Key Points
Question
What is the effect on physical function of adding bimagrumab treatment to optimized standard of care in community-dwelling older adults with sarcopenia?
Findings
In this randomized clinical trial in 180 adults aged 70 years and older meeting the criteria for sarcopenia, there was no statistically significant difference in improvement in physical function between bimagrumab vs placebo. Participants who received bimagrumab had increased lean body mass and reduced fat mass vs placebo.
Meaning
These findings suggest that the addition of bimagrumab does not substantially enhance the effect of adequate diet and habitual light exercise, which can improve physical function and reduce the impact of sarcopenia in older adults.
This randomized clinical trial examines diet and exercise plus bimagrumab vs placebo for physical function in a global population of older adults with sarcopenia.
Abstract
Importance
The potential benefit of novel skeletal muscle anabolic agents to improve physical function in people with sarcopenia and other muscle wasting diseases is unknown.
Objective
To confirm the safety and efficacy of bimagrumab plus the new standard of care on skeletal muscle mass, strength, and physical function compared with standard of care alone in community-dwelling older adults with sarcopenia.
Design, Setting, and Participants
This double-blind, placebo-controlled, randomized clinical trial was conducted at 38 sites in 13 countries among community-dwelling men and women aged 70 years and older meeting gait speed and skeletal muscle criteria for sarcopenia. The study was conducted from December 2014 to June 2018, and analyses were conducted from August to November 2018.
Interventions
Bimagrumab 700 mg or placebo monthly for 6 months with adequate diet and home-based exercise.
Main Outcomes and Measures
The primary outcome was the change in Short Physical Performance Battery (SPPB) score after 24 weeks of treatment. Secondary outcomes included 6-minute walk distance, usual gait speed, handgrip strength, lean body mass, fat body mass, and standard safety parameters.
Results
A total of 180 participants were recruited, with 113 randomized to bimagrumab and 67 randomized to placebo. Among these, 159 participants (88.3%; mean [SD] age, 79.1 [5.3] years; 109 [60.6%] women) completed the study. The mean SPPB score increased by a mean of 1.34 (95% CI, 0.90 to 1.77) with bimagrumab vs 1.03 (95% CI, 0.53 to 1.52) with placebo (P = .13); 6-minute walk distance increased by a mean of 24.60 (95% CI, 7.65 to 41.56) m with bimagrumab vs 14.30 (95% CI, −4.64 to 33.23) m with placebo (P = .16); and gait speed increased by a mean of 0.14 (95% CI, 0.09 to 0.18) m/s with bimagrumab vs 0.11 (95% CI, 0.05 to 0.16) m/s with placebo (P = .16). Bimagrumab was safe and well-tolerated and increased lean body mass by 7% (95% CI, 6% to 8%) vs 1% (95% CI, 0% to 2%) with placebo, resulting in difference of 6% (95% CI, 4% to 7%) (P < .001).
Conclusions and Relevance
This randomized clinical trial found no significant difference between participants treated with bimagrumab vs placebo among older adults with sarcopenia who had 6 months of adequate nutrition and light exercise, with physical function improving in both groups. Bimagrumab treatment was safe, well-tolerated, increased lean body mass, and decreased fat body mass. The effects of sarcopenia, an increasing cause of disability in older adults, can be reduced with proper diet and exercise.
Trial Registration
ClinicalTrials.gov Identifier: NCT02333331; EudraCT number: 2014-003482-25
Introduction
The global population is aging rapidly, leading to an exponential increase in the number of older people and the proportion of those with chronic, noncommunicable diseases.1 Sarcopenia is a chronic, progressive muscle disease characterized by a loss of intrinsic physical capacity that is seen with aging and numerous acute and chronic conditions.2,3,4,5,6 Originally defined as the loss of skeletal muscle mass associated with reduced strength and physical function seen with advanced age,7 sarcopenia is now additionally used to describe adverse body composition changes observed with chronic illnesses and injuries.8,9,10 Sarcopenia is currently defined as the presence of low lean body mass (LBM), based on sex, geographic and body size–adjusted cutoffs, accompanied by muscle weakness and impaired physical performance.2,11,12,13,14 Sarcopenia is common, occurring in up to 13% of older adults.15 Typically manifesting as slower walking speed and difficulty with transitioning from sitting to standing and climbing stairs16 sarcopenia is associated with a greater incidence of falls and fractures, loss of independent living, and death and worse outcomes in those with concurrent chronic illness and injuries.17,18
While there is no universally effective treatment for sarcopenia, potential options are being studied. Recently, international treatment guidelines for sarcopenia19 recommend an improved nutritional status, habitual physical activity, and exercise as first-line therapy, although adoption of these lifestyle behaviors may be difficult.20 Adding to the evolving evidence of the effectiveness of diet and exercise to improve physical function in later life21 is a surge in the evaluation of new and repurposed pharmacotherapies as potential treatments.22,23 One novel approach targets the myostatin/activin type II receptor (ActRII) pathway to induce the systemic hypertrophy of skeletal muscles, with the expectation of increased muscle strength and improved functional capability.23,24,25,26
Bimagrumab (BYM338; Novartis) is a fully human monoclonal antibody that prevents ligand binding and promotes differentiation of human myoblasts.27 Muscle hypertrophy is enhanced by the blockade of both receptor subtypes (ActRIIA and ActRIIB) achieved with bimagrumab, with muscle mass increase approximately 2-fold that seen with myostatin inhibition alone.28 A 2017 proof-of-concept study26 with bimagrumab demonstrated good safety and increased LBM and muscle strength in patients with sarcopenia with gait speeds of less than 1.0 m/s. Clinically relevant improvement in walking performance was observed in a subset of study participants with slower baseline gait speeds (ie, <0.8 m/s). This study was performed to confirm and better define the clinical benefit of bimagrumab treatment in a global population of older adults meeting the most widely accepted criteria for sarcopenia.11,13
Methods
Study Design
This was a 28-week, double-blind, placebo-controlled, parallel-arm, multicenter, phase II randomized clinical trial. All participants, investigators, and sponsor representatives associated with the study were masked to treatment assignment.
