Abstract
Metronomic chemotherapy (MCT) is defined as the rhythmic chemotherapy of low-dose cytotoxic drugs with short or no drug-free breaks over prolonged periods. MCT affects tumor cells and the tumor microenvironment. Particularly, the low-dose schedule impairs the repair process of endothelial cells, resulting in an anti-angiogenesis effect. By stimulating the immune system to eliminate tumor cells, MCT induces immunological activation. Furthermore, combined with targeted therapy, anti-angiogenic drugs enhance the efficacy of MCT. The present review is an overview of phase I, II and III clinical trials focusing on the efficacy, toxicity and mechanism of MCT in patients with non-small cell lung cancer (NSCLC). Furthermore, the prospects of MCT in NSCLC have been discussed. The present review indicated that MCT is an efficacious treatment for selected patients with NSCLC, with acceptable systemic side effects and economic viability for public health.
Keywords: metronomic chemotherapy, non-small cell lung cancer
1. Introduction
Lung cancer is one of the leading causes of cancer-related mortality worldwide, comprising an estimated 12–13% of total new cancer cases and 22–23% of the total cancer-associated deaths in 2020 (1). A total of ~85% of primary lung cancer cases are non-small cell lung cancer (NSCLC) (2) and the majority of patients are diagnosed at an advanced stage. With the exception of a few patients who may have the opportunity to receive immune checkpoint therapy and others with a drive gene mutation who can undergo targeted therapy, almost all patients with NSCLC undergo chemotherapy (3). Conventional chemotherapy attempts to use doses close to the maximum tolerated dose (MTD) to maximize efficacy and eradicate tumor cells directly and has been the cornerstone of standard cancer treatment for several decades (4,5). Even with intensive chemotherapeutics, the overall response rate (ORR) of patients following first-line chemotherapy is <10%, progression-free survival (PFS) time is ~2.5 months and median overall survival (OS) time is ~9 months (6,7). Therefore, a novel treatment that employs the periodic application of low-dose (one-tenth to one-third of MTD) cytotoxic drugs with short or no drug-free breaks (daily, several times a week or weekly) over prolonged periods, known as metronomic chemotherapy (MCT) (Fig. 1), is under investigation and has become a focus of research.
Figure 1.
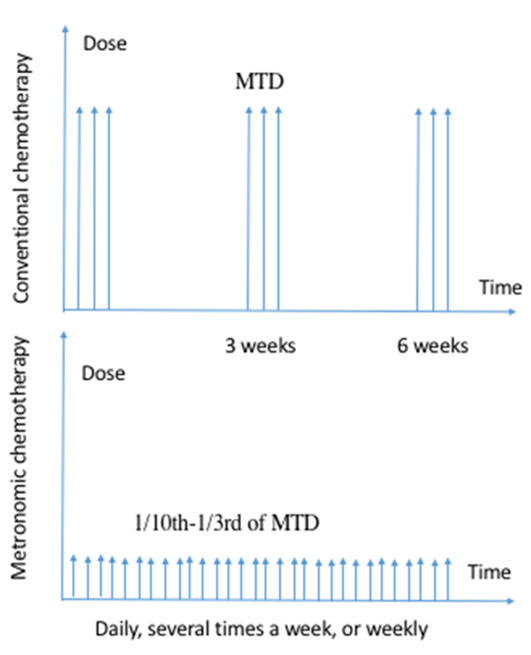
Difference between metronomic chemotherapy and conventional chemotherapy. MTD, maximum tolerated dose.
The present review collected publications using the terms ‘metronomic chemotherapy’, ‘MCT’ and ‘NSCLC’ to search the MEDLINE/PubMed database (http://www.webofknowledge.com/https://pubmed.ncbi.nlm.nih.gov) from January 2000 to December 2019. An overview of phase I, II and III clinical trials focusing on the efficacy, toxicity and mechanism of MCT in patients with NSCLC was conducted. The prospects of MCT in NSCLC have also been discussed. The current review indicated that MCT is an efficacious treatment option for selected patients with NSCLC, with acceptable systemic side effects and economic viability for public health.
2. Preclinical studies of MCT
The term ‘metronomic’ was first proposed by Hanahan et al (7) in 2000 based on studies by Browder et al (8) and Klement et al (9). In 2000, Browder et al established an animal xenograft model that underwent continuous application of low-dose cyclophosphamide, causing endothelial cells in the tumor vascular bed to continue to wither (8). In the same year, Klement et al also confirmed that continuous low-dose vinblastine inhibited tumor angiogenesis, leading to the recession of large, established tumors (9). Following these studies, the effect of MCT using numerous drugs has been gradually explored. The details preclinical drug studies are summarized in Table I.
Table I.
Preclinical studies of metronomic chemotherapy in lung cancer.
| First author, year | Protocol | Dosage | Xenograft | (Refs.) |
|---|---|---|---|---|
| Browder et al, 2000 | Cyclophosphamide | 170 mg/kg every 6 days | Lewis lung carcinoma | (8) |
| Eichhorn et al, 2010 | EndoTAG-1 with cisplatin | 40 and 80 mg/kg/day | Lung cancer | (92) |
| Panigraphy et al, 2010 | Oral etoposide | 40 and 80 mg/kg/day | Lewis lung carcinoma | (93) |
| Wang et al, 2012 | Cyclophosphamide and Endostar | 10 mg/kg of cyclophosphamide and 4 mg/kg of Endostar daily | NSCLC | (44) |
NSCLC, non-small cell lung cancer; EndoTAG-1, cationic lipid complexed paclitaxel.
3. Clinical trials of MCT
During the last decade, numerous clinical trials have been performed to explore MCT in first- and second-line treatment, and to investigate the maintenance of treatment for metastatic NSCLC (10–13). Drugs used by MCT-related clinical trials could be single-drug, two-drug or multiple-drug combinations, and can be combined with targeted drugs, anti-angiogenesis drugs or radiotherapy (Table II).
