Abstract
Radiation-induced lung injury (RILI) is one of the most serious complications of thoracic radiation and TGF-β1 is a central regulator of RILI. However, the molecular mechanism underlying the fine tuning of TGF-β1 signaling in RILI has not been fully understood. In the current study, differentially expressed long non-coding RNAs (LncRNAs) among human lung fibroblasts cell lines HFL-1 and WI-38 treated with TGF-β1, were identified by microarray and validated by real time PCR. LncRNA-RP11 was found to be the most increased LncRNA and it mediated the promotion of fibrogenic activity in human lung fibroblasts after TGF-β1 treatment. Bioinformatic analysis revealed that TGF-β1 may be associated with the component and structure of extracellular matrix in lung fibroblasts cells, and LncRNA-RP11 was predicted and confirmed to be a competing endogenous RNA by directly binding to miR-29a. Functional experiments investigating the biological role of LncRNA-RP11/miR-29a axis in RILI, were then carried out in human fibroblasts. The results showed that radiation promoted the expression of LncRNA-RP11, but regressed the expression of miR-29a. Furthermore, radiation elevated the expression of various common collagenic proteins, which could be abolished by overexpression of miR-29a.
Keywords: LncRNA-RP11, TGF-β1, miR-29a, radiation-induced lung injury
Introduction
Radiotherapy plays an imperative role in treating thoracic tumors and radiation-induced lung injury (RILI) is one of the most common and serious complications during thoracic radiation.1-3 It is characterized by the accumulation of fibroblasts, myofibroblasts, inflammatory cells and extracellular matrix proteins, such as collagen, subsequently forming into scar tissue, which could eventually lead to fatal respiratory insufficiency.1,4 In clinical, RILI remains one of the most important factors limiting the radiation dose that could be delivered to the tumors, which negatively affects the local control rates of various thoracic cancers and patients’ long-term quality of life.1,5
TGF-β1 is a multifunctional regulator of cell growth and differentiation, expressed in response to different kinds of injuries, and plays critical roles in the regulation of radiation-induced normal tissue damage, particularly RILI.6,7 It could promote proliferation of fibroblasts, induce synthesis of collagen, inhibit expression of collagenase and recruit a large amount of pro-inflammation immune cells, leading to the development and exacerbation of RILI.4,8 Hence, inhibiting the TGF-β1 signaling pathway has the potential to reduce the incidence and severity of RILI. However, the underlying mechanisms modulating TGF-β1 signaling in RILI remain exclusive.
Long noncoding RNAs (LncRNAs) are a class of transcripts longer than 200 nucleotides with limited protein coding potential. Recently, many studies have shown that LncRNAs have multiple functions in a wide range of biological processes, including cell proliferation, apoptosis, migration, carcinogenesis, would healing and tissue repair, and they are frequently deregulated in various diseases.9 For instance, several LncRNAs have been identified to participate in the modulation of radiation-induced normal tissue injuries, such as LncRNA HULC,10 Gm14005 and Tmevpg1.11 Nevertheless, LncRNAs related to RILI are seldom reported, thus, we identified the specific LncRNA induced by TGF-β1 in human lung fibroblasts cells by microarray and further preliminarily verified the regulatory effect and molecular mechanism of LncRNA-RP11/miR-29a in RILI cell models, which might provide a potential target for the treatment of RILI in the future.
Materials and Methods
Cell Culture and Radiation
The human lung fibroblasts cell lines HFL1 and WI-38 were purchased from the ATCC (Manassas, VA, USA). HFL1 and WI-38 cell lines were cultured in Ham’s F-12 K and MEM medium, respectively. The 2 medium were both supplemented with FBS (10%), penicillin (100 U/ml), and streptomycin (100 g/ml) in a 37°C humidified incubator with 5% CO2.
