Abstract
Simultaneous pancreas-kidney (SPK) transplantation remains the most effective treatment for providing consistent and long-term euglycemia in patients having type 1 diabetes with renal failure. Thrombosis of the pancreatic vasculature continues to contribute significantly to early graft failure and loss. We compared the rate of thrombosis to graft loss and systematically reviewed risk factors impacting early thrombosis of the pancreas allograft following SPK transplantation. We searched the MEDLINE, EMBASE, The Cochrane Library, and PREMEDLINE databases for studies reporting thrombosis following pancreas transplantation. Identified publications were screened for inclusion and synthesized into a data extraction sheet. Sixty-three studies satisfied eligibility criteria: 39 cohort studies, 22 conference abstracts, and 2 meta-analyses. Newcastle-Ottawa Scale appraisal of included studies demonstrated cohort studies of low bias risk; 1127 thrombi were identified in 15 936 deceased donor, whole pancreas transplants, conferring a 7.07% overall thrombosis rate. Thrombosis resulted in pancreatic allograft loss in 83.3% of reported cases. This review has established significant associations between donor and recipient characteristics, procurement and preservation methodology, transplantation technique, postoperative management, and increased risk of early thrombosis in the pancreas allograft. Further studies examining the type of organ preservation fluid, prophylactic heparin protocol, and exocrine drainage method and early thrombosis should also be performed.
Keywords: simultaneous pancreas-kidney (SPK) transplantation, pancreas transplant, pancreas allograft, thrombosis, graft loss, risk factors
Introduction
The prevalence of type 1 diabetes has been increasing by 2% to 5% each year, with complications resulting from this endemic disease continuing to be a significant cause of morbidity and mortality.1 Poorly controlled, type 1 diabetes can cause progressive damage to the kidneys, and in severe cases can result in end-stage renal failure along with retinopathy, neuropathy, vascular disease, and other long-term health issues. Simultaneous pancreas-kidney (SPK) transplantation is the gold standard of treatment for adults with type 1 diabetes associated with renal failure.2 However, thrombosis of the pancreas after transplantation is a leading cause of relaparotomy and resultant graft loss in the early posttransplant period.2-4 The pathogenesis of thrombosis in the pancreatic vasculature following transplantation is still not well understood.5 Additionally, there is currently no standard protocol consistently proven to prevent thrombosis of the arterial or venous anastomosis sites or within the extension grafts following transplantation. This study aimed to identify risk factors for early (<2 weeks) pancreatic allograft thrombosis following SPK transplantation based on existing evidence-based literature.
Our review of the literature highlighted decreasing technical graft failure and thrombosis rates over the past decade with increasing intervention and rescue of grafts being more common in modern era pancreas transplants.4,6,7 Technical complications, including thrombosis, occur more frequently following pancreas transplantation than after other solid organ transplants.8 Over the past 30 years, numerous retrospective cohort studies have been conducted to try and clarify predictive factors for early pancreas allograft thrombosis. Factors surrounding donor and recipient characteristics as well as procurement, preservation, and transplantation technique have been exhaustively evaluated. Our objective was to systematically collate all relevant studies, compare the findings, and qualitatively report all defined risk factors for thrombosis of the pancreas allograft.
Materials and Methods
Literature Search Strategy
Methods of data extraction and inclusion criteria were specified in advance in a review protocol. The search strategy was formulated in accordance with the Cochrane Handbook for Systematic Reviews of Interventions guidelines9 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.10
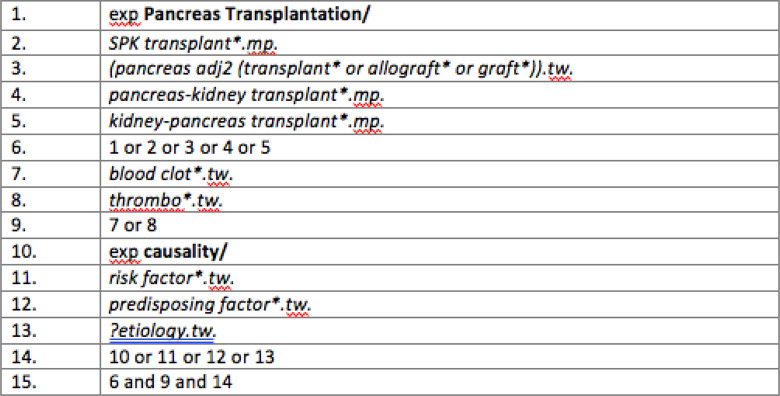
Studies were identified by searching electronic databases, and the reference lists of relevant full-text articles subsequently examined to include all available studies. EMBASE (1947 to present), MEDLINE (1946 to present), and Cochrane Library databases, including Premedline in-process and electronic publications ahead of print citations, were searched. Figure 1 outlines the search strategy utilized.
Figure 1.
Systematic search algorithm using Ovid. MeSH subject headings are highlighted in bold, and keyword searches in italics. Search terms revolved around pancreas transplant, thrombosis, and risk factors.
Eligibility Criteria
This review included meta-analyses, randomized controlled trials, and cohort studies examining thrombosis in the deceased donor, whole pancreas transplantation in humans. Participants of any age undergoing whole SPK, pancreas alone, and pancreas after kidney transplantation were considered eligible for inclusion. All studies reported early (within 2 weeks) partial or complete thrombosis in the pancreas allograft following transplantation. We did not restrict the inclusion of studies by publication date or publication status; however, we only considered studies reported in English. Only clinical data in humans were included.
Study Selection and Data Collection
Two researchers (JB) and (SS) independently screened articles for eligibility based on title and abstract. Any discrepancies were discussed between researchers. Researchers assessed resultant full-text articles for inclusion according to the predetermined eligibility criteria. We extracted variables of interest, including (1) characteristics of study (title, journal, author and year, country, time period), (2) number of transplants (SPK, pancreas after kidney transplant [PAK], pancreas transplant alone [PTA]) and thrombosis (total, venous, and arterial), (3) donor and recipient (donor age, recipient age, donor body mass index [BMI], recipient BMI, recipient gender, cause of donor death, dialysis status), (4) procurement and preservation (preservation fluid, cold ischemia time, total preservation time, administration of steroids to donor), and (5) transplantation and postoperative treatment (exocrine drainage method, arterial reconstruction method, venous management/grafts, pancreatitis, hypotension/vasopressor use, immunosuppression method, prophylactic heparin use). Information from each study was synthesized into the extraction sheet accordingly, and relevant significant risk factors identified can be found in Supplementary Tables 1 to 3.
Bias Appraisal
The Newcastle-Ottawa Quality Assessment Scale for Cohort studies11 was employed to assess the included studies for potential bias (see Table 1).
Table 1.
