Abstract
Background: Sodium hypochlorite (NaOCl) is the most commonly used irrigant in endodontics . The purpose of this study was to evaluate the effect of NaOCl solution (2.5%) and gel (3%) with/without passive ultrasonic irrigation (PUI) on Enterococcus faecalis, Escherichia coli, and their endotoxins, lipopolysaccharide (LPS) and lipoteichoic acid (LTA).
Methods: 40 human lower premolars were contaminated with E. coli (ATCC 25922) for 28 days and E. faecalis (ATCC 29212) for 21 days. Specimens were randomly divided into four groups: (1) 2.5% NaOCl irrigating the canals without PUI activation; (2) 2.5% NaOCl with PUI; (3) 3% NaOCl gel irrigating the canals without PUI; and (4) 3% NaOCl gel with PUI. 40 mL of irrigant was used for each group. PUI activation was carried out using E1-Irrisonic stainless-steel tip at 10% frequency. After treatment, all specimens were filled with 3mL of 17% ethylenediaminetetraacetic acid (EDTA) for 3min and then washed with nonpyrogenic saline solution. Three samples were collected from the canals: S1, at baseline to confirm biofilm formation; S2 after treatment; and S3 after EDTA. Samples were assessed for E. coli and E. faecalis colony forming units, and LPS and LTA were assessed using chromogenic kinetic LAL assay and ELISA, respectively. Data were analyzed by Kruskal-Wallis, Friedmann and Dunn tests with α≤0.05.
Results: All groups were effective in reducing the microbial load of E. coli and E. faecalis after treatment without a significant difference among the groups. NaOCl and NaOCl gel groups had no significant difference in reducing LPS and LTA. Statistically increased reduction was seen for NaOCL + PUI and NaOCl gel + PUI compared for groups without PUI.
Conclusions: NaOCl gel has the same antimicrobial action of NaOCl solution and can partially detoxify endotoxins. PUI improves NaOCl (gel or solution) action over E. faecalis and E. coli and their endotoxins.
Keywords: Sodium hypochlorite, Passive ultrasonic irrigation, Enterococcus faecalis, Escherichia coli, Endotoxins.
Introduction
Sodium hypochlorite (NaOCl) is the most commonly used irrigant in endodontics ( Iqbal, 2012). It has been used since the second half of the 18 th century ( Sedgley, 2004) because it has antimicrobial action ( Luebke, 1967) and dissolves necrotic tissues ( Taylor & Austin, 1918).
Enterococcus faecalis is a Gram-positive bacterium found in the root canal system (RCS) and can be disinfected by NaOCl ( Siqueira et al., 2000). It may also be found in secondary infections of endodontically treated teeth ( Machado et al., 2020). In addition, Escherichia coli, a Gram-negative bacterium, is also found in endodontic infections ( Narayanan & Vaishnavi, 2010).
Bacteria have endotoxins in their outer membrane known as lipoteichoic acid (LTA) in Gram-positive bacteria ( Ginsburg, 2002) and lipopolysaccharide (LPS) in Gram-negative bacteria ( Mergenhagen & Varah, 1963). Endotoxins can be released during the duplication or death of these bacteria in infected RCS and this has a role in developing periapical lesions ( Endo et al., 2012). Endodontic treatment using NaOCl can detoxify endotoxins, but not completely ( Cavalli et al., 2017).
NaOCl is a cytotoxic substance ( Salazar-Mercado et al., 2019); overflow during endodontic treatment can cause diverse exacerbations ( Goswami et al., 2014). Thus, NaOCl gel may be safer due to its minor apical extrusion tendency ( Nesser & Bshara, 2019). Passive ultrasonic irrigation (PUI) improves RCS disinfection ( Plotino et al., 2007), removing the smear layer and vapor lock during endodontic treatment ( Bueno et al., 2019; Dioguardi et al., 2019), and permits greater penetration of irrigants to the dentinal tubules ( Sáinz-Pardo et al., 2014).
The purpose of this study was to evaluate the effect of NaOCl solution (2.5%) and gel (3%) with/without PUI on E. faecalis, E. coli, and their endotoxins, LTA and LPS, respectively.
