Abstract
Following the identification of the nociceptin/orphanin FQ (N/OFQ) peptide (NOP) as an endogenous ligand for the NOP receptor, ample evidence has revealed unique functional profiles of the N/OFQ-NOP receptor system. NOP receptors are expressed in key neural substrates involved in pain and reward modulation. In non-human primates (NHPs), NOP receptor activation effectively exerts antinociception and anti-hypersensitivity at the spinal and supraspinal levels. Moreover, NOP receptor activation inhibits dopaminergic transmission and synergistically enhances mu-opioid peptide (MOP) receptor-mediated analgesia. In this article, we discuss the functional profiles of ligands with dual NOP and MOP receptor agonist activities and highlight their optimal functional efficacy for pain relief and drug abuse treatment. Through coactivation of NOP and MOP receptors, bifunctional NOP/MOP receptor “partial” agonists (e.g., AT-121, BU08028, and BU10038) reveal a wider therapeutic window with fewer side effects. These newly developed ligands potently induce antinociception without MOP receptor agonist-associated side effects such as abuse potential, respiratory depression, itch sensation, and physical dependence. In addition, in both rodent and NHP models, bifunctional NOP/MOP receptor agonists can attenuate reward processing and/or the reinforcing effects of opioids and other abused drugs. While a mixed NOP/opioid receptor “full” agonist cebranopadol is undergoing clinical trials, bifunctional NOP/MOP “partial” agonists exhibit promising therapeutic profiles in translational NHP models for the treatment of pain and opioid abuse. This class of drugs demonstrates the therapeutic advantage of NOP and MOP receptor coactivation, indicating a greater potential for future development.
Keywords: abuse, analgesic, bifunctional ligand, nonhuman primate, NOP receptor, MOP receptor, spinal cord
1. Introduction
Twenty-five years ago, nociceptin/orphanin FQ (N/OFQ), an endogenous 17-amino acid peptide, was identified as a multifunctional ligand for the opioid receptor-like 1 (ORL1) (Meunier et al. 1995; Reinscheid et al. 1995), with ORL1 renamed to N/OFQ peptide (NOP) receptor (Cox et al. 2015). Despite the high homology of the NOP receptor and other classical (i.e., mu-, delta-, and kappa-) opioid receptors (MOP, DOP, and KOP, respectively), N/OFQ does not bind to classical opioid receptors owing to its unique structure (Bunzow et al. 1994; Fukuda et al. 1994; Mollereau et al. 1994; Nishi et al. 1994; Wang et al. 1994). Biochemical and crystal structure analysis revealed that the hydrophobic and hydrophilic features of the binding pockets of the NOP receptor and that of other opioid receptors are different due to key amino acid sequences (Granier et al. 2012; Manglik et al. 2012; Thompson et al. 2012; Wu et al. 2012), underlying the marked differences in the binding selectivity of corresponding ligands between the NOP receptor and classical opioid receptors. Notably, the amino acid sequence of N/OFQ, derived from a precursor peptide prepro-N/OFQ (ppN/OFQ), as well as NOP receptor, is well conserved across mammalians, and ppN/OFQ and N/OFQ are widely distributed in the peripheral and central nervous system (CNS) of both rodents and primates (Berthele et al. 2003; Neal et al. 1999a; Neal et al. 1999b; Peluso et al. 1998; Witta et al. 2004).
The NOP receptor is coupled to the pertussis toxin (PTX)-sensitive Gi/o proteins that inhibit adenylate cyclase and voltage-gated calcium channels and activate inward potassium channels like classical opioid receptors (Hawes et al. 2000; Ma et al. 1997; Margas et al. 2008). On the other hand, PTX-insensitive NOP receptor signaling is mediated through G protein-coupled kinases that lead to beta-arrestin recruitment (Parker and Bruchas 2019; Zhang et al. 2012). These pathways are also associated with activation of protein kinases, whereas the intracellular events following NOP receptor activation can reduce synaptic transmission through presynaptic or postsynaptic actions (Connor et al. 1996a; Connor et al. 1996b; Knoflach et al. 1996). Given that N/OFQ and NOP receptors are abundant in several regions of the brain and spinal cord, the N/OFQ-NOP receptor system exhibits multiple effects in the CNS (Kiguchi et al. 2016; Lambert 2008; Schroder et al. 2014). In fact, it was reported that NOP receptor activation inhibits the release of various neurotransmitters (dopamine, noradrenaline, 5-HT, glutamate, and GABA), and largely contributes to several CNS functions such as reward, emotion, memory, motor function, and sensory processing (Nicol et al. 1998; Nicol et al. 1996; Schlicker and Morari 2000). Although NOP receptor activation drives various intracellular events similar to classical opioid receptors, some of the NOP receptor-mediated effects through G protein and beta-arrestin pathways may be more complicated (Parker and Bruchas 2019). In this review, we highlighted the functional profiles of the N/OFQ-NOP receptor system for modulating pain and reinforcing effects and propose the therapeutic potential of NOP receptor activation for the treatment of pain and drug abuse.
2. Roles of NOP receptor activation in pain and drug abuse
2.1. Expression of N/OFQ and NOP receptor in nervous systems
Ample evidence indicates that NOP receptors are widely distributed in the dorsal root ganglia (DRG), spinal dorsal horn (SDH), and brain areas that are involved in pain processing. Based on histological approaches and reporter fluorescent protein expression in rodents (Anton et al. 1996; Chen and Sommer 2006; Florin et al. 2000; Neal et al. 1999a; Ozawa et al. 2015), NOP receptors are expressed in both small- and large-diameter neurons of the DRG and trigeminal ganglia, found in both peptidergic and nonpeptidergic C-fibers, indicating that activation of the NOP receptor affects thermal and mechanical pain sensitivities (Ozawa et al. 2018; Toll et al. 2019). Consistently, abundant NOP receptor expression was also observed in the superficial area (laminae I and II) of the SDH that receive projections from sensory C-fibers. Importantly, such findings have been translated to NOP receptors present in most small- and large-diameter human DRG neurons (Anand et al. 2016).
Like other classical opioid receptors, N/OFQ and NOP receptors were abundantly expressed in cortical regions (i.e., frontal cortex and cingulate cortex) and other multiple regions of the brain such as periaqueductal gray (PAG), locus coeruleus, and rostral ventromedial medulla (RVM), which directly impact ascending and descending pain pathways (Kiguchi and Ko 2019; Schroder et al. 2014; Toll et al. 2019). Moreover, N/OFQ and NOP receptors were observed in the main mesolimbic pathway consisting of the central amygdala, the bed nucleus of the stria terminals, the ventral tegmental area (VTA), and nucleus accumbens (NAC), and also expressed in the hippocampus, thalamus, striatum, and other brain regions (Gehlert et al. 2006; Letchworth et al. 2000; Neal et al. 1999b; Sim-Selley et al. 2003; Sim and Childers 1997; Slowe et al. 2001). These findings concerning the NOP receptor expression in the brain were also confirmed by additional studies using other animal species, including primates, by neuroanatomical or imaging techniques (Bridge et al. 2003; Kiguchi and Ko 2019; Kimura et al. 2011; Lohith et al. 2014; Lohith et al. 2012). Given the similarities of the N/OFQ-NOP receptor and other opioid receptor systems, NOP receptor activation may largely contribute to pain processing and substance use disorders in rodents and primates.
2.2. Effects of selective NOP agonists in the primary sensory neurons
Based on behavioral assays in rodents, peripherally-administered N/OFQ shows opposite effects in pain processing depending on the dosage. Intraplanter (i.pl.) administration of ultra-low doses (at femtomoles) of N/OFQ produced nociceptive behaviors through substance P release from peripheral nerve endings (Inoue et al. 1998), whereas higher doses (at picomoles) of i.pl. N/OFQ demonstrated antinociceptive effects (Sakurada et al. 2005). Moreover, Ro65–6570, a nonpeptidic NOP receptor agonist, attenuated mechanical hyperalgesia following i.pl. administration in diabetic rats (Schiene et al. 2015). These actions of NOP receptor agonists are consistent with electrophysiological studies showing that NOP receptor activation can inhibit voltage-gated calcium channels (Cav) in small- and medium-diameter DRG neurons via Gi/o protein-dependent pathways (Abdulla and Smith 1998; Winters et al. 2019). N/OFQ preferentially inhibits N-type current to a greater degree than other Cav-mediated current in the DRG neurons (Beedle et al. 2004; Winters et al. 2019). It is worth noting that N/OFQ prevented capsaicin-elicited calcium increases in human DRG neurons (Anand et al. 2016), suggesting that activation of peripheral NOP receptors may produce antinociceptive effects.