Newcastle-Ottawa Scale Assessment of Quality of Individual Studies.
| Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts based on design/analysis | Assessment of outcome | Follow-up was long enough for outcomes to occur | Adequacy of follow-up of cohorts | |
| Agarwal et al12 | a | a | a | a | a | a | ||
| Alonso et al13 | a | a | a | a | a | a | a | a |
| Becker et al14 | a | a | a | a | a | a | a | a |
| Ciancio et al15 | a | a | a | a | a | |||
| Decraemer et al16 | a | a | a | a | a | a | a | a |
| Douzdjian et al17 | a | a | a | a | a | a | ||
| Englesbe et al18 | a | a | a | a | a | a | a | |
| Grewal et al19 | a | a | a | a | a | a | ||
| Gruessner et al20 | a | a | a | a | a | a | ||
| Gruessner and Gruessner6 | a | a | a | a | a | a | a | |
| Hakeem, 201721 | a | a | a | a | a | a | a | a |
| Harbell et al22 | a | a | a | a | a | a | ||
| Hau et al23 | a | a | a | a | a | a | ||
| Hesse et al24 | a | a | a | a | a | a | a | a |
| Humar et al25 | a | a | a | a | a | a | a | a |
| Humar et al8 | a | a | a | a | a | a | a | |
| Humar et al26 | a | a | a | a | a | a | ||
| Ionescu et al27 | a | a | a | a | a | a | ||
| Jimenez et al28 | a | a | a | a | a | a | ||
| Jiminez-Romero, 201829 | a | a | a | a | a | a | ||
| Karam et al30 | a | a | a | a | ||||
| Kopp et al | a | a | a | a | a | a | a | a |
| Lindahl, 201831 | a | a | a | a | a | a | a | a |
| Manrique et al32 | a | a | a | a | ||||
| Martins et al33 | a | a | a | a | a | a | a | a |
| Montiel-Casado et al34 | a | a | a | a | a | a | a | a |
| Okabe et al35 | a | a | a | a | a | a | ||
| Ozaki et al36 | a | a | a | a | a | a | a | |
| Page et al37 | a | a | a | a | a | a | a | |
| Raiha, 201938 | a | a | a | a | a | a | a | |
| Ramessur Chandran et al39 | a | a | a | a | a | a | a | |
| Raveh et al40 | a | a | a | a | a | |||
| Sanchez-Hidalgo et al41 | a | a | a | a | a | a | ||
| Scheffert et al42 | a | a | a | a | a | a | a | a |
| Schneeberger et al43 | a | a | a | a | a | a | a | a |
| Spaggiari et al44 | a | a | a | a | a | |||
| Troppmann et al45 | a | a | a | a | a | a | ||
| Troppmann et al3 | a | a | a | a | a | a | a | |
| Vincent et al46 | a | a | a | a | a | a | ||
Data Abstraction and Outcomes
Primary outcome measures were patient and pancreas graft survival and thrombosis rates. Where possible, long-term outcomes at 5 to 10 years were included. We qualitatively synthesized evidence surrounding donor/recipient selection, recovery procedures, implantation, and immunosuppression to evaluate a role in pancreas outcomes.
Results
Study Characteristics
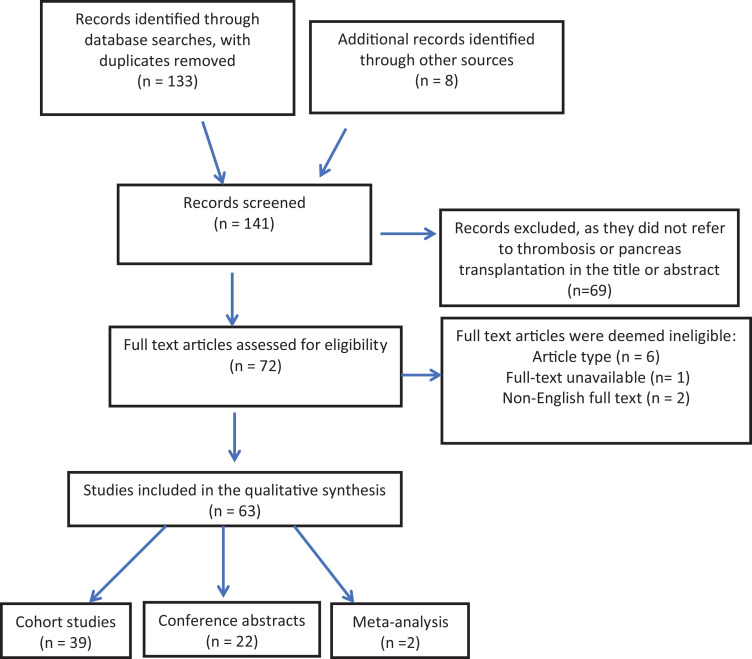
A total of 63 articles were identified for inclusion in the review (Figure 2) from an original total of 133 citations. Screening of reference lists of relevant full-text articles provided 8 additional studies that met inclusion criteria. Of the 141 articles meeting inclusion, 69 did not satisfy the eligibility criteria and were discarded. Upon full-text assessment, 6 citations were excluded due to type of study, 1 for full-text unavailability, and 2 with languages other than English.
Figure 2.
PRISMA CONSORT diagram showing identification and screening of studies for inclusion in the systematic review. PRISMA CONSORT indicates Preferred Reporting Items for Systematic reviews and Meta-Analyses Consolidated Standards of Reporting Trials.
The 63 included articles comprised of 2 meta-analyses,47 39 cohort studies,3,6,8,12-20,23–28,30,32–37,39,41–43,45,46,48 and 22 conference abstracts.4, 7, 49–64 All studies reported thrombosis in pancreas allograft transplantation. The number of pancreas transplants per study ranged from 9 to 10 253; characteristics of each study are provided in Table 2. Multiple citations stemming from single data sets and articles not specifying total thrombus formation were taken into consideration when calculating overall thrombosis rate (see Table 2). With the exclusion of these studies, a total of 1127 thrombi occurred in 15 936 deceased donors, whole pancreas transplants, constituting an overall thrombosis rate of 7.07%. Additionally, 906 grafts were lost secondary to thrombosis in the context of 1087 reported thrombi, representing an 83.3% reported rate of pancreatic allograft loss resulting from thrombosis within the pancreatic vasculature.
Table 2.
Description of Characteristics of Pancreas Transplant Studies Identified, Ordered by Study Design.
| First author | Country | Time period | Number of transplants (SPK, PAK, PTA) | Total thrombosis | Primary outcomes |
|---|---|---|---|---|---|
| Cohort studies | |||||
| Agarwal et al12 | United States | 2003-2005 | 57 SPK 22 PAK 6 PTA |
3 | Immediate pancreatic graft function following preservation with HTK solution, compared with UW solution. Graft and patient survival outcomes analyzed over the follow-up period |
| Alonso et al13 | United States | 2001-2007 | 64 SPK 27 PAK 6 PTA |
6 | Graft survival, patient survival, and complication rates between HTK- and UW-preserved transplants. |
| Becker et al14 | Germany | 2000-2006 | 95 SPK | 4 | 1-, 3-, and 12-month patient and graft survival, and cause of graft loss, of SPK transplants with HTK vs UW preservation solution. |
| Ciancio et al15 | United States | 1994-1997 | 126 SPK | 14 | Incidence, treatment, and outcomes of partial venous thrombosis of the pancreatic allograft |
| Decraemer, et al16 | Belgium | NS | 53 SPK 3 PAK 3 PTA |
4 | Effect of vasopressors and desmopressin use on pancreas graft function (thrombosis or insulin dependence for more than 3 days posttransplantation) |
| Douzdjian et al17 | United States | 1988-1994 | 61 SPK | 5 | Pancreas graft survival at 1 year, and univariate and multivariate analyses of associated factors. |
| Englesbe 200618 | United States | 2002-2004 | 46 SPK 27 PAK 4 PTA |
5 | Pancreas graft outcomes in the HTK era vs UW era, including early complications and 90-day graft survival |
| Grewal et al19,a | United States | 1989-1991 | 34 SPK 5 PTA 2 segmental PAK |
6 | Postimplantation pancreatitis and pancreatic thrombosis in pancreas transplant recipients. Determining potential contributing variables. |
| Gruessner et al20,b | United States | 1986-1994 | 196 SPK 91 PAK 107 PTA |
45 | Surgical complications (infections, graft thrombosis, anastomotic leak) requiring laparotomy and their impact on graft and patient survival. |
| Gruessner and Gruessner6 | United States | 2005-2014 | 8063 SPK 1394 PAK 796 PTA | 553 | Rates, characteristics, and outcomes of pancreas transplants in 2005-2009 compared with 2010-2014 |
| Hakeem, 201721 | United Kingdom | 2009-2014 | 96 SPK 5 PAK |
24 | Incidence, management, and risk factors of pancreas allograft thrombosis with proposed CT grading system and management algorithm. |
| Harbell et al22 | United States | 5-year period NS | 86 SPK 18 PAK 8 PTA |
30 | Prevalence of complete and partial splenic vein thrombosis and clinical significance of nonocclusive thrombosis |
| Hau et al23 | Germany | 2007-2010 | 26 SPK 2 PTA |
2 | Comparison of variables and outcomes surrounding pancreas transplants with organs derived normally and rescued. |
| Hesse et al24 | Belgium | 1999-2002 | 35 SPK 5 PAT |
3 | Effect of perioperative octreotide use on posttransplant complications in enteric-drained pancreas transplantations. |
| Humar et al25,c | United States | 1994-1997 | 100 SPK 80 PAK 33 PTA |
12 | Incidence of early (<3 months posttransplant) surgical complications between 2-time groups; 1985-1994 and 1994-1997. |
| Humar et al8 | United States | 1994-2003 | 327 SPK, 399 PAK, 211 PTA | 64 | Technical graft failure incidence and analysis of potential causes and risk factors in pancreas transplants. |
| Humar et al26,c | United States | 1994-2001 | 232 SPK, 292 PAK, 125 PTA | 57 | Technical failure following deceased donor pancreas transplants and the impact of donor obesity |
