Abstract
Artemisinin and its derivatives have shown broad-spectrum antitumor activities in vitro and in vivo. Furthermore, outcomes from a limited number of clinical trials provide encouraging evidence for their excellent antitumor activities. However, some problems such as poor solubility, toxicity and controversial mechanisms of action hamper their use as effective antitumor agents in the clinic. In order to accelerate the use of ARTs in the clinic, researchers have recently developed novel therapeutic approaches including developing novel derivatives, manufacturing novel nano-formulations, and combining ARTs with other drugs for cancer therapy. The related mechanisms of action were explored. This review describes ARTs used to induce non-apoptotic cell death containing oncosis, autophagy, and ferroptosis. Moreover, it highlights the ARTs-caused effects on cancer metabolism, immunosuppression and cancer stem cells and discusses clinical trials of ARTs used to treat cancer. The review provides additional insight into the molecular mechanism of action of ARTs and their considerable clinical potential.
Keywords: artemisinins, antitumor, mechanism of action, clinical trials, therapeutic approach
Introduction
Cancer is becoming a severe health problem internationally and is one of the most deadly diseases (Siegel et al., 2016). Conventional cancer therapy, especially chemotherapy, provides limited efficacy by DNA damage (Li et al., 2017), but has the problems of formidable side effects and drug resistance. To discover novel potent and safe chemotherapeutic agents or seek better curative methods, medicinal chemists, pharmaceutists and pharmacologists have completed many investigations.
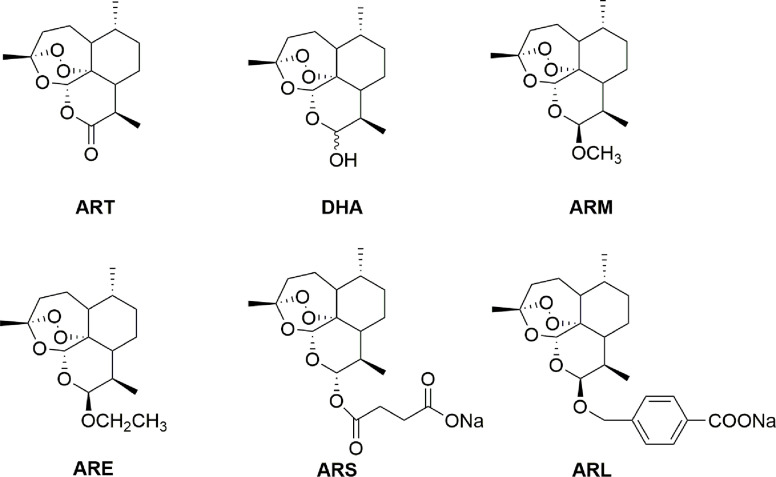
Artemisinin (ART), bearing a peroxide bridge in its sesquiterpene lactone structure, was extracted and separated from Artemisia annua L. (sweet wormwood), which has been used for treatment of fevers and chills as one of the famous Chinese traditional medicines for thousands of years (Klayman, 1985; Li and Wu, 2003; Cui and Su, 2009; Tu, 2016). ART can be reduced to dihydroartemisinin (DHA) by using sodium borohydride in high yields. Thus, this compound was used as a starting material to prepare the first-generation derivatives including artemether (ARM) and arteether (ARE), and sodium artesunate (ARS) as well as sodium artelinate (ARL) collectively termed as artemisinins (ARTs) (Figure 1). As first-line antimalarial medicines, ARTs are safe, low-toxic and well tolerable. However, the neurotoxicity has raised concerns about the safety of ARTs. One of them, ARL was withdrawn from further drug development program because of higher neurotoxicity (Li et al., 2005; Xie et al., 2005). Recently, extensive anticancer effects of ARTs were reported (Ho et al., 2014; Liu X. et al., 2019). More importantly, ARTs have been attractive cancer therapeutic drug candidates with high selectivity, while the neurotoxicity occurred inescapably in clinical studies of cancer at high dosage (Deeken et al., 2018; Von Hagens et al., 2019). Furthermore, the poor solubility of ARTs as well as a short half-life and low bioavailability upon oral administration lead to urgent needs for the finding of novel chemical structures to solve these problems (Crespo-Ortiz and Wei, 2012; Li et al., 2016). To overcome these drawbacks, some novel chemical structures of ARTs have been developed as cancer candidates including mitochondria-targeted derivatives and hybrids while new therapeutic approaches of novel nano-formulations and combination therapy were explored as well (Zhang X. et al., 2015; Zhang et al., 2016; Gao et al., 2018; Suraweera et al., 2018; Hu et al., 2019; Nosrati et al., 2019; Wang Y. et al., 2019; Fröhlich et al., 2020; Zhang et al., 2020).
Figure 1.
Artemisinin and its derivatives.
In addition, the mechanisms of antitumor action are investigated but incompletely elucidated. The mechanism of antitumor action of ARTs mainly involved in apoptotic cell death which has been confirmed by most literatures. Recognized endoperoxide bridge pharmacophore could be reduced by heme or free ferrous iron to generate carbon free radical and reactive oxygen species (ROS) (Firestone and Sundar, 2009; Stockwin et al., 2009; Greenshields et al., 2019). Excess production of ROS is known to cause apoptotic cell death. However, new mechanisms of action in antitumor activity of ARTs by affecting non-apoptotic cell death including oncosis, autophagy and ferroptosis were also found (Du et al., 2010; Zhou et al., 2013; Ooko et al., 2015; Shi et al., 2017; Jiang et al., 2018). Other multiple hallmark events of cancer development and progression were also affected by ARTs including the suppression of cancer cell proliferation, anti-angiogenesis, anti-cancer metastasis and invasion, induction of cell cycle arrest, disruption of cancer signaling pathway and regulation of tumor microenvironment (Efferth, 2017b; Zhang Y. et al., 2018). In tumor microenvironment, there are four types of cells that inhibit immune function including regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages and cancer-associated fibroblasts. As a result, tumor-specific T cells are unable to enter tumor tissues or their functions are impaired after entering the tissues. This indicates that inhibiting immunosuppression will be beneficial for cancer therapy. Furthermore, it is well-known that cancer cells are different from normal cells with rapid proliferation and metabolic changes especially glycolytic metabolism. Fortunately, ARTs showed antitumor activity by affecting immunosuppression and cancer metabolism (Zhang et al., 2014; Cui et al., 2015; Li S. et al., 2019; Zhu et al., 2019; Gao et al., 2020). The pace on the study of mechanisms of action of ARTs still doesn’t stop after receiving these exciting results. Cancer stem cells (CSCs) attract the attention of researchers of ARTs because of their crucial role on tumor occurrence, metastasis and recurrence. Although only a few articles about how ARTs affect CSCs have been reported (Cao et al., 2014; Berte et al., 2016; Tong et al., 2016), these new discoveries might provide a revolutionary approach for cancer therapy.
In this review, recent progress for cancer therapy based on ARTs including the development of novel ARTs derivatives, novel nano-formulations and combination therapy is summarized and the latest findings of the mechanisms of action are analyzed for further understanding this huge potential in cancer treatment and promoting clinical application.
Novel Design Strategies For Developing Anticancer Candidates Based On Arts
The antitumor activity of ARTs has attracted extensive attention (Firestone and Sundar, 2009; Crespo-Ortiz and Wei, 2012; Frohlich et al., 2016; Wong et al., 2017; Zhang Y. et al., 2018; Liu X. et al., 2019). To increase efficacy and reduce toxicity of ARTs, new design strategies for anticancer candidates based on ARTs have been developed. These strategies were raised by researchers from different fields. Medicinal chemists focus more on chemical structural modification to develop novel derivatives whereas pharmacists are more interested in formulation improvement. In our review, we elaborate these novel strategies by focusing on developing novel ART derivatives and manufacturing ART nano-formulations as well as combining ARTs with other drugs for cancer therapy.
Developing Novel Derivatives With Enhanced Antitumor Activity
Mitochondria-Targeted ART Derivatives
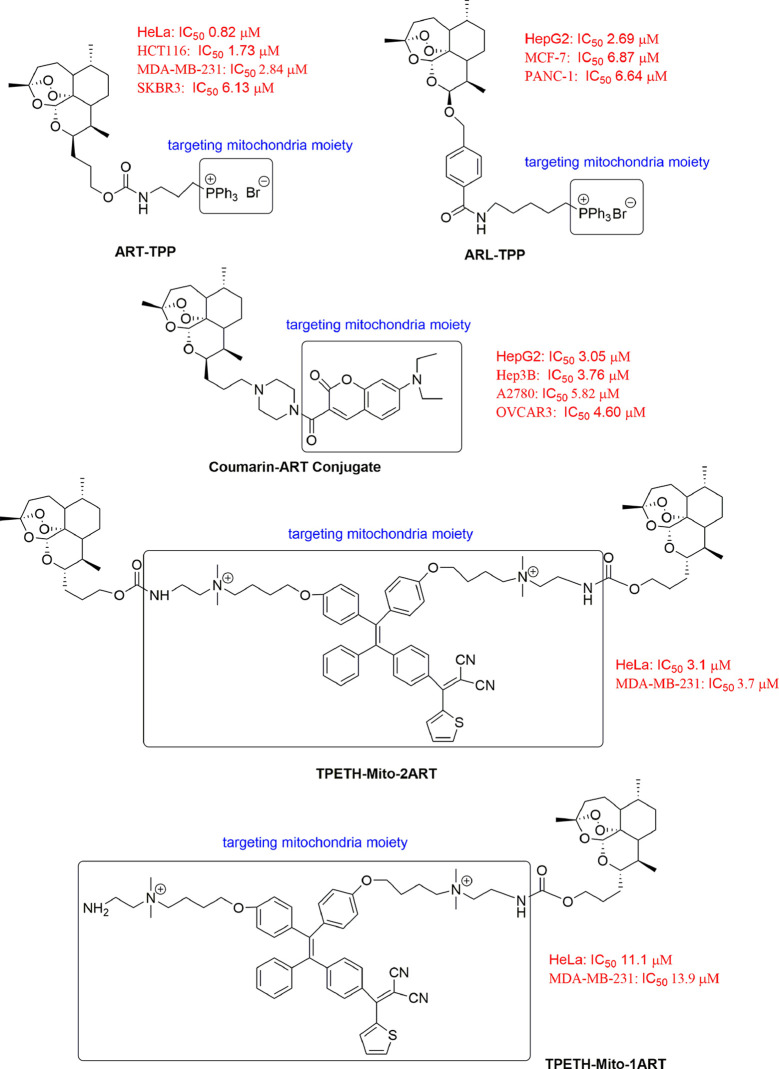
Mitochondria are highly dynamic organelles involved in many cellular functions (Battogtokh et al., 2018). Mitochondria dysfunction has been observed in cancer cells (Gururaja Rao, 2017). Drugs specifically targeting mitochondria are therefore of therapeutic interest. Furthermore, current evidence of the mitochondrial membrane potential (Δψm) difference between normal and cancer cells has offered further confidence for designing drug to target mitochondria (Zielonka et al., 2017). The triphenylphosphonium cation (TPP+), one of delocalized lipophilic cations (DLCs) is used commonly to target parental compounds to the mitochondria (Ye et al., 2017; Jin et al., 2019). Mitochondria-targeted ART derivatives with enhanced antitumor activity have been synthesized as antitumor candidates (Figure 2). Zhang et al. synthesized a mitochondria-targeting ART analogue (ART-TPP) with enhanced anticancer activity to tested cancer cells, with the minimum IC50 value of 0.82 μM for HeLa cells and the maximum IC50 value of 6.13 μM for SKBR3 cells. The cytotoxicity of ART-TPP was more potent than ART alone in tested cells. The principle for the better activity of ART-TPP was confirmed by using a clickable probe. The probe localized well in the mitochondria after cellular uptake and bound to 209 proteins from mitochondria with more potential (Zhang et al., 2016). Sun et al. conjugated ARL to mitochondria-targeting TPP+ to obtain ARL-TPP. ARL-TPP significantly increased cytotoxic activity against MCF-7 cancer cells with an IC50 value of 6.87 μM, PANC-1 cancer cells with an IC50 value of 6.64 μM, HepG2 cancer cells with an IC50 value of 2.69 μM and LoVo cancer cells with an IC50 value of 2.73 μM (Sun et al., 2017). However, because TPP+ are nonemissive, Zhang et al. synthesized another novel class of fluorescent mitochondria-targeted coumarin–artemisinin conjugates to kill cancer cells by linking a mitochondrial dye, coumarin-3-carboximide with ART. These compounds had strong abilities to accumulate in mitochondria with enhanced anticancer activities, then the intracellular ROS levels increased efficiently and cell apoptosis was induced (Zhang X. et al., 2015). Feng et al. synthesized ART and aggregation-induced emission fluorogens (AIEgen) conjugates (TPETH-Mito-1ART and TPETH-Mito-2ART) for mitochondria-targeted and image-guided chemo- and photodynamic therapy for treating cancer. These conjugates largely improved cancer cell ablation efficacy with a synergistic effect by quickly depolarizing mitochondrial membrane and dramatically reducing cancer migration activity (Feng et al., 2018).
