Abstract
Purpose of review:
Uremic pruritus is a highly prevalent and debilitating symptom in patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD). The purpose of this review is to examine current evidence on the mechanisms and treatments of pruritus in CKD and highlight promising areas for future research.
Sources of information:
Published literature, including randomized controlled trials, cohort studies, case reports, and review articles, was searched for evidence pertaining to the pathophysiology and treatment of uremic pruritus.
Methods:
A comprehensive narrative review was conducted to explore the molecular mechanisms underlying uremic pruritus, as well as the evidence (or lack thereof) supporting pharmacological and nonpharmacological treatments for uremic pruritus. The potential role of patient sex in the pathophysiology and management of uremic pruritus is also discussed.
Key findings:
The pathophysiology of uremic pruritus involves a complex interplay of uremic toxins, systemic inflammation, mast cell activation, and imbalance of opioid receptors. Classic treatment strategies for uremic pruritus include optimization of dialysis parameters, amelioration of CKD-related mineral and bone disease, topical emollients and analgesics, antihistamines, the anticonvulsant medications gabapentin and pregabalin, and ultraviolet light B (UV-B) phototherapy. Strong data to support many of these classical treatments for uremic pruritus are limited. Newly evolving treatment approaches for uremic pruritus include opioid receptor modulators, neurokinin-1 inhibitors, and cannabinoids. Further studies regarding their efficacy, pharmacodynamics, and safety in the CKD and ESKD population are needed before these agents are accepted into widespread use. Additional nonpharmacological strategies aimed at treating uremic pruritus include psychotherapy, acupuncture, omega-3 fatty acids, and exercise. Finally, sex differences may exist regarding uremic pruritus, but studies directly addressing sex-specific mechanisms of uremic pruritus remain absent.
Limitations:
High-quality evidence in the management of uremic pruritus remains lacking. Most recommendations are based on expert opinion or studies involving small numbers of patients. In addition, our understanding of the pathophysiological mechanisms behind uremic pruritus is incomplete and continues to evolve over time.
Implications:
Uremic pruritus is a common symptom which reduces quality of life in CKD and ESKD. The identification of novel targeted treatment approaches may ease the burden of uremic pruritus in the future.
Keywords: pruritus, quality of life, sex hormone
Abrégé
Justification:
Le prurit urémique est un syndrome débilitant très prévalent chez les patients atteints d’insuffisance rénale chronique (IRC) et terminale (IRT). Cette revue examine les données probantes actuelles sur les mécanismes et le traitement de cette affection en contexte de néphropathie, et met en évidence les axes de recherche prometteurs.
Sources:
La littérature publiée, soit les essais contrôlés à répartition aléatoire, les études de cohorte, les rapports de cas et les articles de synthèse, a été consultée afin de répertorier les données probantes relatives à la physiopathologie et au traitement du prurit urémique.
Méthodologie:
Une revue narrative complète a été menée afin d’explorer les mécanismes moléculaires sous-tendant le prurit urémique et les données probantes (ou leur absence) appuyant ses traitements pharmacologiques et non pharmacologiques. Le rôle potentiellement joué par le sexe du patient dans la physiopathologie et la gestion de la maladie a également été discuté.
Principaux résultats:
La physiopathologie du prurit urémique implique l’interaction complexe des toxines urémiques, d’une inflammation systémique, de l’activation des mastocytes et d’un déséquilibre des récepteurs opioïdes. Les stratégies classiques de traitement comprennent l’optimisation des paramètres de dialyse, l’apaisement des troubles minéraux osseux liés à l’IRC, les émollients et analgésiques topiques, les antihistaminiques, les anticonvulsivants gabapentine et prégabaline et la photothérapie par UV-B. Les données robustes appuyant ces traitements classiques sont cependant limitées. Parmi les nouvelles approches de traitement, on compte les modulateurs de récepteurs opioïdes, les inhibiteurs de NK-1 et les cannabinoïdes. Des études supplémentaires se penchant sur leur efficacité, leur pharmacodynamie et leur innocuité chez les populations de patients atteints d’IRC et d’IRT sont toutefois nécessaires avant que ces agents ne soient approuvés pour un usage répandu. Les stratégies non pharmacologiques comptent la psychothérapie, l’acupuncture, la prise d’acides gras oméga 3 et l’exercice physique. Enfin, des différences liées au sexe du patient pourraient exister, mais les études portant directement sur les mécanismes sexospécifiques du prurit urémique manquent toujours.
Limites:
Les données probantes concernant la gestion du prurit urémique manquent toujours. La plupart des recommandations sont fondées sur l’avis d’experts ou sur des études portant sur de faibles échantillons. De plus, notre compréhension des mécanismes physiopathologiques causant le prurit urémique est incomplète et en constante évolution.
Conclusion:
Le prurit urémique est un symptôme courant chez les patients atteints d’IRC et d’IRT, dont il réduit la qualité de vie. L’identification de nouvelles approches de traitement ciblées pourrait alléger le fardeau associé au prurit urémique.
Why is this review important?
Uremic pruritus is a common symptom of chronic kidney disease (CKD) that negatively affects quality of life and is a top research priority of patients. This review summarizes current evidence on the mechanisms and treatments for uremic pruritus and highlights promising areas for future research in this field.
What are the key messages?
A number of treatment strategies for uremic pruritus exist (eg, optimization of dialysis parameters, topical emollients and analgesics, antihistamines, ultraviolet light B [UV-B] phototherapy), although the evidence behind their efficacy is limited. Additional exploratory treatment approaches such as opioid receptor modulators, neurokinin-1 inhibitors, cannabinoids, psychotherapy, and exercise are promising avenues for future investigation, as is the role of sex in uremic pruritus.
Introduction
Pruritus refers to an unpleasant itch sensation on the skin that provokes the desire to scratch.1 It results from a systemic condition in which the cross talk between keratinocytes, immune cells, and neurons is perturbed.2 In patients with chronic kidney disease (CKD) and, particularly, patients with end-stage kidney disease (ESKD), uremic pruritis is one of the most common and bothersome manifestations of uremia that signals the ensuing need for renal replacement therapy.3 The Dialysis Outcomes and Practice Patterns Study (DOPPS) of more than 6000 hemodialysis patients across 17 countries from 2012 to 2015 found that 18% of patients reported being very much or extremely bothered by itching.4 Among those patients very much or extremely bothered by itching, 58% reported being depressed about the itching, 45% reported the itching made it hard to work, and 35% reported the itching reduced their desire to be with other people.4 Yet, despite the significant impact on work and social life, 18% of patients very much or extremely bothered by itching reported taking no medication to relieve their symptoms.4
Uremic pruritus therapies remain an unmet clinical need, ranking among patients’ top research priorities. Its molecular underpinnings, however, remain largely unknown. A lack of animal models in which pruritus can be studied in the context of CKD and/or dialysis has limited our capacity to understand and treat this condition.5 Recent advancements in our comprehension of related pruritic diseases (eg, cholestatic pruritus) have translated into novel strategies for pruritus management, some of which have the potential to become effective treatments for uremic pruritus.6,7 However, a clear void remains in bridging the basic understanding of its pathogenesis at the bench with effective therapies that offer relief to patients with CKD at the bedside. In this review, we summarize insights into the pathophysiology of uremic pruritus and provide a comprehensive description of the current and emerging pharmacological and nonpharmacological treatment options. We suggest feasible and innovative means to address related research in a more specific, patient-oriented fashion. Finally, we rationalize patient sex as an overlooked biological variable in uremic pruritus and propose that its consideration in future studies may contribute to more tailored management.
Methods
A comprehensive narrative review was conducted to explore the molecular mechanisms underlying uremic pruritus, as well as the evidence supporting pharmacological and nonpharmacological treatments for uremic pruritus. We used PubMed searches of English, peer-reviewed articles using keywords “uremic pruritus” and “pruritus” and searched for nephrology-related studies of itch. Additional subsearches related to the influence of sex on pruritus were also performed, and a data table was generated. Information was amalgamated into a narrative review, which was internally peer reviewed as part of the Kidney Research Scientist Core Education and National Training (KRESCENT) program prior to external peer review.
Review
Differential Diagnosis of Pruritus in Patients With CKD/ESKD
While uremic pruritus affects a large number of patients with CKD and ESKD, clinicians must consider nonuremic causes of pruritus among this population. Nonuremic causes of pruritus include hypersensitivity reactions (eg, contact dermatitis), primary dermatological conditions (eg, atopic dermatitis, psoriasis), infections (eg, tinea corporis, postherpetic neuralgia), infestations (eg, lice, scabies, bed bugs), cholestatic conditions (eg, viral hepatitis, primary biliary cholangitis), and hematological malignancies (eg, Hodgkin lymphoma, cutaneous T-cell lymphoma). Clinical clues into these nonuremic causes include systemic symptoms, appearance of the skin, itch that is new or suddenly worsening, or itch that is refractory to therapy.
