Abstract
The exogenous application of acetylsalicylic acid (ASA) is stated to increase tolerance of plants against different environmental stresses. Therefore, the present study was planned to get insight into ASA-mediated regulation of growth, secondary metabolism, and oxidative defense in 2 chickpea varieties. Ten seeds of 2 chickpea varieties (DG-89 and Bittle-98) were sown in plastic pots containing sandy loam soil with 3 drought stress levels, i.e. wet conditions or flooded water (100% FC) as recommended control, 75% FC, 50% FC and 25% FC for chickpea. The moisture contents were maintained and regularly monitored through the addition of normal irrigation water. The design of experimental was completely randomized with 3 replicates per treatment. Penultimate leaves were harvested with knife after 20 days of foliar spray to observe the effect of exogenously applied ASA (100 mg/L) on growth, and key-biochemical attributes of chickpea plants (DG-89 and Bittle-98) under drought stress regimes. Drought stress regimes caused a substantial decline in shoot (37% and 35%) and root length (67% and 78%), shoot (80% and 76%) and root (62% and 68%) fresh masses, shoot (71% and 63%) and root (77% and 74%) dry masses, leaf area per plant (77% and 80%), chlorophyll a (7% and 45%), chlorophyll b (57% and 42%), total chlorophyll (30% and 39%), total carotenoids (76% and 54%), total anthocyanins (38%), reducing sugar (10% and 57%), total soluble proteins (77% and 44%), total flavonoids (61% and 59%) and total phenolics (58% and 31%) contents in both DG-89 and Bittle-98, respectively. A significant increase in MDA (25%), H2O2 contents (100% and 62%), proline (145% and 131%), and ascorbic acid (133% and 203%) contents was documented in stressed plants of both varieties, respectively. Additionally, drought stress significantly improved the activities of POD (154% and 76%), CAT (87% and 45%) and SOD (248% and 143%) in both varieties. Exogenous application of ASA reduced drought-mediated oxidative stress by reducing MDA (53% and 14%), and H2O2 (84% and 56%) contents, proline contents (50% and 17%) and enhanced the shoot (6% and 25%) and root (43% and 33%) dry masses, leaf area (9% and 10%), chlorophyll a (7% and 32%), b (82% and 81%), and carotenoids (53% and 33%) in both barley cultivars. When plants of chickpea was treated with ASA had greater total anthocyanins (26% and 35%), free amino acids (48% and 28%), ascorbic acid contents (135% and 179%), total soluble proteins (34% and 23%), total flavonoids (58% and 35%) and phenolic (50% and 69%)contents besides the POD (41% and 64%), CAT (23% and 56%) and SOD (73% and 72%) enzymes activities. Plants of DG-89 showed more tolerance to drought stress than that of Bittle-98 as a manifest from higher plant biomasses. Thus, our results showed that foliar-applied ASA is an effective strategy that can be used to improve the tolerance of chickpea plants to drought stress.
Keywords: acetylsalicylic acid, antioxidants, chlorophyll pigments, drought stress, growth attributes
Introduction
Crop plants have been exposed several ecological stresses that affecting the growth of plants and development of plants.1 Water deficient is studying the very harmful ecological stress that causes a reduction in crop productivity as compared to any abiotic stresses.2 Water deficient in plants impairs the regular growth of plants, interferes water relations and influences (WUE) of plants.3 Biomolecules (nucleic acids, carbohydrates, proteins, hormones, ions and nutrients) inhibited under water deficient condition.4 Drought, salinity, extreme temperatures and metals are identified produce ROS (reactive oxygen species) causing oxidative stress in plants.3,5 Mitochondria, peroxisomes or chloroplasts are the main producers of ROS. When the stomata close and CO2 is limited then electron acceptor of PSI produces O2 - or H2O2. However, transmitted electron from excited chlorophyll to molecular oxygen (O2) produces singlet oxygen.6 Plants develop defense systems consist of an inhibitor to protect themselves against ROS damages and cope with totally different environmental constraints.7 The inhibitor system opposed ROS injury and limits ROS physiological damage in plant cells. The elements of this technique are protein, monohydro ascorbate enzyme (MDHAR), dehydroascorbate reductase (superoxide dismutase (SOD), ascorbate oxidase (AO), catalase (CAT) and non-enzymatic antioxidants, like glutathione (GSH), proline, glutathione reductase (GR), carotenoids. ascorbate oxidase (AO).8 The different antioxidants have similar overlapping functions and may work compensate for another. In this ways, SOD catalyzes the conversion of O2 - into H2O2, which, successively neutralized by CAT and APX. The CAT is one among the accelerator antioxidants with the best turnover rates among all enzymes, and it’s therefore considered the main part of peroxisomes. Many studies revealed that plants as well as mushrooms have the antioxidant abilities.9,10
Acetylsalicylic acid (ASA) is a vital signal molecule concerned within the plants growing processes, flower’s enlargement and plants defense mechanism.11 The Exogenous application of aspirin enhanced the CO2 assimilation rate plant and increased dry masses under drought stress. Foliar application of ASA can regulate important enzyme (comprising mono-dehydroascorbate reductase, MDHA dehydroascorbate reductive, DHAR; GR; GSH peroxidase) and non-enzymatic (including GSH) components of ASA–GSH pathway and glyoxalase system (Gly I and Gly II) and reduce air stress in drought-stressed plants.12
Chickpea (Cicer arietinum L.) is one amongst the significant pulse crops and consumed everywhere in the globe particularly within the Afro-Asian countries. Chickpea is a chief source of super molecule (28.9%) and sugar (62%). Chickpea is a steroid-alcohol free that may be a sensible supply of linoleic, oleic acids, dietary fiber, vitamins (riboflavin, niacin, thiamin, B-complex vitamin and minerals.13,14 The iron, phosphate, calcium, magnesium, manganese, and Zn conjointly gift in chickpea seeds to make and maintain the bone structure strengthen.15 Globally chickpea is full-grown on 11·3 million hectares have a median yield of 849 kg/ha with a production of 9·6 million metric tonnes (FAOSTAT, 2011).16 Pulses like chickpea has potential helpful effects in minimizing the danger of varied lethal diseases though data per individual chickpea played a role part in sickness interference and also the strategies of action are restricted so for.13 This project has been planned to explore the result of foliar applications of ASA in chickpea for a response to drought stress supported some growth, physiological and key-biochemical attributes.
Material and Methodology
Seeds Collection
The seeds of 2 chickpea varieties (DG-89 and Bittle-98) were obtained from the Arid Zone Institute, Bhakkar, Pakistan.