The institutional review board or independent ethics committee at each site approved the study, and each participant gave written informed consent. The study complied with the International Council for Harmonisation Guidelines for Good Clinical Practice, applicable local regulations, and the ethical principles established in the Declaration of Helsinki.29 The Trial Protocol is provided in Supplement 1. An external data monitoring committee reviewed safety data throughout the trial. This study is a primary analysis reported in line with the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Approximately 1 year after the first patient was randomized, the study design was modified in response to communications from health authorities suggesting that the Short Physical Performance Battery (SPPB) was becoming the preferred clinical end point for drug trials in sarcopenia. The original 4-arm dose range (700 mg, 210 mg, 70 mg bimagrumab or placebo every 4 weeks for 24 weeks) finding design was modified to 2 arms (eFigure in Supplement 2) to confirm efficacy of bimagrumab with the SPPB, which had not been used in prior studies. The decision was made to reduce potential risk to patients on the lower 2 dose levels before treatment efficacy was established.
Study Population
Community-living adults aged 70 years and older with gait speeds greater than 4 m of 0.3 m/s or more to less than 0.8 m/s, and appendicular skeletal muscle index (ASMI; kg/m2) values meeting cutoffs for non-Asian11 and Asian13 countries, were enrolled. Race/ethnicity was self-reported. To attempt to control key potential confounders of skeletal muscle anabolism, participants were required to consume at least 20 kcal/kg of body weight and at least 0.8 g protein/kg of body weight daily.30,31 A dietary assessment was administered in person by a dietician or comparably trained professional using an established method.32 Volunteers who did not meet the diet criteria were disqualified or offered nutritional counseling. Those who received nutritional counseling were re-evaluated approximately 4 weeks later and enrolled if they met the dietary criteria or disqualified if they did not. In addition, participants had Mini-Mental State Examination (MMSE) scores of 21 or higher. Key exclusion criteria were the presence of health conditions that could increase a participant’s risk of a safety issue, inability to complete performance outcomes, and concurrent conditions (eg, protein-calorie malabsorption) or medications (eg, prednisone) known to affect skeletal muscle mass or function. Participants with uncontrolled chronic conditions (eg, hypertension) were also excluded.
Interventions
Participants were provided with a personalized exercise program and recording diary, diet analysis and counseling, an oral nutritional supplement, and vitamin D throughout the study period (eAppendix 1 in Supplement 2). The top dose of intravenous bimagrumab, 700 mg bimagrumab every 4 weeks, was chosen for the revised study design to maximize potential improvement with bimagrumab. This study presents data from all participants randomized to receive bimagrumab 700 mg or placebo for 24 weeks (Figure 1).
Figure 1. Study Flow Diagram of Participant Screening and Enrollment.
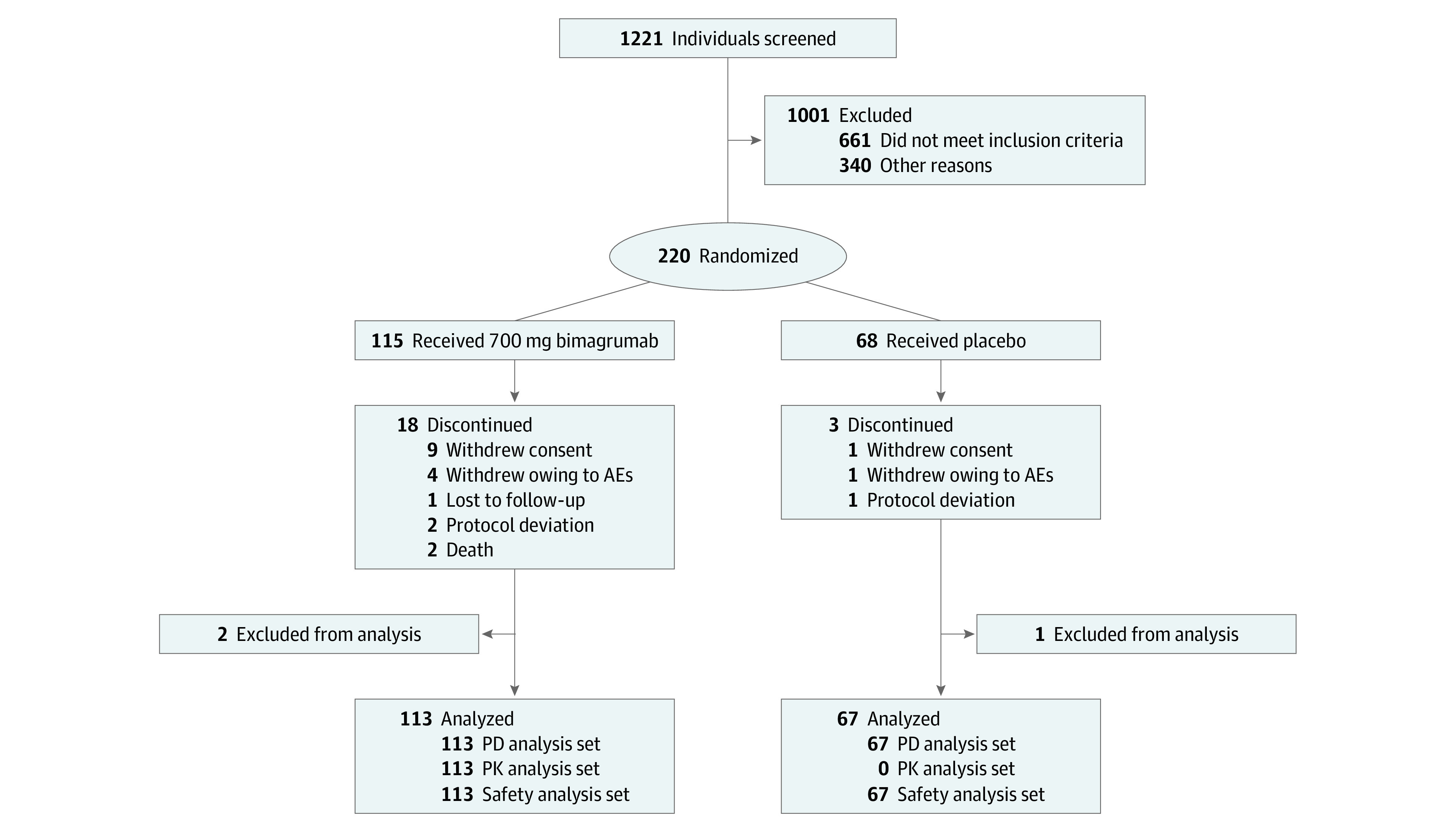
AE indicates adverse events; PD, pharmacodynamics; and PK, pharmacokinetics.
Study Procedures and End Points