Figure 2.
Novel mitochondria-targeted artemisinin derivatives.
Hybrid Derivatives
Pharmacophore hybridization, a classical medicinal chemistry approach, involves combination of two or more functional groups via covalent bonds to produce a novel compound holding preferable biological activities (Meunier, 2008; Muregi and Ishih, 2010; Capci et al., 2019). This approach is often used in searching for safe, effective and specific innovative medicines for cancer therapy (Letis et al., 2017; Yu et al., 2018; Zborovskii et al., 2018). Many studies of ARTs have generated new hybrids, such as ART-derivative-N-heterocyclic carbene (NHC)-gold(I)-hybrids (Zhang et al., 2020), ART–daumone hybrids (Ma et al., 2018), DHA–bile acid hybrids (Letis et al., 2017; Marchesi et al., 2019), DHA–cinnamic acid hybrids (Xu et al., 2016), DHA–coumarin hybrids (Yu et al., 2018) and ART–chemotherapeutic agent conjugates (Li et al., 2016), et al., with more potent activity than with either agent alone (Table 1). We designed and synthesized DHA–cinnamic acid ester derivatives modified at two positions, C-9 and C-12. DHA-37 (namely, compound 17 in previous paper) was considered a candidate for treating lung cancer (Xu et al., 2016). Liu et al. found that DHA-37, different from DHA-induced apoptosis, increased the high mobility group box 1 (HMGB1) expression and induced autophagy by activating the MAPK signal but not PI3K-AKT-mTOR pathway (Liu et al., 2018). Zhang et al. synthesized ART-derivative-NHC-gold(I) hybrids which showed strong anticancer activities on a large panel of representative human cancer cell models with GI50 values in nanomolar (nM) range together with a high selectivity. This high selectivity might be due to inhibition of the redox antioxidant Nrf2 transcription factor. This transcript factor has been confirmed to be strongly associated with aggressiveness and resistance to cancer therapies (Zhang et al., 2020). Ma et al. found that ART–daumone hybrids inhibited cancer cell-mediated osteolysis by upregulating the level of activating transcription factor 3 and downregulating the level of E2F transcription factor 1 and hepatocyte nuclear factor 4 alpha (Ma et al., 2018). Li et al. found that ART-chemotherapeutic conjugates inhibited the growth and proliferation of ovarian cancer cells, resulting in S-phase arrest, apoptosis and epithelial mesenchymal transition (EMT) (Li et al., 2016). Furthermore, Yu et al. found that DHA-coumarin hybrids induced ferroptosis, promoted apoptosis, arrested the cell cycle progression, inhibited cell proliferation and migration (Yu et al., 2018).
Table 1.
Summary of ARTs hybrids for antitumor activity.
| Compound | Cell line | Event/Mechanism | References |
|---|---|---|---|
| ART-derivative-NHC gold(I) hybrids | A549, U-2 OS, MCF-7, T24, LAMA, HL-60, HepG2 | Inhibit Nrf2 transcriptional activity | (Zhang et al., 2020) |
| ART-daumone hybrids | MDA-MB-231, A549 | Tumor suppressive activating transcription factor 3↑, oncogenic E2F transcription factor 1↓, inhibit osteoclast formation, MMP 9↓, cathepsin K↓ | (Ma et al., 2018) |
| DHA-bile acid hybrids | HL-60, HepG2, | Induce apoptosis | (Marchesi et al., 2019) |
| CCRF-CEM, CEM/ADR5000 | Enhance cytotoxicity, low cross resistance | (Letis et al., 2017) | |
| DHA-cinnamic acid hybrids | A549 | HMGB1↑, induce autophagy by activating MAPK signals | (Liu et al., 2018) |
| DHA-coumarin hybrids | HT-29, MDA-MB-231 | Inhibit proliferation, arrest the cell cycle progression, induce both apoptosis and ferroptosis, inhibit migration | (Yu et al., 2018) |
| ART-chemotherapeutic agents conjugates | A2780, OVCAR-3 | S-phase arrest, induce apoptosis and inhibit migration | (Li et al., 2016) |
| Thymoquinone-ART hybrids | CCRF-CEM, CEM/ADR5000 | Better cytotoxicity than doxorubicin | (Fröhlich et al., 2018) |
| HCT116, HT29 | ROS↑, γ-H2AX↑ | (Fröhlich et al., 2017) | |
| ART-triazole hybrids | KB, HepG2 | Exihibit moderate to good cytotoxic activities | (Tien et al., 2016) |
| ART-indole/imidazole hybrids | A549, MCF-7, HepG2, MDA-MB-231, MCF/ADR | Induce cell cycle arrest at G2 phase | (Hu et al., 2019) |
| ART-ferrocene hybrids | CCRF-CEM, CEM/ADR5000 | Show a remarkable anticancer activity | (Reiter et al., 2015) |
| Tamoxifen/Estrogen-ART hybrids | PC-3, MCF-7 | Enhance anticancer activity | (Fröhlich et al., 2020) |
Manufacturing Novel Nano-Formulations With Better Physicochemical Properties
Nano-formulations can achieve a drug-targeted distribution and increase the bioavailability of the drug to improve the curative efficacy, which is a key technology in targeted cancer therapy. To improve physicochemical properties of ARTs, researchers have developed ART-based nano-formulations such as liposomes (Gharib et al., 2014; Leto et al., 2016; Gao et al., 2018; Li H. et al., 2019; Liu J. J. et al., 2019), nanostructured lipid carriers (Emami et al., 2018), micelles (Nosrati et al., 2019; Wang Y. et al., 2019), nanospheres (Chen J. et al., 2015), nanocapsules (Meng et al., 2014; Tran et al., 2015) and multifunctional nanoparticles (Chen et al., 2014; Li et al., 2014; Wang et al., 2016a; Wang et al., 2016b; Pan et al., 2018) (Table 2). These nano-formulations overcame chemotherapeutic resistance with improved selectivity by targeting tumor cells. These novel nano-formulations according to the different design concepts and different delivery systems are summarized below.
Table 2.
Summary of ART-based nano-formulations.
| Nano-formations | Cell line | Event/mechanism | References |
|---|---|---|---|
| Liposomes | A549/R | Increase intracellular ROS generation and cell apoptosis rate | (Gao et al., 2018) |
| MCF-7, MDA-MB-231 | High antiproliferative activity in a magnetic field | (Gharib et al., 2014) | |
| HCT-8 | Enhance anticancer activity | (Leto et al., 2016) | |
| HNSCC | Enhance anticancer activity by increasing DHA-targeted delivery and biocompatibility | (Li H. et al., 2019) | |
| NSCLC | VE-Cad↓, TGF-β1↓, MMP-2↓, HIF-1α↓, inhibit VM channels and tumor metastasis, increase the selective accumulation at tumor sites | (Liu J. J. et al., 2019) | |
| Nanostructured lipid carriers | U87MG | Enhance anticancer activity | (Emami et al., 2018) |
| Micelles | MCF-7, MCF-7/ADR | Enhance antitumor activity and reduce toxicity | (Wang Y. et al., 2019) |
| MCF-7, 4T1 | High efficacy, low toxicity and tumor target | (Nosrati et al., 2019) | |
| CT-26 | Enhance antitumor activity | (Hao et al., 2020) | |
| Nanospheres | A549 | Suppress tumor growth | (Chen J. et al., 2015) |
| Nanocapsules | L1210 and MCF-7 | Decrease antitumor activity by controllable release of ART | (Meng et al., 2014) |
| MCF-7, MDA-MB-231 | Enhance anticancer activity | (Tran et al., 2015) | |
| Multifunctional nanoparticles | HeLa | Enhance antitumor efficacy | (Chen et al., 2014) |
| HeLa, A549 | Significant cytotoxicity and no obvious side effects | (Wang et al., 2016a) | |
| HeLa | Synergy with combined chemo-photothermal therapy | (Wang et al., 2016b) | |
| HeLa, A375, HepG2 | Enhance antiproliferative response compared with free drug | (Pan et al., 2018) | |
| C6, C6 stem cell | Destroy VM channels and induce apoptosis | (Li et al., 2014) |
Based on a Fe2+/Fe3+-mediated Fenton reaction, magnetic DHA nanoliposomes were developed to circumvent cisplatin resistance and enhance targeted delivery and bioefficacy of DHA (Gao et al., 2018; Li H. et al., 2019). Based on transferrin overexpression in tumor cells, magnetic nanoliposomes and transferrin-conjugated liposomes were developed to target tumor in vitro and in vivo (Gharib et al., 2014; Leto et al., 2016). Also, ART-loaded transferrin-conjugated nanostructured lipid carriers were developed to increase water solubility, site specificity, selective targeting, efficient penetration, glioma cell distribution and internalization, as well as effective delivery across the blood–brain barrier with much lower drug concentration, greater therapeutic effect and decreased likelihood of neurotoxicity (Emami et al., 2018).
On the basis of pH-responsive degradation, novel Fe3O4@C@MIL-100(Fe) nanoparticles (Wang et al., 2016a), dual metal-organic-frameworks nanoparticles (Wang et al., 2016b), lipid nanoparticles (Zhang Y. J. et al., 2015), multifunctional nanocarriers (Chen et al., 2014), nanospheres (Chen J. et al., 2015), fluorescent magnet theranostic nanoparticles (Pan et al., 2018) and polymeric micelles (Hao et al., 2020) loading ARTs were fabricated. These nano-formulations possessed pH-responsive property since they relied on acidified tumor microenvironment along with a further acidified endosome/lysosome network. Once these nano-formulations were endocytosized into tumor, they controllably released incorporated drugs to take effect, or Fe2+ ions or Mn2+ ions, which converted ARTs to highly active products to enhance cell killing.
In addition, arginine 8 modified DHA-epirubicin liposomes were developed with ideal physicochemical characteristics for powerful cytotoxicity against A549 cells and these liposomes effectively suppressed vasculogenic mimicry (VM) channels and blocked tumor metastasis (Liu J. J. et al., 2019). mPEG–ART nanocapsules were synthesized based on a mPEGylated ARS pro-drug but conferred decreased cytotoxicity than free ARS (Meng et al., 2014). Fortunately, ARS-loaded chitosan-coated lipid nanocapsules were developed with stronger antitumor activity than free ART (Tran et al., 2015). Doxorubicin and DHA co-loaded Soluplus®-TPGS and biotin-functionalized copolymeric PEG-PCL micelles were developed with higher antitumor activity and lower toxicity (Nosrati et al., 2019; Wang Y. et al., 2019). These newly developed nano-formulations provided novel therapeutic candidates with high efficiency for treating cancer.
Combination Therapy
Combination therapy approach can be used to increase efficacy, reduce toxicity and overcome drug resistance (Suraweera et al., 2018). Resistance to ARTs has already appeared in malaria treatment. New partner drugs of ARTs have been suggested to establish combination-treatment regimen for antimalaria to reduce resistance risk (Wang J. et al., 2019). Successful combinations of ARTs with chemotherapeutics or phytochemicals have been highlighted in recent years (Table 3). These findings provided promising therapeutic approaches for cancer therapy.
