Abstract
Guided by its sight, scent, texture, and taste, animals ingest food. Once ingested, it is up to the gut to make sense of the food’s nutritional value. Classic sensory systems rely on neuroepithelial circuits to convert stimuli into signals that guide behavior. However, sensation of the gut milieu was thought to be mediated only by the passive release of hormones until the discovery of synapses in enteroendocrine cells. These are gut sensory epithelial cells, and those that form synapses are referred as neuropod cells. Neuropod cells provide the foundation for the gut to transduce sensory signals from the intestinal milieu to the brain through fast neurotransmission onto neurons, including those of the vagus nerve. These findings have sparked a new field of exploration in sensory neurobiology—that of gut-brain sensory transduction.
Keywords: neuropod cells, enteroendocrine cells, vagus nerve, sensory transduction, glutamatergic transmission, gut-brain biology
What do we mean by life?
Firstly, a living thing moves about… It moves in response to an inner impulse. It may be stimulated to move, but the driving-force is within…
And not only does it move of itself, but it feeds. It takes up matter from without itself, it changes that matter chemically, and from these changes it gathers the energy for movement.
—H.G. Wells, Julian S. Huxley, and G.P. Wells, “The Science of Life”
INTRODUCTION
By feeding, animals gather not only the energy to thrive but the motivation to transcend. Animals rely on their senses to find, assess, consume, and recall food. And once ingested, it is up to the gut to make sense of the nutritional value of the meal. This point of conversion takes place at the epithelial wall: the place where a given stimulus (e.g., force, temperature, nutrient) is transduced into a electrochemical signal.
The topic of how nutrients are converted by the gut into signals that influence the brain has been discussed from an endocrine perspective since 1902. [The concept of epithelial cell-specific postingestive sensing came about in the early twentieth century when Bayliss & Starling (1902) identified that a gut hormone, secretin, could be secreted following the presentation of luminal acid.] Other landmark review articles have dealt extensively with the traditional aspects of gut endocrinology (Chaudhri et al. 2008, Drucker & Yusta 2014, Gribble & Reimann 2016), and as such, hormones are not the main subject of this text. Instead, this review focuses on recent discoveries that have uncovered the receptors, cells, and neural circuits through which the gut epithelium transduces such stimuli so the brain can guide behavior.
The focus of this review is on gut sensory epithelial cells capable of synapsing with nerves. Although gut sensory epithelial cells include enteroendocrine cells, the term neuropod cell was coined in 2018 to distinguish those that are capable of forming synapses (Kaelberer et al. 2018). Neuropod cells were first uncovered when Bohróquez et al. (2015) discovered that enteroendocrine cells form synapses with nerves in the mucosa of the murine small intestine and colon. The existence of these synapses has since been confirmed by other studies (Bellono et al. 2017, Lu et al. 2019). In 2018, Kaelberer et al. (2018) revealed that neuropod cells synapse with neurons of the vagal nodose to transduce a sense from gut to brain. They do so in milliseconds, using glutamate as a neurotransmitter. This discovery sparked a new area of exploration in sensory neurobiology: the field of gut-brain sensory transduction.
Although several mechanisms for luminal sensing have been described in enteroendocrine cells, the topic of neurotransmission is only beginning to emerge, as such scientific literature is limited. When possible, this text cites from other fields of sensory neurotransmission where details are more abundant. Here, the subject is covered in a linear fashion, starting with how a stimulus is recognized at the intestinal lumen to elicit a signal in the brain that modulates a defined behavior.
SENSING AND STIMULI
In the gastrointestinal tract, sensory stimuli of ingested material begin in the oral cavity and continue throughout the length of the gastrointestinal tract. The idea that the gastrointestinal tract contains specialized areas to sense ingested material was postulated by histologists as early as the 1860s. Schwalbe (1867) and Lovén (1868) observed a clustering of cells on the epithelial surface of the tongue, and Heidenhain (1870) extended these observations to the intestinal epithelium and identified a group of yellow chromate-staining cells. Bayliss & Starling (1902) subsequently found the first signaling molecule, the hormone secretin, and Feyrter (1938) gave rise to the concept of gut endocrinology. But it was not until the late 1990s that the mechanisms of nutrient sensing began to be documented. The discovery of taste receptors in the small intestine (Hofer et al. 1996) sparked interest in both the significance of these receptors following ingestion as well as the similarities between oral sensors and sensors in the gastrointestinal tract. These receptors highlight the role of gut epithelial sensors in nutrient sensing.
Defining Gut Epithelial Sensors
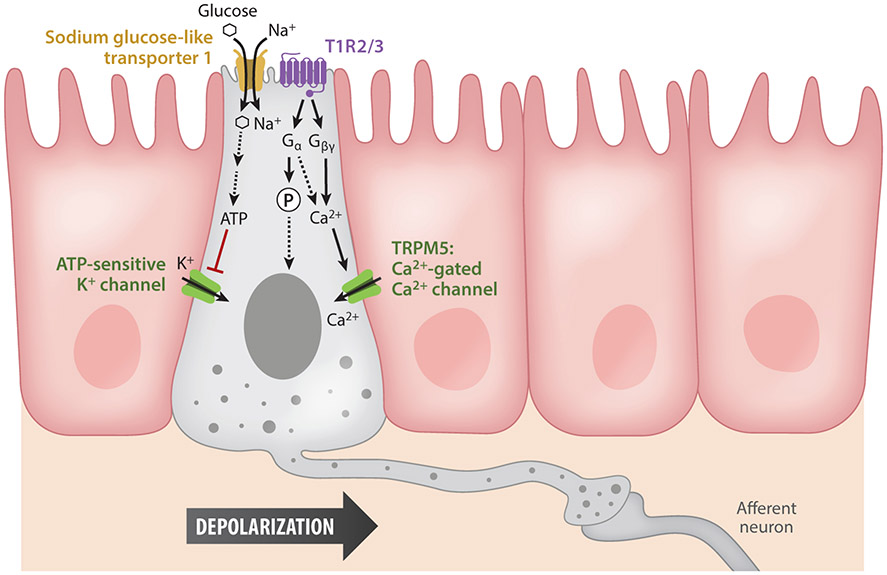
A gut sensory epithelial cell, or for that matter a sensory epithelial cell, is a cell type capable of eliciting electrical activity in response to an external stimulus (Kandel et al. 2000). For cells to be classified as sensors, they must have molecular receptors to sense or detect an input (e.g., nutrients, bacteria) that, once activated, triggers an amplifying intracellular signaling cascade, resulting in a secreted signaling molecule (e.g., neurotransmitter). The most well-studied molecular sensors are the G protein–coupled receptors (GPCRs). A well-known nutrient-sensing class of GPCRs is the mammalian sweet and umami taste T1R receptor family, members of which are found throughout the gastrointestinal epithelium. Sweet and umami tastes have been linked to T1R2/3 and T1R1/3 heterodimers, respectively, and these GPCRs act as functional sensors based on studies of the downstream effector pathways. Binding of taste receptors activates a signaling cascade that ends in the release of intracellular Ca (Margolskee 2002). In both taste cells and enteroendocrine cells, elevated cytoplasmic Ca levels activate the transient receptor potential channel M5 (TRPM5) to trigger membrane depolarization and the additional influx of Ca from voltage-gated channels (Depoortere 2014, Kokrashvili et al. 2009). Stimuli can also be sensed by transporters or channels. Sensing absorbed nutrients can occur either at the site of transport or during subsequent metabolism. Absorption of nutrients is frequently coupled with the uptake of ions, which generates a small depolarizing current, allowing the enteroendocrine cell to sense the nutrient (Figure 1).