The primary end point was the treatment effect of bimagrumab vs placebo on improved physical performance, assessed by the change in SPPB33 score, after 24 weeks of treatment. The SPPB was selected as the primary end point because of its association with adverse health outcomes and mortality in older adults by either a reduction of 1 or more points over time, or a total score of 9 points or fewer.34,35 A change of 1 point on the 0 to 12 scale was considered clinically meaningful, with a higher score reflecting greater function.36 The change from baseline in 6-minute walk distance (6MWD), measured in m,37 and usual gait speed, measured in m/s over 4 m,38 further assessed mobility performance. We quantified LBM and fat body mass (FBM) using standard methods of data acquisition with dual-energy x-ray absorptiometry (DXA), and ASMI was calculated from DXA data.11 Safety was assessed from results of standard laboratory tests, vital signs, electrocardiogram, echocardiography, and reports of adverse events (AEs) and serious AEs.
In addition, we assessed bilateral handgrip strength with a dynamometer (Jamar; Lafayette Instruments),39 the number of self-reported falls, health status by patient-reported outcomes (European Quality of Life-5 Dimensions-5 Levels40 and Short Form–36),41 and differences between participants who improved by 1 or more point on the SPPB score and those who did not improve with treatment.
Statistical Analysis
Change from baseline in SPPB total score, 6MWD, gait speed, and handgrip strength at week 25 was analyzed using a mixed-effect model repeated measure with a covariate for treatment, visit, baseline, visit × baseline, visit × treatment, and region (Asian and non-Asian). No adjustment was made for multiplicity. The ratios of baseline to week 25 of LBM, FBM, and ASMI (DXA parameters) were analyzed by mixed-effect model repeated measure. Data were transformed using natural logarithms and then analyzed using baseline, treatment, subgroup (Asian and non-Asian), and visit as covariates. A saturated covariance structure was used for observations from the same patient. The results were back-transformed to the original scale to present the adjusted geometric mean ratio with 95% CIs, and a P value to reflect the 1-sided evaluation of treatment vs placebo was reported, with significance set at P < .05. The sample size of 135 (85 bimagrumab; 50 placebo) participants was calculated to demonstrate a statistical significance at 1-sided 2.5% level and a point estimate increase (improvement) of at least 1 point in change from baseline SPPB total score compared with placebo.36 Descriptive statistics were used to report an a priori comparison between participants in the bimagrumab and placebo groups who had baseline SPPB scores of 9 points or fewer and no longer met the criteria of lean mass or gait speed or both criteria for sarcopenia. Analysis was conducted using SAS statistical software version 9.4 (SAS Institute). Data were analyzed from August to November 2018.
Results
Population
Between December 2014 and June 2018, 1221 individuals were screened at 58 sites (screen failure rate, 82.0%) (Figure 1). A total of 180 participants were randomized, including 113 assigned to bimagrumab 700 mg and 67 assigned to placebo; of these, 159 participants (88.3%) completed the study, and 89 participants (78.8%) in the bimagrumab group and 62 participants (92.5%) in the placebo group received all 6 doses. The study population had a mean (SD) age of 79.1 (5.3) years, mean (SD) MMSE score of 28 (2.0) points, and 109 participants (60.6%) were women. Baseline characteristics were balanced between groups (Table 1). Pharmacokinetic assessment confirmed that exposure to bimagrumab in this population was comparable to that seen in previous studies and exceeded the threshold needed for consistent target engagement, as further confirmed by changes in LBM and FBM. Adherence to the exercise and diet requirements were maintained throughout the study in both groups. At each clinic visit, participants reviewed their exercise diary and were encouraged to perform their program 2 to 3 times per week. Dietary protein was assessed every 1 to 2 clinic visits, and results suggested that protein intake was maintained above 0.8 g/kg/d in both groups (eTable 1 in Supplement 2).