During radiation, cells were seeded in 60-mm dishes at proper cell densities in duplicate and the culture flasks were placed flat on a treatment bed on the top of a plexi glass plate with a thickness of 1.3 cm. Irradiation was performed using a linear accelerator (Siemens, Munich, Germany) with 6 MV-X ray at 2Gy/min and a source skin distance of 100 cm. The angle of the accelerator during irradiation was 180°, and the rays entered through the bottom of the flask.
Western Blot
Cells were harvested and lysed in cell lysis buffer and analyzed by Western blotting. The antibodies for this study is α-SMA(1:500; abcam, ab5694), Fibronectin(1:500; abcam, ab2413), Vimentin(1:1000; abcam, ab8978), β-Actin(1:1200; bioworld), Collagen I(1:1000; abcam, ab138492), anti-rabbit and anti-mouse IgG horseradish peroxidase conjugated antibody(1:5000; JACKSON) .
RNA Extraction and Microarray Analysis
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The RNA quality was confirmed by formaldehyde agarose gel electrophoresis and quantified by NanoDrop-2000. The samples were used to synthesize double-stranded cDNA, and the cDNA was then labeled and hybridized to the mRNA and LncRNA Expression Microarray (SBC Agilent Human lncRNA array, Shanghai) according to the manufacturer’s protocol. After hybridization, the arrays were washed, and the slides were scanned with an Agilent Microarray Scanner (Agilent p/n G2565BA). Raw data were extracted as pair files using the Agilent Feature Extraction. The random variance model was used to identify the differentially expressed genes. The paired t-test was used to calculate the P-value. The threshold set for up- and down-regulated genes was a fold change ≥4.0 and a p-value ≤0.05, respectively.
GO and KEGG Analysis
To predict the biological roles of differentially expressed mRNAs, GO and KEGG analyses were performed using freely available online MAS system provided by the CapitalBio Corp (Molecule Annotation System, http://bioinfo.capitalbio.com/mas3/). P < 0.05 was considered significant.
Transfection
MiR-29a mimics, miR-29a inhibitor and corresponding negative control were purchased from Ribobio Co. (Guangzhou, China).The plasmid constructs and the corresponding negative control were built by TsingKE Biological technology (Nanjing, China) to silence(shRNA-lncRNA-RP11) or overexpress(pcDNA3.1-RP11) lncRNA-RP11, all of them were transfected with Lipofectamine 2000 (Invitrogen) at a final concentration of 50 nM. The effects of the transient transfection were confirmed by quantitative real-time PCR analysis.
Real-Time PCR
For lncRNA-RP11, cDNA was reverse-transcribed from 2 µg total RNA using a Reverse Transcription Kit (Takara, Biochemical, Tokyo, Japan). cDNA was then amplified with a SYBR Premix Ex Taq II (Perfect Real-Time) kit (Takara). The primer sequences were presented in supplementary Table 1. For miR-29a, cDNA was reverse -transcribed by using all-in-one miRNA qRT-PCR decetion kit(GeneCopoeia, Rockville, MD, USA), Primers for miR-29a and U6 were purchased from GeneCopoeia. The lncRNA-RP11 and miR-29a gene expression levels were calculated using the delta-delta Ct method with β-actin and U6 as an internal control, respectively.
Luciferase Activity Assay
For lncRNA-RP11 mRNA 3′UTR luciferase reporter assays, luciferase reporter plasmid (100 ng) containing the potential binding sequence of 3′UTR of lncRNA-RP11 mRNA (wild type, WT) or mutated sequence (mutant type, MUT) were co-transfected into 293 T and HLF-1 cells in 96-well plate with Hsa-mir 29a mimics, Hsa-mir 29a inhibitor and corresponding NC respectively by using Lipofectamine 2000 (Invitrogen). Luciferase activity assays were performed 48 hours after transfection (Promega, Madison, WI). Firefly luciferase activity was normalized to the corresponding renilla luciferase activity by using the Dual-Glo Luciferase Assay System. All experiments were performed for 3 times.