2.3. Effects of selective NOP agonists in the spinal cord
Despite the early reported dual actions of intrathecal (i.t.) N/OFQ, i.e., N/OFQ at femtomoles produced pain-like behaviors (Inoue et al. 1999; Sakurada et al. 1999), whereas higher doses (at nanomoles) demonstrated antinociceptive effects, a majority of publications reported that spinal NOP receptor activation results in antinociception (Erb et al. 1997; Kiguchi et al. 2016; King et al. 1997; Xu et al. 1996; Yamamoto et al. 1997a). UFP-112, a peptidic NOP receptor agonist, presenting increased agonist potency and decreased peptidase sensitivity, was generated by chemical modifications of N/OFQ, and exerted antinociceptive effects with higher potency and a longer duration than N/OFQ in mice (Calo et al. 2011; Rizzi et al. 2007). Electrophysiological studies revealed that activation of presynaptically and postsynaptically located NOP receptors can inhibit the excitatory glutamatergic transmission via Gi/o protein-dependent intracellular pathways, as well as MOP receptor activation (Ahmadi et al. 2001a; Ahmadi et al. 2001b; Le Cudennec et al. 2002; Liebel et al. 1997; Winters et al. 2019), indicating that NOP receptor activation displays antinociception at the spinal level in rodents. Additionally, NOP receptor activation in the SDH effectively suppressed inflammatory pain, as i.t. N/OFQ inhibited carrageenan- or complete Freund’s adjuvant (CFA)-induced thermal hyperalgesia in rodents (Chen and Sommer 2007; Hao et al. 1998; Yamamoto et al. 1997b). Moreover, i.t. N/OFQ or Ro64–6198, a nonpeptidic NOP receptor agonist, exerts potent alleviation of neuropathic pain (i.e., thermal hyperalgesia and mechanical allodynia) elicited by chronic constriction injury (CCI)- or spinal nerve ligation (SNL) in rodents (Corradini et al. 2001; Courteix et al. 2004; Obara et al. 2005; Yamamoto and Nozaki-Taguchi 1997).
Unlike in rodents, i.t. N/OFQ over a wide dose range (i.e., from femtomoles to nanomoles) only produced NOP receptor antagonist-reversible antinociception and enhanced morphine-induced antinociception in NHPs (Ko and Naughton 2009). Notably, N/OFQ was the most potent endogenous peptide among all opioid peptides, such as β-endorphin and enkephalins, regarding spinal analgesia under inflammatory pain conditions, as i.t. N/OFQ completely inhibited carrageenan-induced hyperalgesia in NHPs (Lee and Ko 2015). Furthermore, the inhibitory effects of i.t. UFP-112 on acute thermal nociception and capsaicin-induced thermal allodynia in NHPs was 10 times more potent than morphine (Hu et al. 2010). Another NOP receptor agonist PWT2-N/OFQ generated by peptide welding technology (PWT) also displayed potent antinociception with a 40-fold higher potency and a markedly prolonged duration in NHPs (Calo et al. 2018; Rizzi et al. 2015). Generally, i.t. administration of MOP receptor agonists, which are gold standard analgesics for severe pain, often causes itch (pruritus) that compromises the analgesic value of spinal opioids (Ganesh and Maxwell 2007; Waxler et al. 2005). Given that i.t. MOP receptor agonists, but not NOP receptor agonists, elicit robust itch-related scratching behaviors in NHPs (Ko and Naughton 2000; Ko et al. 2006), pharmacological studies using NHPs provide a translational bridge to validate novel spinal analgesics without the itch side effect.
2.4. Effects of selective NOP agonists in the brain
In rodents, supraspinal NOP receptor activation often has a pronociceptive role. Intracerebroventricular (i.c.v.) administration of N/OFQ produced mild pronociceptive effects in hot plate and tail-flick tests and i.c.v. N/OFQ attenuated morphine-induced antinociception (King et al. 1998; Meunier et al. 1995; Reinscheid et al. 1995), supported by i.c.v. UFP-101, a peptidic NOP receptor selective antagonist, which demonstrated antinociceptive effects in the formalin test (Rizzi et al. 2006). Hence, supraspinal NOP receptor activation mainly cause hyperalgesic effects under physiological and inflammatory conditions in rodents (Kiguchi et al. 2016; Schroder et al. 2014). On the other hand, with advanced surgical techniques, the supraspinal actions of N/OFQ were determined in NHPs (Ding et al. 2015). It is interesting to note that the intracisternal administration of N/OFQ produced only antinociceptive effects, antagonized by a NOP receptor antagonist. Moreover, supraspinal NOP receptor activation did not attenuate, instead enhanced, morphine-induced antinociception (Ding et al. 2015). These data indicate that there are distinct functional profiles for supraspinal NOP receptor activation between rodents and NHPs, and propose the impact of N/OFQ-NOP receptor system in the CNS for attenuating pain processing in primates.
Based on the classical opioid-like expression patterns and analgesic functions, the role of NOP receptor activation in the reward system has been extensively investigated. Microdialysis experiments revealed that i.c.v. N/OFQ reduced morphine- or cocaine-induced dopamine release in the NAC (Di Giannuario and Pieretti 2000; Lutfy et al. 2001; Murphy et al. 1996), with such effects supported by the microinjection of N/OFQ into NAC or VTA reduced extracellular dopamine levels in the NAC (Murphy and Maidment 1999; Vazquez-DeRose et al. 2013). Furthermore, these findings indicate that the ability of the NOP receptor activation to inhibit dopamine release in the mesolimbic system might counteract the rewarding and/or reinforcing effects of opioids and psychostimulants. In rodents, the conditioned place preference (CPP) test was used to evaluate the suppressive effects of NOP receptor activation on the rewarding effects of opioids. In addition to opioids, psychostimulant-induced reward effects were inhibited by N/OFQ-NOP receptor system, as cocaine- or amphetamine-induced CPP behaviors were attenuated by the administration of NOP receptor agonists (Kotlinska et al. 2003; Murphy and Maidment 1999; Rutten et al. 2010; Sakoori and Murphy 2004; Toll et al. 2016). Furthermore, the administration of peptidic or nonpeptidic NOP receptor agonists suppressed alcohol consumption in rodents (Ciccocioppo et al. 2003; Ciccocioppo et al. 2002; Kuzmin et al. 2007; Kuzmin et al. 2003). It is interesting that not only agonists but also antagonists for NOP receptor reduced the motivation for alcohol (Ciccocioppo et al. 2019). Nevertheless, the majority of publications suggest that NOP receptor activation negatively regulate the abuse potential of drugs. This notion is also supported by a recent study demonstrating that activation of NOP receptor expressing VTA dopamine neurons suppressed motivation for reward (Parker et al. 2019). Notably, Ro64–6198 and SCH221510, which are nonpeptidic NOP receptor agonists, attenuated opioid intake through NOP receptor activation in rats and NHPs, without behavioral selectivity. Intracisternal administration of SCH221510 attenuated the reinforcing effects of not only remifentanil, but also sucrose in rodents (Sukhtankar et al. 2014a). Ro64–6198 attenuated reinforcing effects of remifentanil in NHPs under sedation (Podlesnik et al. 2011). Furthermore, it is also important to note that MOP agonist-induced rewarding and reinforcing effects did not fully require dopamine neurotransmission (Fields and Margolis 2015; Hiranita et al. 2013). Therefore, NOP receptor selective agonists may demonstrate moderate capacity for attenuating the reinforcing effects. Further studies are necessary to understand the interactions between NOP and MOP receptor activation for modulating a drugs’ reinforcing effects and abuse potential.