Figure 1.
Molecular pathways of activation in neuropod cells. A nutrient such as glucose is sensed by neuropod cells in two ways. First, through substrate Na+ transporters, specifically Na+ glucose-like transporter 1, the entry of Na+ depolarizes the cells, leading to vesicle release and the activation of synaptically connected afferent neurons. Glucose is also metabolized, producing ATP that closes on ATP-sensitive K+ channels and further depolarizes the cell. Second, neuropod cells also express the sweet taste receptor T1R2/3, a G protein–coupled receptor. Activated G proteins either phosphorylate transcription factors or release intracellular Ca2+, which activates transient receptor potential channel M5 (TRPM5), the Ca2+-activated Ca2+ channel. The intracellular Ca2+ cascade induces vesicle fusion and the further activation of afferent neurons.
Nutrient Sensors
A meal as simple as an apple is made of a complex arrangement of molecules. Once ingested and digested, individuals macro- and micronutrients, fibers, water, and other molecules form the chyme propelled through the intestine while being absorbed. It is increasingly evident that the gut epithelium has evolved an equally complex array of receptors and transporters to detect specific details about individual molecules. For example, in the case of nutrients, gut sensory epithelial cells can distinguish not only the type of nutrient but also its nutritional value. After all, it is the caloric content of nutrients, at least for sugars, that gives rise to a strong pleasurable outcome, even in the absence of taste (de Araujo et al. 2008).
Sugar.
Digested sugars entering the small intestine trigger an increase in intracellular Ca activity in gut endocrine cells (Reimann et al. 2008). This activity is due to sensing through two different molecular receptors: T1R2/3 and the Na+ glucose-like transporter 1 (SGLT1). Enteroendocrine cells are known to express taste receptors, particularly sweet taste receptors T1R2 and T1R3 (Jang et al. 2007, Reimann et al. 2008). T1R2/3 activation occurs through the GPCR activation pathway, whereas SGLT1 is an active transporter. This active transport system at the apical membrane of enteroendocrine cells transports one molecule of Na+ for one molecule of glucose. The sensing of glucose may also occur following its metabolism via the enzyme glucokinase. Glucokinase couples with ATP-sensitive potassium channels (KATP). Glucose metabolism leads to an increase in ATP/ADP, which causes the closure of KATP channels, leading to cell depolarization. Depolarization then induces voltage-gated Ca channels to open, triggering vesicle fusion and release (Sakura et al. 1998). Thus, both binding of sugar to T1R2/3 and its transport through SGLT1 cause enteroendocrine cell activation in response to sugar.
Lipids.
Most lipids are hydrolyzed into fatty acids and monoacylglycerols in the small intestine. The apical fatty acid translocase, CD36, has been proposed as a sensor of fatty acids. Global CD36 knockout mice demonstrate impaired hormone secretion (Sundaresan et al. 2013). In taste receptor cells, fat translocation through CD36 activates a phospholipase Cβ pathway to mediate intracellular Ca signaling (Sundaresan & Abumrad 2015). This mechanism is thought to function similarly in gut sensory epithelial cells. GPR119 has been identified as a molecular sensor for the long-chain fatty acid monoacylglycerol. In enteroendocrine cells, studies show an enrichment of the GPR119 receptor as well as a link between GPR119 activation and vesicle release of neuropeptides such as glucagon-like peptide 1 and glucagon inhibitory peptide (Engelstoft et al. 2013, Gribble & Reimann 2016). In addition to GPR119, intestinal enteroendocrine cells express FFAR1 and FFAR4, which are GPCRs coupled to a Gαq subunit (Liou et al. 2011b). However, knockout experiments have been unable to abolish long-chain fatty acid-induced incretin secretion in enteroendocrine cells, implying that there are multiple pathways for lipid sensing. Notably, CD36 is much more highly expressed in the proximal small intestine compared to the FFARs, which are more distally located, indicating different lipid-sensing mechanisms along the length of the intestinal tract.
Protein.
Varying degrees of digested protein products are present throughout the lumen of the intestine. Enteroendocrine cells sense both oligopeptides and individual amino acids. Studies using enteroendocrine cell lines have shown that CaSR, GPRC6A, and LPR5 are general protein sensors that induce the secretion of peptides (e.g., cholecystokinin, glucagon-like peptide 1, serotonin, or peptide YY) following receptor binding (Gribble & Reimann 2016, Kokrashvili et al. 2009, Santos-Hernandez et al. 2018, Symonds et al. 2015). Two GPCRs have been identified as being more selective to the specific amino acid l-glutamic acid: mGluR4s and the T1R1/T1R3 heterodimer. mGluR4 is most highly expressed in the proximal colon, whereas the T1R1/T1R3 heterodimer is most prevalent in the ileum. T1R1/T1R3 has also been shown to recognize other individual amino acids, suggesting that it could serve as a more generalized amino acid sensor (Daly et al. 2013, Nelson et al. 2002). However, these studies are limited in their approach, as they occur exclusively in cell lines.
Like glucose transport, amino acid and peptide transporters are also sensors. SNAT2 is a neutral l-amino acid transporter that requires the cotransport of Na+. to elicit Ca activity in the enteroendocrine cell line (STC-1 cells) (Young et al. 2010). PEPT1 is a transporter of dipeptides and tripeptides. When STC-1 cells were stimulated by peptone, PEPT1 elicited downstream phosphorylation pathways (Liou et al. 2011a). B0AT also acts as a neutral amino acid sensor, as suggested by studies in which l-glutamine stimulated the enteroendocrine cell line GLUTag (Gribble & Reimann 2016). The wide diversity of amino acids implicates the existence of a number of amino acid transporters that still need to be identified in gut sensory epithelial cells.
Mechanical Sensors
Besides nutrients, gut sensory epithelial cells also sense mechanical forces due to stretch. As early as the 1950s, it was described that a mechanical stimulus applied to the intestinal lumen elicits the release of serotonin (Bulbring & Crema 1959). Indeed, serotonin release promotes gut motility (Heredia et al. 2009) and the secretion of fluids into the lumen (Sidhu & Cooke 1995). The subset of enteroendocrine cells that release serotonin are called enterochromaffin cells. When mechanically stimulated, enterochromaffin cells release serotonin (Chin et al. 2012). The receptor that senses mechanical stimuli is Piezo2 (Wang et al. 2017), and genetic ablation of Piezo2 impairs the mechanosensitivity of enterochromaffin cells (Alcaino et al. 2018).
