Abstract
Background:
Kidney transplant recipients are given induction therapy to rapidly reduce the immune response and prevent rejection. Guidelines recommend that an interleukin-2 receptor antibody (basiliximab) be the first-line agent and that a lymphocyte-depleting agent (antithymocyte globulin [ATG]) be reserved for those at high immunologic risk.
Objective:
To determine the incidence, risk factors, and outcomes for patients who receive both basiliximab and ATG for induction compared to either agent alone.
Design:
Retrospective cohort study.
Setting:
We used the transplant electronic medical record at the University of Alberta Hospital in Edmonton, Canada.
Patients/samples/participants:
We included incident adult kidney transplant recipients from 2013 to 2018.
Measurements:
We measured baseline characteristics, type, and dose of induction therapy used, estimated glomerular filtration rate (eGFR) at 1-year posttransplant, and outcomes of all-cause graft failure, death-censored graft failure, all-cause mortality, and death with a functioning graft.
Methods:
Differences between induction groups were compared using chi-square test for categorical variables and Kruskal-Wallis tests for continuous variables. We performed multivariable logistic regression modeling with type of induction therapy as the dependent variable and the case-level factors as the predictors (adjusted odds ratio). We estimated the Kaplan-Meier failure functions and used log-rank tests to assess statistical significance of differences in unadjusted incidence across induction therapy types. We compared cumulative incidence functions using a Fine and Gray competing risk regression model.
Results:
In all, 430 kidney transplant recipients were followed for a mean of 3.9 years (standard deviation 1.5). Of these, 71% (n = 305) received basiliximab alone, 22% (n = 93) received ATG alone, and 7% (n = 32) received both basiliximab and ATG. After adjusting for age and sex, compared to the basiliximab alone group, patients were more likely to receive dual-induction therapy if they were sensitized (calculated panel reactive antibody ≥80%), had diabetes mellitus or peripheral vascular disease, or experienced delayed graft function. Compared to the ATG alone group, the dual-induction therapy group had worse graft function at 1 year (mean eGFR 42 vs. 59 mL/min/1.73 m2, P = .0008) and an increased risk of all-cause graft failure (31% vs. 13%, P = .02) and death-censored graft failure (16% vs. 4%, P = .03).
Limitations:
There is a risk of confounding by indication, as patients who received dual-induction therapy likely had worse outcomes due to the indication for dual-induction therapy (such as delayed graft function).
Conclusions:
In our study, 1 out of 10 recipients who were treated with basiliximab also received ATG for induction therapy. These patients experienced worse outcomes than those treated with ATG alone.
Trial registration:
Not applicable (cohort study).
Keywords: basiliximab, immunosuppression, induction, kidney transplant recipient, thymoglobulin
Abrégé
Contexte:
Les receveurs d’une greffe rénale reçoivent un traitement par induction pour réduire la réponse immunitaire et prévenir le rejet. Les lignes directrices recommandent qu’un anticorps du récepteur de l’interleukine-2 (basiliximab) soit l’agent de première ligne et qu’un agent de déplétion immunitaire (immunoglobulines anti-thymocytes [ATG]) soit réservé aux patients présentant un risque immunologique élevé.
Objectif:
Connaître l’incidence d’une double induction (basiliximab + ATG), les facteurs de risque et l’issue des patients l’ayant reçue comparativement aux patients n’ayant reçu qu’un seul des deux agents.
Type d’étude:
Étude de cohorte rétrospective
Cadre:
Nous avons consulté les dossiers médicaux électroniques de transplantation du University of Alberta Hospital d’Edmonton (Canada).
Sujets:
Ont été inclus les adultes receveurs d’une greffe rénale entre 2013 et 2018.
Mesures:
Nous avons mesuré les caractéristiques démographiques initiales, le type et la dose du traitement d’induction administré, le débit de filtration glomérulaire estimé (DFGe) mesuré un an post-greffe, de même que les résultats cliniques de dysfonction du greffon de toutes causes, de dysfonction du greffon censurée par le décès, de mortalité toutes causes ou de décès avec un greffon fonctionnel.
Méthodologie:
Les différences entre les groupes d’induction ont été comparées à l’aide du test de Chi-Deux (variables catégorielles) et des tests de Kruskal-Wallis (variables continues). Nous avons procédé à une modélisation de régression logistique multivariable avec le type de traitement d’induction comme variable dépendante et les facteurs de risque pour chaque cas comme prédicteurs (rapport de cote corrigé). Nous avons évalué les défaillances de fonction avec une courbe de Kaplan-Meier, et des tests logarithmiques par rangs ont été employés pour évaluer la signification statistique des différences dans l’incidence non corrigée entre les types de traitements d’induction administrés. Un modèle de régression des risques concurrents Fine and Gray a été employé pour comparer les fonctions d’incidence cumulée.
Résultats:
En tout, 430 receveurs d’une greffe rénale ont été suivis sur une période moyenne de 3,9 ans (écart type: 1,5 an). De ceux-ci, 71 % (n = 305) n’avaient reçu que du basiliximab, 22 % (n = 93) n’avaient reçu que des ATG et 7 % (n = 32) avaient reçu le double traitement par induction. Après la correction pour l’âge et le sexe, et lorsque comparés aux patients n’ayant reçu que du basiliximab, les patients sensibilisés (allo-anticorps calculés d’au moins 80 %), diabétiques, atteints de maladie vasculaire périphérique ou ayant vécu un délai dans la reprise de la fonction du greffon se sont avérés plus susceptibles de recevoir le double traitement par induction. Le groupe ayant reçu le double traitement par induction présentait une moins bonne fonction du greffon un an après l’intervention (DFGe moyen: 42 vs 59 ml/min/1,73 m2; P = .0008), avaient un risque accru de dysfonction du greffon toutes causes (31 % vs 13 %; P =.02) et un taux de dysfonction du greffon censurée par le décès plus élevé (16 % vs 4 %; P = .03) lorsque comparé au groupe traité par les ATG seulement.
Limites:
Un risque de confusion par indication existe puisque les patients ayant reçu le double traitement par induction ont probablement eu de moins bons résultats, notamment un retard dans la reprise de la fonction du greffon, en raison de cette indication.
Conclusion:
Dans notre étude, un patient sur dix avait reçu le double traitement par induction (basiliximab et ATG). Ces patients ont cependant eu de moins bons résultats de santé que les patients traités aux ATG seulement.
Enregistrement de l’essai:
Sans objet (étude de cohorte).