| Ionescu et al27 | France | 1992-2006 | 27 SPK 3 PTA |
4 | Occurrence of venous thrombosis of the pancreatic graft |
| Jimenez et al28,c | Spain | 1995-2004 | 49 SPK 4 PAK |
7 | Incidence of thrombosis in pancreas grafts using portoiliac and portocaval venous anastomosis |
| Jimenez-Romero, 201829 | Spain | 1995-2016 | 175 SPK 16 PAK |
22 | Donor and recipient characteristics, perioperative variables, and immunosuppression effects on 1-, 3-, and 5-year patient and graft survival. |
| Karam et al30 | France | 1999-2000 | 9 SPK | 1 | Complications and outcomes of Celsior as a preservation solution for multiple organ procurement |
| Kopp et al48 | Netherlands | 2011-2015 | 96 SPK | 39 | DCD vs DBD pancreas donors for 90-day patient and graft survival and associated complications: postperfusion pancreatitis, infection, bleeding, and graft thrombosis |
| Lindahl, 201831 | Norway | 1998-2016 | 229 SPK 67 PTA |
22 | Safety profiles and clinical outcomes (bleeding, thrombosis, relaparotomy) in pancreas transplants with duodenoduodenostomy (DD) vs duodeneojejunostomy (DJ). |
| Manrique et al32 | Spain | 1995-2008 | 109 SPK 9 PAK |
15 | Rate of relaparotomy after pancreas transplantation and associated causes and risk factors |
| Martins et al33 | Portugal | 2000-2013 | 165 SPK | 11 | Impact of dialysis modality on graft failure and patient death following SPK transplantation |
| Montiel-Casado et al34 | Spain | 2007-2011 | 50 SPK 6 PAK 2 PTA |
8 | Venous graft thrombosis and the correlation with peak amylase level in the first 3 days after pancreas transplantation |
| Okabe et al35 | Japan | 2001-2011 | 23 SPK 1 PAK 2 PTA |
2 | Postoperative complications following pancreas transplantation |
| Ozaki et al36 | United States | 1989-1991 | 61 SPK 6 PAK 6 PTA |
3 | Incidence of surgical complications after pancreas transplantation with analysis for corresponding etiology and risk factors |
| Page et al37 | France | 2005-2008 | 61 SPK | 32 | Incidence, causes, and risk factors of early relaparotomy after SPK transplantation |
| Raiha, 201938 | Finland | 2010-2017 | 99 SPK | 0 | Evaluate pretransplant dialysis modality (peritoneal dialysis vs hemodialysis) and risk of postoperative complications following SPK transplantation. |
| Ramessur Chandran et al39 | Australia | 1992-2010 | 117 SPK 1 PAK |
12 | Early pancreas allograft thrombosis, defined as graft loss due to thrombosis within the first postoperative month. |
| Raveh et al40 | United States | 2015-2018 | 88 SPK 4 PAK 3 PTA |
36 | Rates of allograft thrombosis, hemorrhage, graft failure, and death associated with 4 different anticoagulation regimes following pancreas transplantation |
| Sanchez-Hidalgo et al41 | Spain | 2001-2017 | 198 SPK | 13 | Postoperative sodium levels effect on postoperative complications within 2 months after SPK transplantation |
| Scheffert et al42 | United States | 2001-2009 | 109 SPK 31 PAK 12 PTA |
28 | Clinical outcomes associated with early use of low-dose heparin following pancreas transplantation |
| Schneeberger et al43 | Austria | NS (18 months) | 66 SPK 1 PAK 2 PTA |
2 | 6 Months posttransplant graft survival using UW or HTK preservation solution. |
| Spaggiari et al44 | United States | 2007-2017 | 138 PTx | 3 | Pediatric donor pancreatic allograft postoperative complications and long-term outcomes compared with adult pancreas donors. |
| Troppmann et al45 | United States | 1985-1993 | 196 SPK 91 PAK 107 PTA |
45 | Thrombosis rates and corresponding operative and nonoperative variables following bladder-drained and enteric-drained pancreas transplantation. |
| Troppmann, et al3,c | United States | 1986-1994 | 236 SPK 101 PAK 104 PTA |
39 | Surgical complications requiring early relaparotomy (≤3 months posttransplant) after pancreas transplantation. Graft and patient survival following relaparotomy. |
| Vincent et al46 | France | 2004-2009 | 119 SPK 22 PTA |
19 | Early (≤15 days) postoperative complications following pancreas transplant and diagnostic rate of multidetector CT |
| Conference abstracts | |||||
| Cantarovich et al49 | France | 2000-2013 | 255 SPK 68 PTA |
NS | Variables and outcomes of pancreas transplant recipients over time, from 2000 to 2013. |
| Choi et al65 | Korea | 1992-2018 | 178 SPK 47 PAK 134 PTA |
NS | Patient survival, graft survival, and risk factors associated with pancreas transplantation between eras based on case numbers in a single center. |
| Ferrer, 201966 | Spain | 2000-2015 | 272 SPK 23 PAK 3 PTA |
3 | Analyze surgical complication associated with enterically drainage pancreas transplants in a single center |
| Finger et al50,c | United States | 1998-2011 | 1115 PTx | NS | Technical failure (graft loss within 90 days) after pancreas transplantation. |
| Graham et al51 | United States | 2014-2017 | 18 SPK 2 PAK |
3 | Post-pancreas transplantation complication in local and import grafts |
| Gruessner et al4,c | United States | 2004-2012 | 7510 SPK | NS | Overall and specific technical failure (early graft thrombosis, infection/pancreatitis, anastomosis leaks, bleeding) after SPK transplants. |
| Gruessner et al52,a | United States | NS | 9467 SPK | 481 | Early pancreas graft failure following SPK transplant, and impact on kidney graft loss and patient survival |
| Horneland et al53 | Norway | 2006-2010 | 59SPK 2 PTA |
NS | Surgical complications, graft survival, and patient survival following pancreas transplantation |
| Horton et al54 | United States | 2005-2010 | 957 SPK | NS | Pancreas graft failure and patient survival rates after SPK transplantation and correlation with pretransplant insulin requirements. |
| Jimenez-Romero et al55,c | Spain | 1995-2008 | 88 PTx | 9 | Pancreas graft thrombosis, 3-year graft, and patient survival rates following bladder-drained and enteric-drained pancreas transplants. |
| Koyama et al67 | Japan | 2001-2018 | 55 SPK 8 PAK |
4 | Etiologies and risk factors for early- and late-phase pancreas graft loss following SPK or PAK transplants. |
| Kudva et al56 | United States | 1998-2009 | 67 SPK 128 PAK 76 PTA |
29 | Pancreas allograft loss, patient death, and patient survival at 1, 2, 5, and 10 years post-pancreas transplantation. |
| Lin et al57 | United States | 2000-2012 | 214 PTx | 21 | Incidence, risk factors, and associated outcomes of surgical complications after pancreas transplantation. |
| Martins et al58, b | Portugal | 2000-2012 | 150 SPK | NS | Pancreas allograft outcomes/failure and patient death following SPK transplantation. |
| Patil et al59 | United States | 1998-2012 | 192 PTA | 22 | Early partial and complete pancreas thrombosis within 90 days after PTA transplants; analysis of incidence, outcomes, and risk factors. |
| Ramessur et al60 , b | Australia | 1992-2010 | 117 SPK 1 PAK |
12 | Early pancreas allograft thrombosis and associated risk factors |
| Rogers et al61,c | United States | 2002-2010 | 26 SPK | 2 | Pancreas, kidney, and patient survival rates following SPK transplantation in an African American sample using alemtuzumab vs rATG for induction therapy. |
| Rogers et al62 | United States | 2001-2013 | 162 SPK 35 PAK 5 PTA |
15 | Pancreas, kidney, and patient survival rates between African American and non-African American recipients of a pancreas transplantation |
| Scheffert et al63, b | United States | 2001-2009 | 154 PTx | NS | Surgical complications and 30-day graft survival, with evaluation of postoperative heparin use, in pancreas transplantation |
| Shahrestani et al68 | Australia | 2008-2017 | 235 SPK | 41 | Risk factors for pancreas graft thrombosis and resultant loss in SPK transplant recipients. |