Pathophysiology of Uremic Pruritus
Several clinical factors have been associated with uremic pruritus, including hyperparathyroidism, allergic sensitization, neuropathy, and abnormal levels of magnesium, calcium, iron, bile acids, nitric oxide, vitamin A, and parathyroid hormone.8 The development of itch has largely been attributed to systemic immune responses to hemodialysis, leading to nociceptive responses. Uremic toxins and increased serum levels of C-reactive protein, interleukin (IL)-6, and IL-31 in patients on hemodialysis with pruritus support the inflammatory nature of the disease.9-12 Proliferation, degranulation, and histamine release by mast cells are considered key events triggering the itch response.13,14 Interestingly, dialysis membranes may also stress blood cells and induce the release of pruritogenic cytokines,15 further contributing to this condition.
In pruritus, stimulation of dermal itch receptors or peripheral nerve endings generates impulses that are transmitted centrally via C-fibers.16,17 Endogenous opioids activate mast cells, promoting histamine release and undesirable effects such as urticaria and tachycardia.13 An imbalance in the levels and activation of opioid receptors in dermal cells, lymphocytes, peripheral nerves, and brain accentuates itch and predisposes patients to scratch.18 Antihistamine medications often fail to attenuate itch, suggesting a role of histamine-independent mechanisms in uremic pruritus.17,19 In this regard, receptors for morphine, endothelin-1, chloroquine, and IL-13/31 may mediate the itch sensation.9,13
The precise molecular underpinnings that drive the pathophysiology of uremic pruritus remain unclear. The lack of reliable experimental models has resulted in a paucity of research studies investigating the cross talk between mast cells, keratinocytes, and neurons in a more physiological “uremic-like” setting. Thus, it is not currently possible to investigate uremic-specific mechanisms in the laboratory and to differentiate them from mechanisms more generally involved in other itch-related disorders. Encouragingly, some of the “anti-itch” pharmacological and nonpharmacological therapeutic approaches that are described in the following section have shown promising effects in patients with uremic disease. The optimization of dialysis conditions and CKD-related mineral and bone disease (CKD-MBD) parameters is currently the only treatment that directly targets itch pathogenesis. Most of these promising strategies focus on the consequence rather than the cause of uremic pruritus and aim to target the nociceptive phase of the disease. None have shown the capacity to fully ameliorate inflammation, which is likely the trigger to the perpetuation of itch. A better understanding of the current therapeutic approaches to uremic pruritus at the bedside, and the molecular mechanisms targeted by these strategies, is required to identify the key mechanisms and cell types warranting further investigation at the bench.
Therapies for Uremic Pruritus and Their Molecular Mechanisms
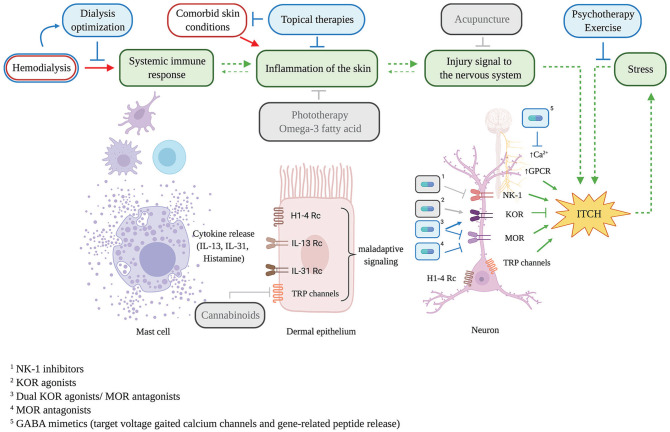
High-quality evidence in the management of uremic pruritus remains lacking, with many recommendations based on expert opinion or studies involving small numbers of patients. Here, we aim to summarize the evidence of classic and emerging pharmacological treatments, their molecular bases where available, and contraindications to their current use in the clinic (summarized jointly in Table 1 and Figure 1) to provide insight into their suitability for patients with CKD and areas warranting further investigation.
Table 1.
Treatment Options for Uremic Pruritus.
| Treatment | Mechanism of action | Limitations/drawbacks |
|---|---|---|
| Dialysis optimization | ||
| ↑ Dialysis dose (↑ Kt/V) | ↑ Clearance of uremic toxins20-23 | |
| High flux dialyzer | ↑ Clearance of uremic toxins24 | |
| Optimization of CKD-MBD parameters | ||
| Parathyroidectomy | Reduction in parathyroid hormone and calcium-phosphate product; mechanism remains unclear25,26 | |
| Topical therapies | ||
| Emollients | Reduce xerosis | |
| Analgesics
(eg, Capsaicin, Pramoxine) |
Analgesia | Insufficient evidence of efficacy of Capsaicin in CKD/ESKD patients27 |
| Immunosuppressant
(eg, Tacrolimus) |
Suppression of immune-mediated exacerbation of dry skin, inflammation, and pruritus | Evidence indicates Tacrolimus is ineffective in CKD/ESKD patients28
FDA warning (risk of dermatological malignancies) |
| Cannabinoids
(THC, CBD) |
Analgesia, ↓ histamine-independent inflammation; exert effects on ionotropic TRPV1-4, TRPA1, and TRPM8 channels29 | Insufficient evidence of efficacy in CKD/ESKD patients30,31
Inconsistent CBD/THC content pharmacokinetics not well understood |
| Systemic pharmacological interventions | ||
| Antihistamines | Block effects of histamine, reducing its contribution to itch | Evidence indicates ineffective in CKD/ESKD patients32,33 |
| Anticonvulsants
(Gabapentin, Pregabalin) |
Negatively modulate voltage-gated calcium channels and calcitonin gene–related peptide release34; possible modulation of μ-opioid receptors35 | Neurological side effects such as dizziness and somnolence reported36 |
| Opioid receptor modulators | ||
| μ-antagonist
(Naltrexone, Naloxone) |
Inhibits μ-opioid receptor, a mediator of itch | Effective in a subset of patients37
Sedation, gastrointestinal complications, among other side effects Dependency risk |
| Selective κ-agonist (Nalfurafine) | Selective central activation of the κ-receptor,38 which contributes to anti-itch sensation (research underway to determine mechanism of this biased agonism39,40) | Only approved in Japan,6,41,42 US randomized controlled trial terminated due to insufficient enrollment43 |
| Peripheral κ-agonist (Difelikefalin) | Activation of peripheral κ-receptors (does not penetrate the blood-brain barrier)7 | Increased diarrhea, dizziness, vomiting No independent trials, not FDA approved |
| Dual κ-agonist/μ-antagonist (Nalbuphine, Butorphanol) | Dual targeting reduces adverse dysphoria that κ-agonism can contribute to or the sedation associated with μ-antagonism | Absent or limited number of controlled, randomized, placebo-controlled trials44-46 |
| Neurokinin-1 inhibitors
(Aprepitant, Serlopitant) |
Blocks substance P-mediated itch sensation in histamine-independent pruritus47 | Interactions of Aprepitant with other medications restrict use in some patients48,49
Limited number of studies in uremic pruritus patients |
Note. CKD = chronic kidney disease; MBD = mineral and bone disorder; ESKD = end-stage kidney disease; FDA = Federal Drug Agency; THC = tetrahydrocannabinol; CBD = cannabidiol; TRP = transient receptor potential.
Figure 1.
Uremic pruritus pathophysiology and management strategies. Hemodialysis may lead to the development of uremic pruritus in a mechanism that involves mast cell activation, maladaptive dermal cell signaling and the induction of the itch sensation via various nociceptive receptors within the peripheral and central nervous systems. Current (blue) and prospective (grey) uremic pruritus management strategies target various junctures of this mechanism, including receptors identified as itch modulators, which can be targeted by systemic pharmacological means (capsules; sharp-ended arrow = activation, flat-ended arrow = inhibition).Note. Ca = calcium; GPCR = G protein-coupled receptor; NK-1 = neurokinin-1; KOR = κ-opioid receptor; MOR = μ-opioid receptors; TRP = transient receptor potential; GABA = gamma-aminobutyric acid.
Dialysis optimization
As it is likely that uremic toxins considerably contribute to the development of uremic pruritus, ensuring that patients are adequately dialyzed often leads to a modest improvement in symptoms. For patients who have progressed to ESKD requiring dialysis, increasing the dose of hemo- or peritoneal dialysis may reduce itch.20-22 For instance, a prospective study among 111 patients on maintenance hemodialysis showed that achieving a Kt/V ≥ 1.5 was associated with a reduction in pruritus intensity compared with a Kt/V < 1.5.22 The use of high-flux versus low-flux dialyzers can further alleviate symptoms.23 Finally, the use of bioincompatible hemodialysis membranes may contribute to uremic pruritus in some patients. In these instances, transition to a biocompatible membrane (eg, polymethylmetacrylate) may reduce its severity.24
Optimization of CKD-MBD parameters
Several small studies have suggested that an elevated calcium-phosphate product and secondary/tertiary hyperparathyroidism contribute to uremic itch.25,26 The largest study included a relatively small number of patients on hemodialysis (37) who underwent parathyroidectomy and experienced significant reductions in both the calcium-phosphate product and parathyroid hormone, with significant reduction in pruritus intensity within 1 week of surgery.25 There remains a lack of evidence that other standard CKD-MBD treatments such as phosphate binders, activated vitamin D analogues, or Cinacalcet are effective in reducing uremic pruritus.