Introduction
Kidney transplant recipients are given induction therapy to rapidly reduce the immune response and prevent acute rejection. The Kidney Disease: Improving Global Outcomes (KDIGO) guideline for the “Care of Kidney Transplant Recipients” recommends that an interleukin-2 receptor antibody (eg, basiliximab) be the first-line induction agent and that a lymphocyte-depleting agent (eg, rabbit antithymocyte globulin [ATG], thymoglobulin®) be reserved for recipients who are at high immunologic risk.1 These recommendations were supported by the Canadian Society of Transplantation and Canadian Society of Nephrology.2 Antithymocyte globulin has a lower risk of rejection compared to basiliximab, but an increased risk of complications (eg, infections, cancers) and higher costs.3,4
Choosing the optimal induction therapy requires clinicians to balance the risk of rejection with the risk of complications, taking into account recipient and donor characteristics. Notably, in the United States, choice of induction agent is driven more strongly by center practices than by patient clinical profile or donor characteristics.5 In difficult cases, recipients may initially be treated with basiliximab, but due to unforeseen events such as delayed graft function, they may be switched to ATG. Alternatively, ATG may be chosen initially for high-risk patients, but due to intolerable side-effects, such as an allergic reaction or pancytopenia, they may be switched to basiliximab. These circumstances result in patients receiving dual-induction therapy, in partial or full dosages. How often this occurs in recipients, as well as their risk factors and outcomes, are currently unknown. Receiving dual-induction therapy may result in complications related to over immunosuppression, including hospitalizations, infections, and cancers. Identifying those at risk for receiving dual-induction therapy may help prevent unnecessary immunosuppression by initiating ATG for induction, while decreasing the risk of delayed graft function. In this study, we examined the unplanned use of dual-induction therapy in kidney transplant recipients at our center.
Materials and Methods
Design and Setting
We conducted a retrospective cohort study at the University of Alberta Hospital in Edmonton, Canada. The University of Alberta Hospital maintains an active multiorgan transplant program and performs approximately 80 to 100 kidney transplantations per year.6,7 We followed guidelines for the reporting of observational studies (Supplemental Table S1)8 and a protocol approved by the research ethics board at the University of Alberta, with a waiver of patient consent.
Data Source
We used the prospectively maintained transplant electronic medical record (OTTR, Organ Transplant Tracking Record) at the University of Alberta Hospital in Edmonton, Canada. We ascertained baseline patient characteristics, covariate information, and outcome data from the OTTR records. The OTTR database contains demographic data, vital statistics, and transplant-related information, including type and dose of induction therapy used, type of kidney donor, degree of human leukocyte antigen (HLA) mismatch, and kidney biopsy pathology results including treatment. Basiliximab is routinely given as an intravenous (IV) infusion of 20 mg on day 0 and 4 posttransplant, whereas ATG is given as a daily IV infusion for a target total dose of 6 to 8 mg/kg divided over the first 3 to 7 days.9 The dose of ATG may be limited by side-effects including cytokine-release syndrome or leukopenia and thus, the cumulative dose of ATG induction therapy can vary per patient based on their weight and drug tolerability.9,10 When missing in OTTR, the cumulative dose of ATG given for induction was retrospectively collected from the kidney transplant recipient’s medical chart. Delayed graft function was defined as the receipt of dialysis within the first week of transplantation (yes/no).
Population
We included all adult kidney transplant recipients (≥18 years old), who received a kidney-only transplant at the University of Alberta Hospital between September 1, 2013, and December 31, 2018, in Edmonton, Canada (Supplemental Figure S1). We excluded pediatric recipients (<18 years old) and those who had received a simultaneous multiorgan transplant (eg, kidney-pancreas), as these patients are primarily managed by services other than the adult Kidney Transplant Service.
Induction Therapy
At the University of Alberta Hospital, basiliximab is considered the first-line induction agent and ATG is reserved for recipients who are at high risk of delayed graft function and/or rejection based on clinical judgment and consideration of recipient and donor characteristics. Induction therapy within the first week of transplant was ascertained from OTTR and categorized as: basiliximab alone, ATG alone, or basiliximab and ATG (dual induction). Standard maintenance immunosuppression consists of combination triple-therapy with prednisone, a calcineurin inhibitor (CNI; typically, tacrolimus), and an antiproliferative agent (typically mycophenolate).
Outcomes
Recipients were followed from their transplant date until death, end of study (July 31, 2019), or the outcome of interest. We determined the frequency of each type of induction therapy regimen and the cumulative dose of basiliximab and ATG, where appropriate. For patients who received dual-induction therapy, we calculated whether they received partial or full dosages of basiliximab and ATG. We compared the median length of hospital stay in days following transplant surgery between the induction groups.
We also compared graft function between the different induction therapy regimens. The estimated glomerular filtration rate (eGFR) at 1-year posttransplant was based on the mean of all serum creatinine values within 3 months before and after the 1-year posttransplant date using the CKD-EPI equation (Chronic Kidney Disease-Epidemiology Collaboration).11 The 1-year eGFR was categorized based on the 2012 KDIGO categories of chronic kidney disease as ≥90, 60 to 89, 45 to 59, 30 to 44, 15 to 29, and <15 mL/min per 1.73 m2.12
We also looked at all-cause graft failure (defined as a composite of death with a functioning graft or return to dialysis or re-transplantation), death-censored graft failure, all-cause mortality, and death with a functioning graft (defined as posttransplant death without evidence of return to dialysis or re-transplantation). Finally, we captured episodes of biopsy-proven rejection and biopsy-proven rejection receiving treatment.
Statistical Analyses
Differences between induction groups were compared using chi-square test for categorical variables and Kruskal-Wallis tests for continuous variables. To quantify the degree to which variance in induction therapy type is explained by recipient and transplant factors, we performed multivariable logistic regression modeling with type of induction therapy as the dependent variable and the case-level factors as the predictors (adjusted odds ratio). We assessed the relative likelihood of using each induction regimen compared with basiliximab alone in pairwise comparisons, since it is considered first-line therapy.
The eGFR and the length of initial hospital stay following transplant between the groups were compared using Kruskal-Wallis tests. We measured time-to-event between the induction groups. If there were multiple episodes of rejection during follow-up, we only considered the first event. We estimated the Kaplan-Meier failure functions and used log-rank tests to assess statistical significance of differences in unadjusted incidence across induction therapy types. In a sensitivity analysis, we compared cumulative incidence functions using a Fine and Gray competing risk regression model to determine whether death and graft failure were important competing risks for the outcomes.13 The proportional hazard assumption was evaluated and satisfied by examining plots of the log-negative-log within-group survivorship functions versus log-time. A 2-tailed P value <.05 was considered statistically significant. Statistical analyses were performed using Statistical Analysis Software (STATA) version 15 (STATA Corporation, College Station, TX).