Bacterial Sensors
In addition to nutrients and mechanical stretch, gut sensory epithelial cells must also survey the resident microbiome. The microbial pattern recognition receptors, Toll-like receptors (TLR-1, 2, 4, 5, and 9), directly sense multiple bacterial components, including peptidoglycan, LPS, flagellin, and CpG. Applying such stimuli on STC-1 cells results in cholecystokinin release and increased activity of NF-κB, TNF, and TGFα expression (Bogunovic et al. 2007, Palazzo et al. 2007). Indirect sensing of the microbiome can also occur through microbial metabolites. Short-chain fatty acids (SCFAs) are some of the most-studied byproducts of microbial metabolism. SCFAs are sensed by gut sensory epithelial cells through several different GPCRs, including OLF78, OLF558, FFAR2 (GPR43), and FFAR3 (GPR41) (Lund et al. 2018). In mice, SCFAs increase glucagon-like peptide 1 and peptide YY plasma blood levels. This effect is abolished when FFAR2 or FFAR3 is genetically ablated in mice (Psichas et al. 2015). Furthermore, SCFA application increases the number of enteroendocrine cells in intestinal organoids. Of interest, these enteroendocrine cells that are capable of sensing microbial metabolites lack the expression of GPCR sensors for macronutrients, suggesting that this class of enteroendocrine cells may be specialized for mechanical and microbial sensing.
TRANSDUCTION AND TRANSMISSION
Neuropeptide and Neurotransmitter Storage in Secretory Vesicles
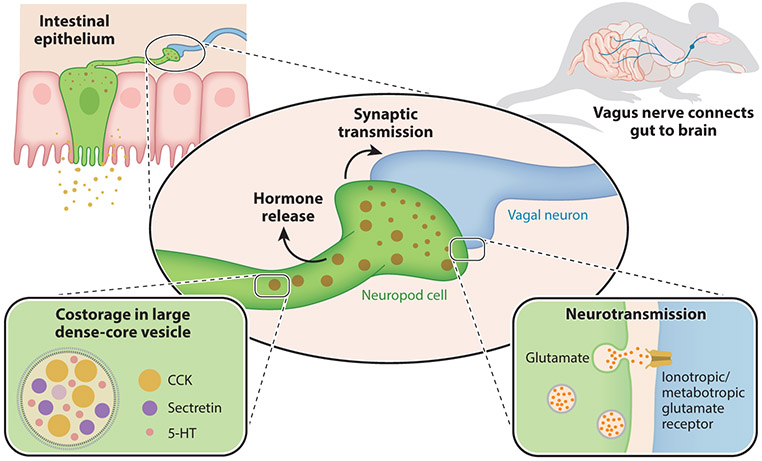
For decades, enteroendocrine cells were thought to contain only one hormone or neuropeptide per cell, indicating that a given stimulus would only cause the release of a single neuropeptide. As technology advanced, so did the ability to resolve what transcripts are expressed at a single-cell level. In 2017, a single-cell RNA sequencing survey analyzed the transcriptome of 533 individual enteroendocrine cells and found that these cells were transcriptionally diverse (Haber et al. 2017). A single cell contains multiple neuropeptide transcripts. In fact, a single cell can contain transcripts for both satiety- and hunger-inducing hormones (Glass et al. 2017). At a subcellular level, these neuropeptides can be co-stored in single vesicles (Cho et al. 2014, Fothergill et al. 2017).
For instance, glucagon-like peptide 1 released from neuropod cells was thought to act on neurons of the nodose ganglia expressing the glucagon-like peptide 1 receptor. However, glucagonlike peptide 1 alone is not sufficient to elicit a vagal response; it requires the presence of ATP (Richards et al. 2014, Williams et al. 2016). ATP is co-stored, along with glucagon-like peptide 1, in vesicles of neuropod cells. Stimulation of these neuropod cells leads to the co-release of both a hormone (glucagon-like peptide 1) and a neurotransmitter (ATP) that then stimulate activity in afferent neurons (Lu et al. 2019). Enterochromaffin cells are associated with the modulation of gut motility via the local release of serotonin. In addition to sensing mechanical stimuli, enterochromaffin cells sense irritants. Irritants elicit the release of synaptic serotonin onto dorsal root ganglion sensory neurons of the spinal cord (Bellono et al. 2017).
Recent findings show that neuropod cells release synaptic glutamate in response to a sugar stimulus. This finding, along with those on serotonin and ATP, has uncovered the possibility that gut sensory epithelial cells use distinct secretory vesicles to store a rich array of signaling molecules (Kaelberer et al. 2018) (Figure 2). When combined, these molecules could serve to transduce distinct properties of the stimuli such as nutritional value, mechanical distension, osmolarity, pH, or temperature.
Figure 2.
Glutamatergic synaptic transmission of neuropod cells. Neuropod cells in the intestinal epithelium contain both large dense-core neuropeptide vesicles and small neurotransmitter vesicles. The large vesicles contain multiple neuropeptides with endocrine functions such as cholecystokinin (CCK), secretin, and serotonin (5-HT) and are co-released with neurotransmitters. Activation of neuropod cells stimulates synaptic vesicle release, including the neurotransmitter glutamate. When this fast neurotransmission acts on afferent vagal neurons, it serves to transduce signals from nutrients directly to the brain in milliseconds. Mouse image adapted from Kaelberer et al. (2018).
Gut Epithelial Cell Innervation
The gut is innervated by several types of extrinsic sensory neurons, which convey information about stomach volume and intestinal contents (Brookes et al. 2013). There have been previous attempts to document the innervation of gut epithelial sensor cells using techniques such as electron microscopy. These studies from the 1970s were unfortunately not successful, partially due to the special organization of enteroendocrine cells, which are sparsely distributed throughout the epithelium. Lundberg et al. (1978) reported one micrograph in which the closest neuron was 100 nm away from an enterochromaffin cell. The second report did not have the resolution to state definitively that there was a synapse, and the authors therefore merely suspected that it was a synapse (Newson et al. 1979). These studies were struggling against the limitations of electron microscopy, in which there is a limited field of view and poor z resolution. Therefore, it was impractical to screen over multiple cells across a large area of epithelium. As the techniques and the proliferation of transgenic mice have advanced, there has been mounting evidence that enteroendocrine cells do make contacts with neurons. Using a monosynaptic rabies virus, Bohórquez et al. (2015) discovered that colonic enteroendocrine cells synapse neurons. In fact, these enteroendocrine cells have both pre- and postsynaptic proteins, suggesting that they could not only send signals via synapses but also receive synaptic inputs from neurons. Then Bellono et al. (2017), using immunohistochemistry, showed that enterochromaffin cells of the small intestine have presynaptic proteins and are adjacent to nerve terminals with postsynaptic proteins. More recently, it was discovered that enteroendocrine cells synapse with neurons largely originated from both the spinal cord dorsal root ganglia and the vagal nodose ganglia (Kaelberer et al. 2018). The nodose ganglia are the sensory ganglia of the vagus nerve and connect the viscera (i.e., heart, lungs, gut, etc.) with the brain.