Table 1. Baseline Characteristics of Older Adults With Sarcopenia.
| Characteristic | Mean (SD) | |
|---|---|---|
| Bimagrumab (n = 113) | Placebo (n = 67) | |
| Age, y | 79.5 (5.46) | 78.3 (5.03) |
| Median (range) | 79 (70-95) | 78 (70-88) |
| Sex, No. (%) | ||
| Men | 47 (41.6) | 24 (35.8) |
| Women | 66 (58.4) | 43 (64.2) |
| Race, No. (%) | ||
| White | 93 (82.3) | 54 (80.6) |
| Asian | 17 (15.0) | 11 (16.4) |
| Other | 0 | 1 (1.0) |
| Ethnicity, No. (%) | ||
| Other | 56 (49.6) | 31 (46.3) |
| Not Hispanic/Latino | 30 (26.5) | 16 (23.9) |
| Hispanic/Latino | 19 (16.8) | 19 (28.4) |
| Height, cm | 163.6 (12.50) | 161.9 (9.55) |
| Median (range) | 162 (138-200) | 160 (145-184) |
| Weight, kg | 65.6 (16.61) | 62.3 (11.17) |
| Median (range) | 63.4 (36-111) | 62.8 (42-94) |
| BMI | 24 (3.55) | 23.6 (2.84) |
| Median (range) | 24 (15-32) | 23.6 (17-31) |
| Total SPPB score | 7.1 (1.73) | 7.3 (1.68) |
| 6MWD, m | 294.3 (83.60) | 312.4 (93.92) |
| Gait speed, m/s | 0.642 (0.1079) | 0.656 (0.0836) |
| Grip strength, kg | ||
| Right hand | 20.4 (7.79) | 19.5 (7.36) |
| Left hand | 19.4 (7.63) | 18.1 (7.09) |
| LBM, kg | 35.4 (8.89) | 33.6 (6.89) |
| FBM, kg | 22.7 (8.74) | 21.2 (7.5) |
| ASMI, kg/m2 | 5.7 (0.82) | 5.5 (0.75) |
| Non-Asia | ||
| Men | 6.64 (0.421) | 6.38 (0.586) |
| Women | 5.13 (0.351) | 5.07 (0.373) |
| Asia | ||
| Men | 6.12 (0.628) | 6.29 (0.527) |
| Women | 4.92 (0.213) | 5.33 (0.265) |
Abbreviations: 6MWD, 6-minute walk distance; ASMI, appendicular skeletal muscle index (calculated as lean mass of upper and lower extremities in kilograms divided by height in meters squared); BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FBM, fat body mass; LBM, lean body mass; SPPB, Short Physical Performance Battery.
Efficacy
No minimum clinically important difference was seen between groups in any performance-based end point. The mean change in total SPPB score from baseline increased at the end of the study by 1.34 (95% CI, 0.90 to 1.77) points with bimagrumab vs 1.03 (95% CI, 0.53 to 1.52) points with placebo (P = .13). Scores improved in both groups at 13 weeks and remained through treatment completion (Figure 2A). Mean changes in 6MWD from baseline to weeks 13 and 25 were below clinically relevant levels of 35 to 54 m for both treatment groups (bimagrumab: 24.60 [95% CI, 7.65 to 41.56] m; placebo: 14.30 [95% CI, −4.64 to 33.23]; (P = .16).37 The mean distance walked improved by week 13 and plateaued in both groups for the remainder of the study (Figure 2B). At week 13, gait speed increased from baseline in both groups by the clinically relevant level of at least 0.1 m/s (bimagrumab: 0.14 [95% CI, 0.09 to 0.18] m/s; placebo: 0.11 [95% CI, 0.05 to 0.16] m/s; P = .16),36 and remained stable for the remainder of the study (Figure 2C). No noteworthy change (ie, <1.4 kg) was seen in the mean bilateral handgrip strength with either treatment, and no clinically meaningful change was seen in patient-reported outcome scores. In addition, no significant differences were seen in participants with improved SPPB scores of at least 1 point compared with those with no improvement (eTable 2 in Supplement 2).
Figure 2. Effect of Bimagrumab Compared With Placebo on Physical Performance Assessed .