Table 3.
Summary of ARTs combinations with chemotherapy drugs and phytochemicals.
| Compound | Cell lines | Event/mechanism | References |
|---|---|---|---|
| DHA-cisplatin | SKOV3/DDP | mTOR inhibition, promote apoptosis | (Feng et al., 2014) |
| A549, A549/DDP | Suppress the expression of HIF-1α and VEGF to effect tumor angiogenesis, increase apoptosis | (Zhang et al., 2013) | |
| A549, LLC | Inhibit tumor growth and metastasis | (Zhou et al., 2010) | |
| ARS-cisplatin | A2780, HO8910 | Downregulate RAD51 | (Wang et al., 2015) |
| DHA-carboplatin | A2780, OVCAR-3 | Death receptor- and mitochondrion-mediated caspase-dependent apoptotic pathways | (Chen et al., 2009) |
| LLC | Inhibit cell proliferation, induce G0/G1 phase cell cycle arrest, increase cell apoptosis, p38 MAPK activation | (Zhang B. et al., 2018) | |
| DHA-5-FU | HCT116 TP53-/- | Inhibit proliferation, induce ROS-mediated apoptosis and Bcl-2/Bax↓ | (Yao et al., 2018) |
| DHA-gemcitabine | NCI-H1975 | G2/M phase cell cycle arrest, cyclin B1↓, cyclin-dependent kinase 1↓, inhibit the migratory and invasive, promote apoptosis p-Akt↓ p-mTOR↓ p-STAT3↓ Bcl-2↓ Bax↑, Akt/mTOR/STAT3 pathway |
(Hong et al., 2017) |
| A549 | Induce apoptosis through bak-mediated intrinsic pathway and fas-caspase-8-mediated extrinsic pathway | (Zhao et al., 2014) | |
| BxPC-3, PANC-1 | Inactivate NF-κB | (Wang et al., 2010) | |
| ARS-cytarabine, DHA-cytarabine | human AML cell lines | Produce initial regression, but did not prolong survival in vivo | (Drenberg et al., 2016) |
| DHA-ABT-263 | BCR-ABL+B-ALL leukemic cells | Down-regulate MCL-1 expression | (Budhraja et al., 2017) |
| Non-small cell lung cancer | Inhibit STAT3 activity, modulate the expression of MCL-1, Survivin and Bim | (Yan et al., 2018) | |
| ARS-erlotinib | glioblastoma multiforme cell lines | Activate EGFR | (Efferth et al., 2004; Efferth, 2017a) |
| ARS-sorafenib | Huh7, SNU-449, and SNU-182 HCC cell lines | Induce ferroptosis | (Li et al., 2020) |
| DHA-curcumin | SKOV3 | Decrease cell viability, arrest cell cycle, promote apoptosis, MK↑, miR-124↑ | (Zhao et al., 2017) |
| lethal(2)giant larvae, [l(2)gl] brain tumor | Prolong life span, restore locomotor activity | (Das et al., 2014) | |
| ARS-triptolide | PANC-1, CFPAC-1 | Inhibit cell growth, induce apoptosis, HSP 20↓, HSP 27↓ | (Liu and Cui, 2013) |
| DHA-dictamnine | A549 | Induce caspase-3–dependent apoptosis | (An et al., 2013) |
| DHA-dexamethasone | multiple myeloma cells | ROS↑, △Ψm↓, induce caspase-medicated apoptosis, overcome resistance | (Chen Y. et al., 2020) |
| ARS-bicalutamide | PC3, 22RV1, LNCaP | Inhibit NF-κB signaling and decreases AR and/or AR-variant 7 expression via ubiquitin-mediated proteasomal degradation, induce oxidative stress and apoptosis via survivin downregulation and caspase-3 activation, resulting in poly-ADP-ribose polymerase cleavage | (Nunes et al., 2017) |
| DHA-doxorubicin | mutant p53 (R248Q)-expressing Hep3B | Decrease P-gp expression via inhibiting the p53 (R248Q)-ERK1/2-NF-κB signaling pathway | (Yang et al., 2019) |
Multiple mechanisms were found in these combinations. The in vivo and in vitro applications of DHA–cisplatin combinations were mainly mediated by inhibiting mTOR (Feng et al., 2014), reducing tumor microvessel density (Zhang et al., 2013) and inhibiting tumor growth and metastasis (Zhou et al., 2010). However, ARS–cisplatin combination was mediated by downregulating RAD51 (Wang et al., 2015). DHA–carboplatin combination was mediated by the mitochondrion and death receptor-mediated caspase-dependent apoptotic pathway (Chen et al., 2009) and p38 mitogen-activated protein kinase (MAPK) activation (Zhang B. et al., 2018). Combinations with the antimetabolite 5-fluorouracil involved ROS (Yao et al., 2018), whereas that with gemcitabine was regulated by the Fas-caspase-8–mediated extrinsic pathway and Bak-mediated intrinsic pathway (Zhao et al., 2014), deactivating gemcitabine-induced NF-kB activation (Wang et al., 2010) or promoting apoptosis via the Akt/mTOR/signal transducer and activator of transcription 3 (STAT3) pathway (Hong et al., 2017). In vivo, Combinations of ARS or DHA with cytarabine produced initial regression but did not prolong survival (Drenberg et al., 2016). Of note, synergy between kinase inhibitors and DHA or ARS involve different mechanisms from those mentioned above, including repressing myeloid cell leukemia 1 (MCL-1) expression (Budhraja et al., 2017; Yan et al., 2018), inhibiting STAT3 activity (Jin et al., 2017; Yan et al., 2018), activating epidermal growth factor receptor (EGFR) (Efferth et al., 2004; Efferth, 2017a) and inducing ferroptosis (Li et al., 2020). DHA–curcumin combination decreased the expression of oncogene midkine and upregulated the expression of the microRNA miR-124 to induce apoptosis (Zhao et al., 2017). However, the ART–curcumin combination prolonged the life span and restored locomotor activity via ROS mediated in Drosophila in brain tumor (Das et al., 2014). In addition, ARS-triptolide combination inhibited pancreatic cancer cell line growth and induced apoptosis, accompanying downregulation of the expression of heat shock proteins (HSP) 20 and HSP 27 (Liu and Cui, 2013). DHA-dictamnine combination dramatically increased apoptotic cell death via a caspase dependent pathway in human lung adenocarcinoma cells (An et al., 2013).
Overcoming drug resistance using combinations of ARTs with other drugs is another advantage for cancer therapy. Chen et al. reported that DHA treatment overcame dexamethasone resistance and enhanced dexamethasone efficacy in multiple myeloma by increasing ROS production and cytochrome C translocation from mitochondria to cytoplasm, resulting in alterations to Δψm and caspase-mediated apoptosis (Chen Y. et al., 2020). Yao et al. reported that DHA effectively restored anticancer activity of 5-FU against 5-FU resistant HCT116 TP53-/- cells through ROS-mediated apoptosis and upregulation of the bcl-2/bax expression ratio (Yao et al., 2018). Nunes et al. reported that combination of ARS and bicalutamide restored sensitivity of castrate-resistant prostate cancer cells to antiandrogens. The mechanisms involved in inhibiting NF-κB signaling, together with decreased expression of androgen receptor (AR) and/or AR-variant 7 and the induction of oxidative stress and apoptosis (Nunes et al., 2017). Mutant p53 (R248Q) can induce doxorubicin resistance in hepatocellular carcinoma (HCC). Yang et al. reported that DHA sensitized mutant p53 (R248Q)-expressing HCC to doxorubicin. The mechanism of action involved in reducing P-gp expression via inhibiting the p53 (R248Q)-ERK1/2-NF-κB signaling pathway (Yang et al., 2019). It is well known that treatment of arsenic trioxide to lung cancer cells easily developed high level of resistance (Chen H. et al., 2017). Chen et al. reported that combination of DHA and arsenic trioxide reduced arsenic trioxide resistance of lung cancer cells by increasing cellular level of ROS and DNA damage. Moreover, treatment of normal human cells with the combination did not result in significant adverse events (Chen H. et al., 2017).
In conclusion, these novel combination approaches based on ARTs improved the antitumor activity and led to the development of new drug partners for clinical application. Furthermore, several newly developed candidates also presented new mechanisms of action including in autophagy (Liu et al., 2018), in ferroptosis (Yu et al., 2018) and in Nrf2 signaling (Zhang et al., 2020), which warrant to be noted.
The Effects of Arts on Cell Signaling Pathways and Modes of Induction of Cell Death
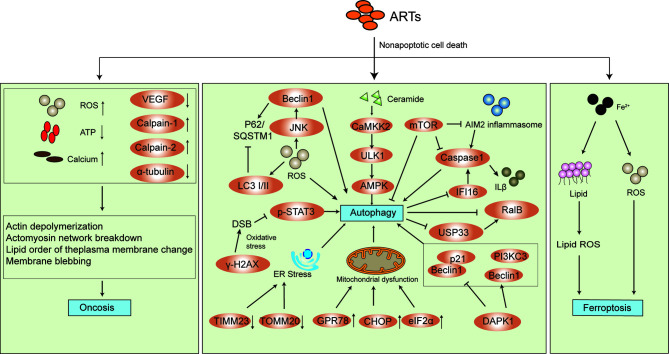
The antitumor activity of ARTs involves multitargets and multipathways as mentioned above. Inducing cell cycle arrest is one of some common ones to inhibit proliferation of tumor cells (Jia et al., 2016; Chen J. et al., 2019), inhibit tumor cell invasion and metastasis (Rasheed et al., 2010; Weifeng et al., 2011), exerting antiangiogenic effects against tumor cells (Wei and Liu, 2017) and inducing cancer cell apoptosis (Jia et al., 2016; Chen J. et al., 2019). More recently, several new mechanisms on non-apoptotic cell death e.g. oncosis, autophagy and ferroptosis have been proposed. As shown in Figure 3, the mechanisms of action involve multiple cell signaling pathways. Besides, reports showed that ARTs regulated cancer cell metabolism including glucose metabolism and lipid metabolism, inhibited immunosuppression and the stemness of cancer stem cells in various cancer cell lines (Chen X. et al., 2017; Cao et al., 2019; Chen X. et al., 2019; Chen S. et al., 2020; Gao et al., 2020). These new findings further highlight the multitarget bioactive properties and complexity of the ART-mediated anticancer effect.
Figure 3.
New mechanisms of action of ARTs-mediated non-apoptotic cell death.
The Effects of ARTs on Non-Apoptotic Cell Death
Oncosis
Oncosis is characterized by rapid cell swelling, organelle swelling, membrane permeability, and cell lysis. It is associated with intercellular events including increased ROS generation, mitochondrial swelling, adenosine triphosphate (ATP) depletion, failure of Ca2+ homeostasis, activation of certain proteases (e.g., calpains and cathepsins), lysosomal disruption, and eventually plasma membrane rupture (Majno and Joris, 1995; Van Cruchten and Van Den Broeck, 2002; Golstein and Kroemer, 2007; D’arcy, 2019). It has been shown that ARS induced oncosis-like cell death in pancreatic cancer and renal cell carcinoma (Du et al., 2010; Jeong et al., 2015). The former cell death occurred with the morphotype characteristics of oncosis and the latter via ROS generation and ATP depletion (Du et al., 2010; Jeong et al., 2015). Interestingly, in gastric cancer, ARS promoted cell oncosis by decreasing the expression of VEGF and increasing the amount of calcium and the expression of calpain-2 (Zhou et al., 2013).