Nutrient Sensory Transduction in the Gut
Paracrine and endocrine actions of hormones are characterized by effects that are detectable minutes to hours after food is ingested. However, studies using fiber photometry Ca activity recordings have shown that hunger neurons in the hypothalamus are inhibited within seconds of nutrients like sugar entering the intestine (Beutler et al. 2017). This highlights the need for the fast synaptic transmission of nutrients to the brain. Although slower hormonal signaling is able to maintain a lasting state of satiety, the faster synaptic transmission of nutrients through the vagus nerve is likely to be signaling the reward of food (Han et al. 2018). Perfusion of sucrose into the intestinal lumen elicits a fast and sustained electrical response. Pharmacological blocking of glutamate neurotransmission ablates the fast onset but does not affect the sustained electrical response, whereas blocking the hormonal transmission ablates the sustained electrical response while the rapid response remains intact (Kaelberer et al. 2018). This bimodal vagal response indicates that the brain is constantly monitoring the luminal contents of the intestine. It does so by using the ability of neuropod cells to engage in fast synaptic neurotransmission as well as slow but sustained hormonal signaling.
Microbial Interactions with Gut Sensory Epithelial Cells
The role of the microbiome in maintaining a healthy central nervous system has been extensively reviewed (Dinan & Cryan 2017). Germ-free mice, devoid of gut microbiota, have a host of neurological deficits (Diaz Heijtz et al. 2011). Interestingly, these symptoms are also mirrored by changes in enteroendocrine cells. Germ-free mice have decreased numbers of chromogranin A–positive enteroendocrine cells in the ileum and increased numbers in the colon (Duca et al. 2012). In addition, the receptor profile of the epithelial layers shifts to decreased numbers of FFAR2 and FFAR3 but increased expression of glucose transporters and taste receptors (Swartz et al. 2012). These studies highlight the interconnected nature of gut sensory function and the microbiome in a healthy animal.
Enteroendocrine cells may sense and interact with microbes in three critical ways: Microbes (a) secrete bacterial ligands, including microbe-associated molecular patterns (MAMPs); (b) interact with luminal nutritional content and release metabolites; and (c) directly interact or infect enteroendocrine cells.
Bacteria constitutively release MAMPs such as lipopolysaccharide (LPS) or flagellin. These patterns have been studied primarily in the context of pathogenic bacterial detection by the immune system, but there has been recent appreciation for the roles of tolerance and commensal monitoring. Both mouse and human enteroendocrine cells have been shown to possess receptors for these molecular patterns, including TLRs (Bogunovic et al. 2007). In mouse enteroendocrine cell lines, application of MAMPs such as LPS activates immune factors such as NF-κB and MAPK (Bogunovic et al. 2007). Moreover, MAMP application increased peptide YY expression in a human cecal cell line in an NF-κB-dependent manner (Larraufie et al. 2017). In vivo, LPS gavage induces activation of enteroendocrine cells to release glucagon-like peptide 1 through a TLR-4-dependent manner (Lebrun et al. 2017). Although MAMPs may cross the epithelial barrier, it is likely that in a healthy animal, enteroendocrine cells—specifically synaptically connected neuropod cells—are detecting signals from the microbiota and relaying the information to the brain.
Gut bacteria also generate a range of metabolites, including SCFAs, bile acids, phenols, indoles, bioactive lipids, and neurotransmitters (Cohen et al. 2017). Most of these compounds act at GPCRs that are known to be expressed in enteroendocrine cells. For example, when SCFA precursors are introduced to the gut lumen, the number of peptide YY enteroendocrine cells that express FFAR2 increases (Kaji et al. 2011). Perfusion of the SCFA butyrate induces colonic motility that is dependent on serotonin signaling from enteroendocrine cells onto vagal afferents (Fukumoto et al. 2003). In addition, the related fatty acid fermentation product isovalerate activates serotonin-expressing neuropod cells to synaptically transmit information onto mucosal afferents (Bellono et al. 2017). Other classes of enteroendocrine cells that coexpress glucagon-like peptide 1 and peptide YY have been shown to be activated by indole (Chimerel et al. 2014) and bile acids (Thomas et al. 2009) and to co-release glucagon-like peptide 1 and ATP (Lu et al. 2019). These studies confirm that neuropod cells play a fundamental role in sensing bacterial metabolites and will likely serve as a future target for manipulating metabolite-mediated behaviors.
Lastly, there are certain pathogenic bacteria that penetrate the mucous layer to directly contact the epithelium. Chlamydia trachomatics, for example, directly infects human enteroendocrine cells (Dlugosz et al. 2011). Intriguingly, upon infection, human enteroendocrine cells upregulate a variety of neurotransmitter transporters, including those for glutamate and GABA (Dlugosz et al. 2014), suggesting that the infected cells are neuropod cells and that infection may target synaptic transmission. Helminth infections, such as Trichinella spiralis, lead to hypophagia, which depends on the presence and number of enteroendocrine cells (Worthington et al. 2013). These studies raise the possibility that direct pathogenic infection of neuropod cells serves as a mechanism for pathogens to gain access to the central nervous system and drive behavior. Moreover, other noninvasive gut microbes affect the central nervous system when delivered intraluminally, and their effects depend on an intact vagus nerve (Bravo et al. 2011, Sgritta et al. 2019). Indeed, some microbes are even moving forward into human trials (Sanchez et al. 2017). However, the mechanism for how microbes interact with the vagus nerve and how this can be used to influence brain behavior remain to be documented.
THE VAGUS NERVE AND THE GUT
The vagus nerve is distributed throughout the body, spanning the brain to viscera. From the brainstem, the vagus nerve travels down the esophagus and branches—wandering—to its visceral organ targets throughout the body. In 1845, Ernst Wilhelm Weber and Edward Weber discovered that vagal stimulation inhibits the heartbeat. By 1875, it was known that the gut and brain are functionally linked by the vagus nerve. Pavlov’s (1910) seminal work in the early 1900s established the vagus nerve as essential for the control of the cephalic phase of gastric acid secretion. Much of the gut-vagus-brain connection remains elusive, and work in this field relied on the early discoveries of anatomists and physiologists until recently, as there has been a surge in technologies and a renewed interest in and new perspective on the role of gut innervation by the vagus nerve.
Vagus Nerve Anatomy
The vagus nerves originates in the medulla. The left and right vagi are composed of efferent and afferent rootlets, which exit the cranium via the jugular foramen, between the temporal and occipital bones (Berthoud & Neuhuber 2000). The cervical vagus nerve runs laterally along the carotid arteries. The left and right vagus pass through the diaphragm along the esophagus. At this point, the left vagus nerve is referred to as the ventral or anterior trunk, and the right vagus nerve is referred to as the dorsal or posterior trunk. As noted by Prechtl & Powley (1990), the vagus nerves are asymmetric, much like the visceral organs they innervate. Most of the dorsal trunk fibers travel to the celiac branch, although a subset travels to the dorsal side of the stomach. The ventral trunk branches to the common hepatic, ventral gastric, and accessory celiac branches to innervate the pylorus, antrum, pancreas, and proximal duodenum.