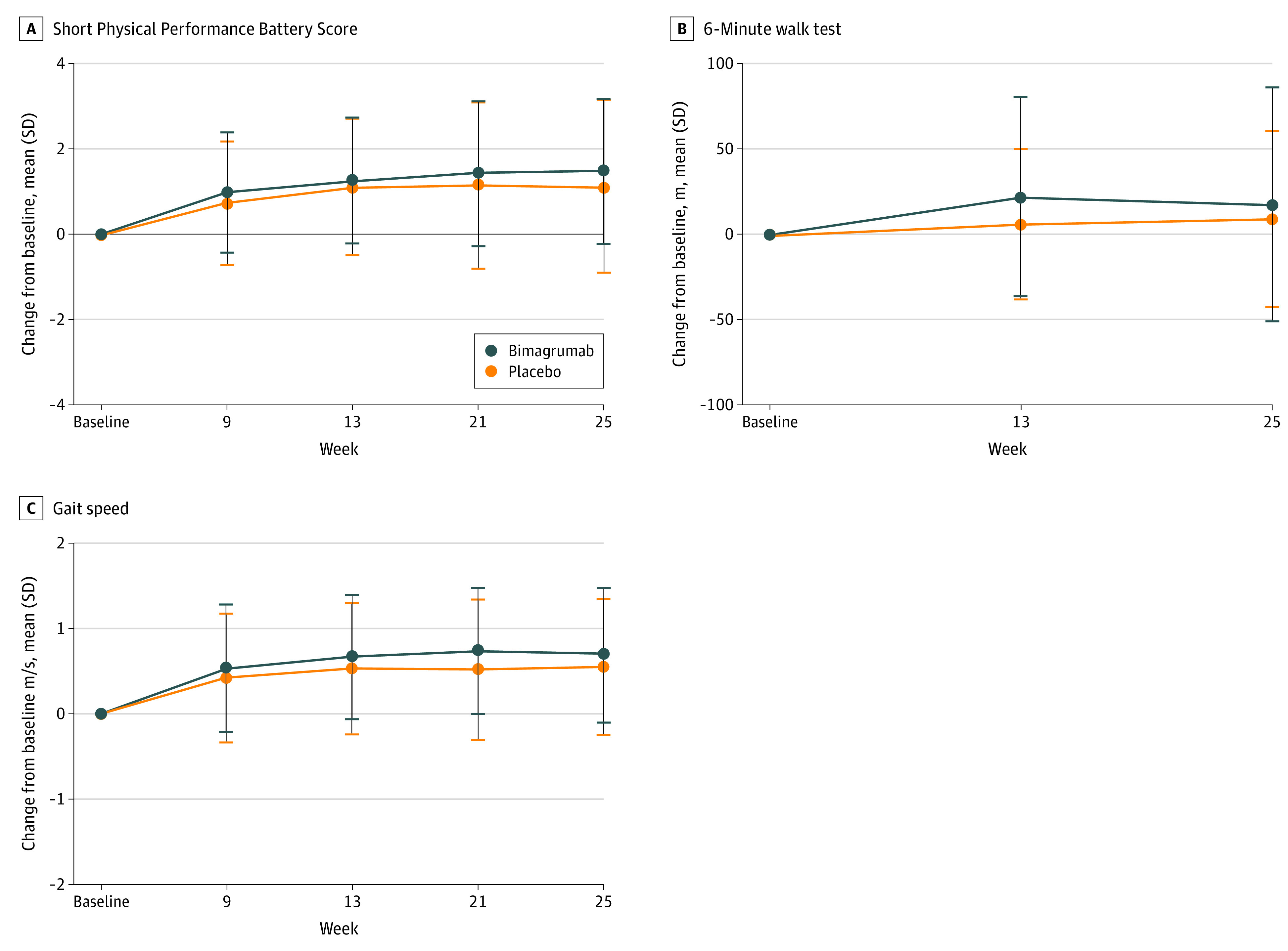
A repeated measure mixed model with a covariate for treatment (placebo or bimagrumab), visit, baseline, visit × baseline, visit × treatment, and region (Asian and non-Asian) was used. No adjustment for multiplicity was made. The baseline value considered for the calculation of the change from baseline is the last value before Day 1.
In a subgroup of the sample with baseline SPPB scores fewer than 9 points, 44 of 63 participants (69.8%) in the bimagrumab group and 23 of 37 participants (62.2%) in the placebo group no longer met the criteria for sarcopenia at the end of the study. However, differences were seen in the patterns of improvement between treatments. Based on the 2 key criteria in the definition of sarcopenia (ie, lean mass as ASMI and gait speed) participants who received bimagrumab improved similarly on 1 or both characteristics (improved gait speed: 19 participants; improved ASMI: 11 participants; or improved ASMI and gait speed: 14 participants). More participants who received the placebo improved on gait speed (18 participants) compared with ASMI (4 participants) or both (1 participant).
There was a significant increase in mean LBM in the bimagrumab group vs placebo at week 13 (mean [SD] change, 1.93 kg [1.74] kg vs 0.37 [1.24] kg; P < .001) and week 25 (mean [SD] change, 2.02 [1.95] vs 0.08 [1.17] kg; P < .001). The increase in LBM with bimagrumab was reflected in the ASMI values, which increased by 7% (95% CI, 6% to 8%) with bimagrumab vs 1% (95% CI, 0% to 2%) with placebo at week 25, resulting in difference of 6% (95% CI, 4% to 7%) (P < .001). Conversely, FBM showed a progressive decrease at week 13 (mean [SD] change, −1.39 [1.8] kg) and week 25 (mean [SD] change, −3.24 [2.5] kg) in the bimagrumab group, compared with no change in the placebo group (mean [SD] change: week 13: 0.1 [1.3] kg; week 25: 0.6 [1.6] kg) (P < .001).
Safety
Bimagrumab 700 mg administered intravenously every 4 weeks over 24 weeks was generally safe and well-tolerated in the study population of older adults with sarcopenia. The most frequently reported AEs were falls, involuntary muscle contractions (eg, muscle spasms), and diarrhea (Table 2).
Table 2. Adverse Events by Reported Description for Both Treatment Groups.
