposide plus cisplatin/carboplatin plus ipilimumab | 0.87 (0.36-2.31) | 2.52 (0.33-20.51) | 1.84 (0.37-9.61) | 1.38 (0.42-4.81) | 1.19 (0.26-5.44) | 0.07 (0.00-11.32) | 0.60 (0.04-4.90) | 0.37 (0.05-2.37) |
| Etoposide plus cisplatin/carboplatin plus PD-L1 | 1.14 (0.56-2.21) | 0.92 (0.12-7.12) | 1.32 (0.39-4.36) | 1.29 (0.53-3.14) | 1.16 (0.39-3.47) | 0.43 (0.01-18.66) | 2.42 (0.32-29.55) | 3.50 (0.56-31.74) |
| Irinotecan plus cisplatin/carboplatin | 2.71 (1.04-7.00) | 1.81 (0.64-4.91) | 3.73 (1.92-7.24) | 1.35 (0.83-2.19) | 2.58 (1.53-4.56) | 0.07 (0.01-0.32) | 0.50 (0.18-1.13) | 0.57 (0.28-1.07) |
| Etoposide plus cisplatin/carboplatin plus bevacizumabc | ||||||||
| Etoposide plus cisplatin/carboplatin plus ipilimumab | 1.36 (0.44-4.72) | 2.37 (0.12-44.51) | 1.63 (0.20-11.85) | NA | 2.36 (0.29-19.73) | 1.47 × 108 (0.09-9.23 × 1032) | 0.92 (0.04-23.56) | 2.59 (0.10-115.96) |
| Etoposide plus cisplatin/carboplatin plus PD-L1 | 1.76 (0.63-5.01) | 0.86 (0.05-16.98) | 1.16 (0.20-6.38) | NA | 2.29 (0.36-13.86) | 8.27 × 108 (0.90-5.60 × 1033) | 4.00 (0.19-116.21) | 27.37 (1.03-1123.70) |
| Irinotecan plus cisplatin/carboplatin | 4.24 (1.26-14.57) | 1.69 (0.16-15.89) | 3.29 (0.80-13.53) | NA | 5.11 (1.09-24.81) | 1.21 × 108 (0.13-5.74 × 1032) | 0.79 (0.07-9.69) | 3.91 (0.30-106.06) |
| Etoposide plus cisplatin/carboplatin plus ipilimumabc | ||||||||
| Etoposide plus cisplatin/carboplatin plus PD-L1 | 1.29 (0.39-4.05) | 0.36 (0.02-6.52) | 0.72 (0.09-5.31) | 0.93 (0.21-4.11) | 0.97 (0.15-6.06) | 5.89 (0.01-3050.86) | 4.15 (0.25-111.54) | 10.07 (0.60-159.51) |
| Irinotecan plus cisplatin/carboplatin | 3.09 (0.81-11.14) | 0.71 (0.07-6.94) | 2.00 (0.36-12.28) | 0.97 (0.26-3.54) | 2.17 (0.45-11.35) | 0.96 (0.00-128.02) | 0.83 (0.08-13.85) | 1.54 (0.20-12.18) |
| Etoposide plus cisplatin/carboplatin plus PD-L1c | ||||||||
| Irinotecan plus cisplatin/carboplatin | 2.37 (0.74-7.93) | 1.94 (0.19-18.69) | 2.81 (0.72-10.94) | 1.05 (0.38-2.88) | 2.23 (0.68-7.81) | 0.16 (0.00-7.94) | 0.20 (0.01-1.75) | 0.16 (0.02-1.13) |
Abbreviations: NA, not applicable; OR, odds ratio; PD-L1, programmed cell death ligand 1; TRAE 3-5, treatment-related adverse event, grades 3 to 5.
An OR greater than 1 indicates that the treatment in the row heading was associated with lower likelihood of adverse events than the treatment it is being compared with.
All comparisons with etoposide plus cisplatin/carboplatin were direct.
All comparisons with etoposide plus cisplatin/carboplatin and bevacizumab, etoposide plus cisplatin/carboplatin and ipilimumab, etoposide plus cisplatin/carboplatin with PD-L1, and irinotecan plus cisplatin/carboplatin are indirect.