Statistical Analysis
Data were expressed at the means ± standard deviation (SD). Between group differences were tested using 1-way ANOVA. Two group comparisons were performed using independent samples Student’s t test. P < 0.05 was considered significant.
Results
TGF-β1 Promotes Fibrogenic Activity in Human Lung Fibroblast via LncRNA-RP11
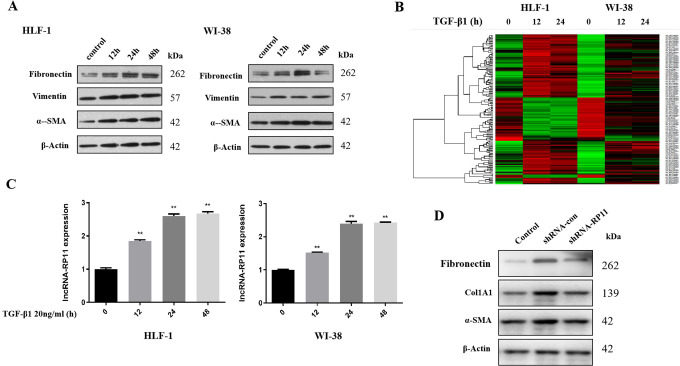
We used 2 different fibroblasts cell lines HLF-1 and WI-38 to test the effect of TGF-β1 in collagenase via co-incubation with 20ng/ml TGF-β1 and found that the expression of collagenic proteins, including Fibronectin, Vitmentin, α-SMA, increased most significantly after 24 hours of co-incubation, in HLF-1 and WI-38 (Figure 1A).
Figure 1.
TGF-β1 promotes fibrogenic activity in human lung fibroblast via LncRNA-RP11. (A) The expression of 3 common collagenic proteins after TGF-β1 treatment. (B) Microarray and hierarchical cluster analyses. (C) The increased expression of LncRNA-RP11 after TGF-β1 treatment.Statistical significance was analyzed by 1-way ANOVA, **p < 0.01 compared to control, n = 3 in each experiment. (D) The expression of collagenic proteins in 3 different conditions. Control, LncRNA-RP11 wild type and without TGF-β1 treatment; shRNA-control, LncRNA-RP11 wild type and with TGF-β1 treatment; shRNA-LncRNA-RP11, LncRNA-RP11 knocking down and with TGF-β1 treatment.
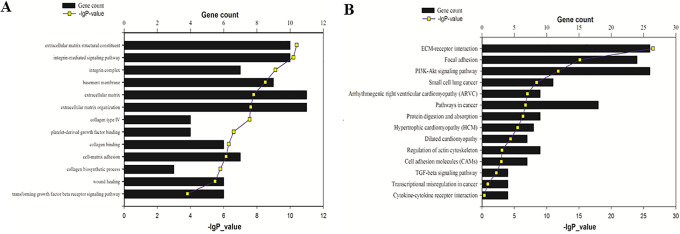
Next, to screen the lncRNAs and mRNAs induced by TGF-β1, we harvested the HLF-1 and WI-38 cells incubated with TGF-β1 for 12 and 24 hours and sent for SBC Agilent Human lncRNA array profiling. There were 97 LncRNAs whose expression increased greater than 2 folds, and 44 LncRNAs whose expression decreased greater than 2 folds after TGF-β1 treatment (Figure 1B). LncRNA-RP11(ENST00000438158.1, RP11-400N13.3-001)was the most increased lncRNAs in both HLF-1 and WI-38 cell lines at 12hours and 24hours groups, with a mean fold change of 3.093 in HLF-1 and 3.145 in WI-38. To predict the potential target induced by TGF-β1, GO and KEGG analyses of differently expressed mRNAs were then carried out. GO analysis found that related genes were associated with extracellular matrix structural constituent and organization, integrin complex and integrin-mediated signaling, basement membrane, collagen synthesis and binding, platelet-derived growth factor binding, cell-matrix adhesion and would healing (Figure 2A). KEGG analysis revealed that the related genes were mainly enriched in extracellular matrix-receptor interaction pathway, focal adhesion pathway, PI3K/Akt signaling pathway, as well as cancer-related pathways (Figure 2B), which indicated that TGF-β1 might regulate the fibrogenic activity in human lung fibroblast cells.