Methods
This study was approved by the research ethics committee of São Paulo State University, Institute of Science and Technology (n o1.504.995). The teeth used in this study were obtained from clinics where teeth are donated during routine procedures and following authorization of the patients. The research team presented the terms of donation by the clinics from which the teeth where obtained to the research ethics committee when submitting the study methodology. A total of 40 human lower premolars were collected (based on dimensional and morphological similarities).
Specimen preparation
To standardize root canal diameter, the teeth were initially instrumented with a #30 K-file (Maillefer, Ballaigues, Switzerland) and irrigated with 5 mL of NaOCl 1% for each file used. The canals were dried with sterile paper points (Dentsply Ind Com LTDA, RJ, Petrópolis, Brazil) and the apical region was sealed with light-cured resin composites (3M Dental Products, St Paul, MN). The outer surfaces of the specimens were covered with two layers of epoxy adhesive (Araldite - Brascola, Sao Paulo, Brazil), except the cervical opening ( de Oliveira et al., 2007). Then they were fixed with chemically activated acrylic resin in 24-well plates and sterilized by gamma radiation with cobalt 60 (20 KGy for 6 hours) ( Csako et al., 1983).
Biofilm formation
Specimens were contaminated with E. coli (ATCC 25922) for 28 days and E. faecalis (ATCC 29212) for 21 days and incubated at 37°±1°C, following the protocol of Maekawa et al. (2013).
Experimental groups
Specimens were divided into four experimental groups (n=10/group) as follows: (1) 2.5% NaOCl (Asfer, São Caetano do Sul, São Paulo, Brazil) irrigating the canals without PUI activation; (2) 2.5% NaOCl irrigating the canals with PUI; (3) 3% NaOCl gel (Ultradent, South Jordan, UT, USA) irrigating the canals without PUI; and (4) 3% NaOCl gel irrigating the canals with PUI. All specimens were instrumented as a part of the biomechanical preparation by Reciproc R40 (VDW, Munich, Germany) following the protocol of each experimental group ( Table 1).
Table 1. Protocols for experimental groups.
NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation.
| Experimental
group |
Irrigation protocol (repeated three times in each
third of the root canal) |
Final wash |
|---|---|---|
| NaOCl | 5 mL of NaOCl 2.5% during instrumentation without
PUI and then 5 mL remained in the canal without any activation. |
10 mL of 2.5% NaOCl solution
without PUI activation. |
| NaOCl + PUI | 5 mL of NaOCl 2.5% during instrumentation without PUI
and then 5 mL activated with PUI |
10 mL of 2.5% NaOCl solution
activated with PUI. |
| NaOCl gel | Filled with 2 mL of 3% NaOCl gel and irrigated with 10
mL of saline solution during instrumentation without PUI. |
Filled with 2 mL of 3% NaOCl
gel and irrigated with 10 mL of saline solution without PUI activation. |
| NaOCl gel + PUI | Filled with 1 mL of 3% NaOCl gel and irrigated with
10mL of saline solution during instrumentation without PUI and then filled with 1 mL of 3% NaOCl gel and irrigated with 10mL of saline solution activated with PUI |
Filled with 2 mL of 3% NaOCl
gel and irrigated with 10 mL of saline solution activated with PUI. |
PUI activation was performed using an E1-Irrisonic stainless-steel tip (Helse, Santa Rosa de Viterbo, Brazil) (0.10mm in diameter) at the working length using CVDente 100 ultrasound activator (CVDentus, São José dos Campos, Brazil) at 10% frequency.
After treatment, all specimens were filled with 17% ethylenediaminetetraacetic acid (EDTA) (Biodinâmica, Ibiporã, PR, Brazil) for 3min and then washed with nonpyrogenic saline solution.
Sample collection
Three samples were collected during the experiment, as in ( Maekawa et al., 2013): S1, at baseline to confirm biofilm formation; S2, immediately after treatment; and S3, after EDTA application.