Table II.
Published clinical studies of metronomic clinical therapy in non-small cell lung cancer.
| First author, year | Metronomic protocol | Study design | Patients (n) | Clinical benefit (%) | Median PFS/TTP (month) | Median OS (month) | (Refs.) |
|---|---|---|---|---|---|---|---|
| Camerini et al, 2015 | Vinorelbine 50 mg 3 times/week (first-line) | Phase II trial | 43 | 58.1 | 5 | 9 | (18) |
| D'Ascanio et al, 2018 | Vinorelbine (30 or 40 mg 3 times/week) | Retrospective study | 44 | 63 | 9 | 12 | (19) |
| Katsaounis et al, 2015 | Vinorelbine (60 mg, every other day) and cisplatin (80 mg/m2) in cycles of 21 days | Study (first-line) | 41 | 65.7 | 4.2 | 12.0 | (25) |
| Jones et al, 2017 | 4-week cycles of Paclitaxel 80 mg/m2 and gemcitabine 300 mg/m2 weekly for 3 weeks and bevacizumab 10 mg/kg every 2 weeks | Pilot phase II study (first-line) | 39 | 56 | 8.5 | 25.5 | (94) |
| Kontopodis et al, 2013 | Vinorelbine 50 mg, 3 times/week (second-line) | Phase II trial | 46 | 30.5 | 2.2 | 9.4 | (27) |
| Hainsworth et al, 2000 | Weekly docetaxel (36 mg/m2) | Phase II trial | 39 | 52 | N/A | 5 | (28) |
| Noronha et al, 2013 | Weekly paclitaxel (80 mg/m2) | Retrospective | 37 | 67.5 | 4 | 7 | (29) |
| Kouroussis et al, 2008 | Temozolomide 75 mg/m2 daily for 21 days every 28 days | Phase II trial | 31 | 16.5 | 2.4 | 3.3 | (30) |
| Correale et al, 2006 | Weekly cisplatin 30 mg/m2 on days 1, 8 and 14 and etoposide 50 mg/m2 on 21 of the 28 days | Pilot phase II | 31 | 58.1 | 9 | 13 | (32) |
| Gorn et al, 2008 | Docetaxel 25 mg/m2 on days 1, 8 and 15, and trofosfamide 50 mg daily every 28 days | Phase II trial | 21 | 61.9 | 2.9 | 6.9 | (11) |
| Chen et al, 2016 | Gefitinib 250 mg daily alone or with daily oral UFT | Randomized phase II trial | 57 | 76 | 8.3 | 23.6 | (39) |
| Tan et al, 2015 | Sorafenib 200 mg 2 times/day for 4 weeks with a fixed metronomic (3 times/week) dose of oral vinorelbine at 60, 90 or 120 mg/week | Phase 1 trial | 48 | 66.7 | 4.4 | 8.2 | (45) |
| Correale et al, 2011 | cisplatin (30 mg/m2, days 1–3), oral Etoposide (50 mg, days 1–15) and bevacizumab (5 mg/kg, day 3) every 3 weeks | Phase II trial | 45 | 86.6 | 9.53 | N/A | (47) |
| Marquette et al, 2013 | Paclitaxel (80 mg/m2 on days 1, 8 and 15), gemcitabine (200–300 mg/m2 on days 1, 8 and 15) and bevacizumab (10 mg/m2 on days 1 and 15) for six cycles | Phase II trial | 33 | 91 | 9 | 30 | (48) |
| Revannasiddaiah et al, 2015 | Cyclophosphamide (50 mg daily) and radiotherapy (20–30 Gy in 5–10 fractions) | Retrospective study | 74 | N/A | 3.1 | N/A | (50) |
| Giorgio et al, 2005 | Temozolomide 150 mg/mq/day for five consecutive days for the first cycle; doses were increased to 200 mg/mq/day for 5 days every 28 days | Phase II trial | 30 | 20 | N/A | N/A | (95) |
| Banna et al, 2018 | Vinorelbine (30 mg 3 times/weekly) | Prospective phase II | 50 | 32 | 2.7 | 7.3 | (96) |
| Bilir et al, 2017 | Vinorelbine (30 mg 3 times/weekly) | Retrospective study | 35 | 69 | 4 | 7 | (97) |
| Mencoboni et al, 2017 | Vinorelbine (50 mg 3 times/weekly) | First-line | 76 | 50 | N/A | 8 | (98) |
N/A, not available; UFT, Tegafur/uracil.
Single agent of MCT in first-line treatment
Despite evidence that carboplatin and paclitaxel significantly improved OS time compared with vinorelbine or gemcitabine monotherapy (median OS, 10.3 months vs. 6.2 months), greater toxicity limited their use (neutropenia, 48% vs. 12%) (10). Single-agent chemotherapy is an option for patients who are less suited for combination chemotherapy, particularly for older patients, and an oral agent may be a more suitable choice of administration (14).
Metronomic oral vinorelbine is an extremely safe treatment for advanced NSCLC with notable clinical benefit, mainly consisting of long-term disease stabilization (15). Camerini et al (16) reported that the median survival of 14 patients with NSCLC who underwent MCT was >30 months. Compared with intravenous administration of vinorelbine, oral administration of vinorelbine has the advantages of convenience and minimal side effects (17). Patients with Union for International Cancer control version 8 stage IIIB-IV NSCLC who were treated with oral vinorelbine 50 mg 3 times/week as first-line chemotherapy had a median time to progression (TTP) of 5 months and a median OS of 9 months (range, 3–29 months), demonstrating good tolerance with rare incidents of serious toxicity (16,18). Patients who were aged >60 years and had stage IIIB or IV, Eastern Cooperative Oncology Group (ECOG) ≥1 and with ≥1 significant comorbidity were treated with oral vinorelbine 30 mg 3 times/week or 40 mg 3 times/week, meaning treatment was administered 1 day on and 1 day off (19). The median OS was 12 months, disease control rate was 63% and median PFS was 9 months. A meta-analysis of metronomic oral vinorelbine encompassing 418 patients reported an OS of 8.7 months (95% confidence interval, 7.6–9.5) (20). A schedule of 20–30 mg every other day without interruption demonstrated good tolerance and clinical benefit (21).