| AEa | No. (%) | |
|---|---|---|
| Bimagrumab (n = 113) | Placebo (n = 67) | |
| Participants with ≥1 AE | 100 (88.5) | 52 (77.6) |
| Fall | 28 (24.8) | 24 (35.8) |
| Muscle spasms | 37 (32.7) | 10 (14.9) |
| Diarrhea | 22 (19.5) | 2 (3.0) |
| Hypertension | 9 (8.0) | 4 (6.0) |
| Contusion | 5 (4.4) | 7 (10.4) |
| Upper respiratory tract infection | 5 (4.4) | 5 (7.5) |
| Back pain | 5 (4.4) | 4 (6.0) |
| Pain in extremity | 6 (5.3) | 3 (4.5) |
| Headache | 5 (4.4) | 3 (4.5) |
| Nausea | 8 (7.1) | 0 |
| Bronchitis | 6 (5.3) | 2 (3.0) |
| Constipation | 6 (5.3) | 1 (1.5) |
| Urinary tract infection | 6 (5.3) | 1 (1.5) |
| Dizziness | 6 (5.3) | 0 |
| Increased lipase | 6 (5.3) | 0 |
| Osteoarthritis | 4 (3.5) | 2 (3.0) |
| Viral infection | 5 (4.4) | 1 (1.5) |
| Decreased appetite | 5 (4.4) | 0 |
| Fatigue | 5 (4.4) | 0 |
| Nasopharyngitis | 5 (4.4) | 0 |
| Rash | 5 (4.4) | 0 |
| Sinusitis | 4 (3.5) | 1 (1.5) |
| Increased amylase | 4 (3.5) | 0 |
| Cough | 4 (3.5) | 0 |
| Dysgeusia | 4 (3.5) | 0 |
| Dyspnea | 4 (3.5) | 0 |
| Vomiting | 4 (3.5) | 0 |
Abbreviation: AE, adverse event.
AEs with more than 3% in bimagrumab group have been listed.
Most AEs were mild or moderate in intensity (Common Terminology Criteria for Adverse Events grade 1).42 Muscle spasms and diarrhea, both associated with bimagrumab in prior studies, were reported more frequently by patients receiving bimagrumab. Five participants discontinued the study owing to AEs, 4 of whom received bimagrumab and 1 of whom received placebo. Fourteen participants (12.4%) in the bimagrumab group and 5 participants (7.5%) participants in the placebo group experienced serious AEs; no serious AEs reported in the bimagrumab group were considered related to the study drug. No clinically meaningful changes in vital signs, echocardiography, or electrocardiogram were observed during the study in either treatment group (eAppendix 2 in Supplement 2). Safety findings were similar to the earlier proof-of-concept study in older adults with sarcopenia.26
Discussion
This randomized clinical trial found that 24 weeks of bimagrumab treatment in adults with sarcopenia, defined as those aged 70 years or older with low LBM for their height and slow gait speed (ie, <0.8 m/s), had no significant effect on physical performance (measured by SPPB) but increased participants’ LBM by a mean of 2.0 kg and decreased FBM by a mean of 3.8 kg compared with placebo. The gain in LBM did not result in clinically relevant treatment differences in muscle strength or mobility. These changes were observed with a background treatment of an optimized diet, vitamin D intake, and exercise, which resulted in improved physical performance and mobility of participants in both groups. The favorable safety profile seen in this trial was consistent with prior studies with bimagrumab in adults receiving the same dose level with a similar or longer duration.43,44
To our knowledge, this is the largest study evaluating a drug targeting the myostatin-ActRII pathway in an international population of community-dwelling older adults with confirmed sarcopenia. Our results are consistent with prior studies in older adults of drugs acting on the myostatin-ActRII pathway. Three mechanisms of action have been evaluated to drug the pathway: antiligand (primarily to myostatin),25,45,46 a soluble ActRIIB,24 and a receptor antagonist,26,27,47,48,49 all of which have increased LBM in adults to varying degrees. Among the 3 approaches evaluated in humans, blocking activity at the ActRIIA and ActRIIB receptors with bimagrumab results in larger increases in LBM compared with inhibiting myostatin alone (2.0 kg vs 0.7 kg).25 However, despite the increase in LBM, no corresponding improvement in clinically relevant muscle strength or physical performance has been reported in adults with sarcopenia or other conditions with reduced muscle mass, strength, or physical function.24,25,48,50 This pattern of muscle hypertrophy without improved function has been seen in other pharmacotherapeutic approaches as well, including agents that target other receptor pathways, such as selective androgen receptor modulators.51 Mixed results have also been reported with testosterone treatment. Data from the HORMA trial52 in men aged 65 to 90 years who received 16 weeks of testosterone or human growth hormone showed a median increase of 1.8 kg in LBM, which corresponded to statistically significant gains in muscle strength and physical function. Other trials with testosterone treatment of a longer duration did not find a sustained positive effect on muscle mass, strength, or function and showed an increased safety risk.53,54 The lack of functional improvement seen with pharmacotherapies is contrary to that seen with exercise, which can improve physical function and strength without muscle hypertrophy.55,56,57 Therefore, future considerations of drug candidates may need to more closely mimic the neuromuscular and metabolic effects of exercise to compensate for the morphological and physiological changes seen with aging, and in sarcopenia in particular.58
Our findings do not confirm the results of a 2017 proof-of-concept study26 evaluating bimagrumab in a similar patient population with sarcopenia that showed significant, clinically relevant improvements in muscle strength, gait speed, and 6MWD after 16 weeks of treatment compared with placebo. Several key differences may explain the discrepancy between studies. The 2017 study26 we performed had the same nutritional intake and oral nutritional supplement requirements as this study but did not include exercise. Our study attempted to control for the variability of exercise participation by providing a personalized, home-based program to promote regular muscle contraction without improving cardiovascular fitness or increasing functional strength. The combination of a high exercise adherence rate and the enhanced nutritional status in this study may have provided the necessary stimuli to improve performance on the functional end points. The other key difference between the studies relates to outcomes. The SPPB was not included in the proof-of-concept study,26 so a comparison of participant responses between studies cannot be made. While performance-based testing administration and training were standardized across both studies, the 2017 study26 was performed in a smaller sample of adults (40 participants) at 5 sites in the United States, compared with our study, which was conducted among 180 participants in 38 sites in 13 countries.