Figure 2.
Molecular function analysis of LncRNA-RP11. (A) GO analysis. (B) KEGG analysis
The expression of LncRNA-RP11 in HLF-1 and WI-38 after TGF-β1 treatment, was further examined by real time PCR. The result showed that LncRNA-RP11 expression increased in a time-dependent manner (2.596 folds at 24hours in HLF-1, 2.393 folds at 24hours in WI-38) after TGF-β1 treatment (Figure 1C). Furthermore, the elevation of the expression levels of 3 collagenic proteins, including Fibronectin, Col1A1 and α-SMA, in HLF-1 after 24 hours of 20ng/ml TGF-β1 incubation, were dramatically reversed by shRNA-LncRNA-RP11 (Figure 1D).
LncRNA-RP11 Directly Targets miR-29a
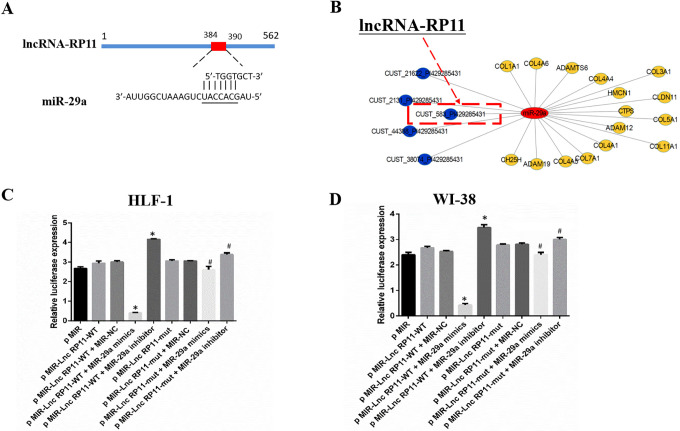
By using bioinformatics analysis, we found that +384 to +390 bp in LncRNA-RP11 is a potential binding site of miRNA-29a (Figure 3A), which is a well-recognized modulator of fibrosis.12 Besides, miRNA-29a was predicted to modulate fibrosis associated genes such as COL3A1, COL1A1 and ADAM (Figure 3B).
Figure 3.
MiR-29a is the direct target of LncRNA-RP11. (A) The predicted binding of LncRNA-RP11 with miR-29a. (B) The predicted targets of miR-29a. (C-D) 3’-UTR luciferase reporter assays in HLF-1 and WI-38. Statistical significance was analyzed by 1-way ANOVA, *p < 0.01 compared to control, #p < 0.01 mutant compared to wild group, n = 3 in each experiment.
To validate the direct binding between miR-29a and LncRNA-RP11 at endogenous levels, the entire wild-type 3’-UTR of miR-29a or the mutant 3’-UTR in the seed region, where located the LncRNA-RP11 binding sites, was cloned downstream of the luciferase gene’s open reading frame. Overexpression of miR-29a suppressed the luciferase activity of luciferase reporter harboring full length LncRNA-RP11, while miR-29a inhibitor elevated the luciferase activity. On the other hand, site-directed mutagenesis of the binding site successfully abolished the above effects in HLF-1 (Figure 3C) and WI-38 (Figure 3D), indicating LncRNA-RP11 could act as a ceRNA of miRNA-29a.