Results
Baseline Characteristics
Between 2013 and 2018, there were 430 adult kidney-only transplantations (302 deceased donor transplants, 128 living donor transplants) performed at our center. The mean follow-up was 3.9 years (standard deviation 1.5). Overall, the median age of the recipients was 54 years (interquartile range [IQR] = 42-62), 30% were female, and 68% were Caucasian (Table 1). Glomerulonephritis was the most common cause of kidney failure (41%), followed by diabetes mellitus (20%). Hemodialysis was the most common modality pretransplant (63%) and recipients had a median dialysis duration of 3.1 years (IQR = 2.2-4.1).
Table 1.
Recipient and Transplant Characteristics According to the Type of Induction Therapy Used.
| Characteristic | Overall (n = 430) | Basiliximab alone (n = 305) | ATG alone (n = 93) | Basiliximab + ATG (n = 32) |
|---|---|---|---|---|
| Recipients factors | ||||
| Age (years) | 54.0 [42.0-62.0] | 53.0 [40.0-62.0] | 57.0 [47.0-63.0]* | 58.0 [44.0-63.5] |
| >65 years | 58 (13.5) | 39 (12.8) | 15 (16.1) | 4 (12.5) |
| Female sex | 128 (29.8) | 85 (27.9) | 35 (37.6) | 8 (25.0) |
| Race | ||||
| Caucasian | 292 (67.9) | 209 (68.5) | 62 (66.7) | 21 (65.6) |
| Black | 16 (3.7) | 9 (3.0) | 5 (5.4) | 2 (6.3) |
| Asian | 28 (6.5) | 21 (6.9) | 7 (7.5) | 0 (0.0) |
| Othera | 87 (20.2) | 60 (19.7) | 18 (19.4) | 9 (28.1) |
| Missing | 7 (1.6) | 6 (2.0) | 1 (1.1) | 0 (0.0) |
| BMI (kg/m2) | 26.6 [23.1-30.3] | 26.5 [23.0-30.1] | 26.1 [23.1-29.2] | 29.0 [24.2-33.5]* |
| Underweight (<18.5) | 14 (3.3) | 10 (3.3) | 3 (3.2) | 1 (3.1) |
| Normal (18.5-24.9) | 148 (34.4) | 107 (35.1) | 32 (34.4) | 9 (28.1) |
| Overweight (25.0-29.9) | 154 (35.8) | 109 (35.7) | 38 (40.9) | 7 (21.9) |
| Obese (≥30) | 113 (26.3) | 78 (25.6) | 20 (21.5) | 15 (46.9)* |
| Missing | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Primary cause of end-stage kidney disease | ||||
| Glomerulonephritis | 178 (41.4) | 138 (45.2) | 34 (36.6) | 6 (18.8)* |
| Diabetes mellitus | 86 (20.0) | 54 (17.7) | 18 (19.4) | 14 (43.8)** |
| Polycystic kidney disease | 48 (11.2) | 32 (10.5) | 13 (14.0) | 3 (9.4) |
| Hypertension | 10 (2.3) | 7 (2.3) | 2 (2.2) | 1 (3.1) |
| Other | 101 (23.5) | 70 (23.0) | 24 (25.8) | 7 (21.9) |
| Missing | 7 (1.6) | 4 (1.3) | 2 (2.2) | 1 (3.1) |
| Pretransplant dialysis modality | ||||
| Hemodialysis | 269 (62.6) | 175 (57.4) | 71 (76.3)** | 23 (71.9) |
| Peritoneal dialysis | 109 (25.3) | 83 (27.2) | 19 (20.4) | 7 (21.9) |
| Pre-emptive | 43 (10.0) | 40 (13.1) | 2 (2.2)* | 1 (3.1) |
| Missing | 9 (2.1) | 7 (2.3) | 1 (1.1) | 1 (3.1) |
| Dialysis duration (years) | 3.1 [2.2-4.1] | 3.1 [2.2-5.2] | 3.0 [2.3-5.0] | 3.1 [2.2-4.4] |
| ABO blood group | ||||
| A | 175 (40.7) | 118 (38.7) | 43 (46.2) | 14 (43.8) |
| B | 63 (14.7) | 41 (13.4) | 19 (20.4) | 3 (9.4) |
| O | 164 (38.1) | 122 (40.0) | 29 (31.2) | 13 (40.6) |
| AB | 28 (6.5) | 24 (7.9) | 2 (2.2) | 2 (6.3) |
| cPRA (%) | 0.0 [0.0-38.0] | 0.0 [0.0-27.0] | 52.0 [0.0-98.0]*** | 2.0 [0.0-36.0] |
| 0 | 209 (48.6) | 171 (56.1) | 24 (25.8)*** | 14 (43.8) |
| 1-9 | 36 (8.4) | 25 (8.2) | 8 (8.6) | 3 (9.4) |
| 10-79 | 111 (25.8) | 79 (25.9) | 24 (25.8) | 8 (25.0) |
| ≥80 | 48 (11.2) | 11 (3.6) | 33 (35.5)*** | 4 (12.5)* |
| Missing | 26 (6.0) | 19 (6.2) | 4 (4.3) | 3 (9.4) |
| Comorbidities | ||||
| Previous organ transplant | 14 (3.3) | 10 (3.3) | 3 (3.2) | 1 (3.1) |
| Hypertension | 382 (88.8) | 273 (89.5) | 80 (86.0) | 29 (90.6) |
| Diabetes mellitus | 138 (32.1) | 93 (30.5) | 28 (30.1) | 17 (53.1)* |
| Myocardial infarction | 26 (6.0) | 17 (5.6) | 5 (5.4) | 4 (12.5) |
| Cerebrovascular accident | 25 (5.8) | 19 (6.2) | 4 (4.3) | 2 (6.3) |
| Peripheral vascular disease | 26 (6.0) | 14 (4.6) | 5 (5.4) | 7 (21.9)** |
| Chronic obstructive pulmonary disease | 30 (7.0) | 20 (6.6) | 7 (7.5) | 3 (9.4) |
| Malignancy | 58 (13.5) | 34 (11.1) | 18 (19.4)* | 6 (18.8) |
| Maintenance immunosuppressionb | ||||
| Tac + MPA/MMF/AZA + Pred | 411 (95.6) | 291 (95.4) | 89 (95.7) | 31 (96.9) |
| Tac + MPA/MMF/AZA | 9 (2.1) | 8 (2.6) | 0 (0.0) | 1 (3.1) |
| Tac or Tac + Pred | 7 (1.6) | 6 (2.0) | 1 (1.1) | 0 (0.0) |
| Other | 1 (0.2) | 0 (0.0) | 1 (1.1) | 0 (0.0) |
| Missing | 2 (0.5) | 0 (0.0) | 2 (2.2)* | 0 (0.0) |
| Transplant factors | ||||
| Transplant era | ||||
| 2013-2015 | 177 (41.2) | 116 (38.0) | 42 (45.2) | 19 (59.4)* |
| 2016-2018 | 253 (58.8) | 189 (62.0) | 51 (54.8) | 13 (40.6)* |
| Donor type | ||||
| Neurological determination of death | 257 (59.8) | 181 (59.3) | 53 (57.0) | 23 (71.9) |
| Donation after cardiac death | 45 (10.5) | 9 (3.0) | 33 (35.5)*** | 3 (9.4) |
| Live donor | 128 (29.8) | 115 (37.7) | 7 (7.5)*** | 6 (18.8)* |
| Human leukocyte antigen mismatches | ||||
| Zero A, B, DR, DQ | 12 (2.8) | 10 (3.3) | 2 (2.2) | 0 (0.0) |
| Zero DR | 50 (11.6) | 33 (10.8) | 14 (15.1) | 3 (9.4) |
| Zero DQ | 82 (19.1) | 57 (18.7) | 21 (22.6) | 4 (12.5) |
| Donor-specific antibody | ||||