2.5. Therapeutic potential of coactivation of NOP/MOP receptors
Even though potent MOP receptor agonists (i.e., morphine, oxycodone, and fentanyl) have been used as essential analgesics to relieve severe pain, the clinical use of such MOP receptor agonists is limited due to several side effects, such as abuse liability, respiratory depression, itch sensation, and constipation. In particular, the recent marked increase in prescription opioid analgesic abuse and opioid overdose mortality has severely impacted our society (Brady et al. 2016; Volkow and McLellan 2016). In spite of improved understanding of the beneficial or undesirable effects of MOP receptor agonists in the past few decades, the lack of alternative analgesics may have contributed to the current opioid crisis (Degenhardt et al. 2014; Gunther et al. 2018; Volkow and Collins 2017). Hence, there is an unmet need for discovering novel potent analgesics devoid of MOP receptor-associated side effects. Notably, unlike MOP receptor agonists, NOP receptor agonists do not demonstrate such dose-limiting side effects, but could produce sedation in NHP (Kiguchi and Ko 2019; Lin and Ko 2013). Based on the unique pharmacological profiles of NOP receptor activation, some NOP receptor agonists have entered human clinical trials for different applications (Calo and Lambert 2018; Tzschentke et al. 2019; Zaveri 2016). Given that NOP receptor activation can inhibit dopaminergic transmission (Di Giannuario and Pieretti 2000; Murphy et al. 1996) and synergistically enhance MOP receptor-mediated analgesia (Cremeans et al. 2012; Hu et al. 2010), it is hypothesized that the coactivation of NOP and MOP receptors may produce analgesia with reduced side effects (Lin and Ko 2013). Specifically, a ligand with dual NOP and MOP agonist actions may have a wider therapeutic window, exerting functional efficacy for pain relief and drug abuse treatment (Lin and Ko 2013; Toll et al. 2016; Zaveri 2016).
3. Therapeutic potential of bifunctional NOP/MOP agonists as spinal analgesics
Since the unique amino acid residue of N/OFQ determines the selectivity for NOP receptors and classical opioid receptors, the introduction of a 2,6-dimethyltyrosine (Dmt) residue at the N-terminal generated a bifunctional peptidic ligand (Preti et al. 2019). [Dmt1]N/OFQ(1–13)-NH2 activated not only the NOP receptor but also the MOP receptor, with the i.t. administration of [Dmt1]N/OFQ(1–13)-NH2 producing antinociceptive effects in mice with acute pain (Kiguchi et al. 2016). On the other hand, BU08028 was developed as a buprenorphine-derived novel orvinol analog demonstrating buprenorphine-like binding profiles to classical opioid receptors (MOP, DOP, and KOP) and a higher binding affinity and efficacy at the NOP receptor (Khroyan et al. 2011). BU08028, i.t.-administered, exerted potent and effective anti-hypersensitive effects in mouse models of neuropathic pain and inflammatory pain (Sukhtankar et al. 2013). Interestingly, i.t. SR16435, a non-morphinan bifunctional NOP/MOP agonist, also reported potent antiallodynic effects in mice. Furthermore, the development of analgesic tolerance to SR16435 following repeated administration was slower in comparison with buprenorphine (Sukhtankar et al. 2013). These lines of evidence indicate that bifunctional NOP/MOP ligands have a strong therapeutic potential as spinal analgesics.
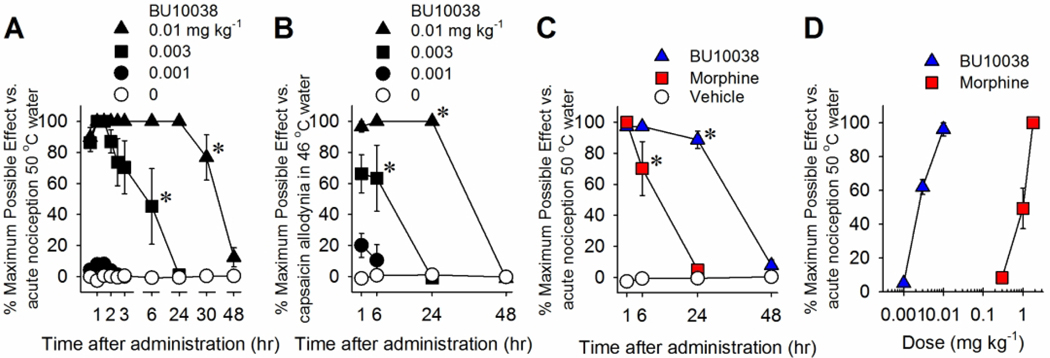
Consistent with findings in rodents, i.t. [Dmt1]N/OFQ(1–13)-NH2 was more potent than N/OFQ in terms of antinociception in NHPs (Molinari et al. 2013). Moreover, PWT-based chemical modification (Calo et al. 2018) developed PWT2-[Dmt1]N/OFQ(1–13)-NH2 that exerted complete antinociceptive effects, demonstrating higher potency and longer duration in NHPs (Cerlesi et al. 2017). Nonetheless, higher doses of [Dmt1]N/OFQ(1–13)-NH2 than the antinociceptive doses caused robust itch sensation in NHPs owing to MOP receptor activation in the SDH. Recently, BU10038 was synthesized as a naltrexone-derived analog with partial agonist activity for both NOP and MOP receptors (Kiguchi et al. 2019). It is worth noting that i.t. BU10038 produced potent and long-lasting antinociceptive and antiallodynic effects compared to morphine in NHP (Fig. 1), and i.t. BU10038 did not cause an itch sensation and tolerance even when daily administered for 4 weeks (Kiguchi et al. 2019). These results suggest that the appropriate balance between NOP and MOP receptor activation display ideal pharmacological profiles (i.e., higher potency without itch sensation and tolerance development), and such ligands demonstrate potential as novel spinal analgesics.
Fig. 1. Effects of systemic administration of BU10038 on nociceptive responses in nonhuman primates.
(A) Antinociception against acute noxious stimulus, 50°C water. (B) Antihypersensitivity against capsaicin-induced allodynia in 46°C water. (C) Comparison of antinociceptive duration of BU10038 (0.01 mg kg−1) and morphine (1.8 mg kg−1). (D) Comparison of antinociceptive potency of BU10038 and morphine. Each data point represents mean ± SEM (n = 4). All compounds were delivered subcutaneously. *P<0.05, significantly different from vehicle condition from the first time point to the corresponding time point. Reprinted with permission from Kiguchi et al. 2019.
4. Therapeutic potential of bifunctional NOP/MOP agonists for treatment of pain and opioid abuse
4.1. Morphinan NOP/MOP partial agonists
Systemically administered NOP receptor agonists demonstrate integrated effects of peripheral, spinal, and supraspinal actions (Kiguchi and Ko 2019). Although the role of the N/OFQ-NOP receptor system in pain processing remains complicated in rodents, it produces only antinociceptive effects, regardless of the sites of action in NHPs (Ko and Naughton 2009; Podlesnik et al. 2011; Sukhtankar et al. 2014b). Given the synergistic antinociceptive effects between NOP receptor agonists and buprenorphine (Cremeans et al. 2012), it has been hypothesized that the systemic administration of bifunctional NOP/MOP agonists may display more potent analgesic effects (Lin and Ko 2013). Based on the unique pharmacological profiles of buprenorphine affecting analgesia and reinforcing effects, BU08028, a buprenorphine analog presenting partial agonist activities for both NOP and MOP receptors might produce beneficial effects in comparison with clinically used opioid analgesics (Khroyan et al. 2011). Interestingly, systemic administration of BU08028 exerted morphine-comparable antinociceptive effects in the tail-flick test in mice, and was enhanced by NOP receptor antagonist SB612111 and attenuated by MOP receptor antagonist naloxone. Notably, similar to morphine, BU08028 increased global activity and caused CPP behavior suggesting the presence of abuse liability (Khroyan et al. 2011). These lines of evidence indicate that MOP receptor-associated activity overpowers NOP receptor-mediated functions regarding the integrated effects of BU08028 in rodents.
In NHPs, BU08028 produced long-lasting (up to 30 h) antinociceptive and antiallodynic effects. BU08028-induced antinociception was antagonized by the NOP receptor and MOP receptor antagonist to the same degree (Ding et al. 2016). In addition, as measured by the progressive-ratio schedule of drug self-administration, BU08028 did not elicit reinforcing effects in comparison with buprenorphine and cocaine in NHPs. BU08028, at higher doses than antinociceptive doses, did not affect respiratory and cardiovascular activities, whereas potent MOP receptor agonists, such as fentanyl, cause respiratory depression in NHPs (Ding et al. 2016). Collectively, there is a translational gap regarding the functions of bifunctional NOP/MOP receptor ligands between rodents and NHPs. Through the coactivation of NOP and MOP receptors, BU08028 provides the first functional evidence for bifunctional NOP/MOP partial agonists as safe and non-addictive analgesics.