Colony forming unit (CFU/mL)
Serial dilutions of all samples were performed with sterile saline solution and aliquots of 30µl of each sample were seeded in two different culture medias: Enterococcosel agar (Becton, Dickinson and Company Sparks, MD, USA) for E. faecalis; and MacConkey agar (Himedia Laboratories, Mumbai, India) for E. coli. The plates were kept at 37°C for 24h and then CFU/mL were counted.
Quantification of LPS/LTA levels
LPS levels in each sample was assessed as in Machado et al. (2020) using kinetic chromogenic limulus amebocyte lysate assay (Lonza, Walkersville, MD, USA). The plates were incubated at 37±1°C for 10 min in a KineticQCL reader, which was coupled to a computer with the WinKQCL software (Lonza). As soon as the kinetic test started, absorbance at 405 nm was read in each microplate well and automatically calculated the log/log linear correlation between reaction time of each standard solution and corresponding endotoxin concentration.
LTA was assessed using enzyme-linked immunosorbent assay using ELISA 96-well plates (Nunc Thermo Scientific, Waltham, MA, USA) sensitized with anti-LTA monoclonal antibody (manufacturer) and kept overnight at relative humidity. Next day, the plates were washed with a wash buffer (PBS with 0.05% Tween 20) and incubated with a blocking buffer (PBS with 2% bovine serum albumin, BSA) for 1 h at room temperature. Then, they were washed with a wash buffer and received 100 μL of the samples collected and 100 μL of the LTA standard followed by serial 2-fold dilutions (standard curve) and maintained for 2 hours at room temperature. Afterwards, the plates were washed again and 100 µL of anti-LTA antibody was added for 1 hour at room temperature. The plates were washed again and 100 μl of horseradish peroxidase HRP conjugated rabbit IgG antibody was added for 1 hour at room temperature. Lastly, the plates were washed, and the reaction was developed using tetramethylbenzidine (TMB). After 20 min under the light, 50 μL of stop solution (2 N sulfuric acid) was added to each well of the plate and optical densities were read in the microtiter plate reader (BioTek Instruments, Inc., Winooski, VT, USA) at 450 nm absorbance ( Machado et al., 2020).
Statistical analysis
Data were analyzed using Kruskal-Wallis, Friedmann and Dunn tests with α≤0.05 byGraphPad Prism 6 (La Jolla, CA, USA)
Results
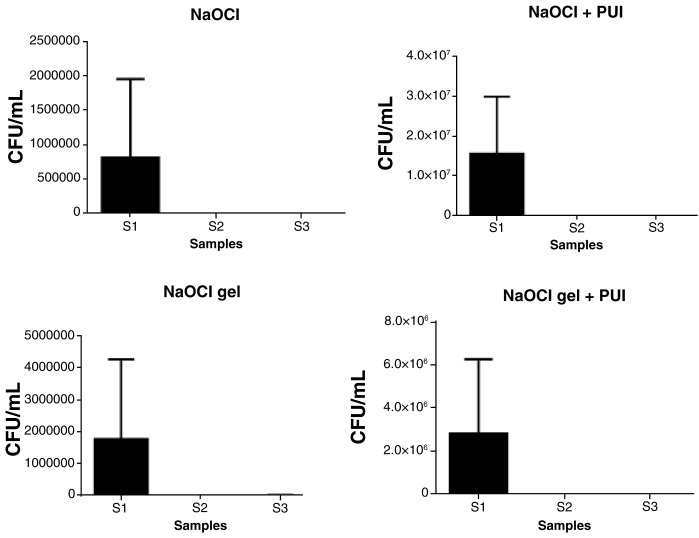
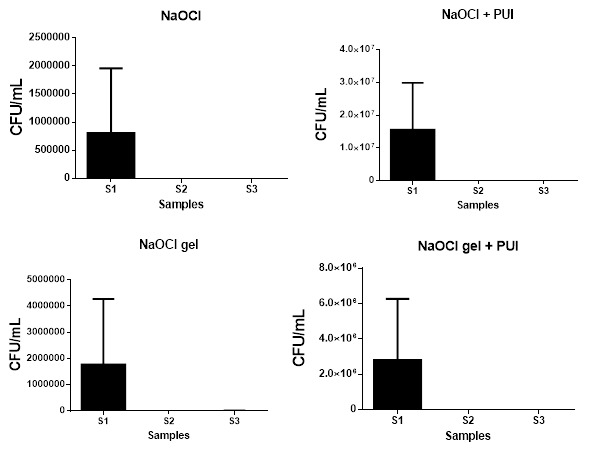
All experimental groups were effective in reducing the microbial load (CFU/mL) of E. coli ( Figure 1) and E. faecalis ( Figure 2) in S2 (from S1 levels). There was no significant difference among the experimental groups for S2 or S3 ( Table 2).