Foliar Application of Acetylsalicylic Acid (ASA)
A pot experiment was conducted at the Department of Botany, Government College University, Faisalabad to find out the difference in the growth, physiological and biochemical attributes in chickpea (Cicer arietinum L.) varieties under drought stress. Ten seeds of 2 varieties were sown in plastic pots containing sandy loam soil supplemented with Hoagland nutrients. The experiment was conducted in plastic pots filled with soil with 3 drought stress levels, i.e. wet conditions or flooded water (100% FC) as recommended control, 75% FC, 50% FC and 25% FC for chickpea. The moisture contents were maintained and regularly monitored by maintain the weight of each pot according to its field capacity (FC), through the addition of normal irrigation water. The design of experimental was completely randomized with 3 replicates per treatment. About 250 mL of ASA solution (100 mgL-1) with 0.5% Tween-20 were sprayed to chickpea plant regularly after 3 days’ intervals for 21 days at vegetative stage. The control plants were watered with the same amount of distilled (DW) water. Penultimate leaves were harvested with knife after 21 days of exogenous applied ASA to exposed the growth and physio-chemical attributes at vegetative stage.
Growth Attributes
Length of shoots was recorded from flag leaf to the soil level. The plants with roots were pulled carefully from soil. Length of roots and shoots was recorded in centimeters. Dry and fresh root and shoot weight was recorded in grams with the help of weighing balance. The leaf area of each plant was recorded.
Physio-Chemical Attributes
Photosynthetic pigments
Yoshida et al17 method was used to determine the chlorophyll a and b, total chlorophylls, total carotenoids, and chlorophyll a: b ratio. Fresh leaves (0.1 g) were crushed in 5 mL of acetone (80%) with the help of mortar and pestle and maintained the volume up to 10 mL. Then centrifuged at 12000×g for 10 minutes. The supernatants absorbance was noted at 663 nm, 645 nm and 480 nm in a spectrophotometer.
Total Anthocyanin Contents
Total anthocyanin content was recorded with the method of Hodges and Nozzolillo.18 Fresh leaves (0.1 g) were crushed in 2 mL of acidified methanol (methanol+1% HCI) by using the mortar and pestle. The homogenized material shifted to the test tube and boiled at 100°C for half an hour in the water bath. Centrifuge the material at 12000×g for 15 minutes. The absorbance was recorded at 536 nm and 600 nm with the help of the spectrophotometer.
Ascorbic Acid Contents
Concentration of ascorbic acid was recorded through the Mukherjee and Choudhuri19 method. Fresh leaves (25 g) was grinded in 10 mL trichloroacetic acid (TCA) (6%) solution. Add 2 mL dinitrophenylhydrazine (2%) in extract (4 mL) and then added 1 drop of thiourea (70% ethanol). The reaction mixture was heated at 100°C for 15 minutes and chilled on an ice bath. Then 5 mL of sulfuric acid 80% (v/v) mixed in the mixture. Then recorded the optical density by using spectrophotometer at 530 nm.
Total Free Amino Acid Contents
Method of Hamilton and Van-Slyke20 was used to estimate the total free amino acid. Fresh leaves (0.1 g) were dipped overnight in the potassium buffer solution. The extracted material (1 mL) was moved to 25 mL test tubes then mixed 0.5 mL ninhydrine solution (10%) and 0.5 mL pyridine solution (2%). Then this material was boiled for 30 minutes at 100ºC. After this, added distilled water in each test tube up to 25 mL to maintain the volume. With spectrophotometer optical density was measured at 570 nm.
Leaf Free Proline Contents
Fresh leaves (0.5 g) of chickpea were grinded in 10 mL of aqueous sulfosalicylic acid (3%) and clarified the mixture by filter paper (Whatman # 2). Two ml of extract was mixed with acid ninhydrin (2 mL) and 2 mL of glacial acetic acid and boiled for at 100°C 1 hour and an ice bathtub used for the termination of the reaction. Then toluene (4 mL) added and stirred for 10-20 seconds. After that chromophore aspirated which contained toluene, keep it in a room. Then optical density was recorded at 520 nm and toluene used as a blank.21
Total Phenolic Contents
Fresh leaves (0.1 g) were grinded in 5 mL acetone (80%) then centrifuged this material for 10 minutes (at 10,000 rpm). The extracted material (100 µL) was added in 2 mL of distilled H2O2, 5 mL sodium carbonate (20%) and 1 milliliter Folin-Phenol Ciocalteus reagent. Volume made up to 10 mL by adding D. H2O2. In leaf, total phenolics recorded using Folin-Phenol Ciocalteus reagent at 750 nm.22
Total Flavonoid Contents
To find out the flavonoid contents, the method of AlCl3 was used. Leaf (0.1 g) was grinded in methanol (80%) and centrifuged. The centrifuged material (1 mL) and NaNO2 (0.3 mL) was added in all test tubes. Keep this mixture for 5 min. Then 0.3 mL of AlCl3 and 2 mL of NaOH (1 M) was mixed and shaken well. Then optical density was noted at 510 nm by following Zhishen et al.23
Total Soluble Proteins
Fresh leaves (0.5 g) were crushed in 10 mL of (50 mM) potassium phosphate buffer on ice tub, centrifuge material at 4°C at 10,000 rpm for 15 min. Bradford24 method was used to measure protein contents. The optical density at 595 nm was recorded after 2 minutes.
Reducing Sugar Contents
Fresh leaves (0.1 g) were grinded in 1 mL of 80% methanol. 100 µL supernatant, 1 mL distilled water and 0.5 mL DNS reagent, 3, 5-dinitrosalicylic acid (1 g), 80 milliliters of 2 M sodium hydroxide, 30 g of KNaC4H4O6·4H2O (sodium potassium tartrate) and 100 mL of dil H2O) was added. After that, this mixture was heated at 100°C for 15 min. Then reaction of the mixture was ended by keeping it on an ice bath. After that optical density was recorded at 540 nm with a spectrophotometer.
Malondialdehyde (MDA) Analysis
Malondialdehyde concentrations were recorded following the method of Heath and Packer.25 Fresh Leaves (0.1 g) were grinded in 1 milliliter TCA (5%). Centrifuge for 15 min at 12,000×g. The extracted material (1 mL) was mixed with an equal volume of TBA (0.5%) in TCA 20% (w/v). The material was heated for a half hour at 95 °C, after that cooled it and centrifuged at 7500×g for 5 min for clarification. The optical density was recorded at 600 nm and 532 nm with the help of a spectrophotometer.
Hydrogen Peroxide (H2O2) Contents
Hydrogen peroxide contents were estimated using the Velikova et al26 method. Fresh leaves (0.2 g) were grinded with 2 mL of TCA (0.1% (w/v)) using mortar and pestle on ice. For 15 minutes, centrifuged it at 12,000×g. Potassium phosphate buffer (pH 7.0; 10 mM; 0.5 mL) and 1 mL of potassium iodide (1 M) were added in 0.5 mL of supernatant. The mixture was vortexed for a few minutes. After that optical density was recorded at 390 nm.