Taxanes (docetaxel and paclitaxel), which are novel microtubule-stabilizing agents, are an integral part of several commonly used chemotherapy regimens in NSCLC (22). Weekly docetaxel and 3-week conventional schemes exhibited similar effects for untreated advanced NSCLC (23,24). In terms of adverse reactions, the febrile neutropenia incidence was significantly lower in the weekly metronomic treatment group compared with the traditional 3-week group.
Multiple MCT drugs in first-line treatment: Vinorelbine and cisplatin (DDP)
To evaluate the safety and efficacy of metronomic vinorelbine in combination with DDP as a first-line treatment for patients with advanced NSCLC, Katsaounis et al (25) conducted a multicenter phase II study, in which a total of 41 patients with advanced NSCLC were treated with oral metronomic vinorelbine (60 mg total dose, every other day) in combination with DDP (80 mg/m2) in cycles of 21 days. The median PFS was 4.2 months, median OS was 12.0 months and 1-year survival rate was 52.6%. Three of these patients exhibited febrile neutropenia and one died due to sepsis. Although the combination schedule was accompanied by myelotoxicity, the treatment was still an effective option in the first-line treatment option for patients with advanced NSCLC. MTDs were reached at an oral dose of 60 mg 3 times/week for vinorelbine and 85 mg/m2 for DDP and ORR was 20.8%. This metronomic strategy was tolerable and effective in patients with NSCLC (21).
Later line treatment
Oral metronomic vinorelbine produced non-negligible survival in elderly or pretreated patients and exhibited stable long-term blood concentrations (21,26,27). Daily oral vinorelbine (30 mg/day) for 21 days with an interruption in treatment for 1 week was well tolerated without dose-limiting toxicity (26). In another study, a schedule of 20–30 mg every other day without interruption provided good tolerability and clinical benefit (21). Additionally, pretreated patients with NSCLC were treated with oral vinorelbine at a dose of 50 mg 3 times/week with a median OS of 9.4 months and a 1-year survival rate of 30.1% (27).
Single agent MCT in later line treatment: Taxane
In 2000, Hainsworth et al (28) concluded that weekly docetaxel (36 mg/m2) was well tolerated in elderly patients with NSCLC, with a median survival time of 5 months and a 1-year survival rate of 27%. In another study, weekly paclitaxel (80 mg/m2) was also well tolerated, effective and safe for recurrent refractory NSCLC, with a median PFS of 4 months and a median OS of 7 months (29).
Single agent MCT in later line treatment: Temozolomide (TMZ)
TMZ, a novel oral alkylating agent, has demonstrated anticancer activity against brain metastases in various solid tumors, including NSCLC (30). TMZ was administered at a dose of 75 mg/m2 daily for 21 days every 28 days, resulting in a median OS of 3.3 months and a 1-year survival rate of 22.5% (30). However, another phase II study reported no objective response to TMZ in patients with chemotherapy-naive advanced NSCLC (31). Treatment discontinuation may be the result of ineffective treatment in patients with NSCLC and a particularly poor prognosis.
Multiple MCT drugs in later line treatment
In 2006, Correale et al (32) conducted a pilot phase II study to evaluate the toxicity and effect of the novel metronomic protocol of weekly DDP 30 mg/m2 on days 1, 8 and 14 and oral daily etoposide (VP16) 50 mg/m2 on day 1–21 of the 28 days of the cycle in high-risk patients with NSCLC with a mean TTP of 9 months and OS of 13 months. However, three deaths were caused by pulmonary embolism. In the same study, 10 patients who underwent MCT exhibited decreased expression of vascular endothelial growth factor (VEGF), indicating that MCT may influence tumor growth and angiogenesis. Generally, MCT is well tolerated and effective, even for patients with poor general condition.
Docetaxel and trofosfamide
Gorn et al (11) conducted a pilot study investigating the combination of docetaxel 25 mg/2 on days 1, 8 and 15, and trofosfamide 50 mg daily every 28 days as a second-line treatment for patients with metastatic NSCLC. The median PFS and median OS were 2.9 and 6.9 months, respectively. Furthermore, no grade IV toxicity or treatment-induced death occurred.
MCT combined with target therapy
Targeted treatments yield higher response rates, longer PFS and prolonged OS compared with traditional cytotoxic chemotherapies (33). However, concurrent administration of gefitinib or erlotinib with standard platinum-doublet chemotherapy in large phase III randomized trials presented no improved survival compared with chemotherapy alone (34–37). It remains unclear whether MCT combined with targeted therapy yields clinical benefit.
Tegafur and gefitinib
Tegafur/uracil (UFT) has an underlying anti-angiogenesis effect and is suitable for MCT (38). This inhibitory effect is more obvious when UFT was administered continuously at low doses (39). For patients with epidermal growth factor receptor (EGFR) mutations, the addition of UFT significantly improved PFS (14.4 vs. 7.6 months) (39). For patients with low microvessel density, the addition of UFT to gefitinib treatment resulted in longer PFS (median PFS, 11.8 vs. 2.8 months). The median OS was 18.3 months in the gefitinib alone group and 23.6 months in the gefitinib with UFT group.