Figure 1. Statistical difference among the samples of each experimental group for E. coli (CFU/mL).
CFU, colony forming units; NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation.
Figure 2. Statistical difference among the samples of each experimental group in E. faecalis (CFU/mL).
CFU, colony forming units; NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation.
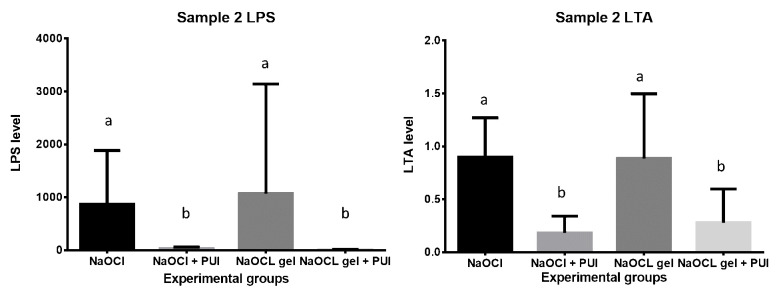
NaOCl and NaOCl gel groups had no significant difference in reducing LPS and LTA at S2. Groups with PUI showed a statistically increased reduction in LPS and LTA compared with groups without PUI ( Figure 3). As a limitation of this study, the LPS and LTA levels were not assessed in S3.
Table 2. Median of colony forming units/mL for E. coli and E. faecalis in all samples (S1, S2 and S3).
NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation.
| E. coli | E. faecalis | |||||
|---|---|---|---|---|---|---|
| Samples | S1 | S2 | S3 | S1 | S2 | S3 |
| NaOCl | 30x10
4
(366x10 4- 10x10 4) A-a |
0
(0-0) B-a |
0
(0-0) B-a |
314.95x10
4
(68x10 5- 56.6x10 4) A-a |
0
(0-0) B-a |
0
(0-0) B-a |
| NaOCl + PUI | 983x10
4
(29x10 6- 3x10 6) A-b |
0
(0-0) B-a |
0
(0-0) B-a |
585x10
4
(79x10 5-26.6x10 4) A-a |
0
(0-0) B-a |
0
(0-0) B-a |
| NaOCl gel | 45x10
4
(756x10 4- 1x10 4) A-a |
0
(0-0) B-a |
0
(0-33667) B-a |
218x10
4
(686x10 4- 1x10 4) A-a |
0
(0-333) B-a |
0
(0-0) B-a |
| NaOCl gel + PUI | 88333
(880x10 4-33x10 6) A-a |
0
(0-0) B-a |
0
(0-0) B-a |
485x10
4
(71x10 5- 18x10 5) A-a |
0
(0-333) B-a |
0
(0-0) B-a |
*Different letters indicate statistically significant differences (p<0.05). Uppercase letters indicate difference in rows (Friedman test; intra-groups) and lowercase letters indicate difference in columns (Kruskal-Wallis test; inter-groups).
Discussion
Well endodontically treated teeth fail mainly because of secondary intraradicular infection ( Siqueira, 2001). E. faecalis and E. coli, among other bacteria, are the most detected microorganisms in periapical lesions ( Geibel et al., 2005).
The use of NaOCl as an irrigant cannot be over-emphasized. In this study, it was effective in disinfecting E. faecalis and E. coli ( Table 2). These results agree with the results of Siqueira et al. (2000), who showed that NaOCl was an effective irrigant over E. faecalis at different concentrations (1%, 2.5%, and 5.25%). In addition, Valera et al. (2009) showed that 1% NaOCl is effective in disinfecting E. faecalis inoculated in the RCS. There have also been more recent studies that have the same results ( Carvalho et al., 2020; Plutzer et al., 2018; Valera et al., 2014). Similarly for E. coli, NaOCl has been reported in the literature as an effective irrigant in disinfecting microorganisms in the RCS lumen at 2.5% concentration ( Maekawa et al., 2011) and in preventing E. coli regrowth ( Valera et al., 2015).