The most common TRAEs for conventional chemotherapy included leukopenia, neutropenia, anemia, thrombocytopenia, diarrhea, vomiting, and nausea. In detail, etoposide-platinum chemotherapy was associated with leukopenia, neutropenia, and anemia with the highest rankings, followed by etoposide-platinum chemotherapy plus bevacizumab (eTable 2 in the Supplement). The addition of bevacizumab to etoposide-platinum chemotherapy was associated with the highest risk of thrombocytopenia, diarrhea, and nausea. The rest of treatments shared similar toxicity profiles (Table 3).
The most frequently reported immune related adverse events were rash, hypothyroidism and hyperthyroidism. Because the incidence of these adverse events was unavailable in the studies that only included chemotherapy regimens, they were not compared in this study.
Rank Probability and Inconsistency Assessment
The ranking profiles of comparable treatments, shown in Figure 2, indicated the probability of each regimen with the best outcomes and safety profiles. Among all first-line treatments for patients with SCLC, bevacizumab plus etoposide-platinum chemotherapy was associated with the highest probability of ranking first for PFS (0.87) and TRAEs of grade 3 or greater (0.89), and PD-L1 inhibitors plus etoposide-platinum chemotherapy was associated with the highest probability of ranking first for DCR (0.97) and OS (0.87). Interestingly, ipilimumab plus etoposide-platinum chemotherapy seemed associated with the highest probability of ranking first for ORR (0.95). eTable 2 in the Supplement summarizes the clinical outcomes, including ORR, DCR, PFS, OS, and TRAEs of grade 3 or greater. eTable 3 in the Supplement shows the results of the evaluation of inconsistency for direct, indirect, and overall effects as well as inconsistency standard deviation.
Figure 2. Rank-Heat Plot of Multiple Therapies in First-Line Treatment of Patients With Extensive-Stage Small Cell Lung Cancer.
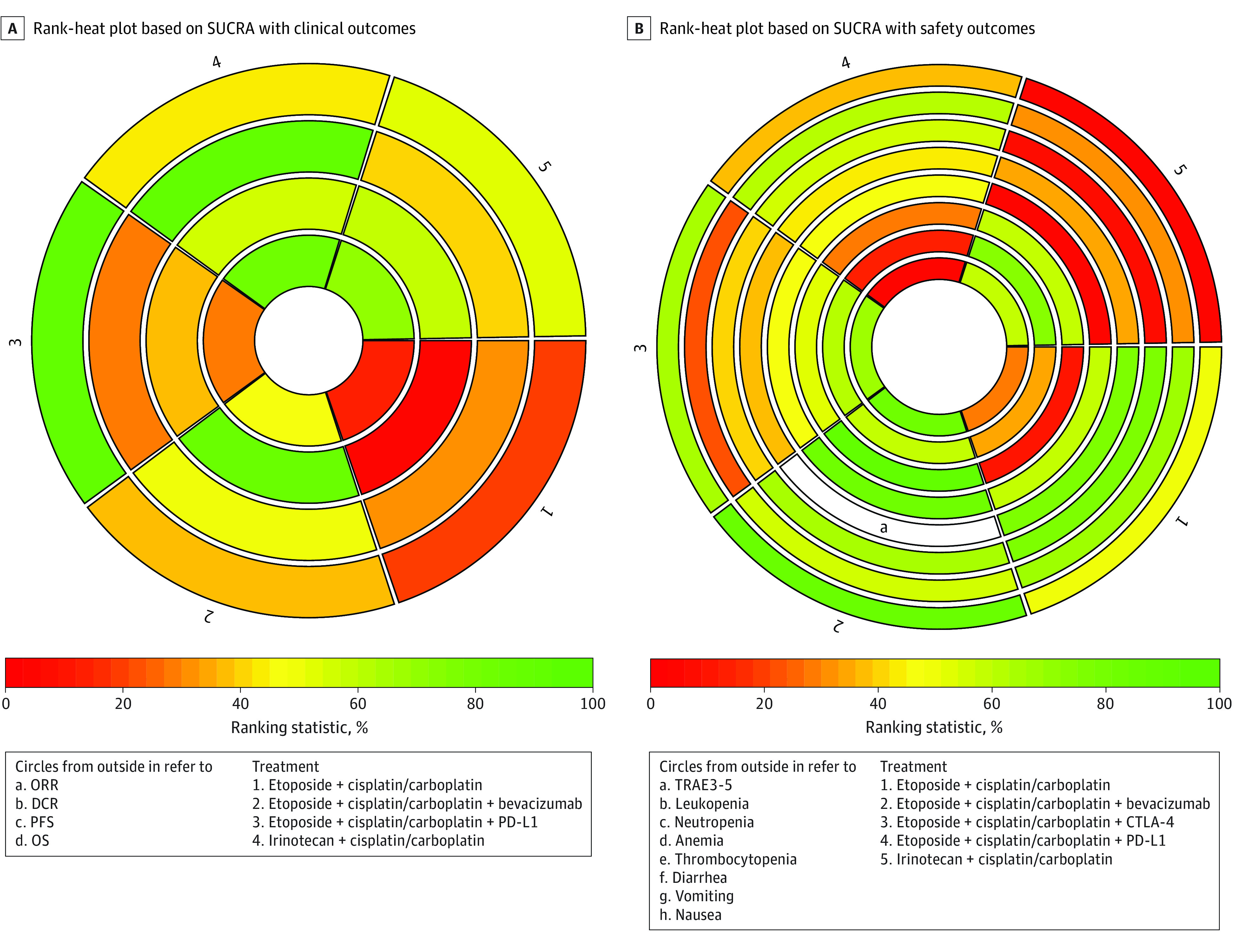
Each sector is colored according to the surface under the cumulative ranking (SUCRA) value of the corresponding treatment and outcome. The scale consists of 3 colors: red, indicating 0% probability of being ranked first; yellow, indicating 50%; and green, indicating 100%. Uncolored sectors indicate that the treatment was not included in the network meta-analyses for the particular outcome. DCR indicates disease control rate; ORR overall response rate; OS, overall survival; PD-L1, programmed cell death ligand 1; PFS, progression-free survival; and TRAE 3-5, treatment-related adverse event, grades 3 to 5.
Discussion