The sensory afferent fibers travel to the nodose ganglia, which contain the pseudounipolar cell bodies, residing at the base of the skull. The nodose ganglia of the mouse contain approximately 2,300 neurons (Ichikawa et al. 2006). A recent single-cell RNA sequencing survey of the nodose ganglia revealed specialized populations of vagal nodose neurons specifically poised for chemosensation, nutrient detection, and mechanosensation (Kupari et al. 2019).
Vagus Nerve Response to Consumption
The vagus nerve is stimulated by eating. Vagal afferents are responsive to meal-derived factors such as mechanical stretch, nutrients, and meal-stimulated neuropeptides. Stimulation of the upper gastrointestinal tract (stomach and duodenum) potently suppresses food intake, which occurs through reduced meal size. Thus, food entering the gastrointestinal tract has traditionally been thought to limit intake and meal size. Vagotomized rats were reported to have decreased food intake (Mordes et al. 1977). Consistent with this, vagus nerve stimulation is thought to reduce food intake, although the vagus nerve has also been shown to increase feeding (Rezek et al. 1975). Individual vagal afferents can be classified by their function and innervation patterns, for example, by mechanosensation or chemosensation. The vagal responses to aspects of feeding are discussed individually below. However, many fibers are polymodal and can respond to an array of gut-derived signals, and this should also be considered.
Stretch.
Paintal (1953) performed electrophysiological experiments in cats and determined that most of the vagal terminals of the stomach end primarily in stretch mechanoreceptors. Using a balloon to distend the stomach, he found a linear relationship to the degree of stomach stretch and the frequency of vagal firing. From these results, he reasoned that distention-elicited vagal firing must cause satiation, and therefore the opposite must be true: The lack of stretch-induced vagal firing must result in hunger, generating the hypothesis that the absence of stomach distension explains hunger pangs. Paintal’s findings have been replicated and expanded. In the rat, gastric loads increase vagal firing (Davison & Clarke 1988) and load-dependently increase vagal firing (Schwartz et al. 1991b). These gastric mechanosensitive vagal afferents do not encode information about the nutrient content of the gastric load (Schwartz et al. 1991a). Recent data have suggested that vagal afferent neurons that are positive for glucagon-like peptide 1 receptor are uniquely responsive to stretch but not to nutrients. Gastric loads suppress meal size, which depends on vagal afferent signaling. In rats equipped with pyloric cuffs, which restrict volume to the stomach, gastric preloads suppressed meal size in a volume-dependent manner (Phillips & Powley 1998). Surgical transection of the hepatic and gastric branches of the vagus nerve attenuated the ability of the gastric preload to suppress meal size. There have been additional reports of duodenal distension activating vagal afferent nerve activity (Schwartz et al. 1995). Together, these data demonstrate that stomach stretch suppresses meal size via vagal afferents.
Nutrients and food intake.
The presence of nutrients in the upper gastrointestinal tract potently and dose-dependently suppresses meal size. Intraduodenal nutrient delivery suppresses meal size in rats in which the food entering the stomach is bypassed using a gastric fistula, called sham feeding (Greenberg et al. 1990). This nutrient-induced suppression of sham feeding is attenuated by vagotomy (Walls et al. 1995, Yox et al. 1991). As the vagal afferents do not directly contact the lumen of the gut, hormones and neurotransmitters relay the status of the luminal contents onto the vagus nerve. Indeed, nutrients stimulate afferent vagal activity (Williams et al. 2016). The presence of sugar in the duodenum increases cervical vagal firing rate, which depends on cholecystokinin and glutamate (Kaelberer et al. 2018). Studies have reported that different vagal fibers are specifically tuned for each macronutrient (Jeanningros 1982, Lal et al. 2001, Mei 1978). The vagal afferents of the more distal gut, which experiences the presence of fat more than sugar, are sensitive to the presence of fat in the ileum and jejunum. Multiunit recordings of the celiac and the cervical vagus nerve have demonstrated increased firing rate following ileal and jejunal infusion of fatty acids (Randich et al. 2000). The presence of nutrients in the gut stimulates vagal activity and suppresses meal size. Thus, the gut-vagus-brain axis has been understood in terms of its negative-feedback control over food intake.
In contrast with negative-feedback control, food in the gut is reinforcing, can condition food preferences, and can stimulate appetite. The role of gut-vagal signaling in this process is unclear. Vagal deafferentation failed to block gut-stimulated conditioning of a neutral sweet flavor (Sclafani et al. 2003). Therefore, it was assumed that hormonal signals, and not vagal signaling, were responsible for gut-induced reward. However, vagal deafferentation can be an incomplete procedure, and vagal neurons can regenerate within a few days. Moreover, it was recently demonstrated that conditioned preference for lipids and amino acids, but not sugars, is specifically disrupted by vagal deafferentation. New precise opto- and chemogenetic approaches should overcome these limitations to uncover the contributions of specific types of vagal neurons to such behaviors.
BRAIN AND BEHAVIOR
Nucleus Tractus Solitarius
The vagal afferents terminate in the brainstem structure identified as the nucleus tractus solitarius (NTS). The study of the brain terminal fields of the vagus nerve has relied on the use of anterograde tracing techniques. Norgren & Smith (1988) described the afferent terminals of the subdiaphragmatic vagus nerve by exposing the nerve and its branches to horseradish peroxidase and evaluating for anterograde transport (Norgren & Smith 1988). The branches of the gutinnervating vagus nerve have distinct projection patterns. The gastric branches terminate in the lateral NTS. Projections from the small intestine terminate in the medial NTS (Zhang et al. 1992). Conversely, the distal small intestine and cecum are innervated by vagal afferents, which project terminals more rostrally in the NTS, in the commissural subnucleus (Altschuler et al. 1991, Zhang et al. 1992).
The relationship between vagal terminals and nutrient stimuli has been studied by using the expression of c-Fos, an immediate early gene that is used as a marker of neuronal activation. Intraduodenal infusions of macronutrients result in significant c-Fos expression in the area postrema (AP) and in the medial, dorsal, and rostral commissural regions of the NTS (Monnikes et al. 1997, Phifer & Berthoud 1998, Zittel et al. 1994). In addition to stretch and nutrients, satiety signals activate NTS neurons. For example, exogenous cholecystokinin administration at doses that suppress meal size activates neurons in the AP and medial NTS (Fraser & Davison 1992, Rinaman et al. 1998). This activation depends on the vagus nerve, as c-Fos activation is nearly absent in these regions in rats with vagal lesions.