In the present study, NaOCl was not effective in detoxifying LPS and LTA completely ( Figure 3). In support of this, the literature has shown that NaOCl is not effective in reducing endotoxin levels in the RCS ( de Oliveira et al., 2007), i.e. it reduces the endotoxin level but does not completely eliminate them ( Neelakantan et al., 2019). However, adding chloride alkali electrolyte-stable anionic surfactant has been shown to improve NaOCl effectivity because it reduces superficial tension ( Valera et al., 2015).
Figure 3. Statistical difference among the experimental groups at S2 for LPS and LTA levels.
NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation; LPS, lipopolysaccharide; LTA, lipoteichoic acid.
NaOCl gel has been suggested as an alternative endodontic irrigant because theoretically it has the same antimicrobial action of NaOCl solution, but with less apical extrusion and could thus be safer ( Nesser & Bshara, 2019). It is effective in reducing the microbial load, but has been shown to be less effective when compared to NaOCl solution of a lower concentration ( Poggio et al., 2010; Zand et al., 2016). In the present study, NaOCl gel was shown to be just as effective as NaOCl solution in reducing microbial load.
To the best of our knowledge, there are no studies in the literature evaluating the effect of NaOCl gel over endotoxins. In this study it was statistically as effective as the solution. But both were more effective when combined with PUI as it increases NaOCl penetration into dentinal tubules ( Faria et al., 2019). The present study is novel as there are no studies evaluating how PUI can affect NaOCl solution or gel action on endotoxins.
PUI is still being studied due to divergence of results in the literature. For example, Paiva et al. (2013) used PUI after instrumentation and showed it was ineffective in reducing microbial load; however, Mohmmed et al. (2018) showed that PUI is effective in biofilm removal from lateral canals. However, this activity may be influenced by the irrigation protocol (irrigation time; irrigant volume; instrument shape and material; and the irrigation frequency and intensity) ( van der Sluis et al., 2007).
In conclusion, our study showed that NaOCl gel has the same antimicrobial action of NaOCl solution and can partially detoxify endotoxins. PUI improves NaOCl (gel or solution) action over E. faecalis and E. coli formation and their endotoxins (LPS and LTA).
Data availability
Underlying data
Harvard Dataverse: Raw Data of NaOCl solution and gel, https://doi.org/10.7910/DVN/JNK3TH ( Abu Hasna, 2020).
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Funding Statement
This work was supported by Pibic-CNPq [2016/ 38890].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- Abu Hasna A: Raw Data of NaOCl solution and gel.Harvard Dataverse, V1,2020. 10.7910/DVN/JNK3TH [DOI] [Google Scholar]
- Bueno CRE, Cury MTS, Vasques AMV, et al. : Cleaning effectiveness of a nickel-titanium ultrasonic tip in ultrasonically activated irrigation: a SEM study. Braz Oral Res. 2019;33:e017. 10.1590/1807-3107bor-2019.vol33.0017 [DOI] [PubMed] [Google Scholar]
- Cavalli D, Toia CC, Flores Orozco EI, et al. : Effectiveness in the removal of endotoxins and microbiological profile in primary endodontic infections using 3 different instrumentation systems: A randomized clinical study. J Endod. 2017;43(8):1237–1245. 10.1016/j.joen.2017.03.032 [DOI] [PubMed] [Google Scholar]
- Carvalho CAT, Hasna AA, Carvalho AS, et al. : Clinical Study of Sodium Hypochlorite, Polymyxin B And Limewater Effect on MMP-3,-8,-9 In Apical Periodontitis. Braz Dent J. 2020;31(2):116–121. 10.1590/0103-6440202003081 [DOI] [PubMed] [Google Scholar]