4.2. Non-morphinan NOP/MOP partial agonists
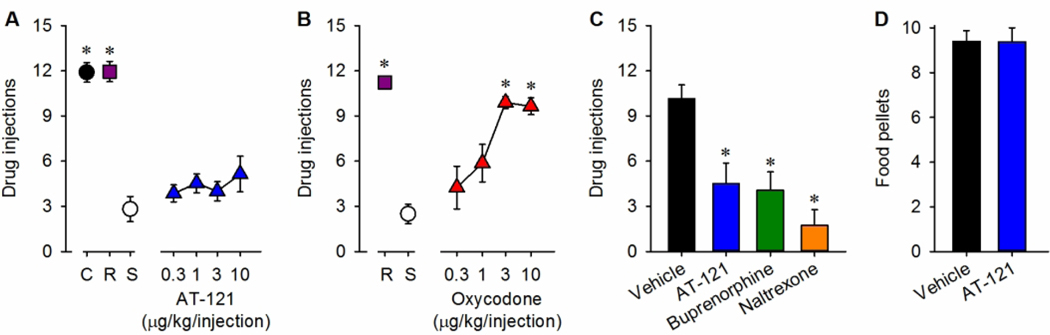
As bifunctional NOP/MOP agonists exhibit potent analgesic effects in rodents and NHPs, researchers have developed several non-morphinan compounds that bind to both NOP and MOP receptors (Zaveri 2011; Zaveri and Meyer 2019). Among the several nonpeptidic NOP receptor ligands, AT-121 has been identified as a non-morphinan bifunctional NOP/MOP agonist showing high binding affinity and partial agonist efficacy for both NOP and MOP receptors (Ding et al. 2018). In NHPs, systemic administration of AT-121 produced morphine-comparable antinociceptive and antiallodynic effects. AT-121-induced antinociception was 100-fold more potent than morphine, and was antagonized by either NOP receptor antagonist J-113397 or MOP receptor antagonist naltrexone (Ding et al. 2018). Moreover, systemic AT-121 elicited neither itch sensation, nor respiratory depression and cardiovascular dysfunctions. Even after repeated administration of AT-121, opioid-induced hyperalgesia, physical dependence and tolerance were not observed in NHPs. Furthermore, AT-121 produced no reinforcing effects and also attenuated the reinforcing effects of oxycodone, an abused prescription opioid, without disrupting food intake (Fig. 2) (Ding et al. 2018). These lines of evidence further support that bifunctional NOP/MOP partial agonists may have a dual therapeutic action for the treatment of pain and opioid abuse.
Fig. 2. Effects of AT-121 on reinforcing effects in nonhuman primates.
(A and B) Number of injections received as a function of dose in monkeys responding to cocaine (C; 0.03 mg/kg per injection), remifentanil (R; 0.3 μg/kg per injection), saline (S; ~0.14 ml/kg per injection), AT-121 (0.3 to 10 μg/kg per injection), or oxycodone (0.3 to 10 μg/kg per injection) under a progressive-ratio schedule of reinforcement. (C) Effects of the vehicle (0.1 ml/kg), AT-121 (0.03 mg/kg), buprenorphine (0.1 mg/kg), or naltrexone (0.01 mg/kg) on the reinforcing effects of oxycodone (3 μg/kg per injection). Each compound was administered intramuscularly 30 min before starting the progressive-ratio schedule of oxycodone. (D) Effect of the vehicle (0.1 ml/kg) or AT-121 (0.03 mg/kg) on the reinforcing effects of food pellets. AT-121 or its vehicle was administered intramuscularly 30 min before starting the fixed-ratio schedule of food pellets. Each data point represents mean ± SEM (n = 4). Data were analyzed by one-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparisons test. *P < 0.05, a significant difference from saline or vehicle. Reprinted with permission from Ding et al. 2018.
4.3. Mixed NOP and opioid receptor agonist
Cebranopadol was discovered as a potent mixed NOP and opioid receptor agonist, revealing high affinities with nearly full agonist activities for NOP and MOP, and partial agonist activity for KOP receptors (Fantinati et al. 2017; Linz et al. 2014; Rizzi et al. 2016). Systemic (intravenous; i.v.) administration of cebranopadol exhibited highly potent and efficacious antinociceptive, anti-hypersensitive effects in rodent models of neuropathic pain with ED50 of 0.5–5.6 μg/kg. Notably, cebranopadol was more than 100-fold potent and long-lasting compared to morphine-induced analgesia. The anti-hypersensitive effects of cebranopadol were partially reversed by J-113397 or naltrexone, indicating that the coactivation of NOP and MOP receptors underlies this activity (Calo and Lambert 2018; Linz et al. 2014; Raffa et al. 2017; Schiene et al. 2018). Unlike morphine, cebranopadol did not affect respiratory and motor functions at analgesic doses. More importantly, the beneficial effects of cebranopadol can be translated to NHPs, as it also produced potent antinociceptive and anti-hypersensitive effects following systemic (1–5.6 μg/kg) or i.t. (1 μg) administration without eliciting itch. Cebranopadol caused reinforcing effects in the fixed-ratio schedule of self-administration, but its reinforcing strength was lower than the MOP receptor full agonist fentanyl (Kiguchi and Ko 2019; Trapella et al. 2018).
Since the oral bioavailability of cebranopadol is 13–23%, it can be orally administered, and the duration of orally-administered cebranopadol was more than 9 h (Calo and Lambert 2018; Linz et al. 2014; Schunk et al. 2014). Given the half-life of 4.5 h and its moderate ability to elicit side effects, there is potential for clinical use to treat severe pain following systemic administration. In phase I and phase II clinical trials of cebranopadol for analgesic indications, pharmacokinetic properties of cebranopadol were demonstrated, as it displayed a 4–6 h of maximum plasma concentration and 14–15 h of half-life in patients (Calo and Lambert 2018; Kleideiter et al. 2018). Of note, even though oral administration of cebranopadol (0.6 mg) produced drug-liking effects, such effects were less or shorter than the usual MOP receptor full agonist hydromorphone (Gohler et al. 2019). These reports suggest that cebranopadol exhibits safer pharmacological profiles than other clinically used opioid analgesics (Calo and Lambert 2018; Tzschentke et al. 2019). The analgesic efficacy of cebranopadol in patients with low back pain was evaluated in randomized, double-blind, placebo-controlled trials, and produced significant analgesic effects over the placebo control, with fewer undesirable side effects (e.g., miosis, respiratory depression, constipation, dizziness, and nausea) (Christoph et al. 2017). In patients with cancer pain, cebranopadol displayed effective and safer profiles (Eerdekens et al. 2019). Collectively, these findings support the notion that the coactivation of NOP and MOP receptors can provide safer pain relief with reduced side effects.
5. Treatment of drug abuse by coactivation of NOP and MOP receptors
Unlike buprenorphine, recently developed bifunctional NOP/MOP partial agonists (i.e., BU08028, BU10038, and AT-121) itself did not exhibit reinforcing effects in NHPs (Ding et al. 2016; Ding et al. 2018; Kiguchi et al. 2019), whereas the mixed NOP and opioid receptor full agonist cebranopadol has moderate reinforcing effects (Kiguchi and Ko 2019; Trapella et al. 2018). In addition, the abuse potential of cebranopadol has been indicated in a recent study reporting that cebranopadol displayed drug-liking effects in humans, but lower than hydromorphone (Gohler et al. 2019). By comparing the reinforcing effects of BU08028, AT-121, and cebranopadol using the same drug-self-administration setting in NHPs, it appears that NOP receptor activation may suppress the reinforcing effects mediated by partial, but not full, MOP agonists. This hypothesis is consistent with a recent report showing that BPR1M97, a NOP/MOP full agonist, exerts potent antinociception without changing reward behavior in mice (Chao et al. 2020). Further studies using several ligands with different intrinsic efficacies at NOP and MOP receptors could determine the functional role of NOP receptor activation in modulating abuse liability based on MOP receptor activation.