Kidney transplantation
Kidney transplantation, which substantially increases clearance of uremic toxins and improves CKD-MBD parameters beyond that of dialysis in most cases, relieves pruritus symptoms in the vast majority of cases. For instance, a prospective cohort study of 49 patients with uremic pruritus (and associated histological skin changes) who underwent successful kidney transplantation showed consistent resolution of the uremic pruritus skin changes following restoration of kidney function.50 Thus, kidney transplantation should be considered in eligible patients suffering from uremic pruritus.
Topical therapies
Emollients and analgesics
Dry skin is exceedingly common in CKD and ESKD.32,51 Emollients, particularly those with a high water content, are effective in reducing pruritus symptoms and improving quality of life.51-53 In addition to emollients, topical analgesics, such as the neuropeptide-releasing agents Capsaicin and Pramoxine, are often prescribed to alleviate pruritus.54-56 These function by blocking both the initiation and conduction of nerve impulses, leading to numbness. However, a systemic review of interventional trials, including 3 of which were for the treatment of hemodialysis-related pruritus, provided insufficient data for the efficacy of Capsaicin as a treatment.27 Similarly, although early evidence suggested that topical Tacrolimus, an immunosuppressant, may be effective in reducing pruritus, its use is now discouraged due to the evidence of lack of efficacy57 and a black box warning from the US Food and Drug Administration related to a potential increased risk of dermatological malignancies.
Cannabinoids
With the recent legalization of recreational marijuana in Canada and several US states, patients increasingly have access to cannabidiol (CBD)- and tetrahydrocannabinol (THC)-containing compounds, making this an important area for new research. A single study of 21 ESKD patients with uremic pruritus suggests a potential benefit of topical creams containing cannabinoids.58 This study reported that after 3 weeks of therapy, 38% of patients experienced complete resolution of their pruritus symptoms while 81% experienced an improvement in their symptoms. The mechanism of action of cannabinoids in treating itch appears to be their ability to target inflammation and pain. The THC and CBD bind ionotropic transient receptor potential (TRP) ion channels (TRPV1-4, TRPA1, and TRPM8), which have been shown to play a role in the complex cutaneous intercellular communication network between epidermal keratinocytes, immune cells, and sensory nerves, leading to itch sensation.29 Although there is promise in the possibility that antagonizing or desensitizing such TRP channels (using well-selected topically applied phytocannabinoids), it is not yet possible to draw conclusions from the few studies performed due to limitations in the reporting percentages of THC and CBD tested, small samples sizes, and short duration of studies.30,31 Furthermore, as the long-term effect of cannabis use, especially in CKD, remains unclear, caution should be made in recommendation of use of its medicinal properties until further independent and controlled studies are undertaken.
Systematic pharmacological therapies
Antihistamines
Despite their common use, there is a lack of data on the efficacy of oral antihistamines in treating uremic pruritus. In fact, the limited data available suggest that these medications may be less effective in the CKD/ESKD population.32,33 In these studies, oral antihistamines provided no benefit above emollients alone, and their antipruritic effects were diminished with advanced renal disease. This is likely due to the increasingly accepted hypothesis that uremic itch is a histamine-independent phenomenon.
Gabapentin/pregabalin
The neuropathic/anticonvulsant agents Gabapentin and Pregabalin are the mostly widely studied systemic medications for treating uremic pruritus. They were initially designed to mimic the neurotransmitter gamma-aminobutyric acid (GABA); however, they do not bind to GABA receptors. Rather, their mechanism of action likely involves negative modulation of the alpha 2 delta subunit of voltage-gated calcium channels and/or inhibition of the release of calcitonin gene–related peptide (a mediator of itch) from primary afferent neurons.34 It has also been hypothesized that modulation of μ-opioid receptors (MORs) may be involved in its anti-itch properties.35 Across numerous studies, Gabapentin and Pregabalin, administered in reduced doses (due to renal insufficiency), have repeatedly been shown to be effective in reducing pruritus in patients undergoing dialysis.57,59-68 When compared with one another, there is no significant difference in efficacy between Gabapentin and Pregabalin60; however, if one of these medications is ineffective, patients may receive benefit from being switched to the other.62 Adherence to these reduced doses in patients with renal insufficiency are essential, as side effects from somnolence and unsteadiness on the feet to mononucleosis have been reported in these populations.36
Opioid receptor modulators
Opioid-based interventions are increasingly recognized as effective in reducing pruritus symptoms, and their refinement remains a frontier in uremic pruritus treatment. Opioid receptors collectively contribute to a wide range of physiological and pathophysiological activities, including mediation of neurological sensations such as pain modulation,69 which occurs collectively through MOR, δ-opioid receptor (DOR), and κ-opioid receptor (KOR) (reviewed thoroughly elsewhere).70 An imbalance of μ and κ receptors has been hypothesized to contribute to uremic pruritus71 as well as other forms of chronic itch.72 When stimulated, the μ-receptor promotes pruritus, whereas the κ-receptor inhibits it. More recently, selective KOR agonists (Nalfurafine [TRK-820]; approved in Japan).6,41,42 and Difelikefalin (CR845; stage III clinical trial complete)7 have become an attractive target for inhibition of itch over MOR antagonists (such as Naltrexone, Naloxone), which appear to be effective in only a subset of patients with frequent adverse effects (primarily gastrointestinal).37,73 A major benefit to the use of KOR agonists is that they are physiologically safe and do not promote euphoria,74 limiting the likelihood of abuse. Recently, a large double-blind, placebo-controlled, randomized controlled, phase 3 trial of intravenous Difelikefalin was completed. Results demonstrated a significant decrease in pruritus based on a numerical rating scale compared with placebo (52% vs 31% improvement) as well as an improvement in quality of life in the Difelikefalin group,7 garnering optimism within the field about its potential impact on treating uremic pruritus. However, independent verification of efficacy and long-term safety data for Difelikefalin is still needed before mainstream use.
The molecular mechanism underlying KOR activity and itch relief is not well understood, but basic research is emerging around the signaling events that contribute to anti-itch action downstream of KOR activation. It has been suggested that the activation of KOR induces an anti-inflammatory response through the downregulation of cytokine, chemokine, and chemokine receptor expression, which may contribute to their antipruritic effects.75 A recent study also found evidence that KOR activation attenuates histamine-independent acute and chronic itch in mice in a mechanism that involves another G protein-coupled receptor (GPCR) that functions in itch sensation, the gastrin-releasing peptide receptor,76-78 via a calcium-independent phospholipase C-protein kinase C-δ pathway.78 To our knowledge, the contribution of gastrin-releasing peptide receptor signaling to the development of uremic pruritus remains fully unexplored and represents a new avenue for future investigations into its treatment.
NK-1 inhibitors
The substance P(SP)/neurokinin-1 (NK-1) pathway is important in histamine-independent pruritus (comprehensive recent review elsewhere).47 Inhibition of NK-1 receptors, the primary receptor of SP, has been shown to decrease the perception of itch. Aprepitant, an NK-1 inhibitor, has demonstrated efficacy in the treatment of other pruritus-related disorders, and it was at one point identified as a promising new option for the treatment of uremic pruritus. However, interactions with other medications restrict its use in some patients. Serlopitant, another NK-1 inhibitor, was successful in a recent phase II clinical trial of reducing pruritus in patients with chronic itch with minimal adverse effects.48 However, enrollment of patients with uremic or cholestatic pruritus was minimized to reduce possible confounding effects from these comorbidities. Thus, it remains unknown whether Serlopitant might be successful for alleviating uremic pruritus. An earlier, smaller study using Serlopitant in patients, including several with CKD,49 showed a strong inhibition of pruritus with few adverse effects. However, the study was a nonrandomized trial and had very few participants, making it difficult to draw conclusions. Increased investigation of the efficacy of NK-1 inhibitors in uremic pruritus will hopefully shed light on its therapeutic potential in the near future.
Nonpharmacological therapies
Research on nonpharmacological therapies for uremic itch in CKD has focused on phototherapy, acupuncture, omega-3 fatty acid intake, aromatherapy, and exercise, although evidence is generally limited to small studies with methodological issues that limit conclusions to be drawn. Phototherapy is believed to be beneficial due to cutaneous immunosuppression at the cellular level, which is beneficial for skin diseases (eg, psoriasis) that have T-cell hyperactivity.79 A systematic review identified 4 randomized controlled trials examining the effects of phototherapy in patients with CKD stage ≥3; the 2 trials examining broadband UV-B therapy found it to be effective compared with ultraviolet light A (UV-A), whereas the other 2 trials examining narrow-band UV-B therapy or far-infrared ray thermal therapy did not report similar benefit.80 Evidence suggesting UV-B therapy is a safe treatment with no increased risk of skin cancer79 indicates it can be safely used in populations with CKD. However, larger randomized controlled trials that address limitations in existing evidence, such as inadequate blinding of participants and personnel and selective reporting of results, are needed to confirm its efficacy.
Acupuncture and acupressure are alternative treatments used to reduce pruritus, possibly via parasympathetic activation and positive functional connectivity of the putamen-posterior midcingulate cortex.81 A systematic review found that, although several randomized controlled trials have reported positive uremic pruritus outcomes associated with acupuncture or acupressure, there was a high risk of bias in the trials82 and a need for further trials with adequate blinding, appropriate control groups, and systematic allocation.