Antioxidant Enzymes Activities
The fresh leaves were grinded in 50 mM phosphate buffer with pH (7.0) and dithiothreitol (1 mM). Then centrifuged for 15 min at 15,000 rpm at 4 oC and saved it at cooled place for measuring the activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) enzymes.27 The activity of SOD activity of fresh leaf tissue was determined with the help of Giannopolitis and Ries.28 The reaction of SOD contained EDTA (75 nm), riboflavin (1.3 μL), methionin (13 mM), potassium phosphate buffer (50 mM; pH 7.5), NBT (50 μL) and 50 µL enzyme extract. The reaction mixture was put in a light box, which has 15 electrical lamps at 78 µmol m-2 s-1 for 15 min. The development of color was noted in response to incubation. The optical density of the illuminated solution at 560 nm was recorded with a spectrophotometer. One unit of SOD activity was defined as the concentration of enzyme that obstructed 50% of NBT chemical reduction. The CAT and POD activities were measured by using the method of Chance & Maehly29 with some modifications. The reaction solution of CAT (3 mL) have phosphate buffer (50 mM; pH 7.0), H2O2 (5.9 mM) and enzyme extract (0.1 mL). Enzyme extract was added to initiate the reaction. Optical density of the reaction solution was observed after every 20 s at 240 nm. One-unit CAT activity was defined as an absorbance change 0f 0.01 units per min. The reaction solution (3 mL) of POD has phosphate buffer (50 mM); pH (5.0), H2O2 (40 mM), guaiacol (20 mM), and enzyme extract (0.1 mL). Optical density of the reaction solution was noted after every 20 s at 470 nm.
Statistical Analysis
Statistical Analysis Averages and standard deviation (SD) values of collected data have been calculated on Microsoft Excel 2010 for Microsoft Windows 2010. The data was analyzed by analysis of variance technique using completely randomized design in factorial arrangement followed by multiple comparison tests to evaluate the significance of the data by using software program Statistix version 8.1.
Results
Growth Attributes
For shoot and root length, data showed a highly significant (p < 0.001) difference in drought, varieties and foliar applied ASA with significant interactions among these factors at vegetative stage of chickpea showed in Table 1. The present study result showed that shoot and root length was decreased in both varieties under different levels of drought (75% FC, 50% FC and 25% FC) as compared to controls. DG-89 showed a more reduction (10-37% and 16-67%) than Bittle-98 (9-35% and 32-78%) in shoot and root length. Shoot and root length was increased when ASA was applied foliarly under different levels of drought stress (100% FC, 75% FC, 50% FC and 25% FC) in both DG-89 (4-20% and 3-41%) Bittle-98 (5-8% and 2-16%), respectively as shown in Figure 1A and B. For leaf area, shoot and root fresh weight, statistical analysis of data showed a highly significant (p < 0.001) difference in drought, varieties (p < 0.05 and p < 0.001) and foliar applied ASA (p < 0.001) with non-significant (p > 0.05) interactions among these factors, respectively at vegetative stage of chickpea showed in Table 1. The present study showed that shoot (39-80% and 35-76%) and root fresh weight (17-62% and 15-68%), and leaf area (32-77% and 34-80%) was reduced in both DG-89 and Bittle-98 than control under different regimes of drought (75% FC, 50% FC and 25% FC), respectively. DG-89 showed a more reduction in respect of shoot and root dry (36-71% and 47-77%) and fresh weight than Bittle-98 (30-63% and 48-74%) under different regimes of drought stress, respectively. Shoot (8-22% and 1-8%) and root fresh weight (2-16% and 8-11%), shoot (0-6% and 2-25%), root dry weights (3-43% and 0.35-33%) and leaf area (2-9% and 2-10%) were increased when ASA was exogenously applied under different levels of drought stress and Bittle-98 in both DG-89 and Bittle-98, respectively (Figure 1C-G).
Table 1.
Mean Square Values From ANOVA Data for Growth and Key-Biochemical Attributes of Chickpea Varieties Treated With Acetylsalicylic Acid (ASA) Under Drought Stress.
| Source of Variance | Drought (D), df = 3 | Varieties (V), df = 1 | Acetylsalicylic acid (ASA), df = 1 | D × V, df = 3 | D × ASA, df = 3 | V × ASA, df = 1 | D × V × ASA, df = 3 | Error, df = 32 |
|---|---|---|---|---|---|---|---|---|
| Shoot length | 177.372*** | 286.408*** | 69.432*** | 27.708*** | 3.402ns | 8.325* | 4.599ns | 2.217 |
| Root length | 237.060*** | 1.554ns | 28.788*** | 0.618ns | 5.451*** | 0.742ns | 2.708* | 0.974 |
| SFW | 53.556*** | 1.006* | 3.724*** | 0.471ns | 0.229ns | 0.803 ns | 0.081ns | 0.3103 |
| RFW | 27.788*** | 1.113*** | 1.948*** | 0.022ns | 0.134*** | 0.0009ns | 0.059*** | 0.0109 |
| SDW | 2.953*** | 0.002 ns | 0.104*** | 0.007 ns | 0.027* | 0.032* | 0.013 ns | 0.007 |
| RDW | 0.417*** | 0.001* | 0.053*** | 0.004*** | 0.017*** | 0.007*** | 0.002** | 0.0004 |
| Leaf area | 12213.4*** | 292.7*** | 110.2*** | 36.4* | 34.1* | 3.4 ns | 17.7 ns | 12.0 |
| Chlorophyll a | 2.160*** | 4.732*** | 3.681*** | 1.169*** | 0.241*** | 1.705*** | 0.135** | 0.03934 |
| Chlorophyll b | 3.116*** | 12.525*** | 4.832*** | 0.032 ns | 0.088 ns | 4.824*** | 0.860*** | 0.0769 |
| Total chlorophyll | 10.42*** | 0.078 ns | 32.657*** | 0.138 ns | 1.093*** | 12.264*** | 1.498*** | 0.1203 |
| Carotenoids | 2.312*** | 3.660*** | 0.119*** | 0.203*** | 0.093*** | 0.551*** | 0.176*** | 0.012 |
| H2O2 | 330.910*** | 665.83*** | 4.906* | 18.477*** | 20.073*** | 0.012 ns | 24.94*** | 1.215 |
| MDA | 511.28*** | 261.34*** | 2517.36*** | 68.61** | 31.95 ns | 833.84*** | 72.41** | 17.47 |
| Ascorbic acid | 36.295*** | 4.205*** | 19.344*** | 1.080*** | 1.080*** | 0.744*** | 0.530*** | 0.024 |
| Total flavonoids | 12.710*** | 6.499*** | 2.086*** | 1.137*** | 0.533** | 0.191 ns | 0.270 ns | 0.1405 |
| Total phenolics | 10.932*** | 13.444*** | 0.046 ns | 1.792*** | 0.928*** | 0.0005 ns | 0.459** | 0.1189 |
| TSP | 9.853*** | 4.378*** | 6.730*** | 0.080* | 2.214*** | 0.996*** | 0.363*** | 0.0288 |
| Free amino acids | 50.926*** | 44.146*** | 0.591*** | 0.566*** | 5.201*** | 10.720*** | 6.013*** | 0.055 |
| Proline | 83.927*** | 2.280** | 208.05*** | 5.686*** | 5.124*** | 3.665** | 3.202*** | 0.413 |
| Anthocyanin | 2.441*** | 2.465*** | 0.249*** | 0.605*** | 0.087*** | 0.843*** | 0.016*** | 0.009 |
| Reducing Sugar | 21.836 | 54.108 | 15.08*** | 6.10*** | 3.381*** | 3.300*** | 0.534** | 0.1085 |
| SOD | 1379.85*** | 439.49*** | 484.69*** | 4.08 ns | 95.10*** | 94.91*** | 15.45 ns | 7.30 |
| Peroxidase | 40144*** | 56331*** | 184565*** | 7488*** | 21300*** | 36364*** | 7599*** | 110 |
| Catalase | 122090*** | 83998*** | 94ns | 7125*** | 15010*** | 15136*** | 2048** | 575 |
***, **, * = significant at 0.001, 0.01 and 0.05 levels, respectively. Abbreviations: SFW=shoot fresh weight; RFW=root fresh weight; SDW=shoot dry weight; RDW= root dry weight; H2O2= hydrogen peroxide; MDA= malondialdehyde; TSP= total soluble protein; SOD= superoxide dismutase
Figure 1.