Autophagy
Autophagy consists of the degradation and recycling of organelles and portions of the cytosol (Parzych and Klionsky, 2014; Saha et al., 2018). As a “self-eating” process and well-known type II programmed cell death, autophagy acts as a double-edged sword because of its association with both cell survival and death. It has been verified that ARTs induced autophagy (Du et al., 2013; Feng et al., 2014; Jia et al., 2014; Qu et al., 2017; Shi et al., 2017; Wang et al., 2017; Cheng et al., 2018; Jiang et al., 2018; Thongchot et al., 2018; Guan and Guan, 2020). However, how autophagy accelerates cell death and enhances the cell survival after ARTs exposure are contradictory. Some evidence showed that autophagy induced by ARS and DHA was to protect cancer cells (Jia et al., 2014; Jiang et al., 2018), whereas autophagy induced by DHA was to kill cancer cells (Qu et al., 2017). Other evidence of autophagy-depended ferroptosis, cell cycle arrest and cell apoptosis induced by ARTs and ARTs-induced autophagy sensitized chemotherapy drugs to enhance the cell death demonstrated the killing effect (Feng et al., 2014; Zhang Z. S. et al., 2015; Cheng et al., 2018; Du et al., 2019; Ma et al., 2020), whereas autophagy inhibitor enhanced the anticancer property of ARTs, indicating the protecting effect of autophagy (Chen S. S. et al., 2015).
The molecular mechanisms of ART-induced autophagy involved accumulation of ROS, which activated JNK pathway in pancreatic cancer cells (Jia et al., 2014), or stimulating de novo synthesis of ceramide and CaMKK2–AMPK–ULK1 axis, which in turn cause the occurrence of autophagy in diffuse large B-cell lymphomas (Cheng et al., 2018), or increasing the expression of death-associated protein kinase 1 (DAPK1), reducing the interaction of beclin1 with bcl-2 and promoting the interaction of beclin1 with PI3KC3 in cholangiocarcinoma (Thongchot et al., 2018). Alternatively, it might cause endoplasmic reticulum stress and mitochondrial dysfunction in human glioblastoma cells (Qu et al., 2017). ARTs-induced autophagy was also mediated by inhibiting the nuclear localization of phosphorylated STAT3 in human tongue squamous cell carcinoma cells (Shi et al., 2017), as well as inhibiting the Akt/mTOR signaling pathway in esophageal cancer cells (Chen X. et al., 2020). Furthermore, ARTs regulated the crosstalk between autophagy and inflammasomes, which induced the activation absent in melanoma 2 (AIM2)/caspase-1 inflammasome to trigger autophagy in hepatocellular carcinoma and induced autophagy with suppressing the activation of IFI16/caspase-1 inflammasome and IL-1β production together with reducing the expression of ubiquitin-specific processing protease 33 (USP33) and Ras like B (RalB) in human laryngeal squamous cell carcinoma (Shi et al., 2019; Shi et al., 2020).
Ferroptosis
Ferroptosis, unlike traditional apoptosis and necrosis, is a novel type of caspase-independent non-apoptotic cell death. It is caused by accumulation of iron-dependent lipid peroxide and is characterized mainly by cell volume shrinkage and enhanced mitochondrial membrane density without typical apoptotic and necrotic manifestations (Dixon et al., 2012; Xie et al., 2016; Lu et al., 2017). ARTs exhibited anticancer activity related to ferric iron and ROS which is similar to ferroptosis. Ooko et al. found that the expression of numerous iron-related genes including genes encoding transferrin, transferrin receptors 1 and 2, cerulopasmin and lactoferrin were significantly correlated to the log10IC50 values for ARTs, indicating ferroptosis-inducing activity of ARTs (Ooko et al., 2015). Subsequently, ARTs-induced ferroptosis was found in various types of cancer cells in vitro and in vivo (Lin et al., 2016; Roh et al., 2017; Du et al., 2019; Wang N. et al., 2019; Chen G. Q. et al., 2020). The underlying molecular mechanisms involved the decrease of the protein levels of glutathione peroxidase 4 (GPX4) and rat sarcoma (Ras) in head and neck squamous cell carcinoma cells (Lin et al., 2016), activation of the Nrf2–antioxidant response element pathway in head and neck cancer cells (Roh et al., 2017) and the ATF4-CHOP-CHAC1 pathway in Burkitt’s lymphoma (Wang N. et al., 2019). Recently, Chen and Wang et al. demonstrated that heat shock protein family A member 5 (HSPA5), also termed GRP78, is a negative regulator of DHA or ARS-induced ferroptosis in KRAS mutant pancreatic cancer cells and glioma cells (Chen Y. et al., 2019; Wang K. et al., 2019).
The Effects of ARTs on Cancer Metabolism
Metabolic changes have been demonstrated in cancer cells compared to normal non-malignant cells. Warburg effect describes a phenomenon in which, despite the presence of oxygen, cancer cells preferentially metabolize glucose by glycolysis to produce lactate as an end product (Warburg, 1956; Hsu and Sabatini, 2008). ARTs inhibited the glycolysis capacity in various tumor cells. Mi et al. first reported that DHA suppressed glucose uptake and glycolysis in non-small-cell lung carcinoma cells and confirmed the effect associated with inhibiting mTOR activity and reducing glucose transporter 1 (GLUT1) expression (Mi et al., 2015). Subsequently, Vatsveen et al. observed that ARS decreased glycolysis capacity and mitochondrial respiration capacity in B-cell lymphoma cells although detailed mechanisms remain to be elucidated (Vatsveen et al., 2018). However, inhibition of glycolysis of DHA was illustrated via inhibiting PI3K/AKT pathway, downregulating HIF-1α expression and down-regulating pyruvate kinase M2 (Li S. et al., 2019; Zhu et al., 2019; Gao et al., 2020). The oxidative pentose phosphate pathway, another catabolic pathway of glucose, is important for tumor growth and cancer cell metabolism. Elf et al. (2017) reported that targeting 6-phosphogluconate dehydrogenase could sensitize leukemia cells to DHA in oxidative pentose phosphate pathway. Besides an effect on glycolytic metabolism, ARS inhibited HCT116 colon cancer cell proliferation by suppressing the fatty acid biosynthetic pathway, mainly downregulating three proteins: acyl-CoA synthetase 5, hydroxyacyl-coenzyme A dehydrogenase and fatty acid synthase (Chen X. et al., 2017).
The Effects of ARTs on Immunosuppression
The immunosuppressive properties of ARTs in tumor cells have been reported. ART hampered 4T1 tumor growth by promoting T cell activation and quelling immunosuppression from Tregs and MDSCs (Cao et al., 2019). After ART treatment, MDSCs and Tregs frequencies were significantly decreased and those of D4+ interferon γ+ T cells and cytotoxic T lymphocytes were significantly increased. The mRNA levels of T-bet, interferon γ, and tumor necrosis factor α were significantly increased and the mRNA level of transforming growth factor β (TGF-β) was significantly decreased. However, the expressions of interleukin 10 (IL-10) and forkhead box P3 (Foxp3) did not change significantly, inconsistent with previous reports (Zhang et al., 2014; Cui et al., 2015). The level of IL-10 was decreased greatly in colorectal cancer and that of Foxp3 decreased in cervical cancer after ARS treatment. In colorectal cancer, ARS downregulated the immunosuppression by decreasing TGF-β1 and IL-10 levels (Cui et al., 2015), which will be beneficial for colorectal tumor patients with higher TGF-β1 and IL-10. In cervical cancer, ARS inhibited prostaglandin production, which in turn led to the decreased expression of Foxp3 in T cells (Zhang et al., 2014). In addition, the expression of miRNAs was affected by ARS treatment, leading to regulation of immunosuppression. In ovarian cancer, ARS up-regulated miR-142 that in turn suppressed Sirt1 level and promoted T helper 1 cell differentiation, thereby enhancing cell apoptosis (Chen X. et al., 2019). In bladder cancer, ARS up-regulated miR-16 expression, which decreased cyclooxygenase 2 expression and prostaglandin production (Jiang et al., 2018).
The Effects of ARTs on Cancer Stem Cells
Cancer stem cells (CSCs) have stem-like properties, with a unique ability for self-renewal, proliferation and differentiation. They play a crucial role in tumor occurrence, metastasis and recurrence (Reya et al., 2001). Recent studies showed that ARTs inhibited the expression of the CSC markers Nanog, Oct3/4, ALDH1, CD44 and Sry-related high mobility group box (SOX2) and cell sphere formation ability (Tong et al., 2016; Chen S. et al., 2020), exhibited anti-CSC proliferation (Cao et al., 2014) and induced CSC apoptosis (Li et al., 2014). They even synergistically enhance anti-CSC proliferation of chemotherapeutic drugs (Berte et al., 2016). Mechanisms included inhibiting p-AKT and activating caspase-3, disturbing mitochondrial metabolism and down-regulating the expression of RAD51, an important component of DNA double-strand break repair (Cao et al., 2014; Berte et al., 2016; Subedi et al., 2016). PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways were involved in the inhibition of cancer cells stemness of ARTs (Tong et al., 2016; Chen S. et al., 2020). These new findings imply that ARTs can use as CSCs inhibitor for cancer therapy.
Clinical Trials
We searched for clinical trials of ARTs at ClinicalTrials.gov. and in PubMed. ClinicalTrials.gov search results by keywords “cancer” and “artesunate” show that some clinical trials of ARS have entered phase II status in recent years. Different modes of administration are used for different cancers. However, no solid final results can be seen yet. In Pubmed, there are only 3 studies in the last 3 years. We summarize the latest research results in 2019 because some previous clinical studies in dogs and humans have been comprehensively reviewed by Efferth and Zhang et al. (Efferth, 2017b; Zhang Y. et al., 2018).
Recent clinical studies for treating cancer focus on ARS. The results showed dose-limiting toxicities could be seen at dosages of 12, 18 and 25 mg/kg by intravenous ARS in patients with advanced solid tumor malignancies and the maximum tolerated dose was 18 mg/kg using day1/day8, 3-week cycle of administration (Deeken et al., 2018). The adverse events of auditory and vestibular system in patients with metastatic or locally advanced breast cancer after 4 weeks of add-on therapy with oral ARS possibly related to the intake of ARS. However, none of audiological results showed any dose-limiting auditory toxicity and adverse events was fully reversible after discontinuation of ARS, indicating that audiological monitoring in further clinical studies with prolonged use of oral ARS in doses up to 200 mg daily is necessary (Konig et al., 2016). Subsequently, a short-term dose-finding study in metastatic breast cancer patients also showed a well-tolerated dose of oral ARS was 200 mg (2.2–3.9 mg/kg/d). Three patients experienced leucopenia, neutropenia, asthenia, anemia, dose-limiting adverse events altogether, possibly related to ARS (Von Hagens et al., 2017). However, short term trials only reveal limited safety information, rarely generally needed for long-term treatment in advanced cancer. Von Hagens et al. reported a long term add-on therapy (compassionate use) study with oral ARS in metastatic breast cancer after participating in a phase I study (ARTIC M33/2) to ensure adequate individual safety and tolerability (Von Hagens et al., 2019). 13 patients continued the add-on therapy as compassionate use. A total of 25 adverse events grade ≥ 2 at least possibly related to ARS long-term add-on therapy were documented, two, six and seventeen in dose groups 100, 150 and 200 mg/d ARS respectively. Six of these adverse events were classified as grade 3, two in patients taking 150 and four in patients on 200 mg/d, indicating the dose-dependent toxicity. However, none of them was probably or certainly related to ARS.
In summary, ARS was well tolerated and safe in patients with solid tumor malignancies (Deeken et al., 2018) and metastatic breast cancer (Konig et al., 2016; Von Hagens et al., 2017; Von Hagens et al., 2019). However, safety monitoring by reminders on ARS administration to detect the occurrence of side-effects must be considered especially when ARS is used in high dose. If necessary, drugs to prevent side-effects should be combined. Moreover, although it is difficult to differentiate between disease-related and drug-induced adverse events in cancer patients, current results show that adverse events were possibly related to the intake of ARS. Whether adverse events are certainly induced by ARS should be determined in future clinical trials. Some factors e.g. mode of administration, dosage and internal and duration of drug administration influence the safety and efficacy. A detailed examination of these factors needs to be taken seriously in future. Finally, currently available phase I clinical trial results of ARTs for treating cancer are still largely limited and the number of participating patients is small. In future, large scale phase II, III and IV clinical trials are needed to provide more convincing evidence for the suitability of ARS in clinical oncology, and more clinical trials of other ARTs such as DHA, ARM and ARE etc. should be advanced.