Omega-3 fatty acid supplementation has been proposed to target uremic pruritus by reducing essential fatty acid deficiency and inflammation.83 Four studies have reported beneficial effects of omega-3 fatty acid supplementation, including 3 small randomized controlled trials, suggesting larger trials are warranted to better understand its efficacy.83 In addition, a small number of exploratory studies have investigated the effects of aromatherapy on uremic pruritus severity and reported beneficial effects84-86; however, randomized controlled trials are needed to address a lack of randomization and blinding in existing evidence.
Finally, a prospective pre-post study investigated the effects of a 12-week aerobic exercise program on symptom burden and reported improvements in symptoms, including pruritus, after the program.87 Randomized controlled trials are therefore needed to better understand the impact of exercise on uremic pruritus, given its various other known benefits in patients with CKD. For example, it would be prudent to investigate the impact of intradialytic exercise on uremic pruritus in hemodialysis patients, given that intradialytic exercise is a more feasible approach to exercise for patients that is associated with a reduction in other symptoms, such as restless legs syndrome and fatigue.88,89 Studies are also needed to understand the mechanisms of the potential effects of exercise.
There are also several unexplored interventions that may have the potential to improve symptom severity and quality of life in people with uremic pruritus. For example, the amplifying effect of stress on the perception of symptoms, including itch, has previously been noted.90 Psychotherapeutic techniques such as cognitive-behavioral therapy, mindfulness meditation, and relaxation training are purported to reduce symptom burden by interrupting maladaptive automatic thought and behavioral reactions that amplify the experience of an unpleasant symptom stimulus.90 These approaches have been studied for their effects on symptoms, including pain, fatigue, and pruritus in non-CKD populations, with several studies suggesting positive effects.91-93 The possibility that stress reduction techniques might be able to reduce the saliency and distress of pruritus therefore warrants further investigation in individuals with CKD. The implementation of routine, systematic symptom assessments in CKD has also been proposed as a way to address underreporting of symptoms in general, identify patients in need of intervention, and trigger more timely and consistent intervention.94 Studies are currently underway that will shed light on the impact of routine symptom assessment protocols on patient outcomes, including pruritus and other common symptoms of CKD.
Sex Differences in Uremic Pruritus
Sex is increasingly recognized as a key variable in the study of diseases,95 including CKD96 and several skin disorders.97-99 Although the role of sex in uremic pruritus has not been a focus of published studies, we have identified 18 reports discussing the role of patient sex on its incidence and severity (Table 2). Five of these studies suggest an association between male sex and the development of uremic pruritus.17,71,100-103 To our knowledge, the largest effort in which the effect of sex was evaluated was a retrospective analysis of 21 075 patients on hemodialysis from 12 countries; Pisoni et al observed that males showed higher odds of having moderate to extreme pruritus.101 Male sex was also identified as a predictor of pruritus in a study of 6137 patients on hemodialysis across 7 countries,103 and as an independent risk factor for severe uremic pruritus in prospective studies of 1773102 and 341 patients on hemodialysis.100 Other studies defend that female sex predisposes to uremic pruritus,22,104-108 but most were limited by small sample size. By contrast, 7 studies concluded that sex does not influence its development.8,109-114 Six of these studies were limited by few patients on dialysis, unadjusted analyses, and/or imbalanced male-to-female ratio.8,110-114
Table 2.
Clinical Studies Discussing the Effect of Sex on UP in Dialysis Patients.
| Study | No. of participants | Dialysis regimen | Country | Sex-related observations |
|---|---|---|---|---|
| Mistik et al100 | 341 | Continuous ambulatory peritoneal dialysis and HD | Turkey | UP ↑ in males |
| Pisoni et al101 | 21 075 | HD only | 12 countries (DOPPS registry) |
UP ↑ in males |
| Narita et al102 | 1773 | HD only | Japan | UP ↑ in males |
| Wikström et al103 | 6137 | HD only | 7 countries (DOPPS registry) |
UP ↑ in males |
| Nahidi et al115 | 26 | HD only | Iran | UP ↑ in males (NS) |
| Szepietowski et al108 | 130 | HD only | Poland | UP ↑ in females |
| Dar et al107 | 100 | HD only | Pakistan | UP ↑ in females |
| Ko et al22 | 111 | HD only | Taiwan | UP ↑ in females |
| Ramakrishnan et al104 | 68 426 | HD and PD | United States | Females tend to report more high itch intensity scores |
| Ersoy et al106 | 181 | HD only | Turkey | UP ↑ in females (NS) |
| Jamal et al105 | 100 | HD only | Saudi Arabia | UP ↑ in females (NS and subjected to age >45 y) |
| Ståhle-Bäckdahl et al114 | 28 | HD only | Sweden | No difference |
| Zucker et al113 | 219 | HD only | Israel | No difference |
| Akhyani et al8 | 167 | HD only | Iran | No difference |
| Razeghi et al112 | 34 | HD only | Iran | No difference |
| Mathur et al111 | 103 | HD only | United States | No difference |
| Hu et al110 | 382 | HD, PD, and chronic kidney disease w/o dialysis | China | No difference |
| Hu et al109 | 11 800 | HD and PD | 17 countries | No difference |
Note. UP = uremic pruritus; HD = hemodialysis; DOPPS = Dialysis Outcomes and Practice Patterns Study; NS = nonsignificant; PD = peritoneal dialysis.
Effect of sex on the pathogenesis of disease
Interest in the inclusion of sex as a consideration in uremic pruritus disease is bolstered by well-established relationships between sex and itch-related mechanisms. Mast cells express androgen and estrogen receptors and respond to sex hormones,116-118 and mast cell number and degranulation are sex-dependent.119,120 Moreover, male and female mast cells are transcriptionally different and respond differently to stress.121 In vitro, testosterone induces IL-33 in male-derived, but not female-derived, mast cells.122 Androgen receptor signaling is similarly linked to IL-33-mediated activation of type 2 innate lymphoid (ILC2) cells via the suppression of tumorigenicity 2 receptor (ST2), a mechanism that might also participate in uremic pruritus.122-124 Upregulation of the IL-33/ST2 axis by androgens stops Th17 immune cells from triggering destructive responses and may be protective in autoimmunity.122,125 However, excessive androgen/IL-33-mediated mast cell degranulation could exacerbate histamine and cytokine secretion and play a pathogenic role in its development. Further exploration of these mechanisms and consideration of their bearing on the sex-related differences in uremic pruritus may provide an opportunity for more target therapies in the future.
Effect of sex on the opioid-cannabinoid system
Levels and localization of MOR, DOR, and KOR vary across sexes,126-128 and sex hormones play a crucial role on their regulation.129 For example, MOR activation by oxycodone administration promotes a sex-specific hippocampal redistribution of opioid receptors,126 and chronic morphine exposure induces MOR mobilization from the membrane to the cytoplasm exclusively in male rat neurons.127 Age-dependent changes in the KOR system are also sex-specific, and more pronounced in males, which were also more altered on administration of the KOR agonist U69593.128 The GPCR signaling (likely involved in antipruritogenic actions of KOR) overlaps with these sex hormone–related mechanisms.130 Estrogens trigger extranuclear signaling on ligation to membrane G-coupled estrogen receptors,131 leading to calcium release, cyclic adenosine monophosphate fluctuations, and induction of extracellular receptor kinase and phosphoinositide 3-kinase/protein kinase B signaling.28,132-134 Reinforcing this concept in relation to the cannabinoid system, estrogens have shown to increase neural TRPV1 expression and sensitization and to prevent capsaicin-induced TRPV1 receptor desensitization. Androgens also induce GPCR-related pathways via the GPCR family member AGPRC6A.38,39 Supporting the link between sex, GPCR, and opioids, gene-by-sex interaction analysis identified that polymorphisms of the G-coupled receptor, adhesion G protein-coupled receptor V1, influenced the risk of opioid dependence in African American men.126 By sitting at the nexus between opioid receptor and sex hormone-induced pathways, GPCR-related mechanisms could represent targets for a sex-directed treatment of uremic pruritus.
Effect of sex on the NK-1 system
Sex affects the levels, distribution, and activation of the SP/NK-1 pathway and pain receptors involved in uremic pruritus.135-140 Female subjects display a higher concentration of SP before and after exposure to allergens.135 In agreement, female rats show increased SP release and NK-1 internalization in sensory neurons compared with male counterparts. This effect was directly linked to the actions of estradiol and may contribute to sex differences in central pain sensitization.136 Female steroids also regulate the sensitivity of mast cells to SP.141 In turn, androgen administration to female rats reduced SP activity in the pituitary gland.142 The pattern of NK-1 distribution is also sex-specific. With increasing age, male sex was associated with a decrease in NK-1 availability in the amygdala and temporal cortex, whereas females had lower NK-1 in the thalamus.138
The effect of sex on the pharmacological modulation of the SP/NK-1 pathway has also been studied.130,143-145 In a mouse model of acute pruritus, the desire to scratch induced by SP administration did not differ between sexes.144 In contrast, data from human studies suggest that females benefit more than males from aprepitant-mediated NK-1 inhibition. In these studies, aprepitant was given to minimize nausea and vomiting after surgery or cisplatin-induced chemotherapy.130,144 Along these lines, antagonism of NK-1, but not NK-2 signaling, attenuated opioid-mediated contact hypersensitivity to a greater extent in female rats.145 The upregulation of SP associated with female sex and estrogens may explain, at least in part, the more evident response to NK-1 inhibition in female subjects. However, additional research is needed to evaluate the role of sex in the pharmacological modulation of itch- and pain-related pathways in the setting of uremic pruritus.