| None | 350 (81.4) | 265 (86.9) | 62 (66.7)*** | 23 (71.9)* |
| Class I only | 5 (1.2) | 0 (0.0) | 5 (5.4)*** | 0 (0.0) |
| Class II only | 4 (0.9) | 1 (0.3) | 3 (3.2)* | 0 (0.0) |
| Class I and II | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 71 (16.5) | 39 (12.8) | 23 (24.7)* | 9 (28.1)* |
| Cytomegalovirus status | ||||
| Donor (−)/recipient (−) | 95 (22.1) | 74 (24.3) | 14 (15.1) | 7 (21.9) |
| Donor (+)/recipient (−) | 76 (17.7) | 57 (18.7) | 13 (14.0) | 6 (18.8) |
| Donor (−/+)/recipient (+) | 259 (60.2) | 174 (57.0) | 66 (71.0)* | 19 (59.4) |
| Ebstein-Barr virus status | ||||
| Donor (−)/recipient (–) | 3 (0.7) | 3 (1.0) | 0 (0.0) | 0 (0.0) |
| Donor (+)/recipient (–) | 21 (4.9) | 21 (6.9) | 0 (0.0)* | 0 (0.0) |
| Donor (−/+)/recipient (+) | 406 (94.4) | 281 (92.1) | 93 (100.0)* | 32 (100.0) |
| Cold ischemia time, hours | 7.6 [3.0-13.5] | 6.2 [2.7-12.1] | 11.5 [5.9-15.9]*** | 7.5 [4.9-15.1] |
| 0-12 | 312 (72.6) | 237 (77.7) | 52 (55.9)*** | 23 (71.9) |
| 13-24 | 111 (25.8) | 64 (21.0) | 39 (41.9)*** | 8 (25.0) |
| >24 | 6 (1.4) | 4 (1.3) | 1 (1.1) | 1 (3.1) |
| Missing | 1 (0.2) | 0 (0.0) | 1 (1.1) | 0 (0.0) |
| Delayed graft functionc | 75 (17.4) | 21 (6.9) | 31 (33.3)*** | 23 (71.9)*** |
Note. Data are presented as number (%) except for age, BMI, dialysis duration, cPRA, and cold ischemia time which are presented as median [interquartile range]. ATG = antithymocyte globulin; BMI = body mass index; cPRA = calculated panel reactive antibody; Tac = tacrolimus; MPA = mycophenolic acid; MMF = mycophenolate mofetil; AZA = azathioprine; Pred = prednisone.
Included Aboriginal, Asian Indian, Filipino, Inuit, Latin American, Metis, Middle Eastern/Arabian, Other/Multiracial, and Pacific Islander.
Maintenance immunosuppression is defined as any of those drugs with an initiation date within 7 days of the transplant.
Defined as receipt of dialysis within the first week of transplant.
P values for pairwise comparison (reference to basiliximab alone): *P < .05–.002; **P = .001–.0001; ***P < .0001.
Of the 430 patients, 71% (n = 305) received basiliximab alone, 22% (n = 93) received ATG alone, and 7% (n = 32) received dual-induction therapy. Of the 32 patients who received dual-induction therapy, 25 patients received basiliximab initially, followed by ATG mostly on postoperative days 1 and 2. Five patients received both basiliximab and ATG on the same day, and 2 patients received ATG initially, followed by basiliximab on postoperative days 3 and 4. The dual-induction group received a similar dose of ATG as the ATG-alone group (mean cumulative dose per patient per kilogram [kg]: 5.8 vs. 6.3 mg/kg, respectively, P = .4; range of cumulative dose per patient per kg: 1.4-9.1 mg/kg vs. 2.2-14.9 mg/kg), and approximately half the dose of basiliximab as the basiliximab alone group (24 mg vs. 40 mg, P < .0001). Cumulative dose data were missing for 1 patient who received basiliximab and 5 patients who received ATG.
Correlates of Dual-Induction Therapy Use
Compared with recipients who received basiliximab alone, recipients who received dual-induction therapy were more often obese (body mass index [BMI] ≥ 30 kg/m2: 47% vs. 26%, P = .01), had diabetes mellitus as the primary cause of kidney failure (44% vs. 18%, P = .0005) and as a co-morbidity (53% vs. 31%, P = .01), had peripheral vascular disease (22% vs. 5%, P = .0001), had a calculated panel reactive antibody level (cPRA) ≥80% (13% vs. 4%, P = .02), and were more likely to experience delayed graft function (72% vs. 7%, p < .0001; Table 1). When comparing recipients of dual-induction therapy versus ATG alone, results were similar except the ATG alone recipients were more likely to have a cPRA ≥80% (36% vs. 13%, P = .01).
After adjusting for age and sex, compared to the basiliximab alone group, recipients were more likely to receive dual induction if they were sensitized (cPRA ≥80%; adjusted odds ratio [aOR] 5.02, 95% confidence interval [CI] 1.38-18.25), had diabetes mellitus (aOR 2.46, 95% CI 1.16-5.21) or peripheral vascular disease (aOR 5.40, 95% CI 1.86-15.68), had missing donor-specific antibody (DSA) results (aOR 2.71, 95% CI 1.15-6.41), or met criteria for delayed graft function (aOR 37.16, 95% CI 14.39-95.97; Table 2). Conversely, they were less likely to receive dual induction if they had glomerulonephritis as the cause of kidney failure versus diabetes mellitus (aOR 0.17, 95% CI 0.06-0.46), if they received a living donor transplant versus neurological determination of death (NDD) donor transplant (aOR 0.17, 95% CI 0.04-0.84), or if they received a transplant in 2016 to 2018 versus 2013 to 2015 (aOR 0.41, 95% CI 0.19-0.88). After adjusting for delayed graft function in addition to age and sex, results were similar, except diabetes mellitus, peripheral vascular disease, and living donor transplant were no longer significant (Supplemental Table S2).
Table 2.