Limitations
Our study has several limitations. While this well-characterized sample is one of the largest in a study of sarcopenia and involved participants from North America, Europe, Asia, and Asia-Pacific, the unbalanced racial distribution limits the broad generalization of the findings. Rather than usual care, which is difficult to quantify and not standardized for an emerging diagnostic entity, like sarcopenia, the study used optimized standard of care as the comparator to determine what clinical benefits bimagrumab might provide when combined with ideal self-care.
Conclusions
Three key conclusions can be drawn from this randomized clinical trial with its well-characterized, international sample of older adults with sarcopenia after 24 weeks of treatment with bimagrumab. First, bimagrumab safely increases LBM within 4 to 8 weeks, and this is maintained with continuous exposure. Second, drugs that promote skeletal muscle hypertrophy exclusively without affecting other aspects of muscle function, such as neuromotor or metabolic activity, likely will not improve muscle strength or functional performance sufficiently as monotherapy to have a clinical impact. Third, adequate nutritional intake (including protein, calories, and vitamin D) and habitual light exercise can improve physical performance in men and women with sarcopenia to the extent that they no longer meet the disease criteria. These data, when combined with clearer diagnostic criteria, a new International Statistical Classification of Diseases, Tenth Revision, Clinical Modification code, and the recent clinical practice treatment guidelines, support the consideration of sarcopenia as a treatable muscle disease with dietary and exercise prescription, and a reversible cause of disability in older adults. Physicians should be encouraged to evaluate older patients for the presence of sarcopenia and provide nutritional and physical therapy prescriptions and referrals to reliable, convenient exercise programs accordingly.
Trial Protocol
eAppendix 1. Supplementary Methods
eAppendix 2. Supplementary Results
eTable 1. Baseline Characteristics and Changes in Outcomes of All Participants Receiving Study Drug
eTable 2. Baseline Characteristics for Responders and Nonresponders
eFigure. Study Design
Data Sharing Statement
References
- 1.He W, Goodkind D, Kowal P.. An Aging World: 2015. US Census Bureau, US Government Publishing Office; 2016. [Google Scholar]
- 2.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 . Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuggle N, Shaw S, Dennison E, Cooper C. Sarcopenia. Best Pract Res Clin Rheumatol. 2017;31(2):218-242. doi: 10.1016/j.berh.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vellas B, Fielding RA, Bens C, et al. Implications of ICD-10 for sarcopenia clinical practice and clinical trials: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2018;7(1):2-9. [DOI] [PubMed] [Google Scholar]
- 5.Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512-514. doi: 10.1002/jcsm.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanker J, Scott D, Brennan-Olsen SL, Duque G. Sarcopenia: a deserving recipient of an Australian ICD-10-AM code. Med J Aust. 2020;212(1):45-45.e1. doi: 10.5694/mja2.50432 [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5)(suppl):990S-991S. doi: 10.1093/jn/127.5.990S [DOI] [PubMed] [Google Scholar]
- 8.Fukushima H, Takemura K, Suzuki H, Koga F. Impact of sarcopenia as a prognostic biomarker of bladder cancer. Int J Mol Sci. 2018;19(10):2999. doi: 10.3390/ijms19102999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70(3):213-218. doi: 10.1136/thoraxjnl-2014-206440 [DOI] [PubMed] [Google Scholar]
- 10.Kim YK, Yi SR, Lee YH, Kwon J, Jang SI, Park SH. Effect of sarcopenia on postoperative mortality in osteoporotic hip fracture patients. J Bone Metab. 2018;25(4):227-233. doi: 10.11005/jbm.2018.25.4.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412-423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults: current consensus definition: prevalence, etiology, and consequences: international working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249-256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95-101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 14.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547-558. doi: 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69(5):584-590. doi: 10.1093/gerona/glu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landi F, Cesari M, Calvani R, et al. ; SPRINTT Consortium . The “Sarcopenia and Physical Frailty in Older People: Multi-Component Treatment Strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res. 2017;29(1):89-100. doi: 10.1007/s40520-016-0715-2 [DOI] [PubMed] [Google Scholar]
- 17.Wong RMY, Wong H, Zhang N, et al. The relationship between sarcopenia and fragility fracture-a systematic review. Osteoporos Int. 2019;30(3):541-553. doi: 10.1007/s00198-018-04828-0 [DOI] [PubMed] [Google Scholar]
- 18.Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):170. doi: 10.1186/s12877-016-0349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dent E, Morley JE, Cruz-Jentoft AJ, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-1161. doi: 10.1007/s12603-018-1139-9 [DOI] [PubMed] [Google Scholar]
- 20.Middleton KR, Anton SD, Perri MG. Long-term adherence to health behavior change. Am J Lifestyle Med. 2013;7(6):395-404. doi: 10.1177/1559827613488867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE study investigators . Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387-2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morley JE. Treatment of sarcopenia: the road to the future. J Cachexia Sarcopenia Muscle. 2018;9(7):1196-1199. doi: 10.1002/jcsm.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rooks D, Roubenoff R. Development of pharmacotherapies for the treatment of Sarcopenia. J Frailty Aging. 2019;8(3):120-130. [DOI] [PubMed] [Google Scholar]
- 24.Attie KM, Borgstein NG, Yang Y, et al. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve. 2013;47(3):416-423. doi: 10.1002/mus.23539 [DOI] [PubMed] [Google Scholar]
- 25.Becker C, Lord SR, Studenski SA, et al. ; STEADY Group . Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3(12):948-957. doi: 10.1016/S2213-8587(15)00298-3 [DOI] [PubMed] [Google Scholar]