MiR-29a Mediates the Pro-Fibrosis Effect of LncRNA-RP11 in Human Lung Fibroblast
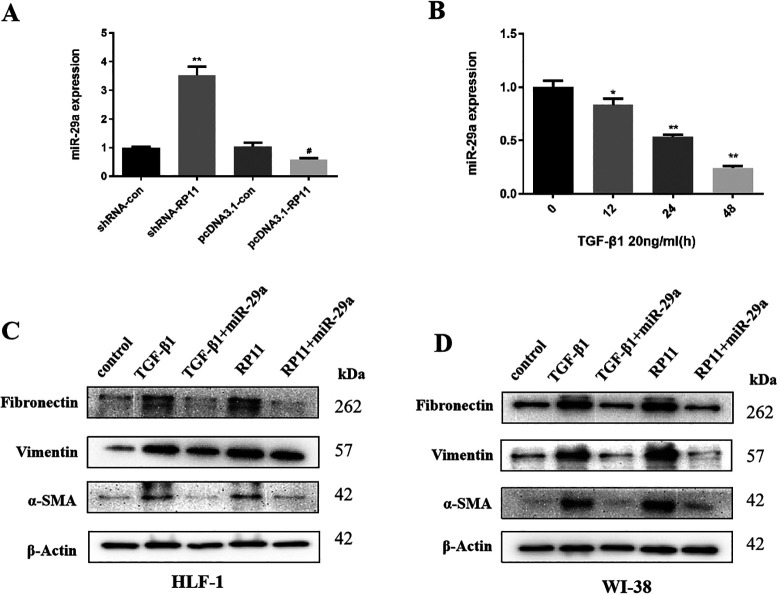
To further verify the relationship between LncRNA-RP11 and miR-29a in human lung fibroblast, the expression of LncRNA-RP11 was knocked-down or overexpressed by using shRNA and pcDNA3.1 plasmid. We found that the expression of miR-29a could be regulated significantly by lncRNA-RP11 in HLF-1 cells (Figure 4A). Next, we incubated HLF-1 cells with 20ng/ml TGF-β1 and tested miR-29a expression by real time-PCR. We found that miR-29a decreased after TGF-β1 incubation in a time-dependent manner (Figure 4B).
Figure 4.
MiR-29a mediates the pro-fibrosis effect of LncRNA-RP11 in human lung fibroblast. (A) Knocking down LncRNA-RP11 significantly increased the expression of miR-29a, while overexpress LncRNA-RP11 decreased the expression of miR-29a.Statistical significance was analyzed by 1-way ANOVA, **p < 0.01 compared to shRNA control, #p < 0.05 compared to pcDNA3.1 control. (B) The expression of miR-29a after TGF-β1 treatment. Statistical significance was analyzed by 1-way ANOVA, *p < 0.05, **p < 0.01 compared to control, n = 3 in each experiment.(C-D) The expression of collagenic proteins after TGF-β1 treatment and with co-expression of miR-29a mimics or pcDNA3.1-RP11 plasmid in HLF-1 and WI-38.
Moreover, in order to examine the biological role of miR-29a in TGF-β1-lncRNA-RP11 promoted fibrogenic activity in human lung fibroblast, a rescue experiment was performed. The result suggested that both TGF-β1 and lncRNA-RP11 could significantly induce the expression of several well-known collagenic proteins such as fibronectin, Vimentin and α-SMA, which are all well reversed by miR-29a in both HLF-1 (Figure 4C) and WI-38 (Figure 4D) cell lines.