| Singh et al64 | England | 1996-2011 | 223 SPK | NS | Causes and risk factors of early technical pancreas graft loss following SPK transplantation |
| Sutherland et al7 | United States | 1978-2008 | 720 SPK 753 PAK 547 PTA |
NS | Pancreas outcomes (failure, function, survival) following pancreas transplants over 30 years, comparing between eras (1978-1994, 1995-2000, 2000-2008) |
| Meta-analysis | |||||
| Shahrestani et al47 | Australia | Database inception to 2015 | 16908 SPK 4334 PAK 2367 PTA |
NS | Complications, 10-year pancreas allograft survival, and patient survival after pancreatic transplantation from donors after cardiac death and donors after brain death. |
| Hameed et al69, b | Australia | Database inception to 2017 | 269 PTx | 15 | Effect of perfusion/preservation fluids on graft survival and complications (thrombosis, pancreatitis). |
Abbreviations: CT, computed tomography; DBD, donor after brain death; DCD, donor after cardiac death; HTK, histidine-tryptophan-ketoglutarate; NS, not specified in the study; PAK, pancreas after kidney; PTA, pancreas transplant alone; PTx, pancreas transplant (nonspecific); rATG, rabbit antithymocyte globulin; SPK, simultaneous pancreas-kidney transplant; UW, University of Wisconsin.
a Includes types of transplants not consistent with the eligibility criteria.
b Identical patients are reported in another study, so this report was not included in summative calculations.
c Significant overlap with another study, so this report was not included in summative calculations.
Risk of Bias Within Studies
The included cohort studies were critically appraised for potential bias, as illustrated in Table 1. Using the Newcastle-Ottawa Quality Assessment Scale, the quality of each study was evaluated based on the selection of study groups, comparability of such groups, and the ascertainment of the outcome of interest.11 The studies demonstrated an adequate selection of study participants, which accurately represented the general population of patients requiring pancreas transplants. A direct comparator group was often not used, as many of the studies did not employ a specific exposure. Instead, retrospective cohorts typically organized complete data sets of transplant variables and conducted multivariate analyses. Lastly, the outcomes of each study were generally well reported, except for addressing follow-up attrition. Overall, included studies were of high quality and low risk of potential bias.
Donor and Recipient Factors
In several studies, donor age was associated with increased pancreas allograft thrombosis6, 20,25,45,68 and early technical failure.50,57,67 Humar et al25 described a thrombosis incidence of 1.8% with donors younger than 20 years, 3.7% with 20- to 40-year-old donors, and 16.2% with donors older than 40 (P = .009). A donor age older than 44 years (vs 30-44) trended toward increased thrombus formation in all 3 types of pancreas transplants included, reaching significant relative risk of 2.03 for PAK (P = .007).6 The impact of recipient age on allograft thrombosis was conflicting with most studies reporting no statistical difference. Noteworthy concession to this included Gruessner et al,6 demonstrating a decreased thrombotic risk with increasing age; recipients older than 44 compared with those 30 to 44 years of age (P = .03 in PAK transplants).
Donor and recipient obesity, measured clinically as a BMI > 30 kg/m2, are both widely considered as risk factors for pancreatic allograft thrombosis.8,39 Findings of this review corroborate this,8,20,26,50 rather, more importantly, one publication even identified an increased thrombotic risk with a donor or recipient BMI ≥ 25 kg/m2 (P < .05).46 Preliminary findings of Shahrestani et al68 identified risk of thrombosis increased by 25.6-fold in male donors compared with females (P = .01). Recipient gender effect on the risk of thrombosis was poorly reported within the literature.19,27,53 A retrospective cohort study by Grewel et al19 in 1993 incurred 5 thromboses in a cohort of 18 male recipients, compared to only 1 thrombosis in the cohort of 23 female recipients (P < .04). This contrasted with more recent nonsignificant findings (P > .05, see Supplementary Table 1) of Ramessur Chandran et al39 of 3 thrombi in 58 males compared to 9 in 60 females, Hakeem et al21 of 17 thrombi in 64 men versus 7 thrombi in 39 females, and Harbell et al22 of thrombosis in 15 of 62 males compared with 15 of 50 females.
Nontraumatic, especially cardiocerebrovascular, cause of donor death increased the risk of subsequent pancreatic allograft thrombosis.6, 8, 20,45,70 Donor after cardiac death (DCD) donor pancreas transplants have significantly higher rates of thrombosis (P = .006).47 This study demonstrated that the use of antemortem heparin in the donor reduced this risk to a nonsignificant level.47 Kopp et al48 concluded that donor after brain death (DBD) and DCD donors presented with comparable pancreatic graft outcomes when normalized to a similar Pancreas Donor Risk Index (PDRI). The DCD donors of equivalent PDRI’s incurred nonsignificant higher rates of partial thrombosis but lower rates of complete thrombosis than those of corresponding DBD donors.48
Pretransplant dialysis status of the recipient influences pancreatic thrombus formation. Recipients receiving dialysis before pancreas transplantation had a significantly lower rate of thrombosis compared with those not yet requiring dialysis treatment.20,25,45 Of recipients receiving pretransplant dialysis, hemodialysis was superior in reducing thrombotic risk than peritoneal dialysis (P = .014).33 Pretransplant panel reactive antibodies (PRA) greater than 20% (vs <20%) we linked to the increased relative risk of thrombosis in SPK, PAK, and PTA transplantations (significant in SPK, P = .01).6 Hypercoagulability (protein C or protein S deficiency or antiphospholipid antibodies) was a predictor of early graft loss resulting from thrombosis (P = .035).42
Procurement and Preservation Factors
Cold ischemic time (CIT) and total preservation times significantly affected graft loss due to thrombosis in several studies. Grewal et al19 found in their study an extended mean CIT for thrombosis of 15.9 hours was associated with a significant risk of pancreas graft thrombosis compared to a mean CIT of 10.8 hours for those with no thrombosis (P < .05). When a 12-hour CIT cutoff was applied to the time of procedure, the CIT of the 6 grafts that thrombosed was all longer than 12 hours (P < .005). More recent studies have confirmed this association,49 with one study detecting significant difference between a mean CIT of 11.5 and 9.4 hours (P = .025).34 Total preservation time greater than 24 hours has been linked to highly significant increased risk of allograft thrombosis in several series.6,8,50
Transplantation and Postoperative Factors
We compared differences in thrombosis rates in bladder drainage and enteric drainage of pancreas transplants due to the potential for increased risk of thrombosis associated with enteric graft leak. Results were distributed between data sets, where some studies identified an increased thrombotic risk with enteric-drained transplants6,8,40 and others with bladder-drained transplants.7,25,55 Finger et al50 noted a significant protective benefit of bladder drainage against graft thrombosis (P = .003). Arterial reconstruction methods other than Y-graft (end-to-side anastomosis, interposition graft, and aortic carrel patch) were associated with increased pancreas allograft thrombosis.45 Portal vein extension did not significantly increase risk19,45,46; however, in the study by Jimenez et al,28 all 7 cases of thrombosis occurred in the 30 transplants with portoiliac venous management versus 0 of 23 portocaval vein grafts, establishing a significant relationship (P < .02).