Upstream of the NTS, vagal tracing and stimulation experiments identify cells receiving gastrointestinal sensory signals in multiple brain regions, including the pontine reticular formation, cerebellum, parabrachial nucleus, lateral hypothalamus, central amygdala, and bed nucleus of the stria terminalis. The labeling is absent when the vagus is severed (Cunningham et al. 2008). Vagal afferents arising in the intestine also transsynaptically project to the dorsal hippocampus (Suarez et al. 2018) and prefrontal cortex (Klarer et al. 2014). Recent reports also suggest that gut vagal afferents project asymmetrically into brain areas. Using a combinatorial viral approach, Han et al. (2018) showed that terminals from the right nodose ganglion project to the ventromedial NTS, whereas those of the left nodose ganglion end in the AP. Thus, the gut-specific left and right nodose ganglia terminate in regionally distinct patterns in the brainstem. The fact that the vagus connects sensory signals arising in the intestinal milieu to multiple brain areas should not surprise anyone, since not only does an animal move of itself, but it feeds. It does so to fulfill this internal motivation guided by the entire brain.
Beyond Food: Reward, Mood, and Memory
Gut feelings are more than a sense of satiety or hunger. The gut vagal afferents can influence reward, mood, and memory.
Reward.
The role of the gut in reward emerged in the 1970s. Phillips & Nikaido (1975) showed that stimulating the LH, a brain area involved in reward, induces voracious feeding, an effect that is abolished when the vagus nerve is severed (Ball 1974). The most common mechanism of reward involves extracellular release of dopamine in the dorsal striatum (de Araujo et al. 2008). Although it was well established that nutrients in the gut are rewarding, it was only recently established that gut vagal neurons are sufficient to induce reward. The gold standard for identifying neurons involved in reward processes is self-stimulation. Using optogenetics, Han et al. (2018) showed that when mice were allowed to nose poke for light stimulation of gut-specific right nodose ganglion neurons, the animals robustly self-stimulated. This result was supported by additional real-time place preference tests in which the optostimulation of the pathway was dependent on the mouse’s location. Mice preferred the areas that caused stimulation over those without stimulation. Stimulation of gut-specific right vagal projections also conditioned a preference for noncaloric flavor. These results demonstrate that right nodose neurons link the viscera to the previously mapped reward neurons in the brain.
Mood.
Gut vagal afferents can also regulate mood. In 1922, the James-Lange theory postulated that emotions are a perceptual representation of the sensory interoceptive state (Lange & James 1922). Indeed, vagal nerve stimulation is used as a treatment for patients with major depression disorder that is resistant to approved pharmacological treatments (Craig 2005). Gut vagal afferents also modulate anxiety and fear in rodents. Rats with vagal deafferentation had reduced anxiety and increased learned fear responses, indicating that these processes are, at least in part, dependent upon gut vagal afferents (Klarer et al. 2014). Thus, it is not surprising that gut microbes influence mood in a vagal-dependent manner.
Memory.
Vagal nerve stimulation is also known to enhance memory (Clark et al. 1995, 1998) and improve working memory performance (Sun et al. 2017). Rats with lesions to the hippocampus, a brain region associated with memory, have impaired memory for food-related cues (Davidson et al. 2010). Vagal afferents arising in the gut are necessary for hippocampal-dependent episodic and spatial memory. Ablating these afferents impairs memory and decreases neurogenesis in the hippocampus (Suarez et al. 2018). Conversely, vagal nerve stimulation increases plasticity, neurogenesis (Follesa et al. 2007), and long-term potentiation in the hippocampus (Zuo et al. 2007).
CLOSING PERSPECTIVE
Not long ago, the gut was regarded more for its products of digestion than for its contributions to the body. But a rapid ascent of molecular technologies, particularly for the study of neural circuits, are allowing us to document the molecules, cells, and circuits that convert stimuli from nutrients into signals that guide behavior. The discovery of synapses in enteroendocrine cells and the neural circuits they form has opened the possibility of understanding how the gut makes sense of complex stimuli in the luminal milieu to guide brain behaviors in real time. Elucidating the circuitry, transmitters, and computing code by which luminal stimuli are conveyed from specific regions of the intestine or colon to distinctive areas of the brain that drive behaviors remains a priority. These paths are likely to contain possibilities of using specific foods, microbes, or other stimuli yet to be designed to treat the brain from the gut.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Elaine B. Bohorquez for her constructive feedback. Funding support was provided by the Duke-NUS 2019 pilot project and National Institutes of Health grants R21AT010818 and DP2 MH122402.
Footnotes
DISCLOSURE STATEMENT
Dr. Bohórquez provides services for the scientific advisory board of Holobiome Inc.
LITERATURE CITED
- Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, et al. 2018. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. PNAS 115:E7632–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler SM, Ferenci DA, Lynn RB, Miselis RR. 1991. Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural subnucleus of the nucleus tractus solitarii in rat. J. Comp. Neurol 304:261–74 [DOI] [PubMed] [Google Scholar]
- Ball GG. 1974. Vagotomy: effect on electrically elicited eating and self-stimulation in the lateral hypothalamus. Science 184:484–85 [DOI] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. 1902. The mechanism of pancreatic secretion. J. Physiol 28:325–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, et al. 2017. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170:185–98.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. 2000. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci 85:1–17 [DOI] [PubMed] [Google Scholar]
- Beutler LR, Chen Y, Ahn JS, Lin YC, Essner RA, Knight ZA. 2017. Dynamics of gut-brain communication underlying hunger. Neuron 96:461–75.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Dave SH, Tilstra JS, Chang DT, Harpaz N, et al. 2007. Enteroendocrine cells express functional Toll-like receptors. Am.J. Physiol. Gastrointest. Liver Physiol 292:G1770–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, et al. 2015. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig 125:782–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, et al. 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. PNAS 108:16050–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. 2013. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol 10:286–96 [DOI] [PubMed] [Google Scholar]
- Bulbring E, Crema A. 1959. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J. Physiol 146:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri OB, Salem V, Murphy KG, Bloom SR. 2008. Gastrointestinal satiety signals. Annu. Rev. Physiol 70:239–55 [DOI] [PubMed] [Google Scholar]
- Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. 2014. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 9:1202–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A, Svejda B, Gustafsson BI, Granlund AB, Sandvik AK, et al. 2012. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am. J. Physiol. Gastrointest. Liver Physiol 302:G397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Robinson ES, Rivera LR, McMillan PJ, Testro A, et al. 2014. Glucagon-like peptide 1 and peptide YY are in separate storage organelles in enteroendocrine cells. Cell Tissue Res. 357:63–69 [DOI] [PubMed] [Google Scholar]
- Clark KB, Krahl SE, Smith DC, Jensen RA. 1995. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol. Learn. Mem 63:213–16 [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. 1998. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol. Learn. Mem 70:364–73 [DOI] [PubMed] [Google Scholar]