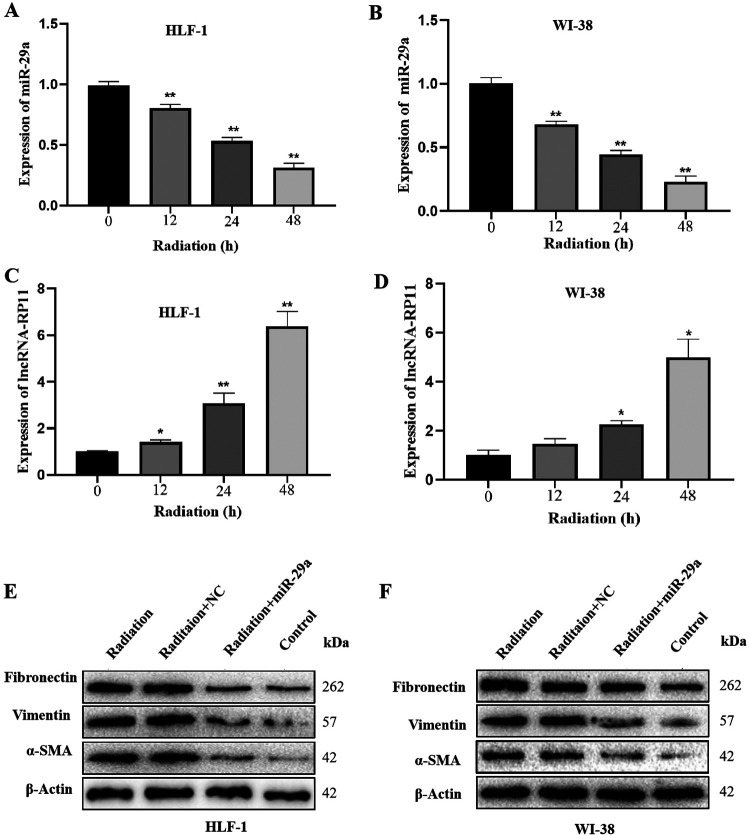
LncRNA-RP11/miR-29a Axis Regulates the Fibrogenic Activity in Response to Radiation
After radiation (20 Gy, 2Gy/min), the expression of LncRNA-RP11 significantly increased over time in HLF-1 (Figure 5A) and WI-38 (Figure 5B). Conversely, the expression of miR-29a significantly decreased over time in HLF-1 (Figure 5C) and WI-38 (Figure 5D).
Figure 5.
LncRNA-RP11/miR-29a axis regulates the fibrogenic activity in response to radiation. (A-B) The expression of LncRNA-RP11 in response to radiation in HLF-1 and WI-38.Statistical significance was analyzed by 1-way ANOVA, **p < 0.01 compared to control, n = 3 in each experiment. (C-D) The expression of miR-29a in response to radiation in HLF-1 and WI-38.Statistical significance was analyzed by 1-way ANOVA, *p < 0.05, **p < 0.01 compared to control, n = 3 in each experiment. (E-F) The expression of collagenic proteins after radiation and with co-expression of miR-29a in HLF-1 and WI-38.
In addition, in order to explore the functional role of miR-29a in radiation-activated fibrogenic activity in human lung fibroblast, a rescue experiment was performed. Unsurprisingly, radiation significantly promoted the expression of several well-known collagenic proteins, and the pro-fibrosis effect of radiation was abolished by overexpression of miR-29a, in both HLF-1 (Figure 5E) and WI-38 (Figure 5F).
Discussion
TGF-β1 plays a central role in the development of RILI, especially radiation induced pulmonary fibrosis.6,8 Multiple clinical studies have supported that TGF-β1 signaling was closely related to RILI.7,13,14 Additionally, several basic studies have investigated the functional roles and biological processes involving TGF-β1 signaling in RILI.15-17 However, the exact molecular mechanisms fine tuning TGF-β1 signaling in RILI remain elusive and our study indicate that LncRNA-RP11 plays a critical role in the modulation of TGF-β1-activated RILI via downregulating miR-29a, especially in terms of fibrogenic activity of human fibroblasts under TGF-β1 treatment or in response to irradiation.
In recent years, noncoding RNA has been found to participate in the biological behavior of various cells by regulating miRNA and other mechanisms, to screening noncoding RNA associated with RILI will help us to understand the mechanism of disease development and explore potential therapeutic targets(Li, 2020). Here, we presented experimental evidence demonstrating that lncRNA-RP11 is an important downstream factor of TGF-β1 signaling in human lung fibroblasts by microarray profiling and validation by real time PCR. Further bioinformatics analysis from differential mRNA revealed that multiple pathways such as extracellular matrix-receptor interaction, focal adhesion may be involved in the activation of pulmonary fibroblasts by TGF-β1. Recent studies have found that extracellular matrix-receptor interaction and focal adhesion are significant pathways enriched by the DEGs that have been proven to be closely related to the regulation of the fibrosis process in lung tissues, besides, well-known collagenic proteins like fibronectin, Col1A1 are all in the extracellular matrix-receptor interaction pathway, the WB blot experiment in this study also confirmed the conjecture of bioinformatics. All the above studies indicate that TGF-β1 is closely involved in the process of pulmonary fibrosis, and lncRNA-RP11 may be an important regulator factor in TGF-β1-fibrosis process.