Effect of foliar applied ASA on growth attributes in Cicer arietinum L. at vegetative stage grown under drought stress (n = 3; Mean ± S.D); A) Shoot length, B) Root length, C) Shoot fresh weight, D) Shoot dry weight, E) Root fresh weight, F) Root dry weight, G) Leaf area.
Chlorophyll Contents and Total Carotenoids
For chlorophyll pigments, data showed a highly significant (p < 0.001) difference in drought, varieties and foliar applied ASA with significant (p < 0.001) interactions among these factors at vegetative stage of chickpea indicated in Table 1. The present study result showed that chlorophyll a (5-7% and 32-45%), chlorophyll b (29-57% and 7-42%), total chlorophyll (15-30% and 19-39%) and total carotenoid (40-76% and 4-55%) contents was reduced in both DG-89 and Bittle-98 varieties than control under different levels of drought (75%-25% FC), respectively. DG-89 showed a more reduction than Bittle-98. Chlorophyll a (2-6% and 5-32%), b (8-52% and 2-51%), total chlorophyll (5-26% and 19-23%) and carotenoid contents (11-99% and 4-45%) was increased when ASA was exogenously applied under different levels of drought stress (100%, 75%, 50% and 25% FC) in DG-89 and Bittle-98, respectively (Figure 2A-D).
Figure 2.
Effect of foliar applied ASA on photosynthetic attributes in Cicer arietinum L. at vegetative stage grown under drought stress (n = 3; Mean ± S.D); A) Chlorophyll a, B) Chlorophyll b, C) Total chlorophyll, D) Total carotenoid contents.
Total Anthocyanin, Ascorbic Acid, Total Free Amino Acid and Total Proline Contents
For anthocyanin contents, data showed a highly significant (p < 0.001) difference in drought, varieties and foliar applied ASA with significant (p < 0.001) interactions among these factors at vegetative stage of chickpea showed in Table 1. The present study result showed that total anthocyanin contents plant was reduced in both varieties than control under different levels of drought (75% FC, 50% FC and 25% FC); DG-89 showed a more reduction (16-38%) than Bittle-98 (15-38%), respectively. Total anthocyanin content was enhanced, when ASA was applied exogenously under different levels of drought stress in DG-89 (1-26%) and Bittle-98 (15-35%), respectively (Figure 3A). For ascorbic contents, data showed a highly significant (p < 0.001) difference in drought (p < 0.001), varieties (p < 0.01) and foliar applied ASA with significant (p < 0.001) interactions among these factors at vegetative stage of chickpea showed in Table.1. Ascorbic acid content was enhanced in both varieties than control under different levels of drought, DG-89 showed a more reduction (11-133%) than Bittle-98 (39-203%), respectively. Total ascorbic acid content was increased when ASA was exogenously applied under different levels of drought stress including control in DG-89 (73-135%) and Bittle-98 (79-179%), respectively (Figure 3B).
Figure 3.
Effect of foliar applied ASA on total anthocyanin, ascorbic acid contents in Cicer arietinum L. at vegetative stage grown under drought stress (n = 3; Mean ± S.D); A) Total anthocyanin, B) Ascorbic acid, C) Free amino acids contents, D) Free proline contents.
For total free amino acid contents, data showed a highly significant (p < 0.001) difference in drought, varieties and foliar applied ASA with a highly significant interactions among these factors at vegetative stage of chickpea showed in Table 1. Total free amino acid content was reduced in both varieties than control under different levels of drought (75% FC, 50% FC and 25% FC); DG-89 showed a more reduction (34-73%) than Bittle-98 (31-42%), respectively. Total free amino acid content was raised, when ASA was exogenously applied under different levels of drought stress including controls in DG-89 (19-48%) and Bittle-98 (3-28%) respectively (Figure 3C). For proline contents, data showed a highly significant (p < 0.001) difference in drought, varieties (p < 0.001) and foliar applied ASA (p < 0.01) with a highly significant (p < 0.001) interactions among these factors at vegetative stage of chickpea showed in Table 1. The proline contents was enhanced in both varieties than control under different levels of drought; DG-89 showed a more reduction (32-145%) than Bittle-98 (50-131%), respectively. Total proline contents was enhanced when ASA was exogenously applied under different levels of drought stress including control in DG-89 (7-50%) and Bittle-98, respectively (Figure 3D).
Total Phenolic, Flavonoid, Reducing Sugar and Total Soluble Protein
For total phenolic, the statistical analysis of data showed a significant (P < 0.001) difference in the drought, foliar applied ASA (P < 0.001), and non-significant difference in varieties (P > 0.05) with significant (P < 0.01) interaction among these factors shown in Table 1. Total phenolic contents was reduced in both DG-89 (27-58%) and Bittle-98 (9-31%) as compared to control under different levels of drought (75% FC, 50% FC and 25% FC). DG-89 showed a more reduction than Bittle-98. Total phenolic was enhanced when ASA was applied foliarly under different levels of drought stress including control in DG-89 (32-50%) and Bittle-98 (7-68%), respectively (Figure 4A). For total flavonoid contents, analysis of data exhibited a significant (P < 0.001) difference in the drought, foliar applied ASA (P < 0.001), and varieties (P < 0.001) with significant (P > 0.05) interaction among these factors shown in Table 1. The flavonoid contents was decreased in both varieties than control under different levels of drought; DG-89 showed a more reduction (18-61%) than Bittle-98 (46-59%), respectively. The flavonoid contents was enhanced when ASA was applied foliarly under different levels of drought stress including control in DG-89 (0.2-61%) and Bittle-98 (5-35%), respectively (Figure 4B).