Notably, daily systemic AT-121 pretreatment significantly attenuated oxycodone-induced reinforcing effects without disrupting food-maintained operant behavior, and the degree of AT-121-induced attenuation was similar to that of buprenorphine (Ding et al. 2018). This effect of AT-121 might be mediated by the coactivation of NOP and MOP receptors with partial agonist activities. This is the first evidence demonstrating the identification of a novel bifunctional NOP/MOP agonist that inhibits abuse liability of prescription opioid oxycodone with behavioral selectivity. Moreover, a recent report demonstrated that BU08028 selectively attenuate alcohol intake without affecting food-maintained operant behaviors in NHPs (Flynn et al. 2019). Acute systemic administration of BU08028 decreased ethanol drinking, and the effective doses of BU08028 were lower than that of buprenorphine. In addition, chronic dosing of BU08028 also attenuated ethanol drinking, with the effects maintained for several weeks without inducing tolerance and adverse effects (Flynn et al. 2019). Given that AT-121 and BU08028 alone did not produce reinforcing effects (i.e., abuse liability) in NHPs, bifunctional NOP/MOP partial agonists may have great therapeutic potential for treating not only pain but also substance use disorders with fewer side effects.
6. Conclusion
Collectively, NOP receptor activation plays a significant role in modulating pain processing and rewarding/reinforcing effects. Given the similarities of anatomical and neurochemical features between human and NHPs, pharmacological studies dissecting the functional significance of ligand-receptor systems using NHPs provide a greater translational platform to facilitate basic research and drug development. Notably, several NOP receptor agonists produce potent antinociceptive and anti-hypersensitive effects, synergistically enhancing morphine-induced antinociception in NHPs without eliciting MOP receptor-associated side effects. Emerging evidence has shown a promising functional profile of bifunctional NOP/MOP partial agonists as safe and non-addictive analgesics (Kiguchi et al. 2016; Kiguchi and Ko 2019; Lin and Ko 2013) (Table 1). Furthermore, recent clinical trial outcomes of cebranopadol support the therapeutic benefits of NOP and MOP receptor coactivation. However, it should be reiterated that cebranopadol produces reinforcing effects in humans and NHPs, indicative of the abuse potential. While coactivation of NOP and MOP receptors may provide a viable treatment option for pain and drug abuse, caution is warranted for bifunctional NOP/MOP “full” agonists. Based on accumulating evidence, bifunctional NOP/MOP “partial” agonists display favorable functional profiles in translational NHP models for the treatment of pain and opioid abuse. This class of drugs demonstrates the therapeutic advantage of coactivation of NOP and MOP receptors and demonstrates a great potential for future development.
Table 1.
Brief summary of functional profiles of compounds with selective MOP agonist or mixed NOP/MOP agonist actions in NHPs.
| Binding Ki NOP/MOP | Intrinsic activity | Analgesia | Itch (pruritus) | Respiratory depression | Tolerance | Physical dependence | Abuse liability | ||
|---|---|---|---|---|---|---|---|---|---|
| MOP | NOP | ||||||||
| Morphine | < 0.001 | 0.83 | − | + | + | + | + | + | + |
| Fentanyl | < 0.001 | 0.97 | − | + | + | + | N.D. | N.D. | + |
| Cebranopadol | 0.78 | 1 | 0.89 | + | − | − | N.D. | N.D. | + |
| AT-121 | 4.49 | 0.14 | 0.41 | + | − | − | − | − | − |
| BU08028 | 0.25 | 0.21 | 0.48 | + | − | − | N.D. | − | − |
| BU10038 | 0.06 | 0.18 | 0.34 | + | − | − | − | − | − |
N.D.: not determined
detected
not detected.
The data of binding affinity and intrinsic activity are based on the studies by Ding et al. 2016; Emmerson et al. 1996; Khroyan et al. 2011; Kiguchi et al. 2019; Linz et al. 2014. The in vivo functional data in NHPs are based on the studies by Ding et al. 2016; Ding et al. 2018; Kiguchi et al. 2019; Sukhtankar et al. 2014b.
Significance statement.
The nociceptin/orphanin FQ peptide (NOP) receptors and mu-opioid peptide (MOP) receptors share similar intracellular events. Interestingly, activation of NOP receptors enhances MOP receptor-mediated analgesia and inhibits dopaminergic transmission. This article summarizes the key findings of ligands with dual NOP/MOP receptor agonist activities. Bifunctional NOP/MOP receptor “partial” agonists not only could be safe, non-addictive analgesics, but also attenuate rewarding and reinforcing effects of opioids and other abused drugs. This class of drugs demonstrates the therapeutic advantage of coactivation of NOP and MOP receptors as a treatment option for pain and opioid abuse.
Acknowledgments
This work was supported by US-NIH grants, DA032568, DA035359, DA040104, and DA044775.
Support or grant information: US, National Institutes of Health, National Institute on Drug Abuse, DA032568, DA035359, DA040104, and DA044775.
Footnotes
Conflict of Interest
All authors report no potential conflict of interest.
References
- Abdulla FA, Smith PA. 1998. Axotomy reduces the effect of analgesic opioids yet increases the effect of nociceptin on dorsal root ganglion neurons. J Neurosci 18(23):9685–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi S, Kotalla C, Guhring H, Takeshima H, Pahl A, Zeilhofer HU. 2001a. Modulation of synaptic transmission by nociceptin/orphanin FQ and nocistatin in the spinal cord dorsal horn of mutant mice lacking the nociceptin/orphanin FQ receptor. Mol Pharmacol 59(3):612–618. [DOI] [PubMed] [Google Scholar]
- Ahmadi S, Liebel JT, Zeilhofer HU. 2001b. The role of the ORL1 receptor in the modulation of spinal neurotransmission by nociceptin/orphanin FQ and nocistatin. Eur J Pharmacol 412(1):39–44. [DOI] [PubMed] [Google Scholar]
- Anand P, Yiangou Y, Anand U, Mukerji G, Sinisi M, Fox M, McQuillan A, Quick T, Korchev YE, Hein P. 2016. Nociceptin/orphanin FQ receptor expression in clinical pain disorders and functional effects in cultured neurons. Pain 157(9):1960–1969. [DOI] [PubMed] [Google Scholar]
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. 1996. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol 368(2):229–251. [DOI] [PubMed] [Google Scholar]
- Beedle AM, McRory JE, Poirot O, Doering CJ, Altier C, Barrere C, Hamid J, Nargeot J, Bourinet E, Zamponi GW. 2004. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci 7(2):118–125. [DOI] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Buttner A, Assmus HP, Wurster K, Zieglgansberger W, Conrad B, Tolle TR. 2003. [3H]-nociceptin ligand-binding and nociceptin opioid receptor mrna expression in the human brain. Neuroscience 121(3):629–640. [DOI] [PubMed] [Google Scholar]
- Brady KT, McCauley JL, Back SE. 2016. Prescription Opioid Misuse, Abuse, and Treatment in the United States: An Update. Am J Psychiatry 173(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge KE, Wainwright A, Reilly K, Oliver KR. 2003. Autoradiographic localization of (125)i[Tyr(14)] nociceptin/orphanin FQ binding sites in macaque primate CNS. Neuroscience 118(2):513–523. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. 1994. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett 347(2–3):284–288. [DOI] [PubMed] [Google Scholar]
- Calo G, Lambert DG. 2018. Nociceptin/orphanin FQ receptor ligands and translational challenges: focus on cebranopadol as an innovative analgesic. Br J Anaesth 121(5):1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo G, Rizzi A, Cifani C, Micioni Di Bonaventura MV, Regoli D, Massi M, Salvadori S, Lambert DG, Guerrini R. 2011. UFP-112 a potent and long-lasting agonist selective for the Nociceptin/Orphanin FQ receptor. CNS Neurosci Ther 17(3):178–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo G, Rizzi A, Ruzza C, Ferrari F, Pacifico S, Gavioli EC, Salvadori S, Guerrini R. 2018. Peptide welding technology - A simple strategy for generating innovative ligands for G protein coupled receptors. Peptides 99:195–204. [DOI] [PubMed] [Google Scholar]