While amylase level defining hyperamylasemia and posttransplant time frames varied between citations, 3 different studies identified postreperfusion pancreatitis as an independent risk factor for graft thrombosis.21,34,45,57 Montiel-Casado et al34 employed a hyperamylasemia threshold of >745 mg/dL (1 standard deviation above mean) within 3 days of transplantation. Three of 8 thromboses and 4 of 50 nonthromboses cases had pancreatitis, conferring an 8.6 times greater risk for vascular thrombosis (P = .032).34 Hypotension, measured by vasopressor use during transplantation, significantly increased the recipients’ risk of allograft thrombosis.39 Intraoperative and onward systolic blood pressures below 95 mm Hg were significant for thrombosis (P = .033 and .007, respectively).39
Intravenous (IV) tacrolimus-based regimen for posttransplant immunosuppression, although rarely used in contemporary pancreas transplants, also appears to be a risk factor for venous thrombosis incidence. Ciancio et al15 acknowledged that all 14 cases of thrombosis in their 126 SPK transplant data set used IV tacrolimus for immunosuppression. Depleting T-cell antibody (vs nondepleting) induction and maintenance protocols of oral tacrolimus and mycophenolate mofetil or sirolimus, all significantly decreased the relative risk of thrombosis.6, 50 Hakeem et al21 corroborate the importance of adequate immunosuppression, demonstrating that acute rejection increased allograft thrombotic risk by 25% (P = .034).
The use of prophylactic heparin posttransplantation has traditionally been thought to lower the incidence of thrombosis as seen in Humar et al25 where recipients who received heparin and aspirin prophylaxis had a lower incidence of thrombosis (4.0% vs 10.8%, P = .06). Multivariable analysis by Scheffert et al42 of graft loss within 30 days due to thrombosis demonstrated a trending protective benefit with the use of prophylactic IV heparin (P = .091). Most recently, Raveh etal40 demonstrated thrombotic risk significantly decreased with postoperative heparin infusion for thromboprophylaxis compared with non-IV heparin regimen (P = .01). The incidence of bleeding increased with IV heparin usage; however, importantly, there was no difference in graft survival for those who required exploration for bleeding.25
Discussion
Pancreas transplantation is associated with an event rate of 7.07% for thrombosis, with resultant graft loss in 83.3% of cases. Findings demonstrate that there are numerous donor and recipient, procurement and preservation, transplantation, and postoperative factors predictive of early thrombosis in the pancreas allograft following transplantation. Although different procedures, SPK, PAK, and PTA transplants inherently are the same vascularized pancreas graft and as such carry the same associated risk factors identified herein and are readily applicable to all forms of pancreas transplantation not just SPK.
Significant factors associated with increased thrombosis rate are donor age (>45), donor and recipient obesity (BMI > 30 kg/m2), nontraumatic and cardiocerebrovascular causes of donor death, high pretransplant PRA (>20%), and inherited hypercoagulable disorders. Recipients not yet requiring dialysis treatment or undergoing peritoneal dialysis are at an increased risk of allograft thrombosis compared to those on hemodialysis treatment at the time of transplantation.6, 25,34,70 This is purported to be due to the uremia-induced platelet dysfunction associated with end-stage renal failure.2 Recipient age of less than 45 years has generally been considered representative of an adequate cardiovascular system for immediate graft success and long-term survival.27 This is highlighted by Shahrestani et al68 in recipients aged between 37 and 42 years, demonstrating a 10.6 times greater risk of thrombosis compared to those <36 years (P = .02). Conversely, findings from Gruessner et al6 identify a decreased thrombotic risk with recipients older than 44 years (vs 30-44 years old), warranting further investigation.
Meticulous procurement of the pancreas is crucial to minimize technical failure posttransplantation. Procurement technique was not evaluated assumedly due to difficulty quantifying the variable levels of trauma sustained during retrieval. Backbench preparation and atraumatic recovery of the pancreas are essential to reducing thrombosis resulting from technical failure.71 This is especially relevant to the pancreas, given its fragile composition and extensive microvasculature.71 At procurement, graft preservation is achieved by flushing the pancreas with a preservation solution that induces hypothermia (4 °C), attenuating the effects of ischemia and reperfusion, and thus extending cold ischemic tolerance.43 The type of preservation solution ideal for pancreas preservation has come under recent debate. University of Wisconsin (UW) solution is considered the reference standard for pancreas preservation; however, in recent years, histidine-tryptophan-ketoglutarate (HTK) solution has become increasingly popular for abdominal organ procurement.43,72 In 2 recent studies, HTK solution has been correlated with increased early postoperative pancreas graft complications (including graft thrombosis) and losses.13,72 It is currently unclear whether the comparatively higher flush volumes recommended with the use of HTK solution are the cause of these findings by inducing hyperperfusion-related injury during organ recovery.13,70,72 Other publications found no significant difference in pancreas allograft complication or survival between HTK and UW solution, indicating further investigation is necessary to evaluate this potential risk factor.12,14,18,43,69 Regardless of preservation solution, small bowel does not tolerate prolonged periods of preservation well, and increasing preservation time increases the risk of leaks and ischemia–reperfusion pancreatitis, which contribute to thrombus formation in the allograft vasculature.34 Cold ischemia time longer than 12 hours and total preservation time greater than 24 hours are well-defined risk factors for early pancreas allograft thrombosis and graft failure.6,8,19
Regarding the transplantation procedure, meticulous arterial Y-graft construction and portal venous drainage should be employed to minimize potential thrombotic risk. Systemic or portal venous drainage has different thrombosis rates, dependent on surgical experience and personal preference. Portal vein extension graft use trended toward increased thrombosis in several studies; however, further investigation is required to clarify this as a potential risk factor. Enteric drainage, although linked to a higher rate of thrombosis than bladder drainage in some series, continues to be used by most significant centres71 and indeed provides better long-term graft survival.65 This is perhaps due to the long-term sequelae of chronic urological complications associated with bladder drainage.71 Conversely, bladder drainage theoretically allows for rapid diagnosis and subsequent treatment of postoperative complications via repeated urinary amylase measurements; however, it is rarely performed in practice.70 Most transplant units have moved to enteric drainage as studies have shown improved graft survival65,73 and significantly lower morbidity.74
The intrinsically low circulatory flow (1.3% of cardiac output) and extensive microvasculature bed of the pancreas lends itself particularly susceptible to hypo/hyperperfusion injury.5 This, in conjunction with inevitable hemodynamic changes associated with transplantation, namely, reperfusion, cell death, edema, and increased local blood flow resistance, represents a significant risk factor for graft thrombosis and ischemia-induced pancreatitis.19 Administration of steroids to the donor prior to procurement was only reported in one study where steroid administration reduced pancreatitis and edema that occurs during aggressive fluid resuscitation of the donor and initiation of graft reperfusion.19 This may reduce reperfusion inflammation and injury, as well as minimizing graft edema, thus maintaining adequate perfusion to prevent early allograft thrombosis.19 This relationship of inadequate perfusion and thrombosis is highlighted by the established association between hypotension/vasopressor use and allograft thrombosis.39 The reduced flow in hypotension promotes stasis and thrombus formation.39 Systemic response to a hypotensive state results in diversion of blood from peripheries to the central organs. With most arterial reconstructions, anastomosing to the common or right external iliac artery reduces perfusion to the pancreas allograft.39
Although it is believed that most transplant centers utilize some form of anticoagulation, there is currently no standardized or universal anticoagulation prophylaxis protocol for pancreas thrombosis. Findings by Scheffert et al42 and Humar et al25 support prophylactic heparin and aspirin use with insignificant influence to bleeding complications. Raveh et al40 consolidate the use of IV heparin thromboprophylaxis in significantly reducing thrombotic risk. Although additionally demonstrating an increased hemorrhage risk, this was primarily in supratherapeutic (activated partial thromboplastin time [aPTT] > 60) cases, suggesting a lower target PTT value may minimize bleeding risk without compromising efficacy.40 Harbell et al22 demonstrate that a protocol based on prophylactic dual-antiplatelet therapy and short-term low-dose heparin infusion (200-400 U/h) followed by treatment with therapeutic heparin for nonocclusive splenic vein thrombi does not significantly increase risk of bleeding complications. In this context, nonocclusive splenic vein thrombosis could be managed safely with anticoagulation alone, thus warranting further prospective randomized studies in this area.