Adjusted Associations of Recipient and Transplant Characteristics With Dual-Induction Therapy Compared to Basiliximab Alone.
| Characteristic | Age and sex-adjusted odds ratio (95% confidence interval) |
|---|---|
| Recipients factors | |
| Age (years) | 1.02 (0.99-1.04) |
| >65 years | 0.97 (0.32-2.94) |
| Female sex | 0.87 (0.38-2.03) |
| Race | |
| Caucasian | Reference |
| Black | 2.00 (0.42-9.44) |
| Asian | N/A |
| Othera | 1.45 (0.63-3.33) |
| Missing | N/A |
| Body mass index (kg/m2) | 1.06 (0.99-1.14) |
| Underweight (<18.5) | Reference |
| Normal (18.5-24.9) | 0.68 (0.08-6.03) |
| Overweight (25.0-29.9) | 0.44 (0.04-4.36) |
| Obese (≥30) | 1.34 (0.15-12.23) |
| Missing | N/A |
| Primary cause of end-stage kidney disease | |
| Glomerulonephritis | 0.17 (0.06-0.46)** |
| Diabetes mellitus | Reference |
| Polycystic kidney disease | 0.36 (0.10-1.36) |
| Hypertension | 0.55 (0.06-4.83) |
| Other | 0.40 (0.15-1.08) |
| Missing | 0.94 (0.10-9.14) |
| Pretransplant dialysis modality | |
| Hemodialysis | Reference |
| Peritoneal dialysis | 0.65 (0.27-1.57) |
| Pre-emptive | 0.19 (0.02-1.48) |
| Missing | 1.20 (0.14-9.91) |
| Dialysis duration (years) | 1.16 (0.91-1.47) |
| ABO blood group | |
| A | Reference |
| B | 0.56 (0.15-2.14) |
| O | 0.86 (0.39-1.91) |
| AB | 0.71 (0.16-3.25) |
| cPRA (%) | 1.01 (1.00-1.02) |
| 0 | Reference |
| 1-9 | 1.65 (0.43-6.38) |
| 10-79 | 1.23 (0.50-3.05) |
| ≥80 | 5.02 (1.38-18.25)* |
| Missing | 1.75 (0.47-6.53) |
| Co-morbidities | |
| Previous organ transplant | 0.93 (0.11-7.66) |
| Hypertension | 1.01 (0.28-3.66) |
| Diabetes mellitus | 2.46 (1.16-5.21)* |
| Myocardial infarction | 2.11 (0.66-6.75) |
| Cerebrovascular accident | 0.91 (0.20-4.08) |
| Peripheral vascular disease | 5.40 (1.86-15.68)** |
| Chronic obstructive pulmonary disease | 1.26 (0.35-4.56) |
| Malignancy | 1.63 (0.61-4.37) |
| Maintenance immunosuppressionb | |
| Tac + MPA/MMF/AZA + Pred | Reference |
| Tac + MPA/MMF/AZA | 1.10 (0.13-9.38) |
| Tac or Tac + Pred | N/A |
| Other | N/A |
| Missing | N/A |
| Transplant factors | |
| Transplant era | |
| 2013-2015 | Reference |
| 2016-2018 | 0.41 (0.19-0.88)* |
| Donor type | |
| Neurological determination of death | Reference |
| Donation after cardiac death | 0.40 (0.10-1.59) |
| Live donor | 0.17 (0.04-0.84)* |
| Human leukocyte antigen mismatches | |
| Zero A, B, DR, DQ | N/A |
| Zero DR | 0.92 (0.26-3.25) |
| Zero DQ | 0.65 (0.21-2.00) |
| Donor-specific antibody | |
| None | Reference |
| Class I only | N/A |
| Class II only | N/A |
| Class I and II | N/A |
| Missing | 2.71 (1.15-6.41)* |
| Cytomegalovirus status | |
| Donor (−)/recipient (−) | Reference |
| Donor (+)/recipient (−) | 1.13 (0.36-3.55) |
| Donor (−/+)/recipient (+) | 1.08 (0.42-2.77) |
| Ebstein-Barr virus status | |
| Donor (−)/recipient (−) | N/A |
| Donor (+)/recipient (−) | N/A |
| Donor (−/+)/recipient (+) | N/A |
| Cold ischemia time, hours | 1.03 (0.98-1.09) |
| 0-12 | Reference |
| 13-24 | 1.20 (0.50-2.89) |
| >24 | 2.32 (0.25-21.55) |
| Missing | N/A |
| Delayed graft functionc | 37.16 (14.39-95.97)*** |
Note. N/A: Unable to estimate odds ratio due to 0 values in the cells. N/A = not available; cPRA = calculated panel reactive antibody; Tac = tacrolimus; MPA = mycophenolic acid; MMF = mycophenolate mofetil; AZA = azathioprine; Pred = prednisone.
Included Aboriginal, Asian Indian, Filipino, Inuit, Latin American, Metis, Middle Eastern/Arabian, Other/Multiracial, and Pacific Islander.
Maintenance immunosuppression is defined as any of those drugs with an initiation date within 7 days of the transplant.
Defined as receipt of dialysis within the first week of transplant.
P values (reference to basiliximab alone): *P < .05–.002; **P = .001–.0001; ***P < .0001.
Outcomes of Dual-Induction Therapy Use
Recipients who were administered dual-induction therapy had a longer median length of hospital stay compared to those who received basiliximab alone (19 vs. 9 days, P = .001) or ATG alone (19 vs. 13 days, P = .1), although the latter did not reach statistical significance (Table 3). Over a median of 2.8 years (IQR 1.7-4.3) of follow-up, there were 43 (10%) all-cause graft failure, 15 (4%) death-censored graft failure, 34 (8%) all-cause mortality, and 28 (7%) deaths with a functioning graft (Table 3). Compared to the ATG alone group, the dual-induction group had worse graft function at 1 year (mean creatinine 226 µmol/L vs. 129 µmol/L, P = .0004; mean eGFR 42 vs. 59 mL/min/1.73 m2, P = .0008), and increased risk of all-cause graft failure (31% vs. 13%, P = .02) and death-censored graft failure (16% vs. 4%, P = .03) after a follow-up of 3 years (Table 3). There was no significant difference in the incidence of biopsy-proven rejection, biopsy-proven rejection receiving treatment, or death (all-cause mortality or death with a functioning graft) between the dual-induction group versus the ATG alone group, although the number of events was small.
Table 3.