Consideration of race in reporting of sex differences in uremic pruritus
A systematic review and meta-analysis of 42 studies revealed that the prevalence of uremic pruritus among adult dialysis patients was higher in China and not influenced by sex.109 It should be noted that this meta-analysis exhibited several key limitations, including inconsistency in the reported number of studies, predominance of studies of small sample size, and inclusion of articles from nonindexed journals. The more predominant representation of Asian countries in the analysis may suggest that ethnicity may influence sex effects on uremic pruritus. Supporting this idea, it is generally accepted that the effect of sex in CKD is influenced by ethnicity and other factors such as age, diabetes/hypertension, and lifestyle.146-149 These observations may explain the discrepancies observed in smaller studies. Nephrologists recognize that patients of all races are susceptible to develop uremic pruritus.150 However, racial differences in the composition and functions of skin have been associated with differences in skin reactivity and susceptibility to pathological stimuli,126 reinforcing the importance of considering the effects of race when studying skin disorders such as uremic pruritus. Some evidence points toward Asians being more sensitive to skin irritation than Blacks, Hispanics, or Caucasians, but the molecular reasoning behind this observation is unclear.127,128 To our knowledge, whether race influences the severity of uremic pruritus has not been yet explored in well-balanced studies. As an example, in a longitudinal study in patients on hemodialysis, Mathur and colleagues reported that race did not play a role in the severity of uremic pruritus. However, Asian ethnicities were not numerically represented in the patient cohort, which was dominated by African American (68%) and Caucasian (32%) individuals.111 Overall, sex104,129,131-133 and race134 differences have been noted on the perception of itch, the emotional impact of pruritus, and the subject ability to report discomfort, and it is possible that both influence data outcomes. Prospective, nation-wide studies should be conducted in race- and sex-balanced cohorts of patients with uremic pruritus to directly evaluate these important variables in an adjusted fashion.
Conclusion and Future Directions
Uremic pruritus is a common comorbidity in patients with CKD and is associated with reduced quality of life and increased mortality. No formal published guidelines for the treatment of uremic pruritus exist, and data supporting present therapies remain limited. Thus, there remains no clear algorithm to guide clinicians on how best to manage this condition. As it is probable that the pathogenesis of uremic pruritus is multifactorial, a combination of pharmacological and nonpharmacological therapies, which remain a largely untapped resource, likely provides the best current treatment strategy for patients.
Recent evidence argues that research efforts focus on further understanding the role of systemic inflammation and pain receptor function in uremic pruritus development and management. Lessons can be learned from innovative research approaches that enable the study of the interplay between different cell types in bioengineered models that mimic physiological environments (eg, organotypic 3-dimensional models of human skin and immune cells).127,128 The development of these in vitro models specific to uremic conditions would serve to enhance our understanding of the cross talk between skin cells, neurons, and patient-derived immune cells and the reasons behind the sex-specific differences seen with uremic pruritus. This may allow for the identification of novel treatment targets specific to the initial inflammatory activation of skin cells, prior to the perpetuation of the itch response.
Efforts toward the understanding of nociceptive responses have already identified roles for cannabinoid-responsive, gastrin-releasing peptide receptor (GRPR), NK-1, and opioid receptors in chronic pruritus. Initial reports have highlighted modulation of many of these pathways as promising uremic pruritus treatments; however, transformation of these preliminary discoveries to genuine advancements at the bedside remains mired by the absence of independent, well-controlled, widespread trials with adequate sample sizes. Given the prevalence of uremic pruritus in patients suffering with CKD, we implore groups to sequel initial efforts on prospective therapies with well-designed trials and to provide retrospective analyses of their efficacy. Only then will we delve deeper than the surface in the treatment of uremic pruritus.
Footnotes
List of Abbreviations: CBD, cannabidiol; CKD, chronic kidney disease; CKD-MBD, chronic kidney disease–metabolic bone disorder; DOR, δ-opioid receptor; ESKD, end-stage kidney disease; GABA, gamma-aminobutyric acid; GPCR, G protein-coupled receptor; HD, hemodialysis; KOR, κ-opioid receptor; MOR, μ-opioid receptor; NK-1, neurokinin-1; PD, peritoneal dialysis; PKC, protein kinase C; PTH, parathyroid hormone; SP, substance P; THC, tetrahydrocannabinol; TRP, transient receptor potential.
Ethics Approval and Consent to Participate: No patient consent was required for this narrative review.
Consent for Publication: The authors have consented publication of this article.
Availability of Data and Materials: No additional data or materials are available for this review. Please contact corresponding author with any requests.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: C.E.M., S.C-F, and J.F.F are supported by a postdoctoral fellowship award, and G.L.H is supported by a New Investigator award from the Kidney Research Scientist Core Education and National Training Program, a national kidney research training partnership of the Kidney Foundation of Canada, the Canadian Society of Nephrology, and the Canadian Institutes of Health Research. C.E.M is also supported by the Canadian Institutes of Health Research Postdoctoral Fellowship.
References
- 1. Shanley KJ. Pathophysiology of pruritus. Vet Clin North Am Small Anim Pract. 1988;18:971-981. [DOI] [PubMed] [Google Scholar]
- 2. Lavery MJ, Kinney MO, Mochizuki H, Craig J, Yosipovitch G. Pruritus: an overview. What drives people to scratch an itch? Ulster Med J. 2016;85:164-173. [PMC free article] [PubMed] [Google Scholar]
- 3. Zemaitis MR, Foris LA, Chandra S, Bashir K. Uremia. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 4. Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12:2000-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bujok J, Walski T, Czerski A, et al. Sheep model of haemodialysis treatment. Lab Anim. 2018;52(2):176-185. [DOI] [PubMed] [Google Scholar]
- 6. Kozono H, Yoshitani H, Nakano R. Post-marketing surveillance study of the safety and efficacy of nalfurafine hydrochloride (Remitch® capsules 2.5 μg) in 3,762 hemodialysis patients with intractable pruritus. Int J Nephrol Renovasc Dis. 2018;11:9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F, KALM-1 Trial Investigators. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382:222-232. [DOI] [PubMed] [Google Scholar]
- 8. Akhyani M, Ganji M-R, Samadi N, Khamesan B, Daneshpazhooh M. Pruritus in hemodialysis patients. BMC Dermatol. 2005;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ko M-J, Peng Y-S, Chen H-Y, et al. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J Am Acad Dermatol. 2014;71(6):1151-1159. [DOI] [PubMed] [Google Scholar]
- 10. Kimmel M, Alscher DM, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant. 2006;21(3):749-755. [DOI] [PubMed] [Google Scholar]
- 11. Chen H-Y, Chiu Y-L, Hsu S-P, et al. Elevated C-reactive protein level in hemodialysis patients with moderate/severe uremic pruritus: a potential mediator of high overall mortality. QJM. 2010;103(11):837-846. [DOI] [PubMed] [Google Scholar]
- 12. Malekmakan L, Malekmakan A, Sayadi M, Pakfetrat M, Sepaskhah M, Roozbeh J. Association of high-sensitive C-reactive protein and dialysis adequacy with uremic pruritus. Saudi J Kidney Dis Transpl. 2015;26(5):890-895. [DOI] [PubMed] [Google Scholar]
- 13. Kremer AE, Feramisco J, Reeh PW, Beuers U, Oude Elferink RP. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochim Biophys Acta. 2014;1842(7):869-892. [DOI] [PubMed] [Google Scholar]
- 14. Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol. 2015;6:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fallahzadeh MK, Roozbeh J, Geramizadeh B, Namazi MR. Interleukin-2 serum levels are elevated in patients with uremic pruritus: a novel finding with practical implications. Nephrol Dial Transplant. 2011;26(10):3338-3344. [DOI] [PubMed] [Google Scholar]