- Csako G, Elin RJ, Hochstein HD, et al. : Physical and biological properties of U.S. standard endotoxin EC after exposure to ionizing radiation. Infect Immun. 1983;41(1):190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira LD, Jorge AOC, Carvalho CAT, et al. : In vitro effects of endodontic irrigants on endotoxins in root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(1):135–142. 10.1016/j.tripleo.2006.11.037 [DOI] [PubMed] [Google Scholar]
- Dioguardi M, Di Gioia G, Illuzzi G, et al. : Passive Ultrasonic Irrigation Efficacy in the Vapor Lock Removal: Systematic Review and Meta-Analysis. ScientificWorldJournal. 2019;2019:6765349. 10.1155/2019/6765349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo MS, Martinho FC, Zaia AA, et al. : Quantification of cultivable bacteria and endotoxin in post-treatment apical periodontitis before and after chemo-mechanical preparation. Eur J Clin MicrobioI Infect Dis. 2012;31(10):2575–2583. 10.1007/s10096-012-1598-6 [DOI] [PubMed] [Google Scholar]
- Faria G, Viola KS, Coaguila-Llerena H, et al. : Penetration of sodium hypochlorite into root canal dentine: effect of surfactants, gel form and passive ultrasonic irrigation. Int Endod J. 2019;52(3):385–392. 10.1111/iej.13015 [DOI] [PubMed] [Google Scholar]
- Geibel MA, Schu B, Callaway AS, et al. : Polymerase chain reaction-based simultaneous detection of selected bacterial species associated with closed periapical lesions. Eur J Med Res. 2005;10(8):333–338. [PubMed] [Google Scholar]
- Ginsburg I: Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2(3):171–179. 10.1016/s1473-3099(02)00226-8 [DOI] [PubMed] [Google Scholar]
- Goswami M, Chhabra N, Kumar G, et al. : Sodium hypochlorite dental accidents. Paediatr Int Child Health. 2014;34(1):66–69. 10.1179/2046905512Y.0000000042 [DOI] [PubMed] [Google Scholar]
- Iqbal A: Antimicrobial irrigants in the endodontic therapy. Int J Health Sci(Qassim). 2012;6(2):186–192. [PMC free article] [PubMed] [Google Scholar]
- Luebke RG: Pulp cavity debridement and disinfection. Dent Clin North Am. 1967;603–613. [PubMed] [Google Scholar]
- Machado FP, Khoury RD, Toia CC, et al. : Primary versus post-treatment apical periodontitis: microbial composition, lipopolysaccharides and lipoteichoic acid levels, signs and symptoms. Clin Oral Investig. 2020. 10.1007/s00784-019-03191-6 [DOI] [PubMed] [Google Scholar]
- Maekawa LE, Valera MC, deOliveira LD, et al. : Effect of Zingiber officinale and propolis on microorganisms and endotoxins in root canals. J Appl Oral Sci. 2013;21(1):25–31. 10.1590/1678-7757201302129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa LE, Valera MC, deOliveira LD, et al. : In vitro evaluation of the action of irrigating solutions associated with intracanal medications on Escherichia coli and its endotoxin in root canals. J Appl Oral Sci. 2011;19(2):106–112. 10.1590/s1678-77572011000200005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenhagen SE, Varah E: Serologically specific lipopolysaccharides from oral veillonella. Arch Oral Biol. 1963;8:31–36. 10.1016/0003-9969(63)90089-x [DOI] [PubMed] [Google Scholar]
- Mohmmed SA, Vianna ME, Penny MR, et al. : Investigations into in situ Enterococcus faecalis biofilm removal by passive and active sodium hypochlorite irrigation delivered into the lateral canal of a simulated root canal model. Int Endod J. 2018;51(6):649–662. 10.1111/iej.12880 [DOI] [PubMed] [Google Scholar]
- Narayanan LL, Vaishnavi C: Endodontic microbiology. J Conserv Dent. 2010;13(4):233–239. 10.4103/0972-0707.73386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan P, Herrera DR, Pecorari VGA, et al. : Endotoxin levels after chemomechanical preparation of root canals with sodium hypochlorite or chlorhexidine: a systematic review of clinical trials and meta-analysis. Int Endod J. 2019;52(1):19–27. 10.1111/iej.12963 [DOI] [PubMed] [Google Scholar]