miR-29a has long been recognized as an important mediator of tissue fibrosis12 and our data suggested that it was downregulated in RILI by LncRNA-RP11. It has been reported that overexpression of miR-29a in murine hepatic stellate cells resulted in the downregulation of collagen expression, including collagen-1α1 and collagen-4α1, by directly targeting the mRNA expression of these extracellular matrix genes.18 Additionally, it has been repeatedly shown that miR-29a could mediate TGFβ1-induced extracellular matrix synthesis through activation of PI3K-AKT pathway or Wnt/β -catenin pathway in human lung fibroblasts.19,20 Moreover, TGF-β1 was found to decrease the expression of miR-29a in various disease models, including hepatic fibrosis and renal fibrosis.21,22 In this study, we found that miR-29a may be involved in the modulation of TGF-β1-activated RILI, which is generally consistent with the above-mentioned studies, highlighting that miR-29a could be a potential therapeutic target in human tissue fibrosis.
However, some limitations exist in our study. Since LncRNA-RP11 doesn’t exist in mice, we could not validate the interaction between TGF-β1 and LncRNA-RP11, as well as between LncRNA-RP11 and miR-29a, in the mice model of RILI, instead in vitro experiments were carried out. Besides, future investigations about the molecular mechanisms concerning the TGF-β1/LncRNA-RP11/miR-29a regulatory axis in RILI are warranted, since the downstream target and pathway of miR-29a were not explored in our study.
Conclusion
Taken together, the present study indicates that in RILI model, TGF-β1 treatment could induce the expression of LncRNA-RP11 in human lung fibroblasts, which may function as a competing endogenous RNA by directly binding to miR-29a. Furthermore, LncRNA-RP11/miR-29a regulatory axis participates in the modulation of collagen synthesis and fibroblast activation after radiation in human lung fibroblasts, which may provided a potential target for treat RILI in the future.
Supplemental Material
Supplemental Material, supplementary_Table_1 for LncRNA-RP11 Modulates TGF-β1-Activated Radiation-Induced Lung Injury Through Downregulating microRNA-29a by Xi Yang, Jianjiao Ni, Yida Li, Liqing Zou, Tiantian Guo, Yuan Li, Li Chu and Zhengfei Zhu in Dose-Response
Abbreviations
- RILI
Radiation-induced lung injury
- LncRNA
long non-coding RNA
- SD
standard deviation
Footnotes
Authors’ Note: Xi Yang and Jianjiao Ni contributed equally.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (No. 81572963, 81872461, 81903253).