Oral vinorelbine and erlotinib
Sutiman et al (40) designed a phase I study of oral vinorelbine in combination with erlotinib using conventional (CSV) and metronomic (MSV) dosing schedules in NSCLC to evaluate the safety, tolerability and pharmacokinetics of treatments. Oral vinorelbine 40 mg/m2 in the CSV group (n=16) and 100 mg/week in the MSV group (n=14) was administered on days 1 and 8. An objective response was achieved in 38 and 29% of the CSV and MSV groups, respectively. In conclusion, the combination of oral vinorelbine with erlotinib was feasible and tolerable in both groups. Neither pharmacokinetic nor pharmacogenetic monitoring appeared to be useful in predicting OS with oral metronomic vinorelbine in advanced NSCLC (41). Metronomic vinorelbine inhibited the phosphorylation of extracellular signal-regulated kinase (ERK)1/ERK2 and protein kinase B, and significantly decreased the expression of cyclin-D1 and ATP-binding cassette super-family G member 2 mRNAs and proteins, sensitizing resistant cells to EGFR tyrosine kinase inhibitors (42).
MCT combined with anti-angiogenic drugs
Anti-angiogenic therapy has become a focus for the treatment of advanced lung cancer. Preclinical studies have demonstrated that the combination of MCT and anti-angiogenesis drugs can further improve efficacy (43,44). Metronomic vinorelbine combined with Endostar significantly enhanced antitumor and anti-angiogenic responses without overt toxicity in a xenograft mouse model of human lung cancer (43).
Vinorelbine and sorafenib
Patients received a starting dose of sorafenib at 200 mg 2 times/day for 4 weeks with a fixed metronomic (3 times/week) dose of oral vinorelbine at 60, 90 or 120 mg/week (45). In patients without dose-limiting toxicities, sorafenib doses were increased to 400 mg 2 times/day for 4 weeks, 600 mg 2 times/day for 4 weeks and, finally, 800 mg 2 times/day. A total of 48 patients were analyzed. The results demonstrated that four (8.3%) patients exhibited a partial response (PR) and seven (14.6%) a cavitary response. The combination of sorafenib and metronomic oral vinorelbine was effective in treating advanced NSCLC. Furthermore, circulating endothelial cell counts were mentioned a promising marker for improved survival.
DDP, VP16 and bevacizumab
A previous study of DDP and oral VP16 MCT combined with bevacizumab in the treatment of advanced NSCLC was performed to evaluate whether MCT increased the efficacy of anti-angiogenesis therapy (46). A total of 40 patients who received combination treatment had an ORR of 77.5%. The stable disease rate was 15%, median TTP was 7.6 months the most common grade 1–2 toxicity was hematological toxicity. The study selected 5 mg/kg as the optimal biological dose of bevacizumab and 7.5 mg/kg as the maximum tolerated dose. Bevacizumab combined with MCT was considered to be safe and feasible with anti-angiogenic activity and significant antitumor effects.
In 2011, Correale et al (47) added bevacizumab to DDP and applied metronomic daily oral VP16 for 45 patients with advanced NSCLC. The patients received DPP (30 mg/m2, days 1–3), oral VP16 (50 mg, days 1–15) and bevacizumab (5 mg/kg, day 3) every 3 weeks. A PR was achieved in 31 (68.9%) patients, NSCLC remained stable in eight (17.8%) and disease progressed in six (13.3%) patients. Furthermore, PFS was 9.53 months. The bio-chemotherapy regimen demonstrated efficacy in advanced NSCLC; however, due to hematological toxicity and gastroenteric toxicity, patients enrolled in future studies should be strictly selected.
Paclitaxel, gemcitabine and bevacizumab
The combination of paclitaxel, gemcitabine and bevacizumab administered in a metronomic schedule was demonstrated to be an effective and tolerable treatment strategy for patients with advanced NSCLC (44). Patients were treated with 4-week cycles of paclitaxel 80 mg/m2 and gemcitabine 300 mg/m2 weekly for three weeks, plus bevacizumab 10 mg/kg every two weeks. The results reported a median PFS of 8.5 months and a median OS of 25.5 months.
A phase II study of MCT with bevacizumab in patients with advanced non-squamous (NS)NSCLC was presented at the 2013 American Society of Clinical Oncology meeting (48). A total of 33 untreated patients with stage 4 NS-NSCLC were administrated a 4-week cycle of paclitaxel (80 mg/m2, days 1, 8 and 15), gemcitabine (200–300 mg/m2, days 1, 8 and 15) and bevacizumab (10 mg/m2, days 1 and 15) for six cycles. The results reported that ORR was 73%, median PFS was 9 months, median OS was 30 months, 1-year survival was 74% and 2-year survival was 55%. The results, although limited by the size of the trial, were consistent with the hypothesis that MCT with bevacizumab may enhance anti-angiogenic effects and clinical benefits in advanced NS-NSCLC.
Metronomic cyclophosphamide with radiotherapy
Improving the outcomes of radiotherapy (RT) in the form of concurrent chemotherapy, anti-angiogenic therapy and anti-growth factor receptor targeted therapies has been a focus of research (49). Comparisons were made between 65 patients treated with palliative RT alone (20–30 Gy in 5–10 fractions) and 74 patients treated with palliative RT and oral metronomic cyclophosphamide (50 mg daily) (50). The PFS was significantly higher when metronomic chemotherapy was added to RT in comparison to treatment with RT alone in patients with adenocarcinoma (3.5 vs. 2.4 months) and no significant differences were observed in patients with squamous cell carcinoma without any measurable hematological toxicity. Radiotherapy combined with metronomic VP16 prolonged OS, indicating that the combination may have a synergistic effect (51).
Maintenance therapy
Based on the characteristics of MCT (low toxicity, high efficacy and low resistance to chemotherapy), its combination with other drugs, including targeted therapy, is a choice of treatment for advanced NSCLC (12,13). Currently, two phase III studies have confirmed the promising efficacy of MCT with maintenance therapy (12,13). After the tumor load is decreased by traditional chemotherapy, MCT may have improved function in inhibiting tumor angiogenesis and adjusting the immune function in maintenance therapy (52,53). However, chemotherapy conversion for NSCLC still requires basic and clinical verification.