This network meta-analysis study included 14 head-to-head phase 2 and 3 randomized clinical trials, with 4838 patients, and compared the benefits and safety profiles of various first-line treatment regimens for patients with ES-SCLC. The results showed that a combination of etoposide-based chemotherapy with other treatments was associated with better antitumor benefits. Among them, the addition of ipilimumab was associated with the best ORR, the addition of PD-L1 inhibitors was associated with the best OS and DCR, and the addition of bevacizumab was associated with the best PFS. Toxicity analyses suggested that combination treatments might cause more TRAEs. Among them, bevacizumab plus etoposide-platinum chemotherapy was associated with the highest toxic effect rate.
The addition of PD-L1 inhibitors (ie, atezolizumab or durvalumab) to the standard etoposide-platinum chemotherapy was associated with the best DCR and OS. This phenomenon could be explained by the fact that patients with SCLC often experience a high variation rate and several autoimmune paraneoplastic syndromes, indicating that they might respond to ICI drugs.19,41,42,43,44 Previous trials45,46,47 have shown that ICI monotherapy has promising antitumor activity with durable response. Furthermore, the improvement of response and prolonged survival in combination regimens may indicate the potential immunogenic ability of chemotherapy to increase the number of cytotoxic lymphocytes and block signal transducer and activator of transcription 6 (STAT6) pathway to enhance antigen cross-presentation.48,49,50 Additional immunotherapy might improve the patient response to standard chemotherapy by developing the antitumor effect of intratumoral T-cells.51 These findings suggest that the addition of PD-L1 inhibitors to etoposide-platinum chemotherapy might provide better clinical benefits to patients with ES-SCLC compared with other treatment options. Our study also found that the addition of ipilimumab to etoposide-based chemotherapy provided was associated with the best benefit in ORR but not in survival outcomes. A possible explanation might be that unlike PD-L1 inhibitors (atezolizumab or durvalumab), ipilimumab could stimulate peripheral T-cells but not those in the tumor microenvironment, showing less antitumor effect in ES-SCLC. Although the addition of bevacizumab to etoposide-based chemotherapy was associated with the best PFS benefit, this treatment modality showed no benefit in other outcome indices, including ORR, DCR, and OS.18,19,52 Moreover, other antiangiogenic small molecules, including cediranib (AZD-2171), vandetanib (ZD-6474), and thalidomide,53,54,55 also showed no additional benefits to patients with ES-SCLC.