A limitation of this review was that several analyzed variables surrounding thrombotic risk were poorly reported within the literature. Recipient gender quantified with associated thrombosis was reported in only 3 studies. Although this identifies an area of potential improvement within the field, the authors of each article were not directly contacted to provide further data that may have been collated but excluded from final reports. This study was also limited by the variable time frames used in included studies for reporting thrombosis. Many studies utilized reporting time frames that extended beyond 2 weeks posttransplant. Although adhering to our predefined eligibility criteria of early thrombosis as within 2 weeks of transplantation, many studies did not clarify exact timing of each thrombosis and as such offers potential that some thrombi reported occurred external to our eligibility period and affected viability for an accurate overall thrombosis rate. Mixed inclusion/lack of delineation of partial, complete, arterial, venous, and functional thrombi between studies adds heterogeneity to this review. This is further confounded by the inconsistent use of postoperative protocol imaging between studies for which subclinical partial/functional thrombi of nonsignificance may have been identified.21,46 Lastly, by employing a broad inclusion criterion, a comprehensive representation of the literature could be collected, limiting selection bias and increasing relevant studies, resulting in an accurate depiction of overall thrombosis rate and graft loss. However, on balance, by having no restriction on publication date, the effects of varying study eras and associated developments in the pretransplant selection, transplantation protocol and technique, postoperative management, and imaging modalities over time may provide potential era bias and an overcalculated thrombosis rate compared to what would be expected from recent studies alone.
Conclusions
Given the intrinsically low parenchymal microvasculature flow of the pancreas and the hypercoagulable state of the diabetic recipient, it is unsurprising that pancreas transplants suffer higher thrombosis rates (9%) and consequent graft loss (7%) than other abdominal organ transplants.2 As such, modifiable risk factors for allograft thrombosis need to be elucidated and taken into careful consideration to optimize outcomes. By doing so, rates of early thrombosis can be reduced, ultimately increasing long-term graft survival and improving patient outcomes. This does, however, need to be balanced against the availability of donors. Too high a restriction to attenuate complications can result in a shortage of potential grafts. Extending donor sources with equivalent risk profiles such as recent evidence supporting pediatric pancreas donors should be further investigated.44 This is especially important in today’s society, where type 1 diabetes is becoming increasingly prevalent.1,75 Furthermore, diabetic patients with end-stage renal failure waiting for an available transplant have a higher mortality rate compared to the first year posttransplantation.76 With this in mind, using the findings of this review, we demonstrate that judicious donor and recipient selection, use of antemortem heparin along with meticulous procurement and preservation, followed by evidence-based transplantation technique and postoperative management are of paramount importance to ongoing and improving success in whole pancreas transplantation. We suggest when all are taken into account and utilized, significant improvements to pancreas graft survival can be achieved. Finally, this review highlights the need for further investigation into improved preservation solutions, exocrine drainage method, standardized anticoagulation, and screening protocols as their effect on thrombosis remain poorly elucidated within the literature.
Supplemental Material
Supplemental Material, SPK_thrombosis_Supporting_Information for Risk Factors for Early Pancreatic Allograft Thrombosis Following Simultaneous Pancreas-Kidney Transplantation: A Systematic Review by Jian Blundell, Sara Shahrestani, Rebecca Lendzion, Henry J. Pleass and Wayne J. Hawthorne in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: JB, primary researcher, performed study design, study selection, data collection/extraction, and wrote the article. SS, secondary researcher, performed study selection, study design, and wrote the article. RL and HP wrote the article. WJH supervised, performed study design, contributed important advice, and wrote the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jian Blundell  https://orcid.org/0000-0002-5011-2727
https://orcid.org/0000-0002-5011-2727
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Chapter 1: epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farney AC, Rogers J, Stratta RJ. Pancreas graft thrombosis: causes, prevention, diagnosis, and intervention. Curr Opin Organ Transplant. 2012;17(1):87–92. [DOI] [PubMed] [Google Scholar]
- 3. Troppmann C, Gruessner AC, Dunn DL, Sutherland DE, Gruessner RW. Surgical complications requiring early relaparotomy after pancreas transplantation: a multivariate risk factor and economic impact analysis of the cyclosporine era. Ann Surg. 1998;227(2):255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gruessner A, Rana A, Porubsky M, Gruessner R. Declining number of simultaneous pancreas/kidney transplants but significant improvement in early technical outcomes. Transplantation. 2014;98:853–854.24914571 [Google Scholar]
- 5. Ashgar F, Hu J, Zhong S, et al. Risk factors of thrombosis of pancreatic graft: a review. J Transplant Technol Res. 2016;6:166. [Google Scholar]
- 6. Gruessner AC, Gruessner RW. Pancreas transplantation of US and Non-US cases from 2005 to 2014 as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). RDS. 2016;13(1):35–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutherland D, Kandaswamy R, Dunn T, et al. Outcomes in >2,000 pancreas (Px) transplants (Tx) at a single institution. Xenotransplantation. 2009;16 (5):290–291. [Google Scholar]
- 8. Humar A, Ramcharan T, Kandaswamy R, et al. Technical failures after pancreas transplants: why grafts fail and the risk factors – a multivariate analysis. Transplantation. 2004;78(8):1188–1192. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JPTGS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; 2011. Updated March 2011. [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 11. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non Randomised Studies in Meta-Analyses. Ottawa Health Research Institute; 1999. [Google Scholar]
- 12. Agarwal A, Murdock P, Pescovitz MD, et al. Follow-up experience using histidine-tryptophan ketoglutarate solution in clinical pancreas transplantation. Transplant Proc. 2005;37(8):3523–3526. [DOI] [PubMed] [Google Scholar]
