Abstract
This cohort study explores whether clinician specialty and patient familiarity with the attending clinician are associated with rates of low-dose computerized tomographic lung cancer screening after shared decision-making visits.
Introduction
The United States Preventive Services Task Force has recommended1 and the Centers for Medicare & Medicaid Services has mandated2 a separate visit in which a clinician and the patient participate in shared decision-making (SDM) prior to deciding on low-dose computerized tomographic (LDCT) screening for lung cancer.
The purpose of this study was to determine whether the rate of subsequent LDCT screening is associated with the type of clinician visited and with whether the clinician had a prior relationship with the patient.
Methods
This cohort study used a 20% random sample of national Medicare data to determine enrollees aged 55 to 80 years who had a separate visit for SDM (Current Procedural Terminology [CPT] code G0296) from January 1, 2016, to September 30, 2018, with complete insurance enrollment 1 year prior. We assessed the clinician specialty from the carrier file and whether that clinician had submitted a bill for services to that patient in the prior 12 months. We assessed receipt of LDCT screening for lung cancer (CPT codes 0297 and S8032) in the following 3 months. We have reported observed rates and odds ratios of receipt of LDCT from generalized linear mixed models with and without clinician characteristics.
The University of Texas Medical Branch institutional review board approved the study, and exempted it from the requirement for informed consent because participants were not identifiable in the data. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. All analyses were performed with SAS Enterprise version 7.1 (SAS Institute) at the CMS Virtual Research Data Center.
Results
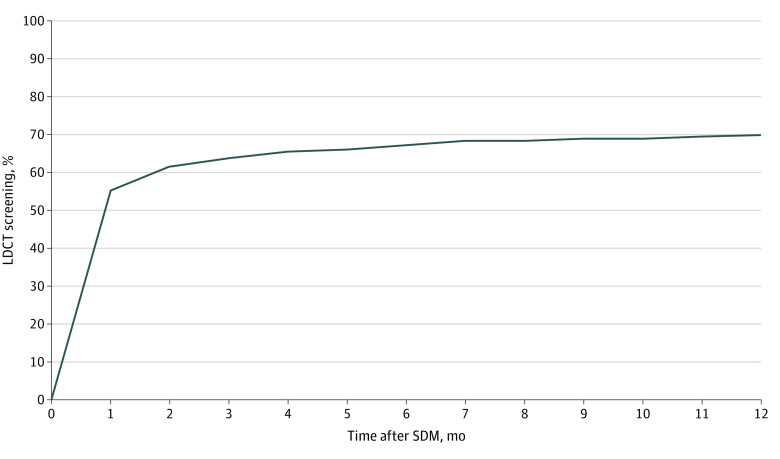
From January 1, 2016, to September 30, 2018, 11 699 enrollees in the cohort had a separate visit for SDM. Of the patients who underwent a subsequent LDCT, 91.6% (4652 of 5081 patients) had done so within 3 months (Figure), so we used that cutoff for the remaining analyses. An SDM visit was followed within 3 months by LDCT in 7522 enrollees (64.3%). The Table reports the unadjusted rates and adjusted odds ratios for receipt of LDCT by enrollee characteristics and type of clinician conducting the SDM.
Figure. Shared Decision-making (SDM) Lung Cancer Screening in Medicare Patients.
The graph represents the cumulative percentage of 7292 enrollees aged 55 to 80 years who participated in SDM in 2016 or 2017 and who subsequently underwent low-dose computerized tomographic (LDCT) screening by month after SDM. Of these 7292 patients who had an SDM visit in 2016 and 2017, 5081 (69.7%) received LDCT within 12 months. Of the enrollees receiving LDCT, 4652 (91.6%) underwent LDCT within 3 months of the SDM visit.