- 16. Yonova D. Pruritus in certain internal diseases. Hippokratia. 2007;11(2):67-71. [PMC free article] [PubMed] [Google Scholar]
- 17. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin. Nephrol. 2015;35:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mettang T, Kremer AE. Uremic pruritus. Kidney Int. 2015;87:685-691. [DOI] [PubMed] [Google Scholar]
- 19. Schwarzacher HG. [Cell aging in vitro (author’s transl)]. Wien Klin Wochenschr. 1975;87:705-709. [PubMed] [Google Scholar]
- 20. Hiroshige K, Kabashima N, Takasugi M, Kuroiwa A. Optimal dialysis improves uremic pruritus. Am J Kidney Dis. 1995;25(3):413-419. [DOI] [PubMed] [Google Scholar]
- 21. Liakopoulos V, Krishnan M, Stefanidis I, et al. Improvement in uremic symptoms after increasing daily dialysate volume in patients on chronic peritoneal dialysis with declining renal function. Int Urol Nephrol. 2004;36(3):437-443. [DOI] [PubMed] [Google Scholar]
- 22. Ko M-J, Wu H-Y, Chen H-Y, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS One. 2013;8(8):e71404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen ZJ, Cao G, Tang WX, et al. A randomized controlled trial of high-permeability haemodialysis against conventional haemodialysis in the treatment of uraemic pruritus. Clin Exp Dermatol. 2009;34(6):679-683. [DOI] [PubMed] [Google Scholar]
- 24. Lin H-H, Liu Y-L, Liu J-H, et al. Uremic pruritus, cytokines, and polymethylmethacrylate artificial kidney. Artif Organs. 2008;32(6):468-472. [DOI] [PubMed] [Google Scholar]
- 25. Chou FF, Ho JC, Huang SC, Sheen-Chen SM. A study on pruritus after parathyroidectomy for secondary hyperparathyroidism. J Am Coll Surg. 2000;190(1):65-70. [DOI] [PubMed] [Google Scholar]
- 26. Massry SG, Popovtzer MM, Coburn JW, Makoff DL, Maxwell MH, Kleeman CR. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. Disappearance of itching after subtotal parathyroidectomy. N Engl J Med. 1968;279:697-700. [DOI] [PubMed] [Google Scholar]
- 27. Gooding SMD, Canter PH, Coelho HF, Boddy K, Ernst E. Systematic review of topical capsaicin in the treatment of pruritus. Int J Dermatol. 2010;49(8):858-865. [DOI] [PubMed] [Google Scholar]
- 28. Duque MI, Yosipovitch G, Fleischer AB, Jr, Willard J, Freedman BI. Lack of efficacy of tacrolimus ointment 0.1% for treatment of hemodialysis-related pruritus: a randomized, double-blind, vehicle-controlled study. J Am Acad Dermatol. 2005;52(3, pt 1):519-521. [DOI] [PubMed] [Google Scholar]
- 29. Xie Z, Hu H. TRP channels as drug targets to relieve itch. Pharmaceuticals (Basel). 2018;11:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tóth KF, Ádám D, Bíró T, Oláh A. Cannabinoid signaling in the skin: therapeutic potential of the “c(ut)annabinoid” system. Molecules. 2019;24:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ho C, Martinusen D, Lo C. A review of cannabis in chronic kidney disease symptom management. Can J Kidney Health Dis. 2019;6:doi: 10.1177/2054358119828391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gilchrest BA, Stern RS, Steinman TI, Brown RS, Arndt KA, Anderson WW. Clinical features of pruritus among patients undergoing maintenance hemodialysis. Arch Dermatol. 1982;118(3):154-156. [PubMed] [Google Scholar]
- 33. Weisshaar E, Dunker N, Röhl F-W, Gollnick H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp Dermatol. 2004;13(5):298-304. [DOI] [PubMed] [Google Scholar]
- 34. Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133-141. [DOI] [PubMed] [Google Scholar]
- 35. Yoon MH, Choi JI, Jeong SW. Spinal gabapentin and antinociception: mechanisms of action. J Korean Med Sci. 2003;18(2):255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoo L, Matalon D, Hoffman RS, Goldfarb DS. Treatment of pregabalin toxicity by hemodialysis in a patient with kidney failure. Am J Kidney Dis. 2009;54(6):1127-1130. [DOI] [PubMed] [Google Scholar]
- 37. Pauli-Magnus C, Mikus G, Alscher DM, et al. Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. J. Am. Soc. Nephrol. 2000;11:514-519. [DOI] [PubMed] [Google Scholar]
- 38. Endoh T, Tajima A, Izumimoto N, et al. TRK-820, a selective kappa-opioid agonist, produces potent antinociception in cynomolgus monkeys. Jpn J Pharmacol. 2001;85(3):282-290. [DOI] [PubMed] [Google Scholar]
- 39. Zhou L, Lovell KM, Frankowski KJ, et al. Development of functionally selective, small molecule agonists at kappa opioid receptors. J. Biol. Chem. 2013;288:36703-36716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brust TF, Morgenweck J, Kim SA, et al. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal. 2016;9:ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25(4):1251-1257. [DOI] [PubMed] [Google Scholar]
- 42. Wikström B, Gellert R, Ladefoged SD, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16(12):3742-3747. [DOI] [PubMed] [Google Scholar]
- 43. Jaiswal D, Uzans D, Hayden J, Kiberd BA, Tennankore KK. Targeting the opioid pathway for uremic pruritus: a systematic review and meta-analysis. Can J Kidney Health Dis. 2016;3. doi: 10.1177/2054358116675345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dawn AG, Yosipovitch G. Butorphanol for treatment of intractable pruritus. J Am Acad. Dermatol. 2006;54:527-531. [DOI] [PubMed] [Google Scholar]
- 45. Hawi A, Alcorn H, Berg J, Hines C, Hait H, Sciascia T. Pharmacokinetics of nalbuphine hydrochloride extended release tablets in hemodialysis patients with exploratory effect on pruritus. BMC Nephrol. 2015;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mathur VS, Kumar J, Crawford PW, Hait H, Sciascia T. TR02 Study Investigators. A multicenter, randomized, double-blind, placebo-controlled trial of nalbuphine ER tablets for uremic pruritus. Am J Nephrol. 2017;46(6):450-458. [DOI] [PubMed] [Google Scholar]
- 47. Pojawa-Gołąb M, Jaworecka K, Reich A. NK-1 receptor antagonists and pruritus: review of current literature. Dermatol Ther (Heidelb). 2019;9(3):391-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yosipovitch G, Ständer S, Kerby MB, et al. Serlopitant for the treatment of chronic pruritus: results of a randomized, multicenter, placebo-controlled phase 2 clinical trial. J Am Acad Dermatol. 2018;78(5):882-891. [DOI] [PubMed] [Google Scholar]
- 49. Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One. 2010;5:e10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Altmeyer P, Kachel HG, Schäfer G, Fassbinder W. [Normalization of uremic skin changes following kidney transplantation]. Hautarzt. 1986;37(4):217-221. [PubMed] [Google Scholar]
- 51. Morton CA, Lafferty M, Hau C, Henderson I, Jones M, Lowe JG. Pruritus and skin hydration during dialysis. Nephrol Dial Transplant. 1996;11:2031-2036. [DOI] [PubMed] [Google Scholar]
- 52. Balaskas E, Szepietowski JC, Bessis D, et al. Randomized, double-blind study with glycerol and paraffin in uremic xerosis. Clin J Am Soc Nephrol. 2011;6(4):748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okada K, Matsumoto K. Effect of skin care with an emollient containing a high water content on mild uremic pruritus. Ther Apher Dial. 2004;8(5):419-422. [DOI] [PubMed] [Google Scholar]
- 54. Breneman DL, Cardone JS, Blumsack RF, Lather RM, Searle EA, Pollack VE. Topical capsaicin for treatment of hemodialysis-related pruritus. J Am Acad Dermatol. 1992;26(1):91-94. [DOI] [PubMed] [Google Scholar]
- 55. Tarng DC, Cho YL, Liu HN, Huang TP. Hemodialysis-related pruritus: a double-blind, placebo-controlled, crossover study of capsaicin 0.025% cream. Nephron. 1996;72(4):617-622. [DOI] [PubMed] [Google Scholar]
- 56. Young TA, Patel TS, Camacho F, et al. A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. J Dermatolog Treat. 2009;20(2):76-81. [DOI] [PubMed] [Google Scholar]
- 57. Amirkhanlou S, Rashedi A, Taherian J, Hafezi AA, Parsaei S. Comparison of gabapentin and ketotifen in treatment of uremic pruritus in hemodialysis patients. Pak J Med Sci. 2016;32(1):22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerol Croat. 2005;13(2):97-103. [PubMed] [Google Scholar]
- 59. Yue J, Jiao S, Xiao Y, Ren W, Zhao T, Meng J. Comparison of pregabalin with ondansetron in treatment of uraemic pruritus in dialysis patients: a prospective, randomized, double-blind study. Int Urol Nephrol. 2015;47(1):161-167. [DOI] [PubMed] [Google Scholar]
- 60. Solak Y, Biyik Z, Atalay H, et al. Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: a prospective, crossover study. Nephrology (Carlton). 2012;17(8):710-717. [DOI] [PubMed] [Google Scholar]
- 61. Shavit L, Grenader T, Lifschitz M, Slotki I. Use of pregabalin in the management of chronic uremic pruritus. J Pain Symptom Manage. 2013;45(4):776-781. [DOI] [PubMed] [Google Scholar]