Overall, this multiple comparison study found that bevacizumab plus etoposide-based chemotherapy was associated with the worst safety profiles and the highest incidences of nausea, diarrhea, and thrombocytopenia. The overall safety profiles in PD-L1 inhibitor plus etoposide-based chemotherapy were similar to standard chemotherapy, with similar frequencies of TRAEs of grade 3 or higher but lower frequencies of other major adverse events such as leukopenia, neutropenia, anemia, thrombocytopenia, and nausea. The better safety profiles may be associated with more cycles of etoposide-platinum treatment for patients in the standard chemotherapy group. In addition, the rate and grade of immune-mediated adverse events were also low, similar to the known toxic effects of ICI drugs.
Unlike previous meta-analyses investigating treatments of patients with ES-SCLC, our network meta-analysis compared more extensive therapy regimens, including durvalumab, atezolizumab, ipilimumab, and bevacizumab, in first-line treatment for this population. Therefore, our study may help clinicians make better decisions from multiple promising treatment regimens for patients with ES-SCLC by taking full consideration of their clinical benefits and toxicity profiles. ICI drugs with etoposide-based chemotherapy were advantageous as the first-line treatment for patients with ES-SCLC. The PD-L1 inhibitor combination appeared to be the best among these treatment regimens. More trials comparing the clinical benefits and safety of combined treatment regimens with these ICI drugs should be conducted.
Limitations
This study has limitations. First, owing to limited data for individual patients, we could not provide subgroup analysis by stratifying patients by sex, smoking status, Eastern Cooperative Oncology Group scores, or other factors that may be associated with the treatment outcomes. These clinical characteristics should be considered in the future studies. Second, some patients underwent second-line or later therapies, but owing to limited data, their potential survival outcome benefits were not considered. Third, because of information sparseness for immune-related adverse events with the combination of ICIs and chemotherapy, we could only analyze the most common events. Fourth, the data for TRAEs of grade 3 or greater were only available in 6 studies, so we could not analyze TRAEs comprehensively. However, the studies that were missing data for TRAEs of grade 3 or greater were all comparisons of etoposide-platinum chemotherapy and irinotecan-platinum chemotherapy. Therefore, the safety data of the combination therapy regimens and etoposide-platinum chemotherapy were adequate. Further studies should investigate the relative safety profiles of all first-line treatments to fill the gaps of our study. Fifth, some indirect comparisons were made in this study on the transitivity assumption. Thus, we distinguished direct and indirect comparisons using different forms in the tables.
Conclusions
The findings of this network meta-analysis suggest that, in general, PD-L1 inhibitors (atezolizumab or durvalumab) plus etoposide-platinum chemotherapy may be an optimal first-line treatment for patients with ES-SCLC; it was associated with the best OS and the fewest toxic effects. Moreover, the addition of bevacizumab to etoposide-platinum chemotherapy was associated with the most TRAEs of grade 3 or higher. These findings could provide recommendations for clinicians in selecting first-line treatments based on their clinical benefits and safety profiles.
eTable 1. Search Strategy
eTable 2. Rank Probabilities With SUCRA Value for Different Outcomes in 5 Kinds of First-line Treatments for Patients With ES-SCLC
eTable 3. Evaluation of Inconsistency
eFigure 1. Flowchart of Study Selection and Design
eFigure 2. Risks of Bias for Included Studies
References
- 1.Oronsky B, Reid TR, Oronsky A, Carter CA. What’s new in SCLC? a review. Neoplasia. 2017;19(10):842-847. doi: 10.1016/j.neo.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17(12):725-737. doi: 10.1038/nrc.2017.87 [DOI] [PubMed] [Google Scholar]
- 3.Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol. 2017;14(9):549-561. doi: 10.1038/nrclinonc.2017.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121(5):664-672. doi: 10.1002/cncr.29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E; ESMO Guidelines Working Group . Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi99-vi105. doi: 10.1093/annonc/mdt178 [DOI] [PubMed] [Google Scholar]
- 6.Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992;10(2):282-291. doi: 10.1200/JCO.1992.10.2.282 [DOI] [PubMed] [Google Scholar]