Summary and Outlook
ARTs which are already established as safe drugs for treating malaria, possess a host of advantages that make them worthy of development as novel anticancer agents. They differ from available anticancer drugs because of the characteristics of high selectivity and efficacity against multiple cancers in cell and biological models as well as more sensitivity to chemoradiotherapy and less susceptibility to resistance. In this review, we summarized novel cancer therapeutic approaches based on ARTs, the latest molecular mechanisms of action and clinical studies. As a whole, ARTs have great potential to be used in clinical oncology, but there are still many problems to be solved.
First, because of poor solubility, short half-life, low bioavailability and toxicity of ARTs, developing novel derivatives and nanodrugs were applied to solve these problems. However, despite the cost savings in drug development by structural modification of ARTs to obtain candidates, updated preclinical and clinical studies of these modified derivatives are still limited. Besides, the aim of developing nanodrugs is to improve the physicochemical property of ARTs, but subsequent pharmacokinetic studies of these nanodrugs are rarely reported. Whether these new preparations actually improve the pharmacokinetic parameters needs to be further clarified.
Second, though the promising anticancer activity of ARTs has been identified in vitro and in vivo, the dose used is at μM level, still high in comparison with nM level of antimalarial activity. The higher the dose, the greater the possible side effects. Dose-dependent neurotoxicity has become the biggest obstacle to develop ARTs as clinical drugs (Genovese et al., 2000; Li et al., 2005). The toxicity to neuron should be considered when screening drugs. Furthermore, monitoring side-effect, especially neurotoxicity must be performed in future to successfully obtain promising drug candidates.
Third, the accurate mechanism of action is still controversial, and the target of action has not been completely elucidated. Current studies show that ARTs can affect tumor progression by multi-approaches and multi-links. Apoptotic cell death and non-apoptotic cell death are both involved in the antitumor activity of ARTs. In addition, ARTs have effects on cancer metabolism, immunosuppression and CSCs. However, the related literature is still relatively scarce. More in-depth research into these aspects is needed.
Finally, clinical study of cancer patients needs to be advanced for not only first-generation derivatives but also newly developed derivatives to obtain more comprehensive information of ARTs. The eventual development of such derivatives into approved drugs for cancer chemotherapy will be enormously important.
Author Contributions
CX and XY conceived, designed, drafted the manuscript, and amended the paper. HZ designed the figures and tables. LM collected the related research articles. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Scientific Research Project of Education Department in Hunan Province (17C0973) to CX; and the National Natural Science Foundation of China (81874212); the Key Laboratory of Study and Discovery of Small Targeted Molecules of Hunan Province, Hunan Normal University (2017TP1020); and Huxiang High-Level Talent Innovation Team (2018RS3072) to XY.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
Akt, protein kinase B; Bak, Bcl-2 homologous antagonist/killer; Bcl-2, B cell lymphoma-2; Bax, Bcl-2–associated X protein; PI3K, phosphatidylinositol 3-kinase; mTOR, mammalian target of rapamycin; LC3, light chain 3; CaMKK2, Ca2+/calmodulin-dependent kinase kinase 2; ULK1, unc-51 like autophagy activating kinase 1; PI3KC3, class III phosphatidylinositol 3-kinase; T-bet, T-box expressed in T cells; MEK, MAPK - mitogen activated protein kinase; ERK, extracellular signal-regulated kinase; Nrf-2, nuclear factor (erythroid‐derived 2)‐like 2; VEGF, vascular endothelial growth factor; JNK, c-Jun-N-terminal kinase; γ-H2AX, phosphorylated histone H2AX; DSB, double-strand break; IFI 16, Interferon-inducible 16; IL, interleukin; ATF4, activating transcription factor 4; CHOP, C/EBP homologous protein; CHAC1, cation transport regulator-like protein 1; ALDH1, aldehyde dehydrogenase-1.
References
- An F. F., Liu Y. C., Zhang W. W., Liang L. (2013). Dihydroartemisinine enhances dictamnine-induced apoptosis via a caspase dependent pathway in human lung adenocarcinoma A549 cells. Asian Pac. J. Cancer Prev. 14, 5895–5900. 10.7314/apjcp.2013.14.10.5895 [DOI] [PubMed] [Google Scholar]
- Battogtokh G., Choi Y. S., Kang D. S., Park S. J., Shim M. S., Huh K. M., et al. (2018). Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: current strategies and future perspectives. Acta Pharm. Sin. B 8, 862–880. 10.1016/j.apsb.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berte N., Lokan S., Eich M., Kim E., Kaina B. (2016). Artesunate enhances the therapeutic response of glioma cells to temozolomide by inhibition of homologous recombination and senescence. Oncotarget 7, 67235–67250. 10.18632/oncotarget.11972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhraja A., Turnis M. E., Churchman M. L., Kothari A., Yang X., Xu H., et al. (2017). Modulation of Navitoclax Sensitivity by Dihydroartemisinin-Mediated MCL-1 Repression in BCR-ABL(+) B-Lineage Acute Lymphoblastic Leukemia. Clin. Cancer Res. 23, 7558–7568. 10.1158/1078-0432.ccr-17-1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Duanmu W., Yin Y., Zhou Z., Ge H., Chen T., et al. (2014). Dihydroartemisinin exhibits anti-glioma stem cell activity through inhibiting p-AKT and activating caspase-3. Pharmazie 69, 752–758. 10.1691/ph.2014.4600. [DOI] [PubMed] [Google Scholar]
- Cao Y., Feng Y. H., Gao L. W., Li X. Y., Jin Q. X., Wang Y. Y., et al. (2019). Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int. Immunopharmacol. 70, 110–116. 10.1016/j.intimp.2019.01.041 [DOI] [PubMed] [Google Scholar]
- Capci A., Lorion M. M., Wang H., Simon N., Leidenberger M., Borges Silva M. C., et al. (2019). Artemisinin-(Iso)quinoline Hybrids by C-H Activation and Click Chemistry: Combating Multidrug-Resistant Malaria. Angew. Chem. Int. Ed. Engl. 58, 13066–13079. 10.1002/anie.201907224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Li M., Zhang R., Wang H. (2009). Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J. Cell Mol. Med. 13, 1358–1370. 10.1111/j.1582-4934.2008.00360.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Guo Z., Wang H. B., Zhou J. J., Zhang W. J., Chen Q. W. (2014). Multifunctional mesoporous nanoparticles as pH-responsive Fe(2+) reservoirs and artemisinin vehicles for synergistic inhibition of tumor growth. Biomaterials 35, 6498–6507. 10.1016/j.biomaterials.2014.04.028 [DOI] [PubMed] [Google Scholar]
- Chen X., He L. Y., Lai S., He Y. (2020). Dihydroartemisinin inhibits the migration of esophageal cancer cells by inducing autophagy. Oncol. Lett. 20, 94. 10.3892/ol.2020.11955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. Q., Benthani F. A., Wu J., Liang D., Bian Z. X., Jiang X. (2020). Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 27, 242–254. 10.1038/s41418-019-0352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Gu S., Dai H., Li X., Zhang Z. (2017). Dihydroartemisinin Sensitizes Human Lung Adenocarcinoma A549 Cells to Arsenic Trioxide via Apoptosis. Biol. Trace Elem. Res. 179, 203–212. 10.1007/s12011-017-0975-5 [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang W., Zhang M., Guo Z., Wang H., He M., et al. (2015). Mn(II) mediated degradation of artemisinin based on Fe3O4@MnSiO3-FA nanospheres for cancer therapy in vivo. Nanoscale 7, 12542–12551. 10.1039/c5nr02402a [DOI] [PubMed] [Google Scholar]
- Chen J., Huang X., Tao C., Xiao T., Li X., Zeng Q., et al. (2019). Artemether Attenuates the Progression of Non-small Cell Lung Cancer by Inducing Apoptosis, Cell Cycle Arrest and Promoting Cellular Senescence. Biol. Pharm. Bull. 42, 1720–1725. 10.1248/bpb.b19-00391 [DOI] [PubMed] [Google Scholar]
- Chen S., Gan S., Han L., Li X., Xie X., Zou D., et al. (2020). Artesunate induces apoptosis and inhibits the proliferation, stemness, and tumorigenesis of leukemia. Ann. Transl. Med. 8, 767. 10.21037/atm-20-4558 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen S. S., Hu W., Wang Z., Lou X. E., Zhou H. J. (2015). p8 attenuates the apoptosis induced by dihydroartemisinin in cancer cells through promoting autophagy. Cancer Biol. Ther. 16, 770–779. 10.1080/15384047.2015.1026477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wong Y. K., Lim T. K., Lim W. H., Lin Q., Wang J., et al. (2017). Artesunate Activates the Intrinsic Apoptosis of HCT116 Cells through the Suppression of Fatty Acid Synthesis and the NF-kappaB Pathway. Molecules, 22, 1272. 10.3390/molecules22081272 [DOI] [PMC free article] [PubMed]
- Chen X., Zhang X. L., Zhang G. H., Gao Y. F. (2019). Artesunate promotes Th1 differentiation from CD4+ T cells to enhance cell apoptosis in ovarian cancer via miR-142. Braz. J. Med. Biol. Res. 52, e7992. 10.1590/1414-431x20197992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Mi Y., Zhang X., Ma Q., Song Y., Zhang L., et al. (2019). Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J. Exp. Clin. Cancer Res. 38, 402. 10.1186/s13046-019-1413-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li R., Zhu Y., Zhong S., Qian J., Yang D., et al. (2020). Dihydroartemisinin Induces Growth Arrest and Overcomes Dexamethasone Resistance in Multiple Myeloma. Front. Oncol. 10:767:767. 10.3389/fonc.2020.00767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Wang T., Song Z., Peng L., Gao M., Hermine O., et al. (2018). Induction of autophagy and autophagy-dependent apoptosis in diffuse large B-cell lymphoma by a new antimalarial artemisinin derivative, SM1044. Cancer Med. 7, 380–396. 10.1002/cam4.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Ortiz M. P., Wei M. Q. (2012). Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J. BioMed. Biotechnol. 2012:247597. 10.1155/2012/247597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Su X. Z. (2009). Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev. Anti Infect. Ther. 7, 999–1013. 10.1586/eri.09.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C., Feng H., Shi X., Wang Y., Feng Z., Liu J., et al. (2015). Artesunate down-regulates immunosuppression from colorectal cancer Colon26 and RKO cells in vitro by decreasing transforming growth factor beta1 and interleukin-10. Int. Immunopharmacol. 27, 110–121. 10.1016/j.intimp.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Das S. S., Nanda G. G., Alone D. P. (2014). Artemisinin and curcumin inhibit Drosophila brain tumor, prolong life span, and restore locomotor activity. IUBMB Life 66, 496–506. 10.1002/iub.1284 [DOI] [PubMed] [Google Scholar]