- 62. Rayner H, Baharani J, Smith S, Suresh V, Dasgupta I. Uraemic pruritus: relief of itching by gabapentin and pregabalin. Nephron Clin Pract. 2012;122(3-4):75-79. [DOI] [PubMed] [Google Scholar]
- 63. Nofal E, Farag F, Nofal A, Eldesouky F, Alkot R, Abdelkhalik Z. Gabapentin: a promising therapy for uremic pruritus in hemodialysis patients: a randomized-controlled trial and review of literature. J Dermatolog Treat. 2016;27:515-519. [DOI] [PubMed] [Google Scholar]
- 64. Naini AE, Harandi AA, Khanbabapour S, Shahidi S, Seirafiyan S, Mohseni M. Gabapentin: a promising drug for the treatment of uremic pruritus. Saudi J Kidney Dis Transpl. 2007;18(3):378-381. [PubMed] [Google Scholar]
- 65. Manenti L, Vaglio A, Costantino E, et al. Gabapentin in the treatment of uremic itch: an index case and a pilot evaluation. J Nephrol. 2005;18(1):86-91. [PubMed] [Google Scholar]
- 66. Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19(12):3137-3139. [DOI] [PubMed] [Google Scholar]
- 67. Foroutan N, Etminan A, Nikvarz N, Shojai Shahrokh Abadi M. Comparison of pregabalin with doxepin in the management of uremic pruritus: a randomized single blind clinical trial. Hemodial Int. 2017;21(1):63-71. [DOI] [PubMed] [Google Scholar]
- 68. Aperis G, Paliouras C, Zervos A, Arvanitis A, Alivanis P. The use of pregabalin in the treatment of uraemic pruritus in haemodialysis patients. J Ren Care. 2010;36(4):180-185. [DOI] [PubMed] [Google Scholar]
- 69. Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012;13(2):230-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Valentino RJ, Volkow ND. Untangling the complexity of opioid receptor function. Neuropsychopharmacology. 2018;43(13):2514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Inan S, Cowan A. Reduced kappa-opioid activity in a rat model of cholestasis. Eur. J. Pharmacol. 2005;518:182-186. [DOI] [PubMed] [Google Scholar]
- 73. Legroux-Crespel E, Clèdes J, Misery L. A comparative study on the effects of naltrexone and loratadine on uremic pruritus. Dermatology. 2004;208(4):326-330. [DOI] [PubMed] [Google Scholar]
- 74. Cowan A, Kehner GB, Inan S. Targeting itch with ligands selective for κ opioid receptors. Handb Exp Pharmacol. 2015;226:291-314. [DOI] [PubMed] [Google Scholar]
- 75. Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol. 2008;252(1-2):146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sun Y-G, Chen Z-F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700-703. [DOI] [PubMed] [Google Scholar]
- 77. Lee H, Ko M-C. Distinct functions of opioid-related peptides and gastrin-releasing peptide in regulating itch and pain in the spinal cord of primates. Sci Rep. 2015;5:11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Munanairi A, Liu X-Y, Barry DM, et al. Non-canonical opioid signaling inhibits itch transmission in the spinal cord of mice. Cell Rep. 2018;23:866-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Want E, Sasaki J, Nakamura M, Koo J. Cutaneous carcinogenic risk of phototherapy: an updated comprehensive review. J Psoriasis and Psoriatic Arth. 2015;1:44-51. doi: 10.1177/247553031500100107. [DOI] [Google Scholar]
- 80. Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70(5):638-655. [DOI] [PubMed] [Google Scholar]
- 81. Min S, Kim K-W, Jung W-M, et al. Acupuncture for histamine-induced itch: association with increased parasympathetic tone and connectivity of putamen-midcingulate cortex. Front Neurosci. 2019;13:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim KH, Lee MS, Kim T-H, Kang JW, Choi T-Y, Lee JD. Acupuncture and related interventions for symptoms of chronic kidney disease. Cochrane Database Syst Rev. 2016:CD009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Panahi Y, Dashti-Khavidaki S, Farnood F, Noshad H, Lotfi M, Gharekhani A. Therapeutic effects of omega-3 fatty acids on chronic kidney disease-associated pruritus: a literature review. Adv Pharm Bull. 2016;6(4):509-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ro Y-J, Ha H-C, Kim C-G, Yeom H-A. The effects of aromatherapy on pruritus in patients undergoing hemodialysis. Dermatol Nurs. 2002;14(4):231-4237. [PubMed] [Google Scholar]
- 85. Shahgholian N, Dehghan M, Mortazavi M, Gholami F, Valiani M. Effect of aromatherapy on pruritus relief in hemodialysis patients. Iran J Nurs Midwifery Res. 2010;15(4):240-244. [PMC free article] [PubMed] [Google Scholar]
- 86. Cürcani M, Tan M. The effect of aromatherapy on haemodialysis patients’ pruritus. J Clin Nurs. 2014;23(23-24):3356-3365. [DOI] [PubMed] [Google Scholar]
- 87. Wilkinson TJ, Watson EL, Gould DW, et al. Twelve weeks of supervised exercise improves self-reported symptom burden and fatigue in chronic kidney disease: a secondary analysis of the “ExTra CKD” trial. Clin Kidney J. 2019;12(1):113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chang Y, Cheng S-Y, Lin M, Gau F-Y, Chao Y-FC. The effectiveness of intradialytic leg ergometry exercise for improving sedentary life style and fatigue among patients with chronic kidney disease: a randomized clinical trial. Int J Nurs Stud. 2010;47(11):1383-1388. [DOI] [PubMed] [Google Scholar]
- 89. Sakkas GK, Hadjigeorgiou GM, Karatzaferi C, et al. Intradialytic aerobic exercise training ameliorates symptoms of restless legs syndrome and improves functional capacity in patients on hemodialysis: a pilot study. ASAIO J. 2008;54(2):185-190. [DOI] [PubMed] [Google Scholar]
- 90. Schut C, Mollanazar NK, Kupfer J, Gieler U, Yosipovitch G. Psychological interventions in the treatment of chronic itch. Acta Derm Venereol. 2016;96(2):157-161. [DOI] [PubMed] [Google Scholar]
- 91. Corbett TK, Groarke A, Devane D, Carr E, Walsh JC, McGuire BE. The effectiveness of psychological interventions for fatigue in cancer survivors: systematic review of randomised controlled trials. Syst Rev. 2019;8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Byrnes KL, Whillier S. Effects of nonpharmaceutical treatments on symptom management in adults with mild or moderate multiple sclerosis: a meta-analysis. J Manipulative Physiol Ther. 2019;42(7):514-531. [DOI] [PubMed] [Google Scholar]
- 93. Warth M, Zöller J, Köhler F, Aguilar-Raab C, Kessler J, Ditzen B. Psychosocial interventions for pain management in advanced cancer patients: a systematic review and meta-analysis. Curr Oncol Rep. 2020;22:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. van der Veer SN, Aresi G, Gair R. Incorporating patient-reported symptom assessments into routine care for people with chronic kidney disease. Clin Kidney J. 2017;10(6):783-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bairey Merz CN, Dember LM, Ingelfinger JR, et al. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol. 2019;15(12):776-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen W, Mempel M, Traidl-Hofmann C, Al Khusaei S, Ring J. Gender aspects in skin diseases. J Eur Acad Dermatol Venereol. 2010;24(12):1378-1385. [DOI] [PubMed] [Google Scholar]
- 98. Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. J Dermatol Sci. 2009;55(3):144-149. [DOI] [PubMed] [Google Scholar]
- 99. Hägg D, Sundström A, Eriksson M, Schmitt-Egenolf M. Severity of psoriasis differs between men and women: a study of the clinical outcome measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish register patients. Am J Clin Dermatol. 2017;18(4):583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mistik S, Utas S, Ferahbas A, et al. An epidemiology study of patients with uremic pruritus. J Eur Acad Dermatol Venereol. 2006;20(6):672-678. [DOI] [PubMed] [Google Scholar]
- 101. Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495-3505. [DOI] [PubMed] [Google Scholar]
- 102. Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626-1632. [DOI] [PubMed] [Google Scholar]
- 103. Wikström B. Itchy skin—a clinical problem for haemodialysis patients. Nephrol Dial Transplant. 2007;22(suppl 5):v3-v7. [DOI] [PubMed] [Google Scholar]
- 104. Ramakrishnan K, Bond TC, Claxton A, et al. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int J Nephrol Renovasc Dis. 2013;7:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jamal A, Subramanian PT. Pruritus among end-stage renal failure patients on hemodialysis. Saudi J Kidney Dis Transpl. 2000;11(2):181-185. [PubMed] [Google Scholar]
- 106. Ersoy NA, Akyar İ. Multidimensional pruritus assessment in hemodialysis patients. BMC Nephrol. 2019;20:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dar NR, Akhter A. Clinical characteristics of uremic pruritus in patients undergoing haemodialysis. J Coll Physicians Surg Pak. 2006;16(2):94-96. [PubMed] [Google Scholar]
- 108. Szepietowski JC, Sikora M, Kusztal M, Salomon J, Magott M, Szepietowski T. Uremic pruritus: a clinical study of maintenance hemodialysis patients. J Dermatol. 2002;29(10):621-627. [DOI] [PubMed] [Google Scholar]