- 7.Rudin CM, Ismaila N, Hann CL, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology endorsement of the American College of Chest Physicians guideline. J Clin Oncol. 2015;33(34):4106-4111. doi: 10.1200/JCO.2015.63.7918 [DOI] [PubMed] [Google Scholar]
- 8.Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. 2007;12(9):1096-1104. doi: 10.1634/theoncologist.12-9-1096 [DOI] [PubMed] [Google Scholar]
- 9.Pietanza MC, Byers LA, Minna JD, Rudin CM. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res. 2015;21(10):2244-2255. doi: 10.1158/1078-0432.CCR-14-2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27(15):2530-2535. doi: 10.1200/JCO.2008.20.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol. 2011;29(16):2215-2222. doi: 10.1200/JCO.2010.29.3423 [DOI] [PubMed] [Google Scholar]
- 12.Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the GOIRC-AIFA FARM6PMFJM Trial. J Clin Oncol. 2017;35(12):1281-1287. doi: 10.1200/JCO.2016.69.4844 [DOI] [PubMed] [Google Scholar]
- 13.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563-567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3(9):1052-1062. doi: 10.1158/2326-6066.CIR-14-0191 [DOI] [PubMed] [Google Scholar]
- 15.Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37(5):533-546. doi: 10.1053/j.seminoncol.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 16.Paz-Ares L, Dvorkin M, Chen Y, et al. ; CASPIAN investigators . Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939. doi: 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 17.Horn L, Mansfield AS, Szczęsna A, et al. ; IMpower133 Study Group . First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 18.Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740-3748. doi: 10.1200/JCO.2016.67.6601 [DOI] [PubMed] [Google Scholar]
- 19.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75-83. doi: 10.1093/annonc/mds213 [DOI] [PubMed] [Google Scholar]
- 20.Han D, Wang G, Sun L, et al. Comparison of irinotecan/platinum versus etoposide/platinum chemotherapy for extensive-stage small cell lung cancer: a meta-analysis. Eur J Cancer Care (Engl). 2017;26(6). doi: 10.1111/ecc.12723 [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Liang X, Zhou X, et al. A meta-analysis of randomized controlled trials comparing irinotecan/platinum with etoposide/platinum in patients with previously untreated extensive-stage small cell lung cancer. J Thorac Oncol. 2010;5(6):867-873. doi: 10.1097/JTO.0b013e3181d95c87 [DOI] [PubMed] [Google Scholar]
- 22.Hu Q, Wang Q, Zhu H, Yao Y, Song Q. Irinotecan compared with etoposide in combination with platinum in previously untreated extensive stage small cell lung cancer: an updated systemic review. J Cancer Res Ther. 2016;12(2):881-887. doi: 10.4103/0973-1482.138002 [DOI] [PubMed] [Google Scholar]
- 23.Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21(16):2313-2324. doi: 10.1002/sim.1201 [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phan K, Tian DH, Cao C, Black D, Yan TD. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg. 2015;4(2):112-122. doi: 10.3978/j.issn.2225-319X.2015.02.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods. 2012;3(2):161-176. doi: 10.1002/jrsm.57 [DOI] [PubMed] [Google Scholar]
- 28.Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279-301. doi: 10.1177/0962280207080643 [DOI] [PubMed] [Google Scholar]
- 29.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80-97. doi: 10.1002/jrsm.1037 [DOI] [PubMed] [Google Scholar]
- 30.Rangraz Jeddi F, Abazari F, Moravveji A, Nadjafi M. Evaluating the ability of hospital information systems to establish evidence-based medicine in Iran. J Med Syst. 2013;37(2):9904. doi: 10.1007/s10916-012-9904-5 [DOI] [PubMed] [Google Scholar]
- 31.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163-171. doi: 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 32.Veroniki AA, Straus SE, Fyraridis A, Tricco AC. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J Clin Epidemiol. 2016;76:193-199. doi: 10.1016/j.jclinepi.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 33.Schmittel A, Fischer von Weikersthal L, Sebastian M, et al. A randomized phase II trial of irinotecan plus carboplatin versus etoposide plus carboplatin treatment in patients with extended disease small-cell lung cancer. Ann Oncol. 2006;17(4):663-667. doi: 10.1093/annonc/mdj137 [DOI] [PubMed] [Google Scholar]