A larger number of severe toxicities, including grade 4 hemoptysis, thromboembolism and pulmonary embolism, were observed in MCT combined with targeted therapy, such as bevacizumab and sunitinib (54). TMZ and VP16 MCT are associated with a risk of myelodysplastic and secondary leukemia (55,56). PFS reached 4 months following second-line metronomic oral vinorelbine-atezolizumab combination for stage-IV NSCLC (57). Additional attention should be paid to the cumulative and long-term toxicity in long-term use of MCT, particularly in children. Furthermore, maintenance therapy with oral metronomic vinorelbine prolonged PFS compared with best supportive care; however, the optimal dose of oral metronomic vinorelbine requires further investigation (58).
4. Mechanism of MCT
Continuous low-dose chemotherapy was shown to achieve more antitumor effects in vivo compared with routine chemotherapy with a completely different mechanism, laying the foundation for MCT (8). In early 2000, a relatively low dose of cyclophosphamide or vinblastine was used to validate the inhibition of angiogenesis inhibition and how that exerts an antitumor effect in an animal model (8). Additionally, several novel mechanisms have been identified, including immune- and stem cell-based mechanisms (Fig. 2) (59–61).
Figure 2.
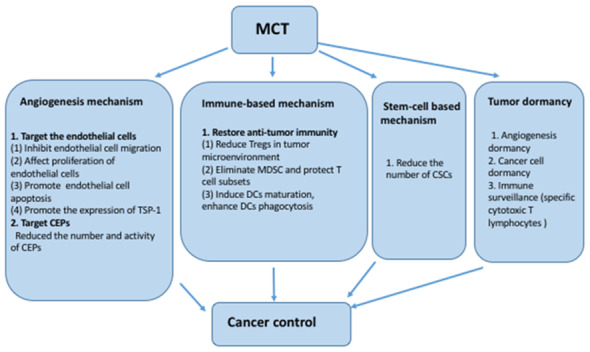
Mechanism of metronomic chemotherapy. MCT, metronomic chemotherapy; TSP-1, thrombospondin 1; CEPs, circulating endothelial progenitors; Tregs, regulatory T cells; MDSC, myeloid-derived suppressor cells; DCs, dendritic cells; CSCs, cancer stem cells.
Angiogenesis mechanism
MCT is hypothesized to target endothelial cells and circulating endothelial progenitors (CEPs) directly with a lack of drug resistance (38,62). Bocci et al (63) reported almost no difference among various endothelial cells and cancer cells during short-term exposure to cytotoxic drugs. However, following long-term continuous exposure, endothelial cells were relatively more sensitive compared with cancer cells (63). Owing to the high sensitivity of tumor endothelial cells, low doses of chemotherapy drugs inhibit their proliferation instead of the proliferation cancer cells (63,64). Additionally, cyclophosphamide MCT reduced the number and activity of CEPs, coinciding with long-term tumor inhibition (65). It has been reported that low-dose MCT may be an effective treatment to prevent CEPs mobilization (66). Furthermore, MCT induces functional normalization of tumor blood vessels, resulting in improved tumor perfusion (66). Consequently, MCT delays both primary and secondary tumor growth by blocking the supply of essential nutrients and removing metabolites (38,67,68). Vinorelbine MCT combined with an angiogenesis inhibitor (Endostar) significantly enhanced anti-angiogenic responses by inhibiting tumor growth by decreasing the expression of cluster of differentiation (CD) 31, VEGF, hypoxia inducible factor 1α and CEPs in a xenograft model of human lung cancer (43).
Immune-based mechanism
In addition to angiogenesis inhibition, MCT also restores antitumor immunity and induces tumor dormancy (69), forming a tumor immune balance from an immunosuppressive state to an immune activation state (70).
Regulatory T cells (Tregs) are CD4+CD25+ forkhead box P3+ lymphocytes and the expression of cytotoxic lymphocyte-associated antigen-4 inhibits cell-specific immune responses (71,72). Tregs inhibit the antitumor immune response, which is mediated by CD8+ lymphocytes, CD4+ T helper cells and natural killer cells (72–74). Increased levels of Tregs are associated with tumor progression and a lack of response to cancer therapy (74). Therefore, the removal of Tregs from patients with NSCLC, particularly those in the tumor microenvironment, is considered important for successful antitumor therapy (74). Banissi et al (74) observed a decreased Treg/CD4+ ratio and attenuated residual Treg immunity suppression ability in a TMZ-resistant glioma model with TMZ MCT (75). Furthermore, Ghiringhelli et al (76) confirmed that Tregs suppress NK cell effector functions in vitro and in vivo, i.e. homeostatic proliferation, cytotoxicity and IL-12-mediated IFN-γ production.
Another subset of tumor suppressor cells, known as myeloid-derived suppressor cells (MDSC), inhibit the activity of T cells in mouse tumor cell line 4T1-Neu (77). The reduction of MDSCs was hypothesized to be associated with the inhibition of p38 mitogen-activated protein kinase activity, decreased levels of tumor necrosis factor-α and NO and inhibition of S100 calcium-binding protein A9 expression (77). Furthermore, MCT has been reported to selectively eliminate MDSCs and the protection of T-cell subsets, thereby enhancing anti-tumor immunity (77). An in vivo study indicated that paclitaxel MCT reduced MDSC infiltration in tumor tissues and that there was no effect on bone marrow hematopoietic stem cells (78).
As antigen-presenting cells, immunotherapy-based dendritic cells (DCs) have been shown to preclinically and clinically induce a strong antitumor immune response (79). This study demonstrated that MCT induced DC maturation, enhanced DC phagocytosis and activated the Rho family of guanosine triphosphate phosphatases on DCs, which regulate cell-cell interactions, cell migration and endocytosis (80). MCT regimens of certain chemotherapeutic drugs, including vinblastine, paclitaxel and VP16, promoted DC maturation at non-toxic concentrations (62). In a clinical setting, optimization of exact dosage and timing may need to be adjusted with respect to the patient's immune response and the type of tumor (81).