Outcomes for Kidney Transplant Recipients According to the Type of Induction Therapy Used.
| Outcomes | Overall (n = 430) | Basiliximab alone (n = 305) | ATG alone (n = 93) | Basiliximab + ATG (n = 32) |
|---|---|---|---|---|
| Median [interquartile range] length of initial hospitalization (days) | ||||
| Length of stay | 10.0 [7.0-61.0] | 9.0 [7.0-42.0] | 13.0 [8.0-111.0] | 19.0 [12.0-93.5] |
| Mean (standard deviation) graft function at 1 yeara | ||||
| Serum creatinine (µmol/L) | 134.5 (92.3) | 127.1 (68.5) | 129.4 (64.7) | 226.1 (231.1)* |
| Missing | 18 (4.2) | 8 (2.6) | 7 (7.5) | 3 (9.4) |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 58.7 (20.4) | 60.2 (19.6) | 59.0 (21.4) | 42.4 (19.3)* |
| ≥90 | 28 (6.5) | 22 (7.2) | 6 (6.5) | 0 (0.0) |
| 60-89 | 174 (40.5) | 129 (42.3) | 39 (41.9) | 6 (18.8)* |
| 45-59 | 105 (24.4) | 80 (26.2) | 17 (18.3) | 8 (25.0) |
| 30-44 | 68 (15.8) | 46 (15.1) | 13 (14.0) | 9 (28.1) |
| 15-29 | 31 (7.2) | 19 (6.2) | 10 (10.8) | 2 (6.3) |
| <15 | 6 (1.4) | 1 (0.3) | 1 (1.1) | 4 (12.5)* |
| Missing | 18 (4.2) | 8 (2.6) | 7 (7.5) | 3 (9.4) |
| Biopsy-proven rejectionb | ||||
| None | 401 (93.3) | 281 (92.1) | 90 (96.8) | 30 (93.8) |
| Overall | 29 (6.7) | 24 (7.9) | 3 (3.2) | 2 (6.3) |
| 0-1 week | 3 (0.7) | 2 (0.7) | 0 (0.0) | 1 (3.1) |
| >1 week-6 months | 19 (4.4) | 16 (5.2) | 2 (2.2) | 1 (3.1) |
| >6 months-1 year | 5 (1.2) | 4 (1.3) | 1 (1.1) | 0 (0.0) |
| >1 year | 2 (0.5) | 2 (0.7) | 0 (0.0) | 0 (0.0) |
| Biopsy-proven rejection receiving treatmentb | ||||
| Any treatment | 27 (6.3) | 23 (7.5) | 2 (2.2) | 2 (6.3) |
| Pulse steroids | 27 (6.3) | 23 (7.5) | 2 (2.2) | 2 (6.3) |
| ATG | 5 (1.2) | 4 (1.3) | 0 (0.0) | 1 (3.1) |
| Intravenous immune globulin | 7 (1.6) | 5 (1.6) | 1 (1.1) | 1 (3.1) |
| Plasmapheresis | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Rituximab | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (0.5) | 1 (0.3) | 1 (1.1) | 0 (0.0) |
| Graft failure | ||||
| All-cause graft failure | 43 (10.0) | 21 (6.9) | 12 (12.9) | 10 (31.3)* |
| Death-censored graft failure | 15 (3.5) | 6 (2.0) | 4 (4.3) | 5 (15.6)* |
| Death | ||||
| All-cause mortality | 34 (7.9) | 17 (5.6) | 10 (10.8) | 7 (21.9) |
| Death with a functioning graft | 28 (6.5) | 15 (4.9) | 8 (8.6) | 5 (15.6) |
Note. Data are presented as number (%) unless otherwise specified. ATG = antithymocyte globulin.
Based on the mean of all serum creatinine values within 3 months of the 1-year posttransplant date.
Although recipients could have more than one episode of biopsy-proven rejection in follow-up, only the first episode of rejection is reported here (23 recipients had 1 rejection, 5 recipients had 2 rejections, and 1 recipient had 3 rejections in follow-up).
P values for pairwise comparison (reference to ATG alone): *P < .05–.002; *P = .001–.0001; ***P < .0001.
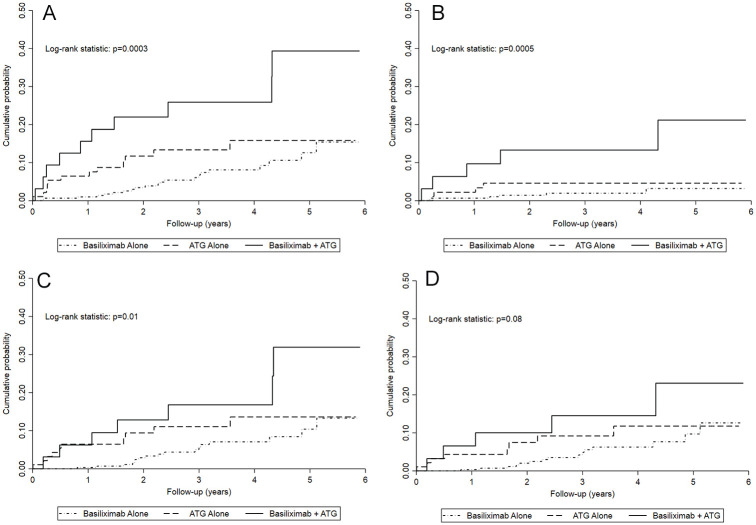
Cumulative probability of all-cause graft failure, death-censored graft failure, and all-cause mortality were significantly different between the dual induction, ATG alone, and basiliximab alone groups, but not for death with a functioning graft (Figure 1). Compared to the basiliximab-alone group, the dual-induction group had a significantly higher cumulative probability of all outcomes (all-cause graft failure, death-censored graft failure, all-cause death, and death with a functioning graft; Supplemental Table S3). Compared to the ATG alone group, the dual-induction group had a significantly higher cumulative probability of all-cause graft failure and death-censored graft failure, but not all-cause death or death with a functioning graft (Supplemental Table S3). Cumulative incidence functions from unadjusted competing risk models for death-censored graft failure and death with a functioning graft showed similar findings (Supplemental Figure S2).
Figure 1.
Kaplan-Meier event curves by type of induction therapy used for (A) all-cause graft failure (composite); (B) death-censored graft failure; (C) all-cause mortality, and (D) death with a functioning graft.
Note. P values from a log-rank statistic to compare the types of induction therapy used. ATG = antithymocyte globulin.
Discussion
In this single-center study, we found that the incidence of dual-induction therapy with basiliximab and ATG is 7%. Approximately 1 out of 10 recipients who were treated with basiliximab induction also received ATG induction, and most of those who received dual-induction therapy started with basiliximab followed by ATG. Development of delayed graft function, presence of diabetes mellitus and peripheral vascular disease, high immunologic risk with cPRA ≥80%, and missing DSA results were associated with receiving dual-induction therapy. Compared to those who received ATG alone, recipients of dual-induction therapy had worse all-cause graft failure and death-censored graft failure.