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Zhengfei Zhu  https://orcid.org/0000-0001-7537-3619
https://orcid.org/0000-0001-7537-3619
References
- 1. Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156(1):150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li M, Gan L, Song A, Xue J, Lu Y. Rethinking pulmonary toxicity in advanced non-small cell lung cancer in the era of combining anti-PD-1/PD-L1 therapy with thoracic radiotherapy. Biochim Biophys Acta Rev Cancer. 2019;1871(2):323–330. [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Zou L, Yang X, et al. Identification of lncRNA, MicroRNA, and mRNA-Associated CeRNA network of radiation-induced lung injury in a mice model. Dose Response. 2019;17(4):1559325819891012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kainthola A, Haritwal T, Tiwari M, et al. Immunological aspect of radiation-induced pneumonitis, current treatment strategies, and future prospects. Front Immunol. 2017;8:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jain V, Berman AT. Radiation pneumonitis: old problem, new tricks. Cancers. 2018;10(7):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anscher MS. Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. oncologist. 2010;15(4):350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang S, Campbell J, Stenmark MH, et al. Plasma levels of IL-8 and TGF-beta1 predict radiation-induced lung toxicity in non-small cell lung cancer: a validation study. Int J Radiat Oncol Biol Phys. 2017;98(3):615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang Y, Zhang W, Yu F, Gao F. The Cellular and molecular mechanism of radiation-induced lung injury. Med Sci Monit. 2017;23:3446–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao L, Man Y, Liu S. Long non-coding RNA HULC promotes UVB-induced injury by up-regulation of BNIP3 in keratinocytes. Biomed Pharmacother. 2018;104:672–678. [DOI] [PubMed] [Google Scholar]
- 11. Aryankalayil MJ, Chopra S, Levin J, et al. Radiation-induced long noncoding RNAs in a mouse model after whole-body. Radiat Res. 2018;189(3):251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Reilly S. miRNA-29a in systemic sclerosis: a valid target. Autoimmunity. 2015;48(8):511–512. [DOI] [PubMed] [Google Scholar]
- 13. Alam A, Mukhopadhyay ND, Ning Y, et al. A preliminary study on racial differences in HMOX1, NFE2L2, and TGFbeta1 gene polymorphisms and radiation-induced late normal tissue toxicity. Int J Radiat Oncol Biol Phys. 2015;93(2):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stenmark MH, Cai XW, Shedden K, et al. Combining physical and biologic parameters to predict radiation-induced lung toxicity in patients with non-small-cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84(2):e217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Illsley MC, Peacock JH, McAnulty RJ, Yarnold JR. Increased collagen production in fibroblasts cultured from irradiated skin and effect of TGF beta(1)- clinical study. Br J Cancer. 2000;83(5):650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim MJ, Ahn J, Yi JY, Kim MH, et al. Induction of galectin-1 by TGF-beta1 accelerates fibrosis through enhancing nuclear retention of Smad2. Exp Cell Res. 2014;326(1):125–135. [DOI] [PubMed] [Google Scholar]
- 17. Xia DH, Xi L, Xv C, et al. The protective effects of ambroxol on radiation lung injury and influence on production of transforming growth factor beta1 and tumor necrosis factor alpha. Med Oncol. 2010;27(3):697–701. [DOI] [PubMed] [Google Scholar]
- 18. Huang YH, Tiao MM, Huang LT, et al. Activation of Mir-29a in activated hepatic stellate cells modulates its profibrogenic phenotype through inhibition of histone deacetylases 4. PloS one. 2015;10(8):e0136453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Liu J, Chen J, Feng T, Guo Q. MiR-29 mediates TGFbeta 1-induced extracellular matrix synthesis through activation of Wnt/beta -catenin pathway in human pulmonary fibroblasts. Technol Health Care. 2015;23(suppl 1):S119–125. [DOI] [PubMed] [Google Scholar]
- 20. Yang T, Liang Y, Lin Q, et al. miR-29 mediates TGFbeta1-induced extracellular matrix synthesis through activation of PI3K-AKT pathway in human lung fibroblasts. J Cell Biochem. 2013;114(6):1336–1342. [DOI] [PubMed] [Google Scholar]
- 21. Ramdas V, McBride M, Denby L, Baker AH, Canonical transforming growth factor-beta signaling regulates disintegrin metalloprotease expression in experimental renal fibrosis via miR-29. Am J Pathol. 2013;183(6):1885–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roderburg C, Urban GW, Bettermann K. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53(1):209–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, supplementary_Table_1 for LncRNA-RP11 Modulates TGF-β1-Activated Radiation-Induced Lung Injury Through Downregulating microRNA-29a by Xi Yang, Jianjiao Ni, Yida Li, Liqing Zou, Tiantian Guo, Yuan Li, Li Chu and Zhengfei Zhu in Dose-Response