- 13. Alonso D, Dunn TB, Rigley T, et al. Increased pancreatitis in allografts flushed with histidine-tryptophan-ketoglutarate solution: a cautionary tale. Am J Transplant. 2008;8(9):1942–1945. [DOI] [PubMed] [Google Scholar]
- 14. Becker T, Ringe B, Nyibata M, et al. Pancreas transplantation with histidine-tryptophan-ketoglutarate (HTK) solution and University of Wisconsin (UW) solution: is there a difference? J Pancreas. 2007;8(3):304–311. [PubMed] [Google Scholar]
- 15. Ciancio G, Cespedes M, Olson L, Miller J, Burke GW. Partial venous thrombosis of the pancreatic allografts after simultaneous pancreas-kidney transplantation. Clin Transplant. 2000;14(5):464–471. [DOI] [PubMed] [Google Scholar]
- 16. Decraemer I, Cathenis K, Troisi R, deHemptinne B, Hesse UJ. The influence of desmopressin and vasopressors in the donor management on graft function following pancreas transplantation. Transplant Proc. 2004;36(4):1042–1044. [DOI] [PubMed] [Google Scholar]
- 17. Douzdjian V, Gugliuzza KG, Fish JC. Multivariate analysis of donor risk factors for pancreas allograft failure after simultaneous pancreas-kidney transplantation. Surgery. 1995;118(1):73–81. [DOI] [PubMed] [Google Scholar]
- 18. Englesbe MJ, Moyer A, Kim DY, et al. Early pancreas transplant outcomes with histidine-tryptophan-ketoglutarate preservation: a multicenter study. Transplantation. 2006;82(1):136–139. [DOI] [PubMed] [Google Scholar]
- 19. Grewal HP, Garland L, Novak K, et al. Risk factors for post implantation pancreatitis and pancreatic thrombosis in pancreas transplant recipients. Transplantation. 1993;56(3):609–612. [DOI] [PubMed] [Google Scholar]
- 20. Gruessner RW, Sutherland DE, Troppmann C, et al. The surgical risk of pancreas transplantation in the cyclosporine era: an overview. J Am Coll Surg. 1997;185(2):128–144. [DOI] [PubMed] [Google Scholar]
- 21. Hakeem A, Chen J, Iype S, et al. Pancreatic allograft thrombosis: suggestion for a CT grading system and management algorithm. Am J Transplant. 2018;18(1):163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harbell JW, Morgan T, Feldstein VA, et al. Splenic vein thrombosis following pancreas transplantation: identification of factors that support conservative management. Am J Transplant. 2017;17(11):2955–2962. [DOI] [PubMed] [Google Scholar]
- 23. Hau HM, Tautenhahn HM, Uhlmann D, et al. Single-center experience using organs after rescue allocation for pancreas transplant in the Eurotransplant region. Exp Clin Transplant. 2014;12(4):351–356. [PubMed] [Google Scholar]
- 24. Hesse U, Meester D, Troisi R, et al. The use of low dose octreotide prophylaxis in pancreatic transplants with enteric drainage. Results of a prospective randomized single center trial. Clin Transplant. 2005;19(3):299–303. [DOI] [PubMed] [Google Scholar]
- 25. Humar A, Kandaswamy R, Granger D, et al. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg. 2000;231(2):269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Humar A, Ramcharan T, Kandaswamy R, et al. The impact of donor obesity on outcomes after cadaver pancreas transplants. Am J Transplant. 2004;4(4):605–610. [DOI] [PubMed] [Google Scholar]
- 27. Ionescu C, Wolf P, Ellero B, Mihaescu G. The venous thrombosis of the pancreatic graft. J Gastro Liver Dis. 2007;16(3):287–292. [PubMed] [Google Scholar]
- 28. Jimenez C, Manrique A, Herrero ML, et al. Incidence of pancreas graft thrombosis in portoiliac and portocaval venous anastomosis. Transplant Proc. 2005;37(9):3977–3978. [DOI] [PubMed] [Google Scholar]
- 29. Jiménez-Romero C, Quinto AM, Municio AM, et al. Simultaneous pancreas-kidney transplantation. Experience of the Doce de Octubre Hospital. Cir Esp. 2018;96(1):25–34. doi: 10.1016/j.ciresp.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 30. Karam G, Compagnon P, Hourmant M, et al. A single solution for multiple organ procurement and preservation. Transpl Int. 2005;18(6):657–663. [DOI] [PubMed] [Google Scholar]
- 31. Lindahl JP, Horneland R, Nordheim E, et al. Outcomes in pancreas transplantation with exocrine drainage through a duodenoduodenostomy versus duodenojejunostomy. Am J Transplant. 2018;18(1):154–162. doi: 10.1111/ajt.14420 [DOI] [PubMed] [Google Scholar]
- 32. Manrique A, Jimenez C, Lopez RM, et al. Relaparotomy after pancreas transplantation: causes and outcomes. Transplant Proc. 2009;41(6):2472–2474. [DOI] [PubMed] [Google Scholar]
- 33. Martins LS, Malheiro J, Pedroso S, et al. Pancreas-kidney transplantation: impact of dialysis modality on the outcome. Transpl Int. 2015;28(8):972–979. [DOI] [PubMed] [Google Scholar]
- 34. Montiel-Casado MC, Fernandez-Burgos I, Perez-Daga JA, et al. Impact of blood amylase peak over vascular graft thrombosis in pancreas transplantation. Transplant Proc. 2012;44(9):2627–2630. [DOI] [PubMed] [Google Scholar]
- 35. Okabe Y, Kitada H, Miura Y, et al. Pancreas transplantation: a single-institution experience in Japan. Surg. 2013;43(12):1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ozaki CF, Stratta RJ, Taylor RJ, et al. Surgical complications in solitary pancreas and combined pancreas-kidney transplantations. Am J Surg. 1992;164(5):546–551. [DOI] [PubMed] [Google Scholar]
- 37. Page M, Rimmele T, Ber CE, et al. Early relaparotomy after simultaneous pancreas-kidney transplantation. Transplantation. 2012;94(2):159–164. [DOI] [PubMed] [Google Scholar]
- 38. Räihä J, Helanterä I, Ekstrand A, Nordin A, Sallinen V, Lempinen M. Effect of pretransplant dialysis modality on outcomes after simultaneous pancreas-kidney transplantation. Ann Transplant. 2019;24:426–431. doi: 10.12659/AOT.916649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramessur Chandran S, Kanellis J, Polkinghorne KR, Saunder AC, Mulley WR. Early pancreas allograft thrombosis. Clin Transplant. 2013;27(3):410–416. [DOI] [PubMed] [Google Scholar]
- 40. Raveh Y, Ciancio G, Burke GW, et al. Susceptibility-directed anticoagulation after pancreas transplantation: a single-center retrospective study. Clin Transplant. 2019;33(7):e13619. [DOI] [PubMed] [Google Scholar]
- 41. Sanchez-Hidalgo JM, Rodriguez-Ortiz L, Arjona-Sanchez A, et al. Pancreas donor hypernatremia: is it really a risk factor for simultaneous pancreas-kidney transplantation? Transplant Proc. 2018;50(2):676–678. [DOI] [PubMed] [Google Scholar]
- 42. Scheffert JL, Taber DJ, Pilch NA, et al. Clinical outcomes associated with the early postoperative use of heparin in pancreas transplantation. Transplantation. 2014;97(6):681–685. [DOI] [PubMed] [Google Scholar]
- 43. Schneeberger S, Biebl M, Steurer W, et al. A prospective randomized multicenter trial comparing histidine-tryptophane-ketoglutarate versus University of Wisconsin perfusion solution in clinical pancreas transplantation. Transpl Int. 2009;22(2):217–224. [DOI] [PubMed] [Google Scholar]
- 44. Spaggiari M, Di Bella C, Di Cocco P, et al. Pancreas transplantation from pediatric donors: a single-center experience. Transplantation. 2018;102(10):1732–1739. [DOI] [PubMed] [Google Scholar]