- Nesser SFA, Bshara NG: Evaluation of the apical extrusion of sodium hypochlorite gel in immature permanent teeth: An in vitro study. Dent Med Probl. 2019;56(2):149–153. 10.17219/dmp/103911 [DOI] [PubMed] [Google Scholar]
- Paiva SSM, Siqueira JF, Rôças IN, et al. : Molecular microbiological evaluation of passive ultrasonic activation as a supplementary disinfecting step: a clinical study. J Endod. 2013;39(2):190–194. 10.1016/j.joen.2012.09.014 [DOI] [PubMed] [Google Scholar]
- Plotino G, Pameijer CH, Grande NM, et al. : Ultrasonics in endodontics: a review of the literature. J Endod. 2007;33(2):81–95. 10.1016/j.joen.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Plutzer B, Zilm P, Ratnayake J, et al. : Comparative efficacy of endodontic medicaments and sodium hypochlorite against Enterococcus faecalis biofilms. Aust Dent J. 2018;63(2):208–216. 10.1111/adj.12580 [DOI] [PubMed] [Google Scholar]
- Poggio C, Arciola CR, Dagna A, et al. : Antimicrobial activity of sodium hypochlorite-based irrigating solutions. Int J Artif Organs. 2010;33(9):654–659. 10.1177/039139881003300911 [DOI] [PubMed] [Google Scholar]
- Sáinz-Pardo M, Estevez R, dePablo ÓV, et al. : Root canal penetration of a sodium hypochlorite mixture using sonic or ultrasonic activation. Braz Dent J. 2014;25(6):489–493. 10.1590/0103-6440201300209 [DOI] [PubMed] [Google Scholar]
- Salazar-Mercado SA, Torres-León CA, Rojas-Suárez JP: Cytotoxic evaluation of sodium hypochlorite, using Pisum sativum L as effective bioindicator. Ecotoxicol Environ Saf. 2019;173:71–76. 10.1016/j.ecoenv.2019.02.027 [DOI] [PubMed] [Google Scholar]
- Sedgley C: Root canal irrigation--a historical perspective. J Hist Dent. 2004;52(2):61–65. [PubMed] [Google Scholar]
- Siqueira JF: Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34(1):1–10. 10.1046/j.1365-2591.2001.00396.x [DOI] [PubMed] [Google Scholar]
- Siqueira JF, Rôças IN, Favieri A, et al. : Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J Endod. 2000;26(6):331–334. 10.1097/00004770-200006000-00006 [DOI] [PubMed] [Google Scholar]
- Taylor HD, Austin JH: The solvent action of antiseptics on necrotic tissue. J Exp Med. 1918;27(1):155–164. 10.1084/jem.27.1.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera MC, daCardoso FGR, Chung A, et al. : Comparison of Different Irrigants in the Removal of Endotoxins and Cultivable Microorganisms from Infected Root Canals. ScientificWorldJournal. 2015;2015:125636. 10.1155/2015/125636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera MC, Maekawa LE, Chung A, et al. : The effect of sodium hypochlorite and ginger extract on microorganisms and endotoxins in endodontic treatment of infected root canals. Gen Dent. 2014;62(3):25–29. [PubMed] [Google Scholar]
- Valera MC, daSilva KCG, Maekawa LE, et al. : Antimicrobial activity of sodium hypochlorite associated with intracanal medication for Candida albicans and Enterococcus faecalis inoculated in root canals. J Appl Oral Sci. 2009;17(6):555–559. 10.1590/s1678-77572009000600003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis LWM, Versluis M, Wu MK, et al. : Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40(6):415–426. 10.1111/j.1365-2591.2007.01243.x [DOI] [PubMed] [Google Scholar]
- Zand V, Lotfi M, Soroush MH, et al. : Antibacterial Efficacy of Different Concentrations of Sodium Hypochlorite Gel and Solution on Enterococcus faecalis Biofilm. Iran Endod J. 2016;11(4):315–319. 10.22037/iej.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]