Table. Observed Rates and Adjusted Odds of Receiving LDCT Lung Cancer Screening Within 90 Days After Medicare Enrollees Had a Visit for SDM.
| Characteristic | Patients, No. | LDCT rate, % (95% CI) | OR (95% CI)a | ||
|---|---|---|---|---|---|
| SDM (n = 11 699) | LDCT (n = 7522) | Model 1 | Model 2 | ||
| Overall | 64.3 | ||||
| Age, y | |||||
| ≥55 to ≤65 | 2772 | 1795 | 64.8 (62.9-66.5) | 1 [Reference] | 1 [Reference] |
| >65 to ≤70 | 4953 | 3329 | 67.2 (65.9-68.5) | 1.10 (0.97-1.25) | 1.10 (0.97-1.25) |
| >70 to ≤75 | 3217 | 2005 | 62.3 (60.6-64.0) | 0.93 (0.81-1.06) | 0.94 (0.82-1.07) |
| >75 to ≤80 | 757 | 393 | 51.9 (48.3-55.5) | 0.69 (0.57-0.85) | 0.70 (0.57-0.86) |
| Year | |||||
| 2016 | 2632 | 1633 | 62.0 (60.2-63.9) | 1 [Reference] | 1 [Reference] |
| 2017 | 4660 | 3019 | 64.8 (63.4-66.2) | 1.13 (0.99-1.28) | 1.10 (0.97-1.24) |
| 2018 | 4407 | 2870 | 65.1 (63.7-66.5) | 1.14 (0.99-1.30) | 1.11 (0.98-1.26) |
| Gender | |||||
| Male | 5893 | 3884 | 65.9 (64.7-67.1) | 1 [Reference] | 1 [Reference] |
| Female | 5806 | 3638 | 62.7 (61.4-63.9) | 0.87 (0.80-0.95) | 0.87 (0.79-0.95) |
| Medicaid | |||||
| No | 9183 | 5964 | 65.0 (64.0-65.9) | 1 [Reference] | 1 [Reference] |
| Yes | 2516 | 1558 | 61.9 (59.9-63.8) | 0.91 (0.81-1.03) | 0.91 (0.80-1.02) |
| Race/ethnicity | |||||
| Non-Hispanic White | 10 639 | 6650 | 64.1 (63.2-65.1) | 1 [Reference] | 1 [Reference] |
| Black | 710 | 447 | 63.0 (59.3-66.5) | 0.87 (0.72-1.06) | 0.87 (0.72-1.06) |
| Hispanic | 229 | 144 | 62.9 (56.3-69.2) | 0.86 (0.62-1.20) | 0.78 (0.56-1.09) |
| Other | 391 | 281 | 71.9 (67.1-76.3) | 1.32 (1.01-1.73) | 1.26 (0.96-1.65) |
| Patient had seen this SDM clinician previously | |||||
| Yes | 6446 | 3577 | 55.5 (54.3-56.7) | NA | 1 [Reference] |
| No | 5253 | 3945 | 75.1 (73.9-76.3) | NA | 1.49 (1.34-1.03) |
| Clinician specialty | |||||
| Family practice | 3234 | 1743 | 53.9 (52.2-55.6) | NA | 1 [Reference] |
| Internal medicine | 2480 | 1514 | 61.1 (59.1-63.0) | NA | 1.18 (1.01-1.36) |
| Pulmonary | 1810 | 1021 | 56.4 (54.1-58.7) | NA | 0.84 (0.70-1.01) |
| Radiologist | 172 | 160 | 93.0 (88.1-96.3) | NA | 9.09 (4.16-19.85) |
| Nurse practitioner | 2738 | 2193 | 80.1 (78.6-81.6) | NA | 1.70 (1.42-2.05) |
| Physician assistant | 734 | 532 | 72.5 (69.1-75.7) | NA | 1.40 (1.08-1.80) |
| Other | 531 | 359 | 67.6 (63.4-71.6) | NA | 1.27 (0.97-1.66) |
Abbreviations: ICC, intraclass correlation coefficient; LDCT, low-dose computerized tomographic; NA, not applicable; OR, odds ratio; SDM, shared decision-making.