- 34.Shi Y, Hu Y, Hu X, Li X, Lin L, Han X. Cisplatin combined with irinotecan or etoposide for untreated extensive-stage small cell lung cancer: a multicenter randomized controlled clinical trial. Thorac Cancer. 2015;6(6):785-791. doi: 10.1111/1759-7714.12303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2038-2043. doi: 10.1200/JCO.2005.04.8595 [DOI] [PubMed] [Google Scholar]
- 36.Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol. 2008;26(26):4261-4267. doi: 10.1200/JCO.2007.15.7545 [DOI] [PubMed] [Google Scholar]
- 37.Kim DW, Kim HG, Kim JH, et al. Randomized phase III trial of irinotecan plus cisplatin versus etoposide plus cisplatin in chemotherapy-naïve Korean patients with extensive-disease small cell lung cancer. Cancer Res Treat. 2019;51(1):119-127. doi: 10.4143/crt.2018.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noda K, Nishiwaki Y, Kawahara M, et al. ; Japan Clinical Oncology Group . Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346(2):85-91. doi: 10.1056/NEJMoa003034 [DOI] [PubMed] [Google Scholar]
- 39.Schmittel A, Sebastian M, Fischer von Weikersthal L, et al. ; Arbeitsgemeinschaft Internistische Onkologie Thoracic Oncology Study Group . A German multicenter, randomized phase III trial comparing irinotecan-carboplatin with etoposide-carboplatin as first-line therapy for extensive-disease small-cell lung cancer. Ann Oncol. 2011;22(8):1798-1804. doi: 10.1093/annonc/mdq652 [DOI] [PubMed] [Google Scholar]
- 40.Zatloukal P, Cardenal F, Szczesna A, et al. A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol. 2010;21(9):1810-1816. doi: 10.1093/annonc/mdq036 [DOI] [PubMed] [Google Scholar]
- 41.Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44(10):1104-1110. doi: 10.1038/ng.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47-53. doi: 10.1038/nature14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633-641. doi: 10.1200/JCO.2017.75.3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koyama K, Kagamu H, Miura S, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14(21):6770-6779. doi: 10.1158/1078-0432.CCR-08-1156 [DOI] [PubMed] [Google Scholar]
- 45.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883-895. doi: 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 46.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 Study. J Clin Oncol. 2017;35(34):3823-3829. doi: 10.1200/JCO.2017.72.5069 [DOI] [PubMed] [Google Scholar]
- 47.Gadgeel SM, Pennell NA, Fidler MJ, et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol. 2018;13(9):1393-1399. doi: 10.1016/j.jtho.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15-25. doi: 10.1038/cdd.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roselli M, Cereda V, di Bari MG, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013;2(10):e27025. published Online First: 2013/12/20. doi: 10.4161/onci.27025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesterhuis WJ, Punt CJ, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011;121(8):3100-3108. doi: 10.1172/JCI43656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu J, Waxman DJ. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018;419:210-221. doi: 10.1016/j.canlet.2018.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pujol JL, Lavole A, Quoix E, et al. ; French Cooperative Thoracic Intergroup (IFCT); French Cooperative Thoracic Intergroup IFCT . Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial. Ann Oncol. 2015;26(5):908-914. doi: 10.1093/annonc/mdv065 [DOI] [PubMed] [Google Scholar]
- 53.Ramalingam SS, Belani CP, Mack PC, et al. Phase II study of Cediranib (AZD 2171), an inhibitor of the vascular endothelial growth factor receptor, for second-line therapy of small cell lung cancer (National Cancer Institute #7097). J Thorac Oncol. 2010;5(8):1279-1284. doi: 10.1097/JTO.0b013e3181e2fcb0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnold AM, Seymour L, Smylie M, et al. ; National Cancer Institute of Canada Clinical Trials Group Study BR.20 . Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol. 2007;25(27):4278-4284. doi: 10.1200/JCO.2007.12.3083 [DOI] [PubMed] [Google Scholar]
- 55.Pujol JL, Breton JL, Gervais R, et al. Phase III double-blind, placebo-controlled study of thalidomide in extensive-disease small-cell lung cancer after response to chemotherapy: an intergroup study FNCLCC cleo04 IFCT 00-01. J Clin Oncol. 2007;25(25):3945-3951. doi: 10.1200/JCO.2007.11.8109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy
eTable 2. Rank Probabilities With SUCRA Value for Different Outcomes in 5 Kinds of First-line Treatments for Patients With ES-SCLC
eTable 3. Evaluation of Inconsistency
eFigure 1. Flowchart of Study Selection and Design
eFigure 2. Risks of Bias for Included Studies