Our data suggest that most of those who received dual-induction therapy were initially deemed to be low immunologic risk and started on basiliximab. Between the first and second dose of basiliximab, they likely developed delayed graft function prompting the conversion to a standard course of ATG induction. This occurred despite no evidence of an increased rate of biopsy-proven rejection within the first week in the dual-induction group. This suggests that the addition of ATG was used to either prevent or treat presumed rejection, or to avoid the need for a biopsy to rule out rejection, rather than for the treatment of biopsy-proven rejection itself. Only a small number of recipients in the dual-induction group were administered basiliximab and ATG on the same day, or with ATG first followed by basiliximab. We suspect that this was likely due to medication intolerance, such as an allergic reaction.
In our study, dual-induction therapy was associated with worse graft function at 1 year, longer hospital stays, and a higher risk of graft failure compared to either agent alone, despite no evidence of increased biopsy-proven rejection. However, the worse outcomes related to dual-induction therapy are likely not due to the added exposure of basiliximab and ATG, but rather to confounding by indication, wherein the adverse events were related to the indication for dual-induction therapy, rather than the induction therapy itself. The high proportion of delayed graft function in recipients treated with dual-induction therapy indicates that this was likely the underlying indication for dual induction. Although there was no significant difference in biopsy-proven rejection between the induction regimens, the total number of events were low. This may be a reflection of under-diagnosis of acute rejection, as recipients treated with dual induction for delayed graft function may not have had a kidney biopsy prior to treatment, or may have had a biopsy after treatment with dual-induction therapy.
In addition, the dual-induction therapy group received similar cumulative doses of ATG as the ATG alone group, but only half the dose of basiliximab as the basiliximab alone group, making the dual-induction therapy itself unlikely to account for the worse clinical outcomes. In fact, some centers have used dual-induction therapy without significant adverse events, namely with a protocol consisting of standard-dose interleukin-2 receptor antibody (ie, basiliximab or daclizumab) but reduced dose of ATG (eg, 1 mg/kg/day × 3 days14 or 200 mg total dose over 3 days).15,16 One pilot randomized study compared basiliximab (20 mg on day 0 and 4) and low-dose ATG (0.5 mg/kg/day × 7 days; n = 17) versus standard-dose ATG alone (2 mg/kg/day × 7 days; n = 16) in 33 high-risk kidney transplant recipients (maintenance therapy consisted of steroids, cyclosporin A, and azathioprine or mycophenolate mofetil).17 At 6 months, patient and graft survival were similar between the 2 groups; however, the basiliximab and low-dose ATG group had fewer complications, such as fever, leukopenia, and cytomegalovirus reactivations, and lower costs (€3000 savings [$3300 USD]) compared to standard-dose ATG alone induction group.
Delayed graft function and acute rejection are important prognostic markers for posttransplant outcomes. Both delayed graft function and acute rejection are associated with an increased risk of graft failure with a reported 5-year graft survival of only 35% when both conditions are present.18,19 Although delayed graft function and acute rejection provide prognostic information, they are of limited clinical use for choosing an appropriate induction therapy since they occur after the induction therapy has already been chosen and administered. Therefore, other predictors for the use of dual-induction therapy are clinically important. In our study, recipients with diabetes mellitus and peripheral vascular disease were associated with receiving dual-induction therapy. Indeed, diabetes mellitus and peripheral vascular disease are known recipient risk factors for delayed graft function, possibly due to acute tubular necrosis.20,21 Therefore, recipients with of diabetes mellitus or peripheral vascular disease may be at higher risk of delayed graft function secondary to acute tubular necrosis, leading to the receipt of dual-induction therapy.
We also found that recipients with cPRA ≥80% and missing DSA results were associated with receiving dual-induction therapy. These recipients may be at higher risk of immune-mediated delayed graft function leading to the use of dual-induction therapy. Despite being sensitized, ATG may have been initially avoided in recipients with cPRA ≥80% if no DSA were being crossed, and/or if there was a perceived increased risk of infection or cancer (eg. EBV mismatch, history of posttransplant lymphoproliferative disorder). It is less likely that the use of dual-induction therapy was planned in partial or full dosages as that is not standard practice at our center for highly sensitized recipients. Most patients who received dual-induction therapy in our study were administered basiliximab first, then ATG. Unfortunately, our clinical transplant database does not capture the reasons for induction therapy choices. It is also important to note that since missing DSA results could be due to the lack of data in our clinical transplant records, it is difficult to infer a direct clinical relationship between missing DSA results and the risk of receiving dual-induction therapy.
Identifying those at risk for receiving dual-induction therapy is clinically important, as it may lead to ATG induction therapy upfront, reducing overall immunosuppression exposure and decreasing length of hospital stay. There are also added costs related to the induction therapies themselves, with the total cost of induction therapy ranging from $5000-$15 000 USD, with ATG being up to twice the cost of basiliximab.22 Note, this cost varies substantially with much higher costs reported at different centers.5,17,23 Most recipients treated with ATG also require prophylaxis against cytomegalovirus at further cost. Thus, dual-induction therapy could add significant costs to the health care system compared to an appropriate single-induction agent. Importantly, our study found that dual-induction therapy was associated with worse all-cause graft failure and death-censored graft failure compared to those who received ATG alone. In addition, compared to basiliximab induction only, ATG has been associated with a 30% decrease in acute rejection at 1 year.4 Whether or not ATG therapy upfront compared to dual-induction therapy can improve posttransplant outcomes requires further interventional studies.
Our study is the largest and first to describe the unplanned use of dual-induction therapy in a contemporary cohort of kidney transplant recipients. It is also the first to compare basiliximab and standard-dose ATG induction with standard-dose basiliximab or ATG alone in kidney-only transplant recipients. There are limitations worth noting. The use of our prospectively collected data in OTTR comprehensively captures transplant-related variables that are not routinely found in national transplant databases, such as dose of induction therapy received, the occurrence of biopsy-proven rejection, and detailed information about treatments. Unfortunately, there may be potential confounders that are not as reliably ascertained from OTTR due to under-reporting. Thus, there may be an underestimation of the burden of disease and confounders in our study. As previously discussed, there is a potential for confounding by indication. Patients who received dual-induction therapy likely had worse outcomes due to the indication for dual-induction therapy (such as delayed graft function), rather than the therapy itself. Donor data were limited, and therefore, we could not assess for any association between the donor quality and outcomes. However, there are still advantages to using observational studies to assess drug efficacy and safety in real-world contemporary practice, particularly for long-term adverse events, such as mortality and graft loss.24 Since these are observational studies, we are only able to describe associations of risk factors and outcomes of dual-induction therapy. We cannot infer that interventions aimed at reducing these risks will improve outcomes. Likewise, it is unclear if the patients in our dual-induction therapy group would have had improved outcomes if they had been initially treated with ATG alone. Finally, given that the study is based on data from a single center with a predominantly Caucasian population, it may not be generalizable to other centers with different patient populations.