- 109. Hu X, Sang Y, Yang M, Chen X, Tang W. Prevalence of chronic kidney disease-associated pruritus among adult dialysis patients: a meta-analysis of cross-sectional studies. Medicine (Baltimore). 2018;97(21):e10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hu T, Wang B, Liao X, Wang S. Clinical features and risk factors of pruritus in patients with chronic renal failure. Exp Ther Med. 2019;18(2):964-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1410-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Razeghi E, Eskandari D, Reza Ganji M, Pasha A., Mansooreh Togha M, Khashayar P. Gabapentin and Uremic Pruritus in Hemodialysis Patients. Ren Fail. 2009;31(2):85-90. [DOI] [PubMed] [Google Scholar]
- 113. Zucker I, Yosipovitch G, David M, Gafter U, Boner G. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. J Am Acad Dermatol. 2003;49(5):842-846. [DOI] [PubMed] [Google Scholar]
- 114. Ståhle-Bäckdahl M. Uremic pruritus. Clinical and experimental studies. Acta Derm Venereol Suppl (Stockh). 1989;145:1-38. [PubMed] [Google Scholar]
- 115. Nahidi Y, Badiee S, Torabi S, Abbasi Shaye Z, Nazemian F, Saki A. Acupuncture Effect on Pruritus in Hemodialysis Patients: A Randomized Clinical Trial. Iran Red Crescent Med J. 2018;20(10). [Google Scholar]
- 116. Chen W, Beck I, Schober W, et al. Human mast cells express androgen receptors but treatment with testosterone exerts no influence on IgE-independent mast cell degranulation elicited by neuromuscular blocking agents. Exp Dermatol. 2010;19(3):302-304. [DOI] [PubMed] [Google Scholar]
- 117. Zaitsu M, Narita S-I, Lambert KC, et al. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol. 2007;44(8):1977-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Clotet S, Soler MJ, Riera M, et al. Stable Isotope Labeling with Amino Acids (SILAC)-based proteomics of primary human kidney cells reveals a novel link between male sex hormones and impaired energy metabolism in diabetic kidney disease. Mol Cell Proteomics. 2017;16(3):368-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Joshi A, Page CE, Damante M, et al. Sex differences in the effects of early life stress exposure on mast cells in the developing rat brain. Horm Behav. 2019;113:76-84. [DOI] [PubMed] [Google Scholar]
- 120. Lenz KM, Pickett LA, Wright CL, Davis KT, Joshi A, McCarthy MM. Mast cells in the developing brain determine adult sexual behavior. J. Neurosci. 2018;38:8044-8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mackey E, Ayyadurai S, Pohl CSD’ D’, Costa S, Li Y, Moeser AJ. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol Sex Differ. 2016;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Russi AE, Ebel ME, Yang Y, Brown MA. Male-specific IL-33 expression regulates sex-dimorphic EAE susceptibility. Proc Natl Acad Sci U S A. 2018;115:E1520-E1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cephus J-Y, Stier MT, Fuseini H, et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21:2487-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Takatori H, Makita S, Ito T, Matsuki A, Nakajima H. Regulatory mechanisms of IL-33-ST2-mediated allergic inflammation. Front Immunol. 2018;9:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Brown MA. Studies of mast cells: adventures in serendipity. Front Immunol. 2018;9:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Berardesca E, Maibach H. Racial differences in skin pathophysiology. J Am Acad Dermatol. 1996;34(4):667-672. [DOI] [PubMed] [Google Scholar]
- 127. Robinson MK. Population differences in acute skin irritation responses: race, sex, age, sensitive skin and repeat subject comparisons. Contact Dermatitis. 2002;46(2):86-93. [DOI] [PubMed] [Google Scholar]
- 128. Kimball AB. Skin differences, needs, and disorders across global populations. J Investig Dermatol Symp Proc. 2008;13(1):2-5. [DOI] [PubMed] [Google Scholar]
- 129. Stumpf A, Burgmer M, Schneider G, et al. Sex differences in itch perception and modulation by distraction—an FMRI pilot study in healthy volunteers. PLoS One. 2013;8(11):e79123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hesketh PJ, Grunberg SM, Herrstedt J, et al. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer. 2006;14(4):354-360. [DOI] [PubMed] [Google Scholar]
- 131. Hartmann EM, Handwerker HO, Forster C. Gender differences in itch and pain-related sensations provoked by histamine, cowhage and capsaicin. Acta Derm Venereol. 2015;95(1):25-30. [DOI] [PubMed] [Google Scholar]
- 132. Schmid Y, Navarini A, Thomas Z-RM, Pfleiderer B, Krähenbühl S, Mueller SM. Sex differences in the pharmacology of itch therapies-a narrative review. Curr Opin Pharmacol. 2019;46:122-142. [DOI] [PubMed] [Google Scholar]
- 133. Stumpf A, Zerey V, Heuft G, Ständer S, Pfleiderer B, Schneider G. Itch perception and skin reactions as modulated by verbal suggestions: role of participant’s and investigator’s sex. Acta Derm Venereol. 2016;96:619-623. [DOI] [PubMed] [Google Scholar]
- 134. Shaw FM, Luk KMH, Chen K-H, Wrenn G, Chen SC. Racial disparities in the impact of chronic pruritus: a cross-sectional study on quality of life and resource utilization in United States veterans. J Am Acad Dermatol. 2017;77(1):63-69. [DOI] [PubMed] [Google Scholar]
- 135. Tomljenovic D, Baudoin T, Megla ZB, Geber G, Scadding G, Kalogjera L. Females have stronger neurogenic response than males after non-specific nasal challenge in patients with seasonal allergic rhinitis. Med Hypotheses. 2018;116:114-118. [DOI] [PubMed] [Google Scholar]
- 136. Nazarian A, Tenayuca JM, Almasarweh F, Armendariz A, Are D. Sex differences in formalin-evoked primary afferent release of substance P. Eur J Pain. 2014;18(1):39-46. [DOI] [PubMed] [Google Scholar]
- 137. Kolodziejski JA, Nelson BS, Smith GT. Sex and species differences in neuromodulatory input to a premotor nucleus: a comparative study of substance P and communication behavior in weakly electric fish. J. Neurobiol. 2005;62:299-315. [DOI] [PubMed] [Google Scholar]
- 138. Engman J, Åhs F, Furmark T, et al. Age, sex and NK1 receptors in the human brain—a positron emission tomography study with [¹¹C]GR205171. Eur Neuropsychopharmacol. 2012;22(8):562-568. [DOI] [PubMed] [Google Scholar]
- 139. Micevych PE, Matt DW, Go VL. Concentrations of cholecystokinin, substance P, and bombesin in discrete regions of male and female rat brain: sex differences and estrogen effects. Exp. Neurol. 1988;100:416-425. [DOI] [PubMed] [Google Scholar]
- 140. DePalatis LR, Khorram O, McCann SM. Age-, sex-, and gonadal steroid-related changes in immunoreactive substance P in the rat anterior pituitary gland. Endocrinology. 1985;117(4):1368-1373. [DOI] [PubMed] [Google Scholar]
- 141. Bradesi S, Eutamene H, Theodorou V, Fioramonti J, Bueno L. Effect of ovarian hormones on intestinal mast cell reactivity to substance P. Life Sci. 2001;68:1047-1056. [DOI] [PubMed] [Google Scholar]
- 142. Shamgochian MD, Leeman SE. Substance P stimulates luteinizing hormone secretion from anterior pituitary cells in culture. Endocrinology. 1992;131(2):871-875. [DOI] [PubMed] [Google Scholar]
- 143. Yamaura K, Tomono A, Suwa E, Ueno K. Sex-related differences in SLIGRL-induced pruritus in mice. Life Sci. 2014;94:54-57. [DOI] [PubMed] [Google Scholar]
- 144. Trimas SJ, Trimas MD. Use of aprepitant and factors associated with incidence of postoperative Nausea and vomiting in patients undergoing facial plastic surgery. JAMA Facial Plast Surg. 2015;17(4):251-255. [DOI] [PubMed] [Google Scholar]
- 145. Elliott JC, Wagner AF, Lysle DT. Neurokinin 1 receptor signaling mediates sex differences in mu and kappa opioid-induced enhancement of contact hypersensitivity. J Neuroimmunol. 2006;181(1-2):100-105. [DOI] [PubMed] [Google Scholar]
- 146. Chang P-Y, Chien L-N, Lin Y-F, Wu M-S, Chiu W-T, Chiou H-Y. Risk factors of gender for renal progression in patients with early chronic kidney disease. Medicine (Baltimore). 2016;95(30):e4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yu MK, Lyles CR, Bent-Shaw LA, Young BA. Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the pathways study. Am J Nephrol. 2012;36(3):245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Clotet S, Riera M, Pascual J, Soler MJ. RAS and sex differences in diabetic nephropathy. Am J Physiol Renal Physiol. 2016;310(10):F945-F957. [DOI] [PubMed] [Google Scholar]
- 149. Clotet-Freixas S, Soler MJ, Palau V, et al. Sex dimorphism in ANGII-mediated crosstalk between ACE2 and ACE in diabetic nephropathy. Lab Invest. 2018;98(9):1237-1249. [DOI] [PubMed] [Google Scholar]
- 150. Berger TG, Steinhoff M. Pruritus and renal failure. Semin Cutan Med Surg. 2011;30:99-100. [DOI] [PMC free article] [PubMed] [Google Scholar]