Stem cell-based mechanism
Cancer stem cells (CSCs) are a group of cells with persistent self-renewal and unlimited proliferation capacities in tumors, triggering resistance to conventional chemotherapy (82). Cyclophosphamide MCT has been demonstrated to significantly impair primary and metastatic tumor formation (83) and decrease the number of CD133+ precursors CD133+/CD44+/CD24+ in a pancreatic cancer xenograft model (84). Furthermore, an in vivo study indicated that the tumorigenicity of these resistant cells' tumorigenicity decreased with significant depletion of parental CD44+ cells, indicating that MCT may target CD44+ CSCs (85).
Tumor dormancy
Previous studies have identified the important effect that tumor cell dormancy serves in inhibiting tumor progression and recurrence (86,87). Tumor dormancy can occur at primary and metastatic tumor sites and is usually divided into three different types: i) Angiogenesis dormancy; ii) cell dormancy and iii) immune surveillance (69,88). Following induced apoptosis of tumor cells by tumor-specific cytotoxic T lymphocytes, residual tumor cells usually survive; however, the immune system ensures that the tumor cells remain in a dormant state until immune escape occurs, followed by dormancy interruption (89).
5. Conclusion
The current review summarized preclinical and clinical trials focused on evaluating MCT either alone or in combination with other treatments in NSCLC management and provided information about the underlying mechanisms. Whether MCT is as efficient as conventional chemotherapy in front-line settings and whether it is a good surrogate for further line treatment in NSCLC has not been not fully demonstrated, particularly in the field of targeted therapy and immune therapy. MCT demonstrated promise in the treatment of NSCLC; however, the current data cannot truly verify its superiority to conventional chemotherapy. Nonetheless, due to lower toxicity, higher tolerability and relatively acceptable efficacy of MCT compared with supportive care, further investigation of MCT in NSCLC is required as MCT may be an optimal option in further line treatments for patients who cannot tolerate conventional chemotherapy, even in front-line settings.
MCT was originally developed to overcome drug resistance by shifting the therapeutic target from tumor cells to the tumor vasculature. The therapy can be personalized as it has been combined with targeted drugs and immune checkpoint inhibitors (90). Notably, ‘personalization’ should be emphasized as precision medicine takes into account the personal, familial and economic aspects of patient care to provide customized treatment (70).
To the best of our knowledge, the current review is the first systematic review of MCT in NSCLC. Although MCT has been a focus of research in recent years, numerous aspects of MCT remain empirical or unresolved. Considering the reported data were extracted from relatively small phase II trials, further studies are required to investigate MCT, including the most suitable drugs, doses of each drug, reasonable frequency, duration and suitable patients. Furthermore, future head-to-head studies are required to determine which scheme is better. Biomarkers are needed to derive the optimal metronomic dosing of cytotoxic agents (91). Monitoring and evaluating the effect of MCT remains worthy of further investigation.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- PFS
progression free survival
- TTP
time to progression
- OS
overall survival
Funding
The current review was funded by the National Natural Science Foundation of China (grant no. 81372660), Key Medical Science Research Fund of Hangzhou (grant no. 2011ZD001), Medical Science Research Fund of Zhejiang Province (grant no. 2013KYA157), Traditional Chinese Medicine Science and Technology Project of Zhejiang Province (grant no. 2013ZA104), Zhejiang Province Public Welfare Technology Application Research Project (grant no. 2017C33200) and Hangzhou Science and Technology Bureau grant no. (20140633B30).
Availability of data and materials
The datasets generated and/or analyzed during the present study are available in the MEDLINE/PubMed database (http://www.webofknowledge.com/https://pubmed.ncbi.nlm.nih.gov).
Author's contributions
YS supervised the project and wrote the original draft of the manuscript. SW conceptualized the current review. SZ reviewed and edited the manuscript. All the authors have read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Tessen HW, Hutzschenreuter U, Steffens CC, et al. The treatment of lung cancer in German outpatient centres. Data from a clinical registry-TLK Registry. Onkologie. 2011;34:153–153. [Google Scholar]
- 3.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 4.Torimura T, Iwamoto H, Nakamura T, Koga H, Ueno T, Kerbel RS, Sata M. Metronomic chemotherapy: Possible clinical application in advanced hepatocellular carcinoma. Transl Oncol. 2013;6:511–519. doi: 10.1593/tlo.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benzekry S, Pasquier E, Barbolosi D, Lacarelle B, Barlési F, André N, Ciccolini J. Metronomic reloaded: Theoretical models bringing chemotherapy into the era of precision medicine. Semin Cancer Biol. 2015;35:53–61. doi: 10.1016/j.semcancer.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Weiss JM, Stinchcombe TE. Second-line therapy for advanced NSCLC. Oncologist. 2013;18:947–953. doi: 10.1634/theoncologist.2013-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Liu J, Cheng Y. Metronomic chemotherapy-A new path to treat advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2015;18:232–239. doi: 10.3779/j.issn.1009-3419.2015.04.08. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 9.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavolé A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–1088. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 11.Görn M, Habermann CR, Anige M, Thöm I, Schuch G, Andritzky B, Brandl S, Burkholder I, Edler L, Hossfeld DK, et al. A pilot study of docetaxel and trofosfamide as second-line ‘metronomic’ chemotherapy in the treatment of metastatic non-small cell lung cancer (NSCLC) Onkologie. 2008;31:185–189. doi: 10.1159/000118626. [DOI] [PubMed] [Google Scholar]