- Cohen LJ, Esterhazy D, Kim SH, Lemetre C, Aguilar RR, et al. 2017. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 549:48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. 2005. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn. Sci 9:566–71 [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Mifflin SW, Gould GG, Frazer A. 2008. Induction of c-Fos and ΔFosB immunoreactivity in rat brain by vagal nerve stimulation. Neuropsychopharmacology 33:1884–95 [DOI] [PubMed] [Google Scholar]
- Daly K, Al-Rammahi M, Moran A, Marcello M, Ninomiya Y, Shirazi-Beechey SP. 2013. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am. J. Physiol. Gastrointest. Liver Physiol 304:G271–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. 2010. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav. Neurosci 124:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison JS, Clarke GD. 1988. Mechanical properties and sensitivity to CCK of vagal gastric slowly adapting mechanoreceptors. Am.J. Physiol 255:G55–61 [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, et al. 2008. Food reward in the absence of taste receptor signaling. Neuron 57:930–41 [DOI] [PubMed] [Google Scholar]
- Depoortere I 2014. Taste receptors of the gut: emerging roles in health and disease. Gut 63:179–90 [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, et al. 2011. Normal gut microbiota modulates brain development and behavior. PNAS 108:3047–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. 2017. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol 595:489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz A, Muschiol S, Zakikhany K, Assadi G, D’Amato M, Lindberg G. 2014. Human enteroendocrine cell responses to infection with Chlamydia trachomatis: a microarray study. Gut Pathog. 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz A, Zakikhany K, Muschiol S, Hultenby K, Lindberg G. 2011. Infection of human enteroendocrine cells with Chlamydia trachomatis: a possible model for pathogenesis in irritable bowel syndrome. Neurogastroenterol. Motil 23:928–34 [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Yusta B. 2014. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu. Rev. Physiol 76:561–83 [DOI] [PubMed] [Google Scholar]
- Duca FA, Swartz TD, Sakar Y, Covasa M. 2012. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLOS ONE 7:e39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, et al. 2013. Seven transmembrane G protein–coupled receptor repertoire of gastric ghrelin cells. Mol. Metab 2:376–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyrter F 1938. Uber diffuse endokrine epitheliale Organe. Leipzig, Ger: J.A. Barth [Google Scholar]
- Follesa P, Biggio F, Gorini G, Caria S, Talani G, et al. 2007. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 1179:28–34 [DOI] [PubMed] [Google Scholar]
- Fothergill LJ, Callaghan B, Hunne B, Bravo DM, Furness JB. 2017. Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology 158:2113–23 [DOI] [PubMed] [Google Scholar]
- Fraser KA, Davison JS. 1992. Cholecystokinin-induced c-fos expression in the rat brain stem is influenced by vagal nerve integrity. Exp. Physiol 77:225–28 [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, et al. 2003. Short chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 284:R1269–76 [DOI] [PubMed] [Google Scholar]
- Glass LL, Calero-Nieto FJ, Jawaid W, Larraufie P, Kay RG, et al. 2017. Single-cell RNA-sequencing reveals a distinct population of proglucagon-expressing cells specific to the mouse upper small intestine. Mol. Metab 6:1296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg D, Smith GP, Gibbs J. 1990. Intraduodenal infusions of fats elicit satiety in sham-feeding rats. Am. J. Physiol 259:R110–18 [DOI] [PubMed] [Google Scholar]
- Gribble FM, Reimann F 2016. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol 78:277–99 [DOI] [PubMed] [Google Scholar]
- Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, et al. 2017. A single-cell survey of the small intestinal epithelium. Nature 551:333–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, et al. 2018. A neural circuit for gut-induced reward. Cell 175:887–88 [DOI] [PubMed] [Google Scholar]
- Heidenhain R 1870. Untersuchungen uber den bau der labdrusen. Arch. Mikrosk. Anat 6:368–406 [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. 2009. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136:1328–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer D, Puschel B, Drenckhahn D. 1996. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. PNAS 93:6631–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H, De Repentigny Y, Kothary R, Sugimoto T. 2006. The survival of vagal and glossopharyngeal sensory neurons is dependent upon dystonin. Neuroscience 137:531–36 [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, et al. 2007. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. PNAS 104:15069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanningros R 1982. Vagal unitary responses to intestinal amino acid infusions in the anesthetized cat: a putative signal for protein induced satiety. Physiol. Behav 28:9–21 [DOI] [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, et al. 2018. A gut-brain neural circuit for nutrient sensory transduction. Science 361:eaat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Karaki S, Tanaka R, Kuwahara A. 2011. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J. Mol. Histol 42:27–38 [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. 2000. Principles of Neural Science. New York: McGraw-Hill [Google Scholar]
- Klarer M, Arnold M, Gunther L, Winter C, Langhans W, Meyer U. 2014. Gut vagal afferents differentially modulate innate anxiety and learned fear. J. Neurosci 34:7067–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokrashvili Z, Rodriguez D, Yevshayeva V, Zhou H, Margolskee RF, Mosinger B. 2009. Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology 137:598–606.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupari J, Haring M, Agirre E, Castelo-Branco G, Ernfors P. 2019. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 27:2508–23.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. 2001. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol 281:G907–15 [DOI] [PubMed] [Google Scholar]
- Lange CG, James W, eds. 1922. A Series of Reprints and Translations, Vol. 1: The Emotions. Baltimore, MD: Williams & Wilkins Co. [Google Scholar]
- Larraufie P, Dore J, Lapaque N, Blottiere HM. 2017. TLR ligands and butyrate increase Pyy expression through two distinct but inter-regulated pathways. Cell Microbiol. 19:e12648. [DOI] [PubMed] [Google Scholar]
- Lebrun LJ, Lenaerts K, Kiers D, Pais de Barros JP, Le Guern N, et al. 2017. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep. 21:1160–68 [DOI] [PubMed] [Google Scholar]
- Liou AP, Chavez DI, Espero E, Hao S, Wank SA, Raybould HE. 2011a. Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am.J. Physiol. Gastrointest. Liver Physiol 300:G895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, et al. 2011b. The G-protein–coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 140:903–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovén C 1868. Beiträge zur kenntniss vom bau der geschmackswarzchen der zunge. Arch. Mikrosk. Anat 4:96–110 [Google Scholar]
- Lu VB, Rievaj J, O’Flaherty EA, Smith CA, Pais R, et al. 2019. Adenosine triphosphate is co-secreted with glucagon-like peptide-1 to modulate intestinal enterocytes and afferent neurons. Nat. Commun 10:1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, et al. 2018. Enterochromaffin 5-HT cells—a major target for GLP-1 and gut microbial metabolites. Mol. Metab 11:70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM, Dahlstrom A, Bylock A, Ahlman H, Pettersson G, et al. 1978. Ultrastructural evidence for an innervation of epithelial enterochromaffine cells in the guinea pig duodenum. Acta Physiol. Scand 104:3–12 [DOI] [PubMed] [Google Scholar]