- 45. Troppmann C, Gruessner AC, Benedetti E, et al. Vascular graft thrombosis after pancreatic transplantation: univariate and multivariate operative and nonoperative risk factor analysis. J Am Coll Surg. 1996;182(4):285–316. [PubMed] [Google Scholar]
- 46. Vincent M, Morla O, Branchereau J, et al. Multi detector computed tomography (MDCT) for the diagnosis of early complications after pancreas transplantation. Abdom Imaging. 2014;39(6):1186–1192. [DOI] [PubMed] [Google Scholar]
- 47. Shahrestani S, Webster AC, Lam VW, et al. Outcomes from pancreatic transplantation in donation after cardiac death: a systematic review and meta-analysis. Transplantation. 2017;101(1):122–130. [DOI] [PubMed] [Google Scholar]
- 48. Kopp WH, Lam HD, Schaapherder AFM, et al. Pancreas transplantation with grafts from donors deceased after circulatory death: 5 years single-center experience. Transplantation. 2018;102(2):333–339 [DOI] [PubMed] [Google Scholar]
- 49. Cantarovich D, Guillen-Gueguin C, Le Borgne F, et al. Pancreas transplantation results are not more progressing and require significant innovations. Transplantation. 2016;100 (7 suppl 1):S10. [Google Scholar]
- 50. Finger EB, Radosevich D, Borja D, et al. Technical failure in pancreas transplantation: a composite model for predicting risk in the current era. Transplantation. 2012;94(10 suppl):30.22706322 [Google Scholar]
- 51. Graham J, Lubetzky M, Chokechanachaisakul A, Kinkhabwala M, Rocca J. Send diabetes on a long trip: the pancreas travels well. Am J Transplant. 2017;17(2):691. [Google Scholar]
- 52. Gruessner AC, Sutherland DE, Gruessner RW. What happens to the kidney after early failure of a simultaneous pancreas graft. Transplantation. 2012;94(10 suppl):33. [Google Scholar]
- 53. Horneland R, Leivestad T, Jenssen T, Oyen O. Higher donor age and male recipient gender have a negative impact on pancreas transplant outcomes (surgical complications and graft survival). Transplantation. 2012;94(10 suppl):692. [Google Scholar]
- 54. Horton P, Schnitzler MA, Dzebisashvili N, et al. High pre-transplant insulin requirements correlates with pancreas failure risk in SPK. Am J Transplant. 2012;12:115.21929643 [Google Scholar]
- 55. Jimenez-Romero CC, Manrique AA, Calvo JJ, et al. Incidence of pancreas graft thrombosis in bladder vs enteric drainage. Xenotransplantation. 2009;16(5):341. [Google Scholar]
- 56. Kudva Y, Dong M, Parsaik A, et al. Systematic study of causes of death and allograft loss in pancreas transplantation in the modern ERA with ATG induction and tacrolimus based triple immunosuppression. Am J Transplant. 2013;13(1):439. [Google Scholar]
- 57. Lin ZX, Kee M, Taber DJ, et al. Incidence, risk factors and associated clinical outcomes of surgical complications following pancreas transplantation. J Surg Res. 2013;179(2): 196 Conference: 8th Annual Academic Surgical Congress of the Association for Academic Surgery, AAS and the Society of University Surgeons, SUS New Orleans, LA United States Conference Publication. [Google Scholar]
- 58. Martins LS, Aguiar P, Dias L, et al. Results from 150 pancreas-kidney transplants—a single centre analysis. Nephrol Dial Transplant. 2013;28(1):i509. [Google Scholar]
- 59. Patil V, Welsch B, Leverson G, et al. Early pancreas thrombosis (within 90 days) after solitary pancreas transplants: a comprehensive study of the incidence, outcomes and risk factors. Transplantation. 2014;98:861. [Google Scholar]
- 60. Ramessur S, Kanellis J, Polkinghorne K, Saunder A, Mulley W. Early pancreatic allograft thrombosis—a single centre review. Nephrology. 2010;15:61–62.20586952 [Google Scholar]
- 61. Rogers J, Farney A, Al-Geizawi S, et al. Simultaneous kidney-pancreas transplantation in African-American recipients: alemtuzumab versus RATG induction. Am J Transplant. 2011;11:436–437. [Google Scholar]
- 62. Rogers J, Farney A, Orlando G, et al. Pancreas transplantation in African American compared to non African American recipients: does recipient ethnicity influence outcomes? Transplantation. 2013;96:S82. [Google Scholar]
- 63. Scheffert JL, Taber DJ, Weimert NA, et al. Risk factors for thrombosis and clinical outcomes associated with post-operative heparin use in pancreas transplantation. Am J Transplant. 2010;10:547.20415898 [Google Scholar]
- 64. Singh RP, Vrakas G, Mamode N, et al. Multivariate analysis of early technical causes of pancreas allograft loss: changing trends. Transplantation. 2012;94:687.22955229 [Google Scholar]
- 65. Choi J, Kim J, Kwon H, et al. Pancreas transplantation outcomes—a single center experience 400 cases. Am J Transplant. 2019;19 (suppl 3):1142. [Google Scholar]
- 66. Ferrer J, Cano-Vargas B, Martinez de la Maza L, et al. Enteric drainage of pancreas transplantation. Clinical impact of intra-abdominal complications. HPB. 2019;21(Supplement 3):S770–S771. [Google Scholar]
- 67. Koyama I, Nakajima I, Iwadoh K, et al. Causes and risk factors of pancreas graft loss: a single center study. Transplantation. 2018;102 (7 suppl 1):S757. [Google Scholar]
- 68. Shahrestani S, Spike E, Hort A, et al. Risk factors for thrombosis in simultaneous pancreas-kidney transplants performed over ten years at Westmead hospital. Transplantation. 2018;102 (7 suppl 1):S240. [Google Scholar]
- 69. Hameed AM, Wong G, Laurence JM, et al. A systematic review and meta-analysis of cold in situ perfusion and preservation for pancreas transplantation. HPB. 2017;19(11):933–943. [DOI] [PubMed] [Google Scholar]
- 70. Troppmann C. Complications after pancreas transplantation. Curr Opin Organ Transplant. 2010;15(1):112–118. [DOI] [PubMed] [Google Scholar]
- 71. Patel SR, Hakim N. Prevention and management of graft thrombosis in pancreatic transplant. Exp Clin Transplant. 2012;10(3):282–289. [DOI] [PubMed] [Google Scholar]
- 72. Stewart ZA, Cameron AM, Singer AL, et al. Histidine-tryptophan-ketoglutarate (HTK) is associated with reduced graft survival in pancreas transplantation. Am J Transplant. 2009;9(1):217–221. [DOI] [PubMed] [Google Scholar]
- 73. Corry RJ, Chakrabarti P, Shapiro R, et al. Comparison of enteric versus bladder drainage in pancreas transplantation. Transplant Proc. 2001;33(1-2):1647–1651. [DOI] [PubMed] [Google Scholar]
- 74. Young CJ. Are there still roles for exocrine bladder drainage and portal venous drainage for pancreatic allografts? Curr Opin Organ Transplant. 2009;14(1):90–94. [DOI] [PubMed] [Google Scholar]
- 75. Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Therapy. 2008;88(11):1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mittal S, Gilbert J, Friend PJ. Donors after circulatory death pancreas transplantation. Curr Opin Organ Transplant. 2017;22(4):372–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, SPK_thrombosis_Supporting_Information for Risk Factors for Early Pancreatic Allograft Thrombosis Following Simultaneous Pancreas-Kidney Transplantation: A Systematic Review by Jian Blundell, Sara Shahrestani, Rebecca Lendzion, Henry J. Pleass and Wayne J. Hawthorne in Clinical and Applied Thrombosis/Hemostasis