- 12.Belani CP, Barstis J, Perry MC, La Rocca RV, Nattam SR, Rinaldi D, Clark R, Mills GM. Multicenter, randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol. 2003;21:2933–2939. doi: 10.1200/JCO.2003.02.563. [DOI] [PubMed] [Google Scholar]
- 13.Schuette W, Blankenburg T, Guschall W, Dittrich I, Schroeder M, Schweisfurth H, Chemaissani A, Schumann C, Dickgreber N, Appel T, Ukena D. Multicenter randomized trial for stage IIIB/IV non-small-cell lung cancer using every-3-week versus weekly paclitaxel/carboplatin. Clin Lung Cancer. 2006;7:338–343. doi: 10.3816/CLC.2006.n.016. [DOI] [PubMed] [Google Scholar]
- 14.Biganzoli L, Lichtman S, Michel JP, Papamichael D, Quoix E, Walko C, Aapro M. Oral single-agent chemotherapy in older patients with solid tumours: A position paper from the International Society of Geriatric Oncology (SIOG) Eur J Cancer. 2015;51:2491–2500. doi: 10.1016/j.ejca.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Camerini A, Banna GL, Cinieri S, Pezzuto A, Mencoboni M, Rosetti F, Figueiredo A, Rizzo P, Ricci A, Langenhoven L, et al. Metronomic oral vinorelbine for the treatment of advanced non-small cell lung cancer: A multicenter international retrospective analysis. Clin Transl Oncol. 2019;21:790–795. doi: 10.1007/s12094-018-1989-y. [DOI] [PubMed] [Google Scholar]
- 16.Camerini A, Valsuani C, Mazzoni F, Siclari O, Puccetti C, Donati S, Rondini M, Tartarelli G, Puccinelli P, Di Costanzo F, Amoroso D. Phase II trial of single-agent oral vinorelbine in elderly (> or =70 years) patients with advanced non-small-cell lung cancer and poor performance status. Ann Oncol. 2010;21:1290–1295. doi: 10.1093/annonc/mdp525. [DOI] [PubMed] [Google Scholar]
- 17.Jassem J, Ramlau R, Karnicka-Mlodkowska H, Krawczyk K, Krzakowski M, Zatloukal P, Lemarié E, Hartmann W, Novakova L, O'Brien M, Depierr A. A multicenter randomized phase II study of oral vs. intravenous vinorelbine in advanced non-small-cell lung cancer patients. Ann Oncol. 2001;12:1375–1381. doi: 10.1023/A:1012539225493. [DOI] [PubMed] [Google Scholar]
- 18.Camerini A, Puccetti C, Donati S, Valsuani C, Petrella MC, Tartarelli G, Puccinelli P, Amoroso D. Metronomic oral vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer: Results of a phase II trial (MOVE trial) BMC Cancer. 2015;15:359. doi: 10.1186/s12885-015-1354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Ascanio M, Pezzuto A, Fiorentino C, Sposato B, Bruno P, Grieco A, Mancini R, Ricci A. Metronomic chemotherapy with vinorelbine produces clinical benefit and low toxicity in frail elderly patients affected by advanced non-small cell lung cancer. Biomed Res Int. 2018;2018:6278403. doi: 10.1155/2018/6278403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujol JL, Coffy A, Camerini A, Kotsakis A, Mencoboni M, Gusella M, Pasini F, Pezzuto A, Banna GL, Bilir C, et al. An individual patient-data meta-analysis of metronomic oral vinorelbine in metastatic non-small cell lung cancer. PLoS One. 2019;14:e0220988. doi: 10.1371/journal.pone.0220988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasini F, Barile C, Caruso D, Modena Y, Fraccon AP, Bertolaso L, Menon D, La Russa F, Crepaldi G, Bononi A, et al. Oral metronomic vinorelbine (OMV) in elderly or pretreated patients with advanced non small cell lung cancer: Outcome and pharmacokinetics in the real world. Invest New Drugs. 2018;36:927–932. doi: 10.1007/s10637-018-0631-8. [DOI] [PubMed] [Google Scholar]
- 22.Huizing MT, Misser VH, Pieters RC, ten Bokkel Huinink WW, Veenhof CH, Vermorken JB, Pinedo HM, Beijnen JH. Taxanes: A new class of antitumor agents. Cancer Invest. 1995;13:381–404. doi: 10.3109/07357909509031919. [DOI] [PubMed] [Google Scholar]
- 23.Gervais R, Ducolone A, Breton JL, Braun D, Lebeau B, Vaylet F, Debieuvre D, Pujol JL, Tredaniel J, Clouet P, Quoix E. Phase II randomised trial comparing docetaxel given every 3 weeks with weekly schedule as second-line therapy in patients with advanced non-small-cell lung cancer (NSCLC) Ann Oncol. 2005;16:90–96. doi: 10.1093/annonc/mdi018. [DOI] [PubMed] [Google Scholar]
- 24.Camps C, Massuti B, Jimenez A, Maestu I, Gómez RG, Isla D, González JL, Almenar D, Blasco A, Rosell R, et al. Randomized phase III study of 3-weekly versus weekly docetaxel in pretreated advanced non-small-cell lung cancer: A Spanish Lung Cancer Group trial. Ann Oncol. 2006;17:467–472. doi: 10.1093/annonc/mdj115. [DOI] [PubMed] [Google Scholar]
- 25.Katsaounis P, Kotsakis A, Agelaki S, Kontopodis E, Agelidou A, Kentepozidis N, Vamvakas L, Christopoulou A, Karachaliou N, Hatzidaki D, Georgoulias V. Cisplatin in combination with metronomic vinorelbine as front-line treatment in advanced non-small cell lung cancer: A multicenter phase II study of the Hellenic Oncology Research Group (HORG) Cancer Chemother Pharmacol. 2015;75:821–827. doi: 10.1007/s00280-015-2707-x. [DOI] [PubMed] [Google Scholar]