- Deeken J. F., Wang H., Hartley M., Cheema A. K., Smaglo B., Hwang J. J., et al. (2018). A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemother. Pharmacol. 81, 587–596. 10.1007/s00280-018-3533-8 [DOI] [PubMed] [Google Scholar]
- Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’arcy M. S. (2019). Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 43, 582–592. 10.1002/cbin.11137 [DOI] [PubMed] [Google Scholar]
- Drenberg C. D., Buaboonnam J., Orwick S. J., Hu S., Li L., Fan Y., et al. (2016). Evaluation of artemisinins for the treatment of acute myeloid leukemia. Cancer Chemother. Pharmacol. 77, 1231–1243. 10.1007/s00280-016-3038-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J.-H., Zhang H.-D., Ma Z.-J., Ji K.-M. (2010). Artesunate induces oncosis-like cell death in vitro and has antitumor activity against pancreatic cancer xenografts in vivo. Cancer Chemother. Pharmacol. 65, 895–902. 10.1007/s00280-009-1095-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X. X., Li Y. J., Wu C. L., Zhou J. H., Han Y., Sui H., et al. (2013). Initiation of apoptosis, cell cycle arrest and autophagy of esophageal cancer cells by dihydroartemisinin. BioMed. Pharmacother. 67, 417–424. 10.1016/j.biopha.2013.01.013 [DOI] [PubMed] [Google Scholar]
- Du J., Wang T., Li Y., Zhou Y., Wang X., Yu X., et al. (2019). DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic. Biol. Med. 131, 356–369. 10.1016/j.freeradbiomed.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Efferth T., Ramirez T., Gebhart E., Halatsch M. E. (2004). Combination treatment of glioblastoma multiforme cell lines with the anti-malarial artesunate and the epidermal growth factor receptor tyrosine kinase inhibitor OSI-774. Biochem. Pharmacol. 67, 1689–1700. 10.1016/j.bcp.2003.12.035 [DOI] [PubMed] [Google Scholar]
- Efferth T. (2017. a). Cancer combination therapy of the sesquiterpenoid artesunate and the selective EGFR-tyrosine kinase inhibitor erlotinib. Phytomedicine 37, 58–61. 10.1016/j.phymed.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Efferth T. (2017. b). From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 46, 65–83. 10.1016/j.semcancer.2017.02.009 [DOI] [PubMed] [Google Scholar]
- Elf S., Lin R., Xia S., Pan Y., Shan C., Wu S., et al. (2017). Targeting 6-phosphogluconate dehydrogenase in the oxidative PPP sensitizes leukemia cells to antimalarial agent dihydroartemisinin. Oncogene 36, 254–262. 10.1038/onc.2016.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami J., Yousefian H., Sadeghi H. (2018). Targeted Nanostructured Lipid Carrier for Brain Delivery of Artemisinin: Design, Preparation, Characterization, Optimization and Cell Toxicity. J. Pharm. Pharm. Sci. 21, 225s–241s. 10.18433/jpps30117 [DOI] [PubMed] [Google Scholar]
- Feng X., Li L., Jiang H., Jiang K., Jin Y., Zheng J. (2014). Dihydroartemisinin potentiates the anticancer effect of cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer cells: involvement of apoptosis and autophagy. Biochem. Biophys. Res. Commun. 444, 376–381. 10.1016/j.bbrc.2014.01.053 [DOI] [PubMed] [Google Scholar]
- Feng G., Liu J., Zhang C. J., Liu B. (2018). Artemisinin and AIEgen Conjugate for Mitochondria-Targeted and Image-Guided Chemo- and Photodynamic Cancer Cell Ablation. ACS Appl. Mater. Interfaces 10, 11546–11553. 10.1021/acsami.8b01960 [DOI] [PubMed] [Google Scholar]
- Firestone G. L., Sundar S. N. (2009). Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev. Mol. Med. 11, e32. 10.1017/s1462399409001239 [DOI] [PubMed] [Google Scholar]
- Frohlich T., Capci Karagoz A., Reiter C., Tsogoeva S. B. (2016). Artemisinin-Derived Dimers: Potent Antimalarial and Anticancer Agents. J. Med. Chem. 59, 7360–7388. 10.1021/acs.jmedchem.5b01380 [DOI] [PubMed] [Google Scholar]
- Fröhlich T., Ndreshkjana B., Muenzner J. K., Reiter C., Hofmeister E., Mederer S., et al. (2017). Synthesis of Novel Hybrids of Thymoquinone and Artemisinin with High Activity and Selectivity Against Colon Cancer. ChemMedChem 12, 226–234. 10.1002/cmdc.201600594 [DOI] [PubMed] [Google Scholar]
- Fröhlich T., Reiter C., Saeed M. E. M., Hutterer C., Hahn F., Leidenberger M., et al. (2018). Synthesis of Thymoquinone-Artemisinin Hybrids: New Potent Antileukemia, Antiviral, and Antimalarial Agents. ACS Med. Chem. Lett. 9, 534–539. 10.1021/acsmedchemlett.7b00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich T., Mai C., Bogautdinov R. P., Morozkina S. N., Shavva A. G., Friedrich O., et al. (2020). Synthesis of Tamoxifen-Artemisinin and Estrogen-Artemisinin Hybrids Highly Potent Against Breast and Prostate Cancer. ChemMedChem 15, 1473–1479. 10.1002/cmdc.202000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Li Y., You C., Sun K., An P., Sun C., et al. (2018). Iron Oxide Nanocarrier-Mediated Combination Therapy of Cisplatin and Artemisinin for Combating Drug Resistance through Highly Increased Toxic Reactive Oxygen Species Generation. ACS Appl. Bio Mater. 1, 270–280. 10.1021/acsabm.8b00056 [DOI] [PubMed] [Google Scholar]
- Gao P., Shen S., Li X., Liu D., Meng Y., Liu Y., et al. (2020). Dihydroartemisinin Inhibits the Proliferation of Leukemia Cells K562 by Suppressing PKM2 and GLUT1 Mediated Aerobic Glycolysis. Drug Des. Devel. Ther. 14, 2091–2100. 10.2147/dddt.s248872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese R. F., Newman D. B., Brewer T. G. (2000). Behavioral and neural toxicity of the artemisinin antimalarial, arteether, but not artesunate and artelinate, in rats. Pharmacol. Biochem. Behav. 67, 37–44. 10.1016/s0091-3057(00)00309-9 [DOI] [PubMed] [Google Scholar]
- Gharib A., Faezizadeh Z., Mesbah-Namin S. A., Saravani R. (2014). Preparation, characterization and in vitro efficacy of magnetic nanoliposomes containing the artemisinin and transferrin. Daru 22, 44. 10.1186/2008-2231-22-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein P., Kroemer G. (2007). Cell death by necrosis: towards a molecular definition. Trends Biochem. Sci. 32, 37–43. 10.1016/j.tibs.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Greenshields A. L., Fernando W., Hoskin D. W. (2019). The anti-malarial drug artesunate causes cell cycle arrest and apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3 breast cancer cells. Exp. Mol. Pathol. 107, 10–22. 10.1016/j.yexmp.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Guan X., Guan Y. (2020). Artemisinin induces selective and potent anticancer effects in drug resistant breast cancer cells by inducing cellular apoptosis and autophagy and G2/M cell cycle arrest. J. buon 25, 1330–1336. [PubMed] [Google Scholar]
- Gururaja Rao S. (2017). Mitochondrial Changes in Cancer. Handb. Exp. Pharmacol. 240, 211–227. 10.1007/164_2016_40 [DOI] [PubMed] [Google Scholar]
- Hao D. L., Xie R., De G. J., Yi H., Zang C., Yang M. Y., et al. (2020). pH-Responsive Artesunate Polymer Prodrugs with Enhanced Ablation Effect on Rodent Xenograft Colon Cancer. Int. J. Nanomed. 15, 1771–1786. 10.2147/ijn.s242032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W. E., Peh H. Y., Chan T. K., Wong W. S. (2014). Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol. Ther. 142, 126–139. 10.1016/j.pharmthera.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Hong J., Ai-Ying J., Han W., Yong C., Yan W., Xiao-Feng J. (2017). Dihydroartemisinin and gefitinib synergistically inhibit NSCLC cell growth and promote apoptosis via the Akt/mTOR/STAT3 pathway. Mol. Med. Rep. 16 (3), 3475–3481. 10.3892/mmr.2017.6989 [DOI] [PubMed] [Google Scholar]
- Hsu P. P., Sabatini D. M. (2008). Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707. 10.1016/j.cell.2008.08.021 [DOI] [PubMed] [Google Scholar]
- Hu Y., Li N., Zhang J., Wang Y., Chen L., Sun J. (2019). Artemisinin-indole and artemisinin-imidazole hybrids: Synthesis, cytotoxic evaluation and reversal effects on multidrug resistance in MCF-7/ADR cells. Bioorg. Med. Chem. Lett. 29, 1138–1142. 10.1016/j.bmcl.2019.02.021 [DOI] [PubMed] [Google Scholar]
- Jeong D. E., Song H. J. J., Lim S., Lee S. J. J., Lim J. E., Nam D.-H., et al. (2015). Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget 6, 33046–33064. 10.18632/oncotarget.5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Kong R., Ma Z. B., Han B., Wang Y. W., Pan S. H., et al. (2014). The activation of c-Jun NH(2)-terminal kinase is required for dihydroartemisinin-induced autophagy in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 33, 8. 10.1186/1756-9966-33-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J., Qin Y., Zhang L., Guo C., Wang Y., Yue X., et al. (2016). Artemisinin inhibits gallbladder cancer cell lines through triggering cell cycle arrest and apoptosis. Mol. Med. Rep. 13, 4461–4468. 10.3892/mmr.2016.5073 [DOI] [PubMed] [Google Scholar]
- Jiang F., Zhou J. Y., Zhang D., Liu M. H., Chen Y. G. (2018). Artesunate induces apoptosis and autophagy in HCT116 colon cancer cells, and autophagy inhibition enhances the artesunateinduced apoptosis. Int. J. Mol. Med. 42, 1295–1304. 10.3892/ijmm.2018.3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Jiang A. Y., Wang H., Cao Y., Wu Y., Jiang X. F. (2017). Dihydroartemisinin and gefitinib synergistically inhibit NSCLC cell growth and promote apoptosis via the Akt/mTOR/STAT3 pathway. Mol. Med. Rep. 16, 3475–3481. 10.3892/mmr.2017.6989 [DOI] [PubMed] [Google Scholar]
- Jin L., Dai L., Ji M., Wang H. (2019). Mitochondria-targeted triphenylphosphonium conjugated glycyrrhetinic acid derivatives as potent anticancer drugs. Bioorg. Chem. 85, 179–190. 10.1016/j.bioorg.2018.12.036 [DOI] [PubMed] [Google Scholar]
- Klayman D. L. (1985). Qinghaosu (artemisinin): an antimalarial drug from China. Science 228, 1049–1055. 10.1126/science.3887571 [DOI] [PubMed] [Google Scholar]
- Konig M., Von Hagens C., Hoth S., Baumann I., Walter-Sack I., Edler L., et al. (2016). Investigation of ototoxicity of artesunate as add-on therapy in patients with metastatic or locally advanced breast cancer: new audiological results from a prospective, open, uncontrolled, monocentric phase I study. Cancer Chemother. Pharmacol. 77, 413–427. 10.1007/s00280-016-2960-7 [DOI] [PubMed] [Google Scholar]
- Letis A. S., Seo E. J., Nikolaropoulos S. S., Efferth T., Giannis A., Fousteris M. A. (2017). Synthesis and cytotoxic activity of new artemisinin hybrid molecules against human leukemia cells. Bioorg. Med. Chem. 25, 3357–3367. 10.1016/j.bmc.2017.04.021 [DOI] [PubMed] [Google Scholar]
- Leto I., Coronnello M., Righeschi C., Bergonzi M. C., Mini E., Bilia A. R. (2016). Enhanced Efficacy of Artemisinin Loaded in Transferrin-Conjugated Liposomes versus Stealth Liposomes against HCT-8 Colon Cancer Cells. ChemMedChem 11, 1745–1751. 10.1002/cmdc.201500586 [DOI] [PubMed] [Google Scholar]
- Li Y., Wu Y. L. (2003). An over four millennium story behind qinghaosu (artemisinin)–a fantastic antimalarial drug from a traditional chinese herb. Curr. Med. Chem. 10, 2197–2230. 10.2174/0929867033456710 [DOI] [PubMed] [Google Scholar]
- Li Q., Xie L. H., Si Y., Wong E., Upadhyay R., Yanez D., et al. (2005). Toxicokinetics and hydrolysis of artelinate and artesunate in malaria-infected rats. Int. J. Toxicol. 24, 241–250. 10.1080/10915810591007201 [DOI] [PubMed] [Google Scholar]