- 26.Rooks D, Praestgaard J, Hariry S, et al. Treatment of sarcopenia with bimagrumab: results from a phase II, randomized, controlled, proof-of-concept study. J Am Geriatr Soc. 2017;65(9):1988-1995. doi: 10.1111/jgs.14927 [DOI] [PubMed] [Google Scholar]
- 27.Lach-Trifilieff E, Minetti GC, Sheppard K, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014;34(4):606-618. doi: 10.1128/MCB.01307-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morvan F, Rondeau JM, Zou C, et al. Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy. Proc Natl Acad Sci U S A. 2017;114(47):12448-12453. doi: 10.1073/pnas.1707925114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 30.Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542-559. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 31.Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929-936. doi: 10.1016/j.clnu.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dao MC, Subar AF, Warthon-Medina M, et al. Dietary assessment toolkits: an overview. Public Health Nutr. 2019;22(3):404-418. doi: 10.1017/S1368980018002951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94. doi: 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 34.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221-M231. doi: 10.1093/gerona/55.4.M221 [DOI] [PubMed] [Google Scholar]
- 35.Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14(1):215. doi: 10.1186/s12916-016-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 37.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 38.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51(3):314-322. doi: 10.1046/j.1532-5415.2003.51104.x [DOI] [PubMed] [Google Scholar]
- 39.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423-429. doi: 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 40.Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717-1727. doi: 10.1007/s11136-012-0322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57(12):M772-M777. doi: 10.1093/gerona/57.12.M772 [DOI] [PubMed] [Google Scholar]
- 42.Department of Health and Human Services . Common Terminology Criteria for Adverse Events (CTCAE): version 5.0. Accessed May 16, 2020. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- 43.Coleman L, Heymsfield S, Miller M, et al. Bimagrumab, an activin receptor antagonist, for treatment of obesity and type 2 diabetes [T-P-LB-3714]. Poster presented at: ObesityWeek; November 3-7, 2019; Las Vegas, NV. [Google Scholar]
- 44.Hanna MG, Badrising UA, Benveniste O, et al. ; RESILIENT Study Group . Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial. Lancet Neurol. 2019;18(9):834-844. doi: 10.1016/S1474-4422(19)30200-5 [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharya I, Pawlak S, Marraffino S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of domagrozumab (PF-06252616), an antimyostatin monoclonal antibody, in healthy subjects. Clin Pharmacol Drug Dev. 2018;7(5):484-497. doi: 10.1002/cpdd.386 [DOI] [PubMed] [Google Scholar]
- 46.Latres E, Pangilinan J, Miloscio L, et al. Myostatin blockade with a fully human monoclonal antibody induces muscle hypertrophy and reverses muscle atrophy in young and aged mice. Skelet Muscle. 2015;5:34. doi: 10.1186/s13395-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rooks DS, Laurent D, Praestgaard J, Rasmussen S, Bartlett M, Tankó LB. Effect of bimagrumab on thigh muscle volume and composition in men with casting-induced atrophy. J Cachexia Sarcopenia Muscle. 2017;8(5):727-734. doi: 10.1002/jcsm.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polkey MI, Praestgaard J, Berwick A, et al. Activin type II receptor blockade for treatment of muscle depletion in chronic obstructive pulmonary disease. a randomized trial. Am J Respir Crit Care Med. 2019;199(3):313-320. doi: 10.1164/rccm.201802-0286OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amato AA, Sivakumar K, Goyal N, et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83(24):2239-2246. doi: 10.1212/WNL.0000000000001070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fielding RA, Ralston SH, Rizzoli R. Emerging impact of skeletal muscle in health and disease. Calcif Tissue Int. 2015;96(3):181-182. doi: 10.1007/s00223-015-9964-x [DOI] [PubMed] [Google Scholar]
- 51.Papanicolaou DA, Ather SN, Zhu H, et al. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. 2013;17(6):533-543. doi: 10.1007/s12603-013-0335-x [DOI] [PubMed] [Google Scholar]
- 52.Sattler F, Bhasin S, He J, et al. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: the HORMA trial. J Gerontol A Biol Sci Med Sci. 2011;66(1):122-129. doi: 10.1093/gerona/glq183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Storer TW, Basaria S, Traustadottir T, et al. Effects of testosterone supplementation for 3 years on muscle performance and physical function in older men. J Clin Endocrinol Metab. 2017;102(2):583-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122. doi: 10.1056/NEJMoa1000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beaudart C, Dawson A, Shaw SC, et al. ; IOF-ESCEO Sarcopenia Working Group . Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28(6):1817-1833. doi: 10.1007/s00198-017-3980-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson ME, Rejeski WJ, Blair SN, et al. ; American College of Sports Medicine; American Heart Association . Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094-1105. doi: 10.1161/CIRCULATIONAHA.107.185650 [DOI] [PubMed] [Google Scholar]
- 57.Olsen PO, Termannsen AD, Bramming M, Tully MA, Caserotti P. Effects of resistance training on self-reported disability in older adults with functional limitations or disability—a systematic review and meta-analysis. Eur Rev Aging Phys Act. 2019;16:24. doi: 10.1186/s11556-019-0230-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016;23(6):1034-1047. doi: 10.1016/j.cmet.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Supplementary Methods
eAppendix 2. Supplementary Results
eTable 1. Baseline Characteristics and Changes in Outcomes of All Participants Receiving Study Drug
eTable 2. Baseline Characteristics for Responders and Nonresponders
eFigure. Study Design
Data Sharing Statement