In conclusion, we found that patients who received dual-induction therapy had worse graft function at 1 year and worse all-cause and death-censored graft failure. This may be due to confounding by indication, as delayed graft function was a strong predictor for receiving dual-induction therapy. Other predictors for requiring dual-induction therapy, specifically before the development of delayed graft function, may be useful for choosing ATG upfront. In our study, these predictors included presence of diabetes mellitus, peripheral vascular disease, cPRA ≥80%, and missing DSA results. With more medically complex transplants being performed, it is important to select the optimal induction therapy for recipients. Further research is needed to develop robust risk stratification and monitoring tools to assist in clinical decision making and improve short- and long-term outcomes for kidney transplant recipients.
Supplemental Material
Supplemental material, RTx_Dual_Induction_-_Supplementary_Material_-_20200807 for Incidence, Risk Factors, and Outcomes of Kidney Transplant Recipients Treated With Both Basiliximab and Antithymocyte Globulin by Rachel Jeong, Robert R. Quinn, Krista L. Lentine, Pietro Ravani, Feng Ye, Patricia Campbell, Kevin Wen, Chris Broscheit, Sita Gourishankar and Ngan N. Lam in Canadian Journal of Kidney Health and Disease
Acknowledgments
This study is based in part, on data provided by Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of Alberta Health Services. Alberta Health Services does not express any opinion in relation to this study.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval was obtained from the research ethics board at the University of Alberta, with a waiver of patient consent.
Consent for Publication: All authors reviewed the final manuscript and consented for publication.
Availability of Data and Materials: Data and materials may be made available upon written request to the corresponding author.
Authors’ Note: N.N.L. conceived of the study. R.J. and N.N.L. drafted the manuscript. F.Y. performed the statistical analyses and created the figures. All authors interpreted the results, revised the manuscript, and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Pietro Ravani  https://orcid.org/0000-0001-6973-8570
https://orcid.org/0000-0001-6973-8570
Ngan N. Lam  https://orcid.org/0000-0002-0129-7091
https://orcid.org/0000-0002-0129-7091
Supplemental Material: Supplemental material for this article is available online.
References
- 1. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(suppl 3):S1-S155. [DOI] [PubMed] [Google Scholar]
- 2. Knoll GA, Blydt-Hansen TD, Campbell P, et al. Canadian Society of Transplantation and Canadian Society of Nephrology commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis. 2010;56(2):219-246. [DOI] [PubMed] [Google Scholar]
- 3. Brennan DC, Daller JA, Lake KD, et al. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967-1977. [DOI] [PubMed] [Google Scholar]
- 4. Webster AC, Ruster LP, McGee RG, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev. 2010; CD003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dharnidharka VR, Naik AS, Axelrod DA, et al. Center practice drives variation in choice of US kidney transplant induction therapy: a retrospective analysis of contemporary practice. Transpl Int. 2018;31(2):198-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. University of Alberta. Renal transplantation. https://www.ualberta.ca/department-of-medicine/education/residency-programs/nephrology/rotations/renal-transplantation.html. Accessed May 11, 2020.
- 7. Edmonton Sun. University of Alberta hospital celebrates 50 years of kidney transplants. https://edmontonsun.com/2017/01/26/university-of-alberta-hospital-celebrates-50-years-of-kidney-transplants/wcm/a5d71d09-1cb9-4e61-ab1a-c475b2e32c13. Accessed May 11, 2020.
- 8. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [DOI] [PubMed] [Google Scholar]
- 9. Chapman TM, Keating GM. Basiliximab. Drugs. 2003;63:2803-2835. [DOI] [PubMed] [Google Scholar]
- 10. Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715-2729. [DOI] [PubMed] [Google Scholar]
- 11. Shaffi K, Uhlig K, Perrone RD, et al. Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis. 2014;63(6):1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kidney Disease: improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. [Google Scholar]
- 13. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. [Google Scholar]
- 14. Ciancio G, Gaynor JJ, Guerra G, et al. Randomized trial of rATg/daclizumab vs rATg/Alemtuzumab as dual induction therapy in renal transplantation: Results at 8 years of follow-up. Transpl Immunol. 2017;40:42-50. [DOI] [PubMed] [Google Scholar]
- 15. Favi E, Gargiulo A, Spagnoletti G, et al. Induction with basiliximab plus thymoglobulin is effective and safe in old-for-old renal transplantation: six-month results of a prospective clinical study. Transplant Proc. 2010;42(4):1114-1117. [DOI] [PubMed] [Google Scholar]
- 16. Spagnoletti G, Salerno MP, Calia R, Romagnoli J, Citterio F. Thymoglobuline plus basiliximab a mixed cocktail to start? Transpl Immunol. 2017;43-44:1-2. [DOI] [PubMed] [Google Scholar]
- 17. Ruggenenti P, Codreanu I, Cravedi P, Perna A, Gotti E, Remuzzi G. Basiliximab combined with low-dose rabbit anti-human thymocyte globulin: a possible further step toward effective and minimally toxic T cell–targeted therapy in kidney transplantation. Clin J Am Soc Nephrol. 2006;1(3):546-554. [DOI] [PubMed] [Google Scholar]
- 18. Gaston RS, Fieberg A, Hunsicker L, et al. Late graft failure after kidney transplantation as the consequence of late versus early events. Am J Transplant. 2018;18(5):1158-1167. [DOI] [PubMed] [Google Scholar]
- 19. Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968-974. [DOI] [PubMed] [Google Scholar]
- 20. Mannon RB. Delayed graft function: the AKI of kidney transplantation. Nephron. 2018;140(2):94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danovitch GM. Handbook of Kidney Transplantation. 6th ed. Philadelphia, PA: Wolters Kluwer; 2017. [Google Scholar]
- 22. James A, Mannon RB. The cost of transplant immunosuppressant therapy: is this sustainable? Curr Transplant Reports. 2015;2:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sageshima J, Ciancio G, Guerra G, et al. Prolonged lymphocyte depletion by single-dose rabbit anti-thymocyte globulin and alemtuzumab in kidney transplantation. Transpl Immunol. 2011;25(2-3):104-111. [DOI] [PubMed] [Google Scholar]
- 24. Suissa S, Henry D, Caetano P, et al. CNODES: the Canadian Network for Observational Drug Effect Studies. Open Med. 2012;6:e134-e140. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, RTx_Dual_Induction_-_Supplementary_Material_-_20200807 for Incidence, Risk Factors, and Outcomes of Kidney Transplant Recipients Treated With Both Basiliximab and Antithymocyte Globulin by Rachel Jeong, Robert R. Quinn, Krista L. Lentine, Pietro Ravani, Feng Ye, Patricia Campbell, Kevin Wen, Chris Broscheit, Sita Gourishankar and Ngan N. Lam in Canadian Journal of Kidney Health and Disease