- Cerlesi MC, Ding H, Bird MF, Kiguchi N, Ferrari F, Malfacini D, Rizzi A, Ruzza C, Lambert DG, Ko MC, Calo G, Guerrini R. 2017. Pharmacological studies on the NOP and opioid receptor agonist PWT2-[Dmt(1)]N/OFQ(1–13). Eur J Pharmacol 794:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PK, Chang HF, Chang WT, Yeh TK, Ou LC, Chuang JY, Tsu-An Hsu J, Tao PL, Loh HH, Shih C, Ueng SH, Yeh SH. 2020. BPR1M97, a dual mu opioid receptor/nociceptin-orphanin FQ peptide receptor agonist, produces potent antinociceptive effects with safer properties than morphine. Neuropharmacology 166:107678. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sommer C. 2006. Nociceptin and its receptor in rat dorsal root ganglion neurons in neuropathic and inflammatory pain models: implications on pain processing. J Peripher Nerv Syst 11(3):232–240. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sommer C. 2007. Activation of the nociceptin opioid system in rat sensory neurons produces antinociceptive effects in inflammatory pain: involvement of inflammatory mediators. J Neurosci Res 85(7):1478–1488. [DOI] [PubMed] [Google Scholar]
- Christoph A, Eerdekens MH, Kok M, Volkers G, Freynhagen R. 2017. Cebranopadol, a novel first-in-class analgesic drug candidate: first experience in patients with chronic low back pain in a randomized clinical trial. Pain 158(9):1813–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Borruto AM, Domi A, Teshima K, Cannella N, Weiss F. 2019. NOP-Related Mechanisms in Substance Use Disorders. Handb Exp Pharmacol 254:187–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Massi M. 2003. The nociceptin/orphanin FQ/NOP receptor system as a target for treatment of alcohol abuse: a review of recent work in alcohol-preferring rats. Physiol Behav 79(1):121–128. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Polidori C, Antonelli L, Salvadori S, Guerrini R, Massi M. 2002. Pharmacological characterization of the nociceptin receptor which mediates reduction of alcohol drinking in rats. Peptides 23(1):117–125. [DOI] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Chieng B, Christie MJ. 1996a. Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol 119(8):1614–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Yeo A, Henderson G. 1996b. The effect of nociceptin on Ca2+ channel current and intracellular Ca2+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol 118(2):205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini L, Briscini L, Ongini E, Bertorelli R. 2001. The putative OP(4) antagonist, [Nphe(1)]nociceptin(1–13)NH(2), prevents the effects of nociceptin in neuropathic rats. Brain Res 905(1–2):127–133. [DOI] [PubMed] [Google Scholar]
- Courteix C, Coudore-Civiale MA, Privat AM, Pelissier T, Eschalier A, Fialip J. 2004. Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain 110(1–2):236–245. [DOI] [PubMed] [Google Scholar]
- Cox BM, Christie MJ, Devi L, Toll L, Traynor JR. 2015. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br J Pharmacol 172(2):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ, Ko MC. 2012. Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther 343(1):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Charlson F, Mathers B, Hall WD, Flaxman AD, Johns N, Vos T. 2014. The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction 109(8):1320–1333. [DOI] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S. 2000. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides 21(7):1125–1130. [DOI] [PubMed] [Google Scholar]
- Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, Husbands SM, Ko MC. 2016. A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci U S A 113(37):E5511–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Hayashida K, Suto T, Sukhtankar DD, Kimura M, Mendenhall V, Ko MC. 2015. Supraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primates. Br J Pharmacol 172(13):3302–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, Czoty PW, Kishioka S, Zaveri NT, Ko MC. 2018. A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med 10(456). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerdekens MH, Kapanadze S, Koch ED, Kralidis G, Volkers G, Ahmedzai SH, Meissner W. 2019. Cancer-related chronic pain: Investigation of the novel analgesic drug candidate cebranopadol in a randomized, double-blind, noninferiority trial. Eur J Pain 23(3):577–588. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. 1996. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther 278(3):1121–1127. [PubMed] [Google Scholar]
- Erb K, Liebel JT, Tegeder I, Zeilhofer HU, Brune K, Geisslinger G. 1997. Spinally delivered nociceptin/orphanin FQ reduces flinching behaviour in the rat formalin test. Neuroreport 8(8):1967–1970. [DOI] [PubMed] [Google Scholar]
- Fantinati A, Bianco S, Guerrini R, Salvadori S, Pacifico S, Cerlesi MC, Calo G, Trapella C. 2017. A diastereoselective synthesis of Cebranopadol, a novel analgesic showing NOP/mu mixed agonism. Sci Rep 7(1):2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Margolis EB. 2015. Understanding opioid reward. Trends Neurosci 38(4):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin S, Meunier J, Costentin J. 2000. Autoradiographic localization of [3H]nociceptin binding sites in the rat brain. Brain Res 880(1–2):11–16. [DOI] [PubMed] [Google Scholar]
- Flynn SM, Epperly PM, Davenport AT, Cami-Kobeci G, Husbands SM, Ko MC, Czoty PW. 2019. Effects of stimulation of mu opioid and nociceptin/orphanin FQ peptide (NOP) receptors on alcohol drinking in rhesus monkeys. Neuropsychopharmacology 44(8):1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T. 1994. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett 343(1):42–46. [DOI] [PubMed] [Google Scholar]
- Ganesh A, Maxwell LG. 2007. Pathophysiology and management of opioid-induced pruritus. Drugs 67(16):2323–2333. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Shaw JL. 2006. Distribution of nociceptin and Ro64–6198 activated [35S]-GTPgammaS binding in the rat brain. Neuropeptides 40(2):95–105. [DOI] [PubMed] [Google Scholar]
- Gohler K, Sokolowska M, Schoedel KA, Nemeth R, Kleideiter E, Szeto I, Eerdekens MH. 2019. Assessment of the Abuse Potential of Cebranopadol in Nondependent Recreational Opioid Users: A Phase 1 Randomized Controlled Study. J Clin Psychopharmacol 39(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. 2012. Structure of the delta-opioid receptor bound to naltrindole. Nature 485(7398):400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther T, Dasgupta P, Mann A, Miess E, Kliewer A, Fritzwanker S, Steinborn R, Schulz S. 2018. Targeting multiple opioid receptors - improved analgesics with reduced side effects? Br J Pharmacol 175(14):2857–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JX, Xu IS, Wiesenfeld-Hallin Z, Xu XJ. 1998. Anti-hyperalgesic and anti-allodynic effects of intrathecal nociceptin/orphanin FQ in rats after spinal cord injury, peripheral nerve injury and inflammation. Pain 76(3):385–393. [DOI] [PubMed] [Google Scholar]
- Hawes BE, Graziano MP, Lambert DG. 2000. Cellular actions of nociceptin: transduction mechanisms. Peptides 21(7):961–967. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL. 2013. Stimulants as specific inducers of dopamine-independent sigma agonist self-administration in rats. J Pharmacol Exp Ther 347(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Calo G, Guerrini R, Ko MC. 2010. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain 148(1):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Kobayashi M, Kozaki S, Zimmer A, Ueda H. 1998. Nociceptin/orphanin FQ-induced nociceptive responses through substance P release from peripheral nerve endings in mice. Proc Natl Acad Sci U S A 95(18):10949–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, Ueda H. 1999. Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J Pharmacol Exp Ther 291(1):308–313. [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Cami-Kobeci G, Husbands SM, Zaveri NT, Toll L. 2011. The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-meth oxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther 336(3):952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Cami-Kobeci G, Sukhtankar DD, Czoty PW, DeLoid HB, Hsu FC, Toll L, Husbands SM, Ko MC. 2019. BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates. Br J Anaesth 122(6):e146–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Ko MC. 2016. Central N/OFQ-NOP Receptor System in Pain Modulation. Adv Pharmacol 75:217–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ko MC. 2019. Effects of NOP-Related Ligands in Nonhuman Primates. Handb Exp Pharmacol 254:323–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Fujita M, Hong J, Lohith TG, Gladding RL, Zoghbi SS, Tauscher JA, Goebl N, Rash KS, Chen Z, Pedregal C, Barth VN, Pike VW, Innis RB. 2011. Brain and whole-body imaging in rhesus monkeys of 11C-NOP-1A, a promising PET radioligand for nociceptin/orphanin FQ peptide receptors. J Nucl Med 52(10):1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Chang A, Pasternak GW. 1998. Functional blockade of opioid analgesia by orphanin FQ/nociceptin. Biochem Pharmacol 55(9):1537–1540. [DOI] [PubMed] [Google Scholar]