Figure 4.
Effect of foliar applied ASA on total phenolic, flavonoid content, reducing sugar and total soluble protein contents in Cicer arietinum L. at vegetative stage grown under drought stress (n = 3; Mean ± S.D); A) Total phenolic content, B) Total flavonoid content; C) reducing sugar content, D) Total soluble protein content.
For reducing sugar contents, the statistical analysis of data showed a significant (P < 0.001) difference in the drought, varieties (P < 0.001) and foliar applied ASA (P < 0.001) with a significant (P < 0.01) interaction among these factors shown in Table 1. Reducing sugar contents was reduced in both varieties than control under different levels of drought (75% FC, 50% FC and 25% FC). DG-89 showed a more reduction (2-10%) than Bittle-98 (8-57%), respectively. Reducing sugar contents was improved, when ASA was applied foliarly under different levels of drought stress including control in DG-89 (9-64%) and Bittle-98 (22-86%), respectively (Figure 4C). For total soluble protein contents, the analysis of data showed a significant (P < 0.001) difference in the drought, varieties and foliar applied ASA with a significant (P < 0.001) interaction among these factors shown in Table 1. Total soluble protein content was reduced in both varieties than control under different levels of drought; DG-89 showed a less reduction (2-10%) than Bittle-98 (8-57%), respectively. Total soluble protein content was increased, when ASA was applied exogenously under different levels of drought stress including control in DG-89 (9-64%) and Bittle-98 (22-86%), respectively (Figure 4D).
MDA and H2O2 Content
For MDA and H2O2 contents, the statistical analysis of data showed a significant (P < 0.001) difference in the drought, foliar applied ASA, and in varieties with a significant interaction among these factors shown in Table 1. MDA and H2O2 contents of the plant was increased in both varieties than control under different levels of drought (75% FC, 50% FC and 25% FC). More MDA contents were produced in DG-89 (19-25%) than Bittle-98 (1-25%), respectively. In the context of H2O2 contents, DG-89 also showed more contents (15-124%) than Bittle-98 (4-82%), respectively. MDA and H2O2 contents were reduced, when ASA was applied foliarly under different levels of drought stress including control in both varieties. Maximum reduction was observed in DG-89 (20-108%) than Bittle-98 (36-57%) as shown in Figure 5A-B.
Figure 5.

Effect of foliar applied ASA on MDA and hydrogen peroxide content in Cicer arietinum L. at vegetative stage grown under drought stress (n = 3; Mean ± S.D); A) MDA content, B) Total hydrogen peroxide content.
Antioxidant Enzymes Activities (SOD, POD and CAT Contents)
For SOD, POD and CAT contents, the statistical analysis of data showed a significant (P < 0.001) difference in the drought, foliar applied ASA and varieties with interaction (P < 0.001 and P > 0.05) among these factors, respectively as shown in Table 1. Both POD (43-154% and 68-76%) CAT (3-87% and 19-45%) contents were increased in both DG-89 and Bittle-98, compared to control under different levels of drought (75% FC, 50% FC and 25% FC). Similarly, SOD contents was also increased in both varieties; DG-89 showed a more increased (25-248%) than Bittle-98 (38-143%), respectively. The SOD, POD and CAT contents were enhanced by using ASA as spray under different levels of drought stress including control in DG-89 (4-23%, 8-40% and 3-73%) and Bittle-98 (8-56%, 36-64% and 50-72%), respectively (Figure 6A-C).
Figure 6.
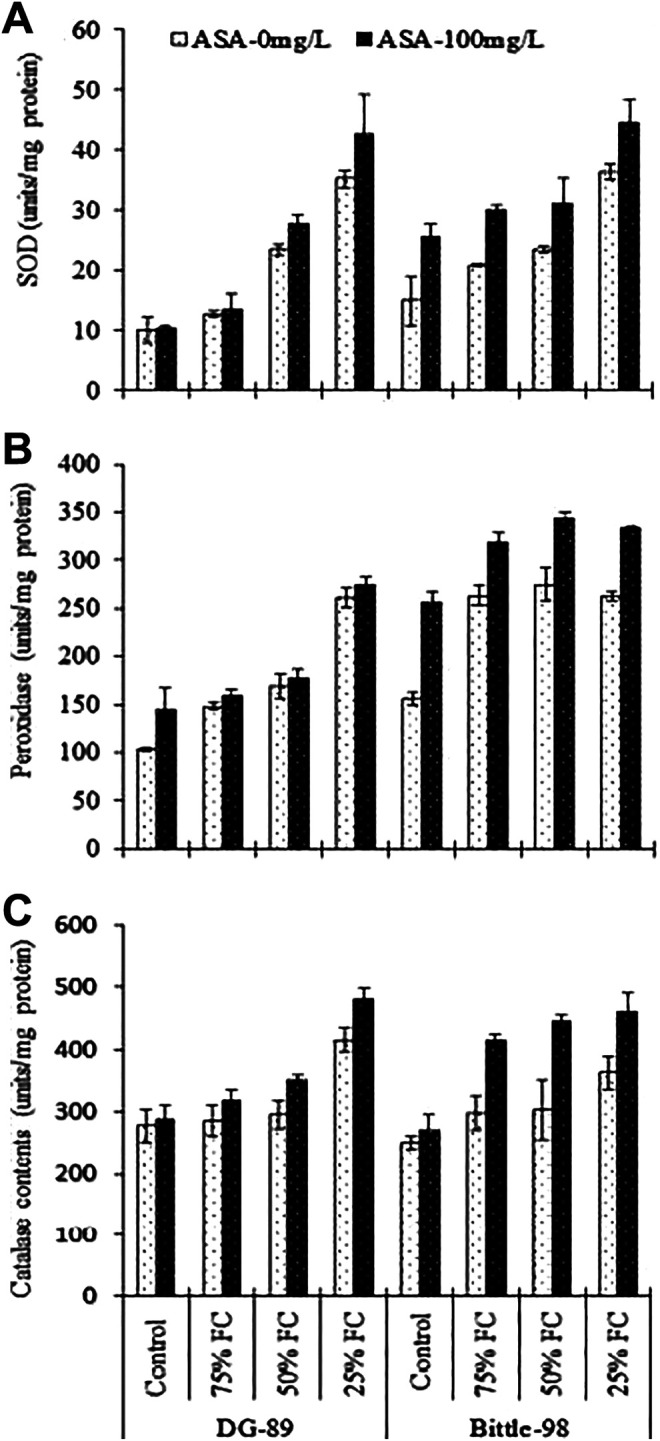
Effect of foliar applied ASA on superoxide dismutase (SOD), peroxidase and catalase acitvities in Cicer arietinum L. at vegetative stage grown under drought stress (n = 3; Mean ± S.D); A) SOD B), Peroxidase, C) Catalase.