- 26.Bozec A, Fischel JL, Milano G. Epidermal growth factor receptor/angiogenesis dual targeting: preclinical experience. Curr Opin Oncol. 2006;18:330–334. doi: 10.1097/01.cco.0000228737.78003.06. [DOI] [PubMed] [Google Scholar]
- 27.Kontopodis E, Hatzidaki D, Varthalitis I, Kentepozidis N, Giassas S, Pantazopoulos N, Vardakis N, Rovithi M, Georgoulias V, Agelaki S. A phase II study of metronomic oral vinorelbine administered in the second line and beyond in non-small cell lung cancer (NSCLC): A phase II study of the Hellenic Oncology Research Group. J Chemotherapy. 2013;25:49–55. doi: 10.1179/1973947812Y.0000000050. [DOI] [PubMed] [Google Scholar]
- 28.Hainsworth JD, Burris HA, III, Litchy S, Morrissey LH, Barton JH, Bradof JE, Greco FA. Weekly docetaxel in the treatment of elderly patients with advanced nonsmall cell lung carcinoma. A Minnie Pearl Cancer Research Network Phase II Trial. Cancer. 2000;89:328–333. doi: 10.1002/1097-0142(20000715)89:2<328::AID-CNCR17>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Noronha V, Patil VM, Joshi A, Prabhash K. Efficacy and safety of metronomic administration of paclitaxel for advanced recurrent non-small-cell lung cancer. Indian J Cancer. 2013;50:122–127. doi: 10.4103/0019-509X.117032. [DOI] [PubMed] [Google Scholar]
- 30.Kouroussis C, Vamvakas L, Vardakis N, Kotsakis A, Kalykaki A, Kalbakis K, Saridaki Z, Kentepozidis N, Giassas S, Georgoulias V. Continuous administration of daily low-dose temozolomide in pretreated patients with advanced non-small cell lung cancer: A phase II study. Oncology. 2009;76:112–117. doi: 10.1159/000192586. [DOI] [PubMed] [Google Scholar]
- 31.Dziadziuszko R, Ardizzoni A, Postmus PE, Smit EF, Price A, Debruyne C, Legrand C, Giaccone G, EORTC Lung Cancer Group Temozolomide in patients with advanced non-small cell lung cancer with and without brain metastases: A phase II study of the EORTC Lung Cancer Group (08965) Eur J Cancer. 2003;39:1271–1276. doi: 10.1016/S0959-8049(03)00234-X. [DOI] [PubMed] [Google Scholar]
- 32.Correale P, Cerretani D, Remondo C, Martellucci I, Marsili S, La Placa M, Sciandivasci A, Paolelli L, Pascucci A, Rossi M, et al. A novel metronomic chemotherapy regimen of weekly platinum and daily oral etoposide in high-risk non-small cell lung cancer patients. Oncol Rep. 2006;16:133–140. doi: 10.3892/or.16.1.133. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch FR, Suda K, Wiens J, Bunn PA., Jr New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388:1012–1024. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 34.Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial-INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial-INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 36.Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 37.Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Milanowski J, Karnicka-Mlodkowski H, Pesek M, Serwatowski P, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 38.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 39.Chen YM, Fan WC, Tsai CM, Liu SH, Shih JF, Chou TY, Wu CH, Chou KT, Lee YC, Perng RP, et al. A Phase II randomized trial of gefitinib alone or with tegafur/uracil treatment in patients with pulmonary adenocarcinoma who had failed previous chemotherapy. J Thorac Oncol. 2011;6:1110–1116. doi: 10.1097/JTO.0b013e3182121c09. [DOI] [PubMed] [Google Scholar]
- 40.Sutiman N, Zhang ZX, Tan EH, Ang MK, Tan SW, Toh CK, Ng QS, Chowbay B, Lim WT. Phase I study of oral vinorelbine in combination with erlotinib in advanced non-small cell lung cancer (NSCLC) using two different schedules. PLoS One. 2016;11:e0154316. doi: 10.1371/journal.pone.0154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gusella M, Pasini F, Caruso D, Barile C, Modena Y, Fraccon AP, Bertolaso L, Menon D, Crepaldi G, Bononi A, et al. Clinical outcomes of oral metronomic vinorelbine in advanced non-small cell lung cancer: Correlations with pharmacokinetics and MDR1 polymorphisms. Cancer Chemother Pharmacol. 2019;83:493–500. doi: 10.1007/s00280-018-3751-0. [DOI] [PubMed] [Google Scholar]
- 42.Orlandi P, Di Desidero T, Salvia G, Muscatello B, Francia G, Bocci G. Metronomic vinorelbine is directly active on non small cell lung cancer cells and sensitizes the EGFR(L858R/T790M) cells to reversible EGFR tyrosine kinase inhibitors. Biochem Pharmacol. 2018;152:327–337. doi: 10.1016/j.bcp.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Qin RS, Zhang ZH, Zhu NP, Chen F, Guo Q, Hu HW, Fu SZ, Liu SS, Chen Y, Fan J, Han YW. Enhanced antitumor and anti-angiogenic effects of metronomic Vinorelbine combined with Endostar on Lewis lung carcinoma. BMC Cancer. 2018;18:967. doi: 10.1186/s12885-018-4738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang R, Qin S, Chen Y, Li Y, Chen C, Wang Z, Zheng R, Wu Q. Enhanced anti-tumor and anti-angiogenic effects of metronomic cyclophosphamide combined with Endostar in a xenograft model of human lung cancer. Oncol Rep. 2012;28:439–445. doi: 10.3892/or.2012.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan EH, Tan DS, Li WY, Haaland B, Ang MK, Chau NM, Toh CK, Tan IB, Koh TS, Thng CH, et al. Metronomic vinorelbine (oral) in combination with sorafenib in advanced non-small cell lung cancer. Lung Cancer. 2015;88:289–296. doi: 10.1016/j.lungcan.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Correale P, Remondo C, Carbone SF, Ricci V, Migali C, Martellucci I, Licchetta A, Addeo R, Volterrani