- Li X. Y., Zhao Y., Sun M. G., Shi J. F., Ju R. J., Zhang C. X., et al. (2014). Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials 35, 5591–5604. 10.1016/j.biomaterials.2014.03.049 [DOI] [PubMed] [Google Scholar]
- Li X., Zhou Y., Liu Y., Zhang X., Chen T., Chen K., et al. (2016). Preclinical Efficacy and Safety Assessment of Artemisinin-Chemotherapeutic Agent Conjugates for Ovarian Cancer. EBioMedicine 14, 44–54. 10.1016/j.ebiom.2016.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang Y., Zhou Y., Li J., Chen K., Zhang L., et al. (2017). Cooperative effect of chidamide and chemotherapeutic drugs induce apoptosis by DNA damage accumulation and repair defects in acute myeloid leukemia stem and progenitor cells. Clin. Epigenet. 9, 83. 10.1186/s13148-017-0377-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. J., Dai H. Q., Huang X. W., Feng J., Deng J. H., Wang Z. X., et al. (2020). Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol. Sin. 10.1038/s41401-020-0478-3 [DOI] [PMC free article] [PubMed]
- Li H., Li X., Shi X., Li Z., Sun Y. (2019). Effects of magnetic dihydroartemisinin nano-liposome in inhibiting the proliferation of head and neck squamous cell carcinomas. Phytomedicine 56, 215–228. 10.1016/j.phymed.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Li S., Huang P., Gan J., Ling X., Du X., Liao Y., et al. (2019). Dihydroartemisinin represses esophageal cancer glycolysis by down-regulating pyruvate kinase M2. Eur. J. Pharmacol. 854, 232–239. 10.1016/j.ejphar.2019.04.018 [DOI] [PubMed] [Google Scholar]
- Lin R., Zhang Z., Chen L., Zhou Y., Zou P., Feng C., et al. (2016). Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 381, 165–175. 10.1016/j.canlet.2016.07.033 [DOI] [PubMed] [Google Scholar]
- Liu Y., Cui Y. F. (2013). Synergism of cytotoxicity effects of triptolide and artesunate combination treatment in pancreatic cancer cell lines. Asian Pac. J. Cancer Prev. 14, 5243–5248. 10.7314/apjcp.2013.14.9.5243 [DOI] [PubMed] [Google Scholar]
- Liu X., Wu J., Fan M., Shen C., Dai W., Bao Y., et al. (2018). Novel dihydroartemisinin derivative DHA-37 induces autophagic cell death through upregulation of HMGB1 in A549 cells. Cell Death Dis. 9, 1048. 10.1038/s41419-018-1006-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. J., Tang W., Fu M., Gong X. Q., Kong L., Yao X. M., et al. (2019). Development of R8 modified epirubicin-dihydroartemisinin liposomes for treatment of non-small-cell lung cancer. Artif. Cells Nanomed. Biotechnol. 47, 1947–1960. 10.1080/21691401.2019.1615932 [DOI] [PubMed] [Google Scholar]
- Liu X., Cao J., Huang G., Zhao Q., Shen J. (2019). Biological Activities of Artemisinin Derivatives Beyond Malaria. Curr. Top. Med. Chem. 19, 205–222. 10.2174/1568026619666190122144217 [DOI] [PubMed] [Google Scholar]
- Lu B., Chen X. B., Ying M. D., He Q. J., Cao J., Yang B. (2017). The Role of Ferroptosis in Cancer Development and Treatment Response. Front. Pharmacol. 8, 992. 10.3389/fphar.2017.00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G. T., Lee S. K., Park K. K., Park J., Son S. H., Jung M., et al. (2018). Artemisinin-Daumone Hybrid Inhibits Cancer Cell-Mediated Osteolysis by Targeting Cancer Cells and Osteoclasts. Cell Physiol. Biochem. 49, 1460–1475. 10.1159/000493449 [DOI] [PubMed] [Google Scholar]
- Ma Q., Liao H., Xu L., Li Q., Zou J., Sun R., et al. (2020). Autophagy-dependent cell cycle arrest in esophageal cancer cells exposed to dihydroartemisinin. Chin. Med. 15, 37. 10.1186/s13020-020-00318-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Joris I. (1995). Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 146, 3–15. [PMC free article] [PubMed] [Google Scholar]
- Marchesi E., Chinaglia N., Capobianco M. L., Marchetti P., Huang T. E., Weng H. C., et al. (2019). Dihydroartemisinin-Bile Acid Hybridization as an Effective Approach to Enhance Dihydroartemisinin Anticancer Activity. ChemMedChem 14, 779–787. 10.1002/cmdc.201800756 [DOI] [PubMed] [Google Scholar]
- Meng H., Xu K., Xu Y., Luo P., Du F., Huang J., et al. (2014). Nanocapsules based on mPEGylated artesunate prodrug and its cytotoxicity. Colloids Surf. B Biointerf. 115, 164–169. 10.1016/j.colsurfb.2013.11.039 [DOI] [PubMed] [Google Scholar]
- Meunier B. (2008). Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem. Res. 41, 69–77. 10.1021/ar7000843 [DOI] [PubMed] [Google Scholar]
- Mi Y. J., Geng G. J., Zou Z. Z., Gao J., Luo X. Y., Liu Y., et al. (2015). Dihydroartemisinin inhibits glucose uptake and cooperates with glycolysis inhibitor to induce apoptosis in non-small cell lung carcinoma cells. PloS One 10, e0120426. 10.1371/journal.pone.0120426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muregi F. W., Ishih A. (2010). Next-Generation Antimalarial Drugs: Hybrid Molecules as a New Strategy in Drug Design. Drug Dev. Res. 71, 20–32. 10.1002/ddr.20345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrati H., Barzegari P., Danafar H., Kheiri Manjili H. (2019). Biotin-functionalized copolymeric PEG-PCL micelles for in vivo tumour-targeted delivery of artemisinin. Artif. Cells Nanomed. Biotechnol. 47, 104–114. 10.1080/21691401.2018.1543199 [DOI] [PubMed] [Google Scholar]
- Nunes J. J., Pandey S. K., Yadav A., Goel S., Ateeq B. (2017). Targeting NF-kappa B Signaling by Artesunate Restores Sensitivity of Castrate-Resistant Prostate Cancer Cells to Antiandrogens. Neoplasia 19, 333–345. 10.1016/j.neo.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooko E., Saeed M. E., Kadioglu O., Sarvi S., Colak M., Elmasaoudi K., et al. (2015). Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 22, 1045–1054. 10.1016/j.phymed.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Pan U. N., Sanpui P., Paul A., Chattopadhyay A. (2018). Synergistic Anticancer Potential of Artemisinin When Loaded with 8-Hydroxyquinoline-Surface Complexed-Zinc Ferrite Magnetofluorescent Nanoparticles and Albumin Composite. ACS Appl. Bio Mater. 1, 1229–1235. 10.1021/acsabm.8b00358 [DOI] [PubMed] [Google Scholar]
- Parzych K. R., Klionsky D. J. (2014). An overview of autophagy: morphology, mechanism, and regulation. Antioxid. Redox Signal 20, 460–473. 10.1089/ars.2013.5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C., Ma J., Liu X., Xue Y., Zheng J., Liu L., et al. (2017). Dihydroartemisinin Exerts Anti-Tumor Activity by Inducing Mitochondrion and Endoplasmic Reticulum Apoptosis and Autophagic Cell Death in Human Glioblastoma Cells. Front. Cell Neurosci. 11, 310. 10.3389/fncel.2017.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S. A., Efferth T., Asangani I. A., Allgayer H. (2010). First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int. J. Cancer 127, 1475–1485. 10.1002/ijc.25315 [DOI] [PubMed] [Google Scholar]
- Reiter C., Fröhlich T., Zeino M., Marschall M., Bahsi H., Leidenberger M., et al. (2015). New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids. Eur. J. Med. Chem. 97, 164–172. 10.1016/j.ejmech.2015.04.053 [DOI] [PubMed] [Google Scholar]
- Reya T., Morrison S. J., Clarke M. F., Weissman I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature 414, 105–111. 10.1038/35102167 [DOI] [PubMed] [Google Scholar]
- Roh J. L., Kim E. H., Jang H., Shin D. (2017). Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 11, 254–262. 10.1016/j.redox.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Panigrahi D. P., Patil S., Bhutia S. K. (2018). Autophagy in health and disease: A comprehensive review. BioMed. Pharmacother. 104, 485–495. 10.1016/j.biopha.2018.05.007 [DOI] [PubMed] [Google Scholar]
- Shi X., Wang L., Li X., Bai J., Li J., Li S., et al. (2017). Dihydroartemisinin induces autophagy-dependent death in human tongue squamous cell carcinoma cells through DNA double-strand break-mediated oxidative stress. Oncotarget 8, 45981–45993. 10.18632/oncotarget.17520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Wang L., Ren L., Li J., Li S., Cui Q., et al. (2019). Dihydroartemisinin, an antimalarial drug, induces absent in melanoma 2 inflammasome activation and autophagy in human hepatocellular carcinoma HepG2215 cells. Phytother. Res. 33, 1413–1425. 10.1002/ptr.6332 [DOI] [PubMed] [Google Scholar]
- Shi X., Li S., Wang L., Li H., Li Z., Wang W., et al. (2020). RalB degradation by dihydroartemisinin induces autophagy and IFI16/caspase-1 inflammasome depression in the human laryngeal squamous cell carcinoma. Chin. Med. 15, 64. 10.1186/s13020-020-00340-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2016). Cancer Statistics 2016. CA: A Cancer J. Clinicians 66, 7–30. 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Stockwin L. H., Han B., Yu S. X., Hollingshead M. G., Elsohly M. A., Gul W., et al. (2009). Artemisinin dimer anticancer activity correlates with heme-catalyzed reactive oxygen species generation and endoplasmic reticulum stress induction. Int. J. Cancer 125, 1266–1275. 10.1002/ijc.24496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi A., Futamura Y., Nishi M., Ryo A., Watanabe N., Osada H. (2016). High-throughput screening identifies artesunate as selective inhibitor of cancer stemness: Involvement of mitochondrial metabolism. Biochem. Biophys. Res. Commun. 477, 737–742. 10.1016/j.bbrc.2016.06.128 [DOI] [PubMed] [Google Scholar]
- Sun C., Cao Y., Zhu P., Zhou B. (2017). A mitochondria-targeting artemisinin derivative with sharply increased antitumor but depressed anti-yeast and anti-malaria activities. Sci. Rep. 7, 45665. 10.1038/srep45665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraweera A., O’byrne K. J., Richard D. J. (2018). Combination Therapy With Histone Deacetylase Inhibitors (HDACi) for the Treatment of Cancer: Achieving the Full Therapeutic Potential of HDACi. Front. Oncol. 8, 92. 10.3389/fonc.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongchot S., Vidoni C., Ferraresi A., Loilome W., Yongvanit P., Namwat N., et al. (2018). Dihydroartemisinin induces apoptosis and autophagy-dependent cell death in cholangiocarcinoma through a DAPK1-BECLIN1 pathway. Mol. Carcinog. 57, 1735–1750. 10.1002/mc.22893 [DOI] [PubMed] [Google Scholar]
- Tien D. D., Giang L. N. T., Anh D. T. T., Dung N. T., Ha T. N., Ha N. T. T., et al. (2016). Synthesis and Cytotoxic Evaluation of Artemisinin-triazole Hybrids. Nat. Prod. Commun. 11, 1789–1792. 10.1177/1934578X1601101204. [DOI] [PubMed] [Google Scholar]
- Tong Y., Liu Y., Zheng H., Zheng L., Liu W., Wu J., et al. (2016). Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/beta-catenin signaling. Oncotarget 7, 31413–31428. 10.18632/oncotarget.8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T. H., Nguyen T. D., Poudel B. K., Nguyen H. T., Kim J. O., Yong C. S., et al. (2015). Development and Evaluation of Artesunate-Loaded Chitosan-Coated Lipid Nanocapsule as a Potential Drug Delivery System Against Breast Cancer. AAPS PharmSciTech 16, 1307–1316. 10.1208/s12249-015-0311-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y. (2016). Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 55, 10210–10226. 10.1002/anie.201601967 [DOI] [PubMed] [Google Scholar]