Discussion
Chickpea is an important pulse crop grown and consumed all over the world due to good source of protein, protein quality and carbohydrates. It is favored mostly arid and marginal lands for growth where it faces water deficit at different growth stages. Water deficit seriously affects the crop at different growth stages. Yield declined depends on areas and duration of the season. Water deficit effects more on flowering stage than vegetative stage formation. initial pods are reduce in length and size, all generative parts of the plant owing to water deficit that result in damaging of crop. The consequences may be considered for both quantitative as well as qualitative parameters together with seed evidence,30 seedling maintenance,31 physio1ogica1 parameters and biochemical parameters,32 solute balance,33 stimulation of reactive oxygen species, level of ascorbic acid and phenolics,34 molecular regulation,35 formation of nodule36 and yield components. Water deficit seriously affects chemistry of different pathways in chickpea and ultimately synthesis of different biomolecule stopped.
Under drought conditions, shoot length and root length was decreased due to suppression of plant growth. Studies also reported that water potential decreases with increase of drought stress.37 By application of ASA plant showed a maximum increase in plant height of chickpea, but maximum increase was recorded in DG-89 (Figure 1A and B). In our experimental results chickpea varieties showed reduction in root and shoot biomass production under drought stress. Acetylsalicylic acid improved the plant biomass (Figure 1C-G). Biomass production of plant supposed area for selecting low plants yielding genotypes and plant cultivars give better results with increased biomass probably to improved yield.38 Drought stress conditions cause a reduction in fresh and dry biomass of various crops such as rice, bent grass and wheat.39,40 Chlorophyll contents were decreased due to water deficient. Carotenoids decreased under water deficient condition due to production of ROS in the thylakoids that protect plants from oxidative damage.41 In this study, ASA improved the physiological activities (photosynthetic pigments) in chickpea under drought stress by protecting the chlorophyll contents from oxidative injury by improving the carotenoids level (Figure 2A-D); similar results have been reported for wheat cultivars.42 Studies had revealed that the ascorbic acid content of plants was decreased due to drought stress.43,44 Different study reported that a decrease in ascorbic acid content was due to damages of oxygen species and plant defense mechanism.45 Ascorbic acid is one of important metabolites that considerably intervenes the abiotic stress in plants.46 The results are in agreement with finding by Hussain et al,47 who recorded increase in ascorbic acid content in maize plants exposed to high temperature stress. The present study revealed that activity of ascorbic acid was increased due to ASA (Figure 3B). Some secondary metabolites such as flavonoids and total phenolic contents that increase the drought tolerance in plants.48 Phenolics and flavonoid contents are strong antioxidants, which play important role in scavenging of free radicals against drought induced oxidative damage. Accumulation of total phenolic and flavonoids contents in plants have been correlated with stress tolerance. Total phenolics and flavonoid contents were reduced under water deficient condition in our study. Acetylsalicylic acid showed a positive effect on total phenolics and flavonoid contents under water stress condition (4A-B). The results are in agreement with finding by Hussain et al.47
Osmotic adjustment is also an important way to resist adverse stress in plants. The osmotic adjustment substances, such as soluble protein and proline play vital roles in maintaining the osmotic equilibrium and integrity of membranes.49 Degradation of soluble protein is one of most marked signs in plants exposed to metal stress. In current study, drought stress significantly reduced the soluble protein content in leaves of chickpea (Figure 4D); similar results have been reported for radish seedlings.50 The application of ASA increased the soluble protein content in drought treated wheat plants. This increase in soluble protein may possibly be due to increase of nitrate reductase biosynthesis with the inhibition of their degradation,51 thus enhanced the accumulation of soluble protein in leaves of chickpeat plants. Proline mediates stress signaling, scavenges ROS and maintains osmolarity of cells under stressful conditions. In present study, ASA mediated enhancement in proline reduced the oxidative damage by strengthening antioxidant system.52 Increased proline protects the photosystem II functioning by eliminating the ROS generated in chloroplast due to stress.53 Exogenous ASA mediated improvement in proline accumulation under normal and drought stress conditions may have resulted from its effects on its metabolizing pathways. Stress-mediated increase in proline contents results from enhanced synthesis and reduced catabolism of proline54 and ASA application may have imparted differential regulation on proline accumulation. Similar to our results, Choudhary et al55 have also demonstrated improved accumulation of proline in drought stressed chickpea plants (Figure 3D).
Drought stress caused the addition of more free amino acids in plant cell that maintain stability of membrane structure and osmotic adjustment in plant cell.56 Water stress, decreased the free amino acids in plant cells. Acetylsalicylic acid increases the accumulation of amino acids in plants.57 Acetylsalicylic acid plays role as a defensive mechanism due to OH radicals’ production in plant. In the present study, H2O2 and MDA were reduced in DG-89 and Bittle-98, when acetylsalicylic acid was applied under water deficient condition. Reducing sugar contents were decreased under water stress (Figure 5A and B). It has been suggested that sugars act as osmoregulator and provide protection to plants against environmental stresses (Jouve, L. et al, 2004).58 Sugars act as respiratory substrate and maintain cellular homeostasis due to their function as osmolyte.59 Plants under drought stress accumulated reducing sugars that could have played significant part in osmoprotection.60 Likewise, our results showed that ASA foliar application enhanced the reducing sugar content as drought levels were increased. Anthocyanin and flavonoids content was decreased due to presence of metabolites under water deficient condition.46 These metabolites act as an osmoprotectant.61,62 Foliar applied ASA enhanced the anthocyanin contents under drought stress (Figure 3A). Drought stress can promote the production of reactive oxygen species (ROS) thus causing oxidative stress in plants.48 Increased activities of antioxidant enzymes also contribute significantly to the antioxidant potential of plants.63 Oxidative damage increases due to the disequilibrium between oxidant and antioxidant.64 Catalase activity of different plants showed a maximum increase under drought stress.65 The activity of CAT enhanced because CAT and POD enzymes was included in detoxifying hydrogen peroxide process in Solanum tuberosum.66 Our findings showed that these enzymatic antioxidants are increased under water deficient condition due to application of ASA (Figure 6A-C). Peroxidase activity increased under water deficient conditions in the study of Zoz et al.67 Our results showed that increase in POD content was due to the application of acetylsalicylic acid. Water deficient increased the activity of SOD due to production of active oxygen species in sunflowers,68 and poplars.69 From all the above, it can be predicted that drought stress causes a reduction in plant biochemical, physiological & morphological attributes. However, ASA enhances the plant defense mechanism to cope with drought stress. Plant show a positive impact of ASA on plant yield overall.