- Margolskee RF. 2002. Molecular mechanisms of bitter and sweet taste transduction. J. Biol. Chem 277:1–4 [DOI] [PubMed] [Google Scholar]
- Mei N 1978. Vagal glucoreceptors in the small intestine of the cat. J. Physiol 282:485–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnikes H, Lauer G, Bauer C, Tebbe J, Zittel TT, Arnold R. 1997. Pathways of Fos expression in locus ceruleus, dorsal vagal complex, and PVN in response to intestinal lipid. Am. J. Physiol 273:R2059–71 [DOI] [PubMed] [Google Scholar]
- Mordes JP, Herrera MG, Silen W. 1977. Decreased weight gain and food intake in vagotomized rats. Proc. Soc. Exp. Biol. Med 156:257–60 [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, et al. 2002. An amino-acid taste receptor. Nature 416:199–202 [DOI] [PubMed] [Google Scholar]
- Newson B, Ahlman H, Dahlstrom A, Das Gupta TK, Nyhus LM. 1979. On the innervation of the ileal mucosa in the rat—a synapse. Acta Physiol. Scand 105:387–89 [DOI] [PubMed] [Google Scholar]
- Norgren R, Smith GP. 1988. Central distribution of subdiaphragmatic vagal branches in the rat. J. Comp. Neurol 273:207–23 [DOI] [PubMed] [Google Scholar]
- Paintal AS. 1953. Impulses in vagal afferent fibres from stretch receptors in the stomach and their role in the peripheral mechanism of hunger. Nature 172:1194–95 [DOI] [PubMed] [Google Scholar]
- Palazzo M, Balsari A, Rossini A, Selleri S, Calcaterra C, et al. 2007. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J. Immunol. 178:4296–303 [DOI] [PubMed] [Google Scholar]
- Pavlov IP. 1910. The Work of the Digestive Glands. London: Griffin [Google Scholar]
- Phifer CB, Berthoud HR. 1998. Duodenal nutrient infusions differentially affect sham feeding and Fos expression in rat brain stem. Am.J. Physiol 274:R1725–33 [DOI] [PubMed] [Google Scholar]
- Phillips AG, Nikaido RS. 1975. Disruption of brain stimulation–induced feeding by dopamine receptor blockade. Nature 258:750–51 [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. 1998. Gastric volume detection after selective vagotomies in rats. Am. J. Physiol 274:R1626–38 [DOI] [PubMed] [Google Scholar]
- Prechtl JC, Powley TL. 1990. The fiber composition of the abdominal vagus of the rat. Anat. Embryol 181:101–15 [DOI] [PubMed] [Google Scholar]
- Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, et al. 2015. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes 39:424–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, Tyler WJ, Cox JE, Meller ST, Kelm GR, Bharaj SS. 2000. Responses of celiac and cervical vagal afferents to infusions of lipids in the jejunum or ileum of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol 278:R34–43 [DOI] [PubMed] [Google Scholar]
- Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. 2008. Glucose sensing in L cells: a primary cell study. Cell Metab. 8:532–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezek M, Vanderweele DA, Novin D. 1975. Stages in the recovery of feeding following vagotomy in rabbits. Behav. Biol 14:75–84 [DOI] [PubMed] [Google Scholar]
- Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, et al. 2014. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 63:1224–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. 1998. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am. J. Physiol 275:R262–68 [DOI] [PubMed] [Google Scholar]
- Sakura H, Ashcroft SJ, Terauchi Y, Kadowaki T, Ashcroft FM. 1998. Glucose modulation of ATP-sensitive K-currents in wild-type, homozygous and heterozygous glucokinase knock-out mice. Diabetologia 41:654–59 [DOI] [PubMed] [Google Scholar]
- Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, et al. 2017. Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients 9:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Hernandez M, Miralles B, Amigo L, Recio I. 2018. Intestinal signaling of proteins and digestion-derived products relevant to satiety. J. Agric. Food Chem 66:10123–31 [DOI] [PubMed] [Google Scholar]
- Schwalbe G 1867. Das epithel der papillae vallatae. Arch. Mikrosk. Anat 3:504–8 [Google Scholar]
- Schwartz GJ, McHugh PR, Moran TH. 1991a. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am. J. Physiol 261:R64–69 [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Netterville LA, McHugh PR, Moran TH. 1991b. Gastric loads potentiate inhibition of food intake produced by a cholecystokinin analogue. Am. J. Physiol. 261:R1141–46 [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Tougas G, Moran TH. 1995. Integration of vagal afferent responses to duodenal loads and exogenous CCK in rats. Peptides 16:707–11 [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K, Schwartz GJ. 2003. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol. Behav 78:285–94 [DOI] [PubMed] [Google Scholar]
- Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, et al. 2019. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 101:246–59.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu M, Cooke HJ. 1995. Role for 5-HT and ACh in submucosal reflexes mediating colonic secretion. Am. J. Physiol 269:G346–51 [DOI] [PubMed] [Google Scholar]
- Suarez AN, Hsu TM, Liu CM, Noble EE, Cortella AM, et al. 2018. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun 9:2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Perakyla J, Holm K, Haapasalo J, Lehtimaki K, et al. 2017. Vagus nerve stimulation improves working memory performance. J. Clin. Exp. Neuropsychol 39:954–64 [DOI] [PubMed] [Google Scholar]
- Sundaresan S, Abumrad NA. 2015. Dietary lipids inform the gut and brain about meal arrival via CD36-mediated signal transduction. J. Nutr 145:2195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan S, Shahid R, Riehl TE, Chandra R, Nassir F, et al. 2013. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 27:1191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz TD, Duca FA, de Wouters T, Sakar Y, Covasa M. 2012. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br. J. Nutr 107:621–30 [DOI] [PubMed] [Google Scholar]
- Symonds EL, Peiris M, Page AJ, Chia B, Dogra H, et al. 2015. Mechanisms of activation of mouse and human enteroendocrine cells by nutrients. Gut 64:618–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, et al. 2009. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10:167–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls EK, Phillips RJ, Wang FB, Holst MC, Powley TL. 1995. Suppression of meal size by intestinal nutrients is eliminated by celiac vagal deafferentation. Am. J. Physiol 269:R1410–19 [DOI] [PubMed] [Google Scholar]
- Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, et al. 2017. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol 595:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. 2016. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166:209–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington JJ, Klementowicz JE, Rahman S, Czajkowska BI, Smedley C, et al. 2013. Loss of the TGFβ-activating integrin αvβ8 on dendritic cells protects mice from chronic intestinal parasitic infection via control of type 2 immunity. PLOS Pathog. 9:e1003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SH, Rey O, Sternini C, Rozengurt E. 2010. Amino acid sensing by enteroendocrine STC-1 cells: role of the Na+-coupled neutral amino acid transporter 2. Am. J. Physiol. Cell Physiol 298:C1401–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yox DP, Stokesberry H, Ritter RC. 1991. Vagotomy attenuates suppression of sham feeding induced by intestinal nutrients. Am.J. Physiol 260:R503–8 [DOI] [PubMed] [Google Scholar]
- Zhang X, Fogel R, Renehan WE. 1992. Physiology and morphology of neurons in the dorsal motor nucleus of the vagus and the nucleus of the solitary tract that are sensitive to distension of the small intestine. J. Comp. Neurol 323:432–48 [DOI] [PubMed] [Google Scholar]
- Zittel TT, De Giorgio R, Sternini C, Raybould HE. 1994. Fos protein expression in the nucleus of the solitary tract in response to intestinal nutrients in awake rats. Brain Res. 663:266–70 [DOI] [PubMed] [Google Scholar]
- Zuo Y, Smith DC, Jensen RA. 2007. Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol. Behav 90:583–89 [DOI] [PMC free article] [PubMed] [Google Scholar]