- Van Cruchten S., Van Den Broeck W. (2002). Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat. Histol. Embryol. 31, 214–223. 10.1046/j.1439-0264.2002.00398.x [DOI] [PubMed] [Google Scholar]
- Vatsveen T. K., Myhre M. R., Steen C. B., Walchli S., Lingjaerde O. C., Bai B., et al. (2018). Artesunate shows potent anti-tumor activity in B-cell lymphoma. J. Hematol. Oncol. 11, 23. 10.1186/s13045-018-0561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hagens C., Walter-Sack I., Goeckenjan M., Osburg J., Storch-Hagenlocher B., Sertel S., et al. (2017). Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2). Breast Cancer Res. Treat 164, 359–369. 10.1007/s10549-017-4261-1 [DOI] [PubMed] [Google Scholar]
- Von Hagens C., Walter-Sack I., Goeckenjan M., Storch-Hagenlocher B., Sertel S., Elsasser M., et al. (2019). Long-term add-on therapy (compassionate use) with oral artesunate in patients with metastatic breast cancer after participating in a phase I study (ARTIC M33/2). Phytomedicine 54, 140–148. 10.1016/j.phymed.2018.09.178 [DOI] [PubMed] [Google Scholar]
- Wang S. J., Gao Y., Chen H., Kong R., Jiang H. C., Pan S. H., et al. (2010). Dihydroartemisinin inactivates NF-kappaB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett. 293, 99–108. 10.1016/j.canlet.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Wang B., Hou D., Liu Q., Wu T., Guo H., Zhang X., et al. (2015). Artesunate sensitizes ovarian cancer cells to cisplatin by downregulating RAD51. Cancer Biol. Ther. 16, 1548–1556. 10.1080/15384047.2015.1071738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhou J., Chen R., Shi R., Xia G., Zhou S., et al. (2016. a). Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4@C@MIL-100(Fe) nanoparticles. Biomaterials 107, 88–101. 10.1016/j.biomaterials.2016.08.039 [DOI] [PubMed] [Google Scholar]
- Wang D., Zhou J., Chen R., Shi R., Zhao G., Xia G., et al. (2016. b). Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 100, 27–40. 10.1016/j.biomaterials.2016.05.027 [DOI] [PubMed] [Google Scholar]
- Wang Z. C., Liu Y., Wang H., Han Q. K., Lu C. (2017). Research on the relationship between artesunate and Raji cell autophagy and apoptosis of Burkitt’s lymphoma and its mechanism. Eur. Rev. Med. Pharmacol. Sci. 21, 2238–2243. [PubMed] [Google Scholar]
- Wang J., Xu C., Liao F. L., Jiang T., Krishna S., Tu Y. (2019). A Temporizing Solution to “Artemisinin Resistance”. N Engl. J. Med. 380, 2087–2089. 10.1056/NEJMp1901233 [DOI] [PubMed] [Google Scholar]
- Wang K., Zhang Z., Wang M., Cao X., Qi J., Wang D., et al. (2019). Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des. Devel. Ther. 13, 2135–2144. 10.2147/dddt.s199459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Zeng G. Z., Yin J. L., Bian Z. X. (2019). Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt’s Lymphoma. Biochem. Biophys. Res. Commun. 519, 533–539. 10.1016/j.bbrc.2019.09.023 [DOI] [PubMed] [Google Scholar]
- Wang Y., Ding Y., Zhao J., Wang C., Gao M., Chi X., et al. (2019). Dihydroartemisinin and doxorubicin co-loaded Soluplus((R))-TPGS mixed micelles: formulation characterization, cellular uptake, and pharmacodynamic studies. Pharm. Dev. Technol. 24, 1125–1132. 10.1080/10837450.2019.1641726 [DOI] [PubMed] [Google Scholar]
- Warburg O. (1956). On the origin of cancer cells. Science 123, 309–314. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- Wei T., Liu J. (2017). Anti-angiogenic properties of artemisinin derivatives (Review). Int. J. Mol. Med. 40, 972–978. 10.3892/ijmm.2017.3085 [DOI] [PubMed] [Google Scholar]
- Weifeng T., Feng S., Xiangji L., Changqing S., Zhiquan Q., Huazhong Z., et al. (2011). Artemisinin inhibits in vitro and in vivo invasion and metastasis of human hepatocellular carcinoma cells. Phytomedicine 18, 158–162. 10.1016/j.phymed.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Wong Y. K., Xu C., Kalesh K. A., He Y., Lin Q., Wong W. S. F., et al. (2017). Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med. Res. Rev. 37, 1492–1517. 10.1002/med.21446 [DOI] [PubMed] [Google Scholar]
- Xie L. H., Johnson T. O., Weina P. J., Si Y., Haeberle A., Upadhyay R., et al. (2005). Risk assessment and therapeutic indices of artesunate and artelinate in Plasmodium berghei-infected and uninfected rats. Int. J. Toxicol. 24, 251–264. 10.1080/10915810591007229 [DOI] [PubMed] [Google Scholar]
- Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., et al. (2016). Ferroptosis: process and function. Cell Death Differ. 23, 369–379. 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C. C., Deng T., Fan M. L., Lv W. B., Liu J. H., Yu B. Y. (2016). Synthesis and in vitro antitumor evaluation of dihydroartemisinin-cinnamic acid ester derivatives. Eur. J. Med. Chem. 107, 192–203. 10.1016/j.ejmech.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Yan X., Li P., Zhan Y., Qi M., Liu J., An Z., et al. (2018). Dihydroartemisinin suppresses STAT3 signaling and Mcl-1 and Survivin expression to potentiate ABT-263-induced apoptosis in Non-small Cell Lung Cancer cells harboring EGFR or RAS mutation. Biochem. Pharmacol. 150, 72–85. 10.1016/j.bcp.2018.01.031 [DOI] [PubMed] [Google Scholar]
- Yang Y., He J., Chen J., Lin L., Liu Y., Zhou C., et al. (2019). Dihydroartemisinin Sensitizes Mutant p53 (R248Q)-Expressing Hepatocellular Carcinoma Cells to Doxorubicin by Inhibiting P-gp Expression. BioMed. Res. Int. 2019, 8207056. 10.1155/2019/8207056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Bhandari A., Wang Y., Pan Y., Yang F., Chen R., et al. (2018). Dihydroartemisinin potentiates antitumor activity of 5-fluorouracil against a resistant colorectal cancer cell line. Biochem. Biophys. Res. Commun. 501, 636–642. 10.1016/j.bbrc.2018.05.026 [DOI] [PubMed] [Google Scholar]
- Ye Y., Zhang T., Yuan H., Li D., Lou H., Fan P. (2017). Mitochondria-Targeted Lupane Triterpenoid Derivatives and Their Selective Apoptosis-Inducing Anticancer Mechanisms. J. Med. Chem. 60, 6353–6363. 10.1021/acs.jmedchem.7b00679 [DOI] [PubMed] [Google Scholar]
- Yu H., Hou Z., Tian Y., Mou Y., Guo C. (2018). Design, synthesis, cytotoxicity and mechanism of novel dihydroartemisinin-coumarin hybrids as potential anti-cancer agents. Eur. J. Med. Chem. 151, 434–449. 10.1016/j.ejmech.2018.04.005 [DOI] [PubMed] [Google Scholar]
- Zborovskii Y. L., Orysyk V. V., Golovynska I., Dzhus O. I., Garmanchuk L. V., Stepanov Y. V., et al. (2018). Novel Hybrid Compound 4-[(E)-2-phenylethenesulfonamido]-N-hydroxybutanamide with Antimetastatic and Cytotoxic Action: Synthesis and Anticancer Screening. Anticancer Agents Med. Chem. 18, 1495–1504. 10.2174/1871520618666180313151503 [DOI] [PubMed] [Google Scholar]
- Zhang B., Zhang Z., Wang J., Yang B., Zhao Y., Rao Z., et al. (2018). Dihydroartemisinin sensitizes Lewis lung carcinoma cells to carboplatin therapy via p38 mitogen-activated protein kinase activation. Oncol. Lett. 15, 7531–7536. 10.3892/ol.2018.8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. L., Wang Z., Hu W., Chen S. S., Lou X. E., Zhou H. J. (2013). DHA regulates angiogenesis and improves the efficiency of CDDP for the treatment of lung carcinoma. Microvasc. Res. 87, 14–24. 10.1016/j.mvr.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Zhang L. X., Liu Z. N., Ye J., Sha M., Qian H., Bu X. H., et al. (2014). Artesunate exerts an anti-immunosuppressive effect on cervical cancer by inhibiting PGE2 production and Foxp3 expression. Cell Biol. Int. 38, 639–646. 10.1002/cbin.10244 [DOI] [PubMed] [Google Scholar]
- Zhang C. J., Wang J., Zhang J., Lee Y. M., Feng G., Lim T. K., et al. (2016). Mechanism-Guided Design and Synthesis of a Mitochondria-Targeting Artemisinin Analogue with Enhanced Anticancer Activity. Angew. Chem. Int. Ed. Engl. 55, 13770–13774. 10.1002/anie.201607303 [DOI] [PubMed] [Google Scholar]
- Zhang C., Fortin P. Y., Barnoin G., Qin X., Wang X., Fernandez Alvarez A., et al. (2020). An Artemisinin-Derivative-(NHC)Gold(I) Hybrid with Enhanced Cytotoxicity through Inhibition of NRF2 Transcriptional Activity. Angew. Chem. Int. Ed. Engl. 59, 12062–12068. 10.1002/anie.202002992 [DOI] [PubMed] [Google Scholar]
- Zhang X., Ba Q., Gu Z., Guo D., Zhou Y., Xu Y., et al. (2015). Fluorescent Coumarin-Artemisinin Conjugates as Mitochondria-Targeting Theranostic Probes for Enhanced Anticancer Activities. Chemistry 21, 17415–17421. 10.1002/chem.201502543 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xu G., Zhang S., Wang D., Saravana Prabha P., Zuo Z. (2018). Antitumor Research on Artemisinin and Its Bioactive Derivatives. Nat. Prod. Bioprospect. 8, 303–319. 10.1007/s13659-018-0162-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. J., Zhan X., Wang L., Ho R. J., Sasaki T. (2015). pH-responsive artemisinin dimer in lipid nanoparticles are effective against human breast cancer in a xenograft model. J. Pharm. Sci. 104, 1815–1824. 10.1002/jps.24407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. S., Wang J., Shen Y. B., Guo C. C., Sai K. E., Chen F. R., et al. (2015). Dihydroartemisinin increases temozolomide efficacy in glioma cells by inducing autophagy. Oncol. Lett. 10, 379–383. 10.3892/ol.2015.3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Gao W., Chen T. (2014). Synergistic induction of apoptosis in A549 cells by dihydroartemisinin and gemcitabine. Apoptosis 19, 668–681. 10.1007/s10495-013-0953-0 [DOI] [PubMed] [Google Scholar]
- Zhao J., Pan Y., Li X., Zhang X., Xue Y., Wang T., et al. (2017). Dihydroartemisinin and Curcumin Synergistically Induce Apoptosis in SKOV3 Cells Via Upregulation of MiR-124 Targeting Midkine. Cell Physiol. Biochem. 43, 589–601. 10.1159/000480531 [DOI] [PubMed] [Google Scholar]
- Zhou H. J., Zhang J. L., Li A., Wang Z., Lou X. E. (2010). Dihydroartemisinin improves the efficiency of chemotherapeutics in lung carcinomas in vivo and inhibits murine Lewis lung carcinoma cell line growth in vitro. Cancer Chemother. Pharmacol. 66, 21–29. 10.1007/s00280-009-1129-z [DOI] [PubMed] [Google Scholar]
- Zhou X., Sun W.-J., Wang W.-M., Chen K., Zheng J.-H., Lu M.-D., et al. (2013). Artesunate inhibits the growth of gastric cancer cells through the mechanism of promoting oncosis both in vitro and in vivo. Anti-cancer Drugs 24, 920–927. 10.1097/cad.0b013e328364a109 [DOI] [PubMed] [Google Scholar]
- Zhu W., Li Y., Zhao D., Li H., Zhang W., Xu J., et al. (2019). Dihydroartemisinin suppresses glycolysis of LNCaP cells by inhibiting PI3K/AKT pathway and downregulating HIF-1α expression. Life Sci. 233, 116730. 10.1016/j.lfs.2019.116730 [DOI] [PubMed] [Google Scholar]
- Zielonka J., Joseph J., Sikora A., Hardy M., Ouari O., Vasquez-Vivar J., et al. (2017). Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 117, 10043–10120. 10.1021/acs.chemrev.7b00042 [DOI] [PMC free article] [PubMed] [Google Scholar]