Model 1 includes patient characteristics and Model 2 adds clinician characteristics from a 2-level hierarchical generalized linear mixed model (enrollee and clinician). ICC for model 1 was 27.42%; ICC for model 2 was 24.70%. The adjusted odds of receiving LDCT and 95% CIs were calculated from a 2-level hierarchical generalized linear mixed model with the clinician as a random effect. The ICCs estimated the outcome associated with adding the clinician.6
The highest rates of subsequent LDCT screenings were following SDM visits with radiologists (93.0%; 95% CI, 88.1%-96.3%), followed by nurse practitioners (80.1%; 95% CI, 78.6%-81.6%), with lower rates from pulmonary specialists (56.4%; 95% CI, 54.1%-58.7%) and family physicians (53.9%; 95% CI, 52.2%-55.6%). The adjusted odds ratio of undergoing LDCT screening after an SDM with a radiologist was 9.09 (95% CI, 4.16-19.85); a nurse practitioner, 1.70 (95% CI, 1.42-2.05); and a pulmonary specialist, 0.84 (95% CI, 0.70-1.01) (family physicians were used as a reference for odds ratios). Approximately 55% of enrollees (6446 of 11 699 [55.1%]) had seen the SDM clinician in the year prior to the SDM visit. In these patients, the rate of LDCT was 55.5% (95% CI, 54.3%-56.7%) vs 75.1% (95% CI, 73.9%-76.3%) in those who had not previously seen the clinician.
The intraclass correlation coefficient (ICC) for model 1 in the Table was 27.4%, indicating that 27% of the variation in whether patients received LDCT after SDM was associated with which clinician they saw.
Discussion
The decision to undergo LDCT lung cancer screening varies substantially by the clinician participating in the SDM. Ideally in SDM, one might expect that all the variation in choice of LDCT would be because of differences between individual enrollees in perceived risk of cancer, willingness to undergo surgery if recommended, and personal values.
We found that SDM with a clinician with prior experience with the patient led to substantially lower rates of LDCT (55% vs 75%). Possible explanations include a greater familiarity with patient values and more trust. Some of the SDM clinicians previously unknown to the patient might have been affiliated with LDCT screening centers, which may be biased toward LDCT.3
This study had several limitations. No information on which clinicians were affiliated with LDCT programs and whether an SDM tool was used were available. Also, there is no ideal rate of LDCT after SDM because the decision is supposed to be based on patient-specific factors. As previously reported,4,5 the overall rate of SDM visits is very low, a surprising finding given that the Centers for Medicare & Medicaid Services made a separate SDM visit a requirement for reimbursement for LDCT.2
In this study, clinician specialty was associated with the outcome of an SDM visit. SDM with clinicians known to the patient was associated with lower LDCT rates.
References
- 1.Moyer VA; US Preventive Services Task Force . Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi: 10.7326/M13-2771 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Medicare & Medicaid Services Decision memo for screening for lung cancer with low-dose computed tomography (LDCT) (CAG-0043N). Published February 5, 2015. Accessed April 26, 2018. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- 3.Clark SD, Reuland DS, Enyioha C, Jonas DE. Assessment of lung cancer screening program websites. JAMA Intern Med. 2020;180(6):824-830. doi: 10.1001/jamainternmed.2020.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision making for lung cancer screening. JAMA Intern Med. 2018;178(10):1311-1316. doi: 10.1001/jamainternmed.2018.3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin JS, Nishi S, Zhou J, Kuo YF. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179(5):716-718. doi: 10.1001/jamainternmed.2018.6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musca SC, Kamiejski R, Nugier A, Méot A, Er-Rafiy A, Brauer M. Data with hierarchical structure: impact of intraclass correlation and sample size on type-I error. Front Psychol. 2011;2:74. doi: 10.3389/fpsyg.2011.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]