Conclusions
Taken together, ASA significantly mitigated the drought effects in chickpea reflected in the form of improved growth characteristics, chlorophyll and anthocyanin synthesis. Acetylsalicylic acid increased oxidative defense system that protected plants from drought-induced oxidative damage measured in terms of H2O2 and MDA levels. Proline accumulation alongside higher levels of total soluble proteins and total free amino acids showed growth promotive impact of ASA in chickpea under drought stress. Acetylsalicylic acid treated plants also described significant increase in endogenous levels of phenolics, flavonoids and ascorbic acid. Therefore, ASA application can be exploited to improve drought tolerance in chickpea by its active involvement in key regulatory functioning.
Abbreviations
- SOD
superoxide dismutase
- POD
peroxidase
- CAT
catalase
- MDA
Malondialdehyde
- OD
optical density.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Iqbal Hussain  https://orcid.org/0000-0002-8449-0494
https://orcid.org/0000-0002-8449-0494
Muhammad Akram  https://orcid.org/0000-0002-7457-8572
https://orcid.org/0000-0002-7457-8572
Jaweria Nisar  https://orcid.org/0000-0002-5451-9137
https://orcid.org/0000-0002-5451-9137
References
- 1. Farooq M, Bramley H, Palta JA, Siddique KH. Heat stress in wheat during reproductive and grain-filling phases. Crit Rev Plant Sci. 2011;30(6):491–507. [Google Scholar]
- 2. Salehi-Lisar SY, Bakhshayeshan-Agdam H. Drought stress in plants: causes, consequences, and tolerance In: Hossain MA, Wani SH, Bhattacharjee S, Burritt DJ, Tran LP, eds. Drought Stress Tolerance in Plants. Springer; 2016; 1:1–16. [Google Scholar]
- 3. Fahad S, Bajwa AA, Nazir U, et al. Crop production under drought and heat stress: plant responses and management options. Front Plant Sci. 2017;8:1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boutraa T, Akhkha A, Al-Shoaibi AA, Alhejeli AM. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J Taibah Univ Sci. 2010;3(1):39–48. [Google Scholar]
- 5. Shahid M, Dumat C, Pourrut B, Abbas G, Shahid N, Pinelli E. Role of metal speciation in lead-induced oxidative stress to Vicia faba roots. Russ J Plant Physiol. 2015;62(4):448–454. [Google Scholar]
- 6. Petrov EG, Robert B, Lin SH, Valkunas L. Theory of triplet excitation transfer in the donor-oxygen-acceptor system: application to cytochrome b6 f. Biophys J. 2015;109(8):1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rao A, Ahmad SD, Sabir SM, et al. Potential antioxidant activities improve salt tolerance in ten varieties of wheat (Triticum aestivum L.). Am J Plant Sci. 2013;4(6A):69–76. [Google Scholar]
- 8. Laxa M, Liebthal M, Telman W, Chibani K, Dietz KJ. The role of the plant antioxidant system in drought tolerance. Antioxidants. 2019;8(4):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nisar J, Mustafa I, Anwar H, et al. Shiitake culinary-medicinal mushroom, Lentinus edodes (Agaricomycetes): a species with antioxidant, immunomodulatory, and hepatoprotective activities in hypercholesterolemic rats. Int J Med Mushrooms. 2017;19(11):981–990. [DOI] [PubMed] [Google Scholar]
- 10. Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci. 2015;11(8):982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ashraf M, Akram NA, Arteca RN, Foolad MR. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit Rev Plant Sci. 2010;29(3):162–190. [Google Scholar]
- 12. Hasanuzzaman M, Nahar K, Bhuiyan TF, et al. Salicylic acid: an all-rounder in regulating abiotic stress responses in plants In: El-Esawi MA, ed. Phytohormones-Signaling Mechanisms and Crosstalk in Plant Development and Stress Responses. 2017:31–75. [Google Scholar]
- 13. Jukanti AK, Gaur PM, Gowda CLL, Chibbar RN. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): a review. Br J Nutr. 2012;108(S1):S11–S26. [DOI] [PubMed] [Google Scholar]
- 14. Wallace TC, Murray R, Zelman KM. The nutritional value and health benefits of chickpeas and hummus. Nutrients. 2016;8(12):766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muszyński S, Tomaszewska E, Dobrowolski P, et al. Analysis of bone osteometry, mineralization, mechanical and histomorphometrical properties of tibiotarsus in broiler chickens demonstrates a influence of dietary chickpea seeds (Cicer arietinum L.) inclusion as a primary protein source. PLoS One. 2018;13(12):e0208921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. FAOSTAT (2011) http://faostat.fao.org/site/567/DesktopDefault.aspx. Accessed 12 December 2011. [Google Scholar]
- 17. Yoshida S, Forma DA, Cock JK, Gomez KA. Laboratory Manual for Physiological Studies of Rice. IRRI; 1976. [Google Scholar]
- 18. Hodges DM, Nozzolillo C. Anthocyanin and anthocyanoplast content of cruciferous seedlings subjected to mineral nutrient deficiencies. J Plant Physiol. 1996;147(6):749–754. [Google Scholar]
- 19. Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant. 1983;58(2):166–170. [Google Scholar]
- 20. Hamilton PB, Van Slyke DD. Amino acid determination and metal accumulation by Brassica juncea L. Int J Plant Prod. 1943;3(1):1735–8043. [Google Scholar]
- 21. Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- 22. Julkenen-Titto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem. 1985;33(2):213–217. [Google Scholar]
- 23. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- 24. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. [DOI] [PubMed] [Google Scholar]
- 25. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophysics. 1968;125(1):189–198. [DOI] [PubMed] [Google Scholar]
- 26. Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66. [Google Scholar]
- 27. Dixit V, Pandey V, Shyam R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J Exp Bot. 2001;52(358):1101–1109. [DOI] [PubMed] [Google Scholar]
- 28. Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiology. 1977;59(2):309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chance B, Maehly AC. Assay of catalase and peroxidases. Method Enzymol. 1955;2:764–775. [Google Scholar]
- 30. Hosseini M, Hassibi P. Effects of water deficit stress on several quantitative and qualitative characteristics of canola (Brassica napus L.) cultivars. Not Sci Biol. 2011;3(3):120–125. [Google Scholar]
- 31. Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C. How tree roots respond to drought. Front Plant Sci. 2015;6:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mafakheri A, Siosemardeh AF, Bahramnejad B, Struik PC, Sohrabi Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci. 2010;4(8):580. [Google Scholar]
- 33. Turner NC. Agronomic options for improving rainfall-use efficiency of crops in dryland farming systems. J Exp Bot. 2004;55(407):2413–2425. [DOI] [PubMed] [Google Scholar]