- King MA, Rossi GC, Chang AH, Williams L, Pasternak GW. 1997. Spinal analgesic activity of orphanin FQ/nociceptin and its fragments. Neurosci Lett 223(2):113–116. [DOI] [PubMed] [Google Scholar]
- Kleideiter E, Piana C, Wang S, Nemeth R, Gautrois M. 2018. Clinical Pharmacokinetic Characteristics of Cebranopadol, a Novel First-in-Class Analgesic. Clin Pharmacokinet 57(1):31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach F, Reinscheid RK, Civelli O, Kemp JA. 1996. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J Neurosci 16(21):6657–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. 2000. An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology 92(3):795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. 2009. Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain 10(5):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Wei H, Woods JH, Kennedy RT. 2006. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther 318(3):1257–1264. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Rafalski P, Biala G, Dylag T, Rolka K, Silberring J. 2003. Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur J Pharmacol 474(2–3):233–239. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. 2007. The nociceptin/orphanin FQ receptor agonist Ro 64–6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology 32(4):902–910. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. 2003. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther 304(1):310–318. [DOI] [PubMed] [Google Scholar]
- Lambert DG. 2008. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov 7(8):694–710. [DOI] [PubMed] [Google Scholar]
- Le Cudennec C, Suaudeau C, Costentin J. 2002. Evidence for a localization of [(3)H]nociceptin binding sites on medullar primary afferent fibers. J Neurosci Res 68(4):496–500. [DOI] [PubMed] [Google Scholar]
- Lee H, Ko MC. 2015. Distinct functions of opioid-related peptides and gastrin-releasing peptide in regulating itch and pain in the spinal cord of primates. Sci Rep 5:11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Mathis JP, Rossi GC, Bodnar RJ, Pasternak GW. 2000. Autoradiographic localization of (125)I[Tyr(14)]orphanin FQ/nociceptin and (125)I[Tyr(10)]orphanin FQ/nociceptin(1–11) binding sites in rat brain. J Comp Neurol 423(2):319–329. [PubMed] [Google Scholar]
- Liebel JT, Swandulla D, Zeilhofer HU. 1997. Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br J Pharmacol 121(3):425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP, Ko MC. 2013. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci 4(2):214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, Schroder W, Kogel BY, Beier H, Englberger W, Schunk S, De Vry J, Jahnel U, Frosch S. 2014. Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther 349(3):535–548. [DOI] [PubMed] [Google Scholar]
- Lohith TG, Zoghbi SS, Morse CL, Araneta MD, Barth VN, Goebl NA, Tauscher JT, Pike VW, Innis RB, Fujita M. 2014. Retest imaging of [11C]NOP-1A binding to nociceptin/orphanin FQ peptide (NOP) receptors in the brain of healthy humans. Neuroimage 87:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohith TG, Zoghbi SS, Morse CL, Araneta MF, Barth VN, Goebl NA, Tauscher JT, Pike VW, Innis RB, Fujita M. 2012. Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J Nucl Med 53(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT. 2001. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 154(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Cheng ZJ, Fan GH, Cai YC, Jiang LZ, Pei G. 1997. Functional expression, activation and desensitization of opioid receptor-like receptor ORL1 in neuroblastoma x glioma NG108–15 hybrid cells. FEBS Lett 403(1):91–94. [DOI] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. 2012. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 485(7398):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margas W, Sedeek K, Ruiz-Velasco V. 2008. Coupling specificity of NOP opioid receptors to pertussis-toxin-sensitive Galpha proteins in adult rat stellate ganglion neurons using small interference RNA. J Neurophysiol 100(3):1420–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. 1995. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377(6549):532–535. [DOI] [PubMed] [Google Scholar]
- Molinari S, Camarda V, Rizzi A, Marzola G, Salvadori S, Marzola E, Molinari P, McDonald J, Ko MC, Lambert DG, Calo G, Guerrini R. 2013. [Dmt1]N/OFQ(1–13)-NH2: a potent nociceptin/orphanin FQ and opioid receptor universal agonist. Br J Pharmacol 168(1):151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. 1994. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341(1):33–38. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Ly HT, Maidment NT. 1996. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience 75(1):1–4. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Maidment NT. 1999. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem 73(1):179–186. [DOI] [PubMed] [Google Scholar]
- Neal CR Jr., Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ Jr., 1999a. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol 412(4):563–605. [PubMed] [Google Scholar]
- Neal CR Jr., Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ Jr., 1999b. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol 406(4):503–547. [PubMed] [Google Scholar]
- Nicol B, Lambert DG, Rowbotham DJ, Okuda-Ashitaka E, Ito S, Smart D, McKnight AT. 1998. Nocistatin reverses nociceptin inhibition of glutamate release from rat brain slices. Eur J Pharmacol 356(2–3):R1–3. [DOI] [PubMed] [Google Scholar]
- Nicol B, Lambert DG, Rowbotham DJ, Smart D, McKnight AT. 1996. Nociceptin induced inhibition of K+ evoked glutamate release from rat cerebrocortical slices. Br J Pharmacol 119(6):1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Takeshima H, Mori M, Nakagawara K, Takeuchi T. 1994. Structure and chromosomal mapping of genes for the mouse kappa-opioid receptor and an opioid receptor homologue (MOR-C). Biochem Biophys Res Commun 205(2):1353–1357. [DOI] [PubMed] [Google Scholar]
- Obara I, Przewlocki R, Przewlocka B. 2005. Spinal and local peripheral antiallodynic activity of Ro64–6198 in neuropathic pain in the rat. Pain 116(1–2):17–25. [DOI] [PubMed] [Google Scholar]
- Ozawa A, Brunori G, Cippitelli A, Toll N, Schoch J, Kieffer BL, Toll L. 2018. Analysis of the distribution of spinal NOP receptors in a chronic pain model using NOP-eGFP knock-in mice. Br J Pharmacol 175(13):2662–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa A, Brunori G, Mercatelli D, Wu J, Cippitelli A, Zou B, Xie XS, Williams M, Zaveri NT, Low S, Scherrer G, Kieffer BL, Toll L. 2015. Knock-In Mice with NOP-eGFP Receptors Identify Receptor Cellular and Regional Localization. J Neurosci 35(33):11682–11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE, Bruchas MR. 2019. NOP Receptor Signaling Cascades. Handb Exp Pharmacol 254:131–139. [DOI] [PubMed] [Google Scholar]
- Parker KE, Pedersen CE, Gomez AM, Spangler SM, Walicki MC, Feng SY, Stewart SL, Otis JM, Al-Hasani R, McCall JG, Sakers K, Bhatti DL, Copits BA, Gereau RW, Jhou T, Kash TJ, Dougherty JD, Stuber GD, Bruchas MR. 2019. A Paranigral VTA Nociceptin Circuit that Constrains Motivation for Reward. Cell 178(3):653–671 e619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gaveriaux-Ruff C. 1998. Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol 81(1–2):184–192. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Ko MC, Winger G, Wichmann J, Prinssen EP, Woods JH. 2011. The effects of nociceptin/orphanin FQ receptor agonist Ro 64–6198 and diazepam on antinociception and remifentanil self-administration in rhesus monkeys. Psychopharmacology (Berl) 213(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti D, Calo G, Guerrini R. 2019. NOP-Targeted Peptide Ligands. Handb Exp Pharmacol 254:17–36. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Burdge G, Gambrah J, Kinecki HE, Lin F, Lu B, Nguyen JT, Phan V, Ruan A, Sesay MA, Watkins TN. 2017. Cebranopadol: novel dual opioid/NOP receptor agonist analgesic. J Clin Pharm Ther 42(1):8–17. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr., , Civelli O. 1995. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270(5237):792–794. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Cerlesi MC, Ruzza C, Malfacini D, Ferrari F, Bianco S, Costa T, Guerrini R, Trapella C, Calo G. 2016. Pharmacological characterization of cebranopadol a novel analgesic acting as mixed nociceptin/orphanin FQ and opioid receptor agonist. Pharmacol Res Perspect 4(4):e00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Nazzaro C, Marzola GG, Zucchini S, Trapella C, Guerrini R, Zeilhofer HU, Regoli D, Calo G. 2006. Endogenous nociceptin/orphanin FQ signalling produces opposite spinal antinociceptive and supraspinal pronociceptive effects in the mouse formalin test: pharmacological and genetic evidences. Pain 124(1–2):100–108. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Spagnolo B, Wainford RD, Fischetti C, Guerrini R, Marzola G, Baldisserotto A, Salvadori S, Regoli D, Kapusta DR, Calo G. 2007. In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. Peptides 28(6):1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Sukhtankar DD, Ding H, Hayashida K, Ruzza C, Guerrini R, Calo G, Ko MC. 2015. Spinal antinociceptive effects of the novel NOP receptor agonist PWT2-nociceptin/orphanin FQ in mice and monkeys. Br J Pharmacol 172(14):3661–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, Tzschentke TM. 2010. Effects of the NOP receptor agonist Ro65–6570 on the acquisition of opiate- and psychostimulant-induced conditioned place preference in rats. Eur J Pharmacol 645(1–3):119–126. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. 2004. Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology (Berl) 172(2):129–136. [DOI] [PubMed] [Google Scholar]
- Sakurada T, Katsuyama S, Sakurada S, Inoue M, Tan-No K, Kisara K, Sakurada C, Ueda H, Sasaki J. 1999. Nociceptin-induced scratching, biting and licking in mice: involvement of spinal NK1 receptors. Br J Pharmacol 127(7):1712–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada T, Komatsu T, Moriyama T, Sasaki M, Sanai K, Orito T, Sakurada C, Sakurada S. 2005. Effects of intraplantar injections of nociceptin and its N-terminal fragments on nociceptive and desensitized responses induced by capsaicin in mice. Peptides 26(12):2505–2512. [DOI] [PubMed] [Google Scholar]
- Schiene K, Schroder W, Linz K, Frosch S, Tzschentke TM, Jansen U, Christoph T. 2018. Nociceptin/orphanin FQ opioid peptide (NOP) receptor and micro-opioid peptide (MOP) receptors both contribute to the anti-hypersensitive effect of cebranopadol in a rat model of arthritic pain. Eur J Pharmacol 832:90–95. [DOI] [PubMed] [Google Scholar]
- Schiene K, Tzschentke TM, Schroder W, Christoph T. 2015. Mechanical hyperalgesia in rats with diabetic polyneuropathy is selectively inhibited by local peripheral nociceptin/orphanin FQ receptor and micro-opioid receptor agonism. Eur J Pharmacol 754:61–65. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Morari M. 2000. Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides 21(7):1023–1029. [DOI] [PubMed] [Google Scholar]
- Schroder W, Lambert DG, Ko MC, Koch T. 2014. Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol 171(16):3777–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunk S, Linz K, Hinze C, Frormann S, Oberborsch S, Sundermann B, Zemolka S, Englberger W, Germann T, Christoph T, Kogel BY, Schroder W, Harlfinger S, Saunders D, Kless A, Schick H, Sonnenschein H. 2014. Discovery of a Potent Analgesic NOP and Opioid Receptor Agonist: Cebranopadol. ACS Med Chem Lett 5(8):857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Vogt LJ, Childers SR, Vogt BA. 2003. Distribution of ORL-1 receptor binding and receptor-activated G-proteins in rat forebrain and their experimental localization in anterior cingulate cortex. Neuropharmacology 45(2):220–230. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Childers SR. 1997. Anatomical distribution of mu, delta, and kappa opioid- and nociceptin/orphanin FQ-stimulated [35S]guanylyl-5’-O-(gamma-thio)-triphosphate binding in guinea pig brain. J Comp Neurol 386(4):562–572. [DOI] [PubMed] [Google Scholar]
- Slowe SJ, Clarke S, Lena I, Goody RJ, Lattanzi R, Negri L, Simonin F, Matthes HW, Filliol D, Kieffer BL, Kitchen I. 2001. Autoradiographic mapping of the opioid receptor-like 1 (ORL1) receptor in the brains of mu-, delta- or kappa-opioid receptor knockout mice. Neuroscience 106(3):469–480. [DOI] [PubMed] [Google Scholar]
- Sukhtankar DD, Lagorio CH, Ko MC. 2014a. Effects of the NOP agonist SCH221510 on producing and attenuating reinforcing effects as measured by drug self-administration in rats. Eur J Pharmacol 745:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Lee H, Rice KC, Ko MC. 2014b. Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology (Berl) 231(7):1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Zaveri NT, Husbands SM, Ko MC. 2013. Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/mu-opioid receptor ligands in mouse models of neuropathic and inflammatory pain. J Pharmacol Exp Ther 346(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G, Roth BL, Cherezov V, Stevens RC. 2012. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 485(7398):395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT. 2016. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol Rev 68(2):419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Ozawa A, Cippitelli A. 2019. NOP-Related Mechanisms in Pain and Analgesia. Handb Exp Pharmacol 254:165–186. [DOI] [PubMed] [Google Scholar]
- Trapella C, Ding H, Kiguchi N, Calo G, Ko MC. 2018. Reinforcing and antinociceptive effects of a mixed opioid and NOP receptor agonist, cebranopadol, in non-human primates. The 17th world congress on pain (IASP) Meeting abstract:PST538. [Google Scholar]
- Tzschentke TM, Linz K, Koch T, Christoph T. 2019. Cebranopadol: A Novel First-in-Class Potent Analgesic Acting via NOP and Opioid Receptors. Handb Exp Pharmacol 254:367–398. [DOI] [PubMed] [Google Scholar]
- Vazquez-DeRose J, Stauber G, Khroyan TV, Xie XS, Zaveri NT, Toll L. 2013. Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur J Pharmacol 699(1–3):200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Collins FS. 2017. The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377(4):391–394. [DOI] [PubMed] [Google Scholar]
- Volkow ND, McLellan AT. 2016. Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N Engl J Med 374(13):1253–1263. [DOI] [PubMed] [Google Scholar]
- Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR. 1994. cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett 348(1):75–79. [DOI] [PubMed] [Google Scholar]
- Waxler B, Dadabhoy ZP, Stojiljkovic L, Rabito SF. 2005. Primer of postoperative pruritus for anesthesiologists. Anesthesiology 103(1):168–178. [DOI] [PubMed] [Google Scholar]
- Winters BL, Christie MJ, Vaughan CW. 2019. Electrophysiological Actions of N/OFQ. Handb Exp Pharmacol 254:91–130. [DOI] [PubMed] [Google Scholar]
- Witta J, Palkovits M, Rosenberger J, Cox BM. 2004. Distribution of nociceptin/orphanin FQ in adult human brain. Brain Res 997(1):24–29. [DOI] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. 2012. Structure of the human kappa-opioid receptor in complex with JDTic. Nature 485(7398):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Wiesenfeld-Hallin Z. 1996. Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. Neuroreport 7(13):2092–2094. [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N. 1997. Effects of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, and N-methyl-D-aspartate receptor antagonists on the thermal hyperalgesia induced by partial sciatic nerve injury in the rat. Anesthesiology 87(5):1145–1152. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. 1997a. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience 81(1):249–254. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. 1997b. Effects of intrathecally administered nociceptin, an opioid receptor-like1 (ORL1) receptor agonist, on the thermal hyperalgesia induced by carageenan injection into the rat paw. Brain Res 754(1–2):329–332. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. 2011. The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr Top Med Chem 11(9):1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT. 2016. Nociceptin Opioid Receptor (NOP) as a Therapeutic Target: Progress in Translation from Preclinical Research to Clinical Utility. J Med Chem 59(15):7011–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT, Meyer ME. 2019. NOP-Targeted Nonpeptide Ligands. Handb Exp Pharmacol 254:37–67. [DOI] [PubMed] [Google Scholar]
- Zhang NR, Planer W, Siuda ER, Zhao HC, Stickler L, Chang SD, Baird MA, Cao YQ, Bruchas MR. 2012. Serine 363 is required for nociceptin/orphanin FQ opioid receptor (NOPR) desensitization, internalization, and arrestin signaling. J Biol Chem 287(50):42019–42030. [DOI] [PMC free article] [PubMed] [Google Scholar]