- 34. Maqbool MA, Aslam M, Ali H. Breeding for improved drought tolerance in chickpea (Cicer arietinum L.). Plant Breed. 2017;136(3):300–318. [Google Scholar]
- 35. Hu H, Xiong L. Genetic engineering and breeding of drought-resistant crops. Annu Rev Plant Biol. 2014;65:715–741. [DOI] [PubMed] [Google Scholar]
- 36. Nishiyama R, Watanabe Y, Fujita Y, et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell. 2011;23(6):2169–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atteya AM. Alteration of water relations and yield of corn genotypes in response to drought stress. Bulg J Plant Physiol. 2003;29(1-2):63–76. [Google Scholar]
- 38. El-Mageed TA, Semida WM, Mohamed GF, Rady MM. Combined effect of foliar-applied salicylic acid and deficit irrigation on physiological–anatomical responses, and yield of squash plants under saline soil. S Afr J Bot. 2016;106:8–16. [Google Scholar]
- 39. DaCosta M, Huang B. Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. J Am Soc Hortic Sci. 2007;132(3):319–326. [Google Scholar]
- 40. Anjum SA, Xie XY, Wang LC, Saleem MF, Man C, Lei W. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res. 2011;6(9):2026–2032. [Google Scholar]
- 41. Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161(11):1189–1202. [DOI] [PubMed] [Google Scholar]
- 42. Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot. 2016;105:306–312. [Google Scholar]
- 43. Jaleel CA, Riadh K, Gopi R, et al. Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant. 2009;31(3):427–436. [Google Scholar]
- 44. Nair AS, Abraham TK, Jaya DS. Studies on the changes in lipid peroxidation and antioxidants in drought stress induced cowpea (Vigna unguiculata L.) varieties. J Environ Biol. 2008;29(5):689–691. [PubMed] [Google Scholar]
- 45. Abdul Qadir DZ. Drought and gibberellic acid-dependent oxidative stress: effect on antioxidant defense system in two lettuce cultivars. Pak J Biol Sci. 2001;4(9):1138–1143. [Google Scholar]
- 46. Chao YY, Hong CY, Kao CH. The decline in ascorbic acid content is associated with cadmium toxicity of rice seedlings. Plant Physiol Biochem. 2010;48(5):374–381. [DOI] [PubMed] [Google Scholar]
- 47. Hussain I, Ashraf MA, Rasheed R, Iqbal M, Ibrahim M, Ashraf S. Heat shock increases oxidative stress to modulate growth and physico-chemical attributes in diverse maize cultivars. Int Agrophys. 2016;30(4):519–531. [Google Scholar]
- 48. Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. [DOI] [PubMed] [Google Scholar]
- 49. Wu S, Hu C, Tan Q, Nie Z, Sun X. Effects of molybdenum on water utilization, antioxidative defense system and osmotic-adjustment ability in winter wheat (Triticum aestivum) under drought stress. Plant Physiol Biochem. 2014;83:365–374. [DOI] [PubMed] [Google Scholar]
- 50. Choudhary SP, Bhardwaj R, Gupta BD, et al. Epibrassinolide induces changes in indole-3-acetic acid, abscisic acid and polyamine concentrations and enhances antioxidant potential of radish seedlings under copper stress. Physiol Plant. 2010;140(3):280–296. [DOI] [PubMed] [Google Scholar]
- 51. Ashfaque F, Inam A, Iqbal S, Sahay S. Response of silicon on metal accumulation, photosynthetic inhibition and oxidative stress in chromium-induced mustard (Brassica juncea L.). S Afr J Bot. 2017;111:153–160. [Google Scholar]
- 52. Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59(2):206–216. [Google Scholar]
- 53. Esfandiari E, Shekari F, Shekari F, Esfandiari M. The effect of salt stress on antioxidant enzymes activity and lipid peroxidationon the wheat seedling. Not Bot Horti Agrobo Cluj-Napoca. 2007;35:48–56. [Google Scholar]
- 54. Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem. 2019;137:144–153. doi:10.1016/j.plaphy.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 55. Choudhary M, Jetley UK, Khan MA, Zutshi S, Fatma T. Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol Environ Saf. 2007;66(2):204–209. [DOI] [PubMed] [Google Scholar]
- 56. Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav. 2012;7(11):1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naggar AD, Schjørring JK. Interactions between uptake of amino acids and inorganic nitrogen in wheat plants. Biogeosciences. 2012;9(4):1509–1518. [Google Scholar]
- 58. Jouve L, Hoffmann L, Hausman JF. Polyamine, carbohydrate, and proline content changes during salt stress exposure of aspen (Populus tremula L.): involvement of oxidation and osmoregulation metabolism. Plant Biol. 2004;6(1):74–80. [DOI] [PubMed] [Google Scholar]
- 59. Gupta AK, Kaur N. Sugar signaling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J Biosci. 2005;30(5):761–776. [DOI] [PubMed] [Google Scholar]
- 60. Qureshi FF, Ashraf MA, Rasheed R, et al. Organic chelates decrease phytotoxic effects and enhance chromium uptake by regulating chromium-speciation in castor bean (Ricinus communis L.). Sci Total Environ. 2020;716:137061. [DOI] [PubMed] [Google Scholar]
- 61. Liang LH, Mei X, Lin F, Xia J, Liu SJ, Wang JH. Effect of low temperature stress on tissue structure and physiological index of cashew young leaves. Ecol Environ Sci. 2009;18(1):317–320. [Google Scholar]
- 62. Hatier JH, Gould KS. Foliar anthocyanins as modulators of stress signals. J Theor Biol. 2008;253(3):625–627. [DOI] [PubMed] [Google Scholar]
- 63. Mahmud JA, Hasanuzzaman M, Nahar K, Bhuyan MHMB, Fujita M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L: coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol Environ Saf. 2018;147:990–1001. [DOI] [PubMed] [Google Scholar]
- 64. Hasanuzzaman M, Nahar K, Öztürk M. eds. Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes. Springer; 2019. [Google Scholar]
- 65. Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot. 2005;56(411):417–423. [DOI] [PubMed] [Google Scholar]
- 66. Benevides MP, Marconi PL, Gallego SM, Comba ME, Tomaro ML. Relationship between antioxidant defence systems and salt tolerance in Solanum tuberosum. Funct Plant Biol. 2000;27(3):273–278. [Google Scholar]
- 67. Zoz T, Steiner F, Guimarães VF, Castagnara DD, Meinerz CC, Fey R. Peroxidase activity as an indicator of water deficit tolerance in soybean cultivars. Biosci J. 2013;29(5):1664–1671. [Google Scholar]
- 68. Gunes A, Pilbeam DJ, Inal A, Coban S. Influence of silicon on sunflower cultivars under drought stress, I: growth, antioxidant mechanisms, and lipid peroxidation. Commun Soil Sci Plant Anal. 2008;39(13-14):1885–1903. [Google Scholar]
- 69. Xiao X, Xu X, Yang F. Adaptive responses to progressive drought stress in two Populus cathayana populations. Silva Fenn. 2008;42(5):705–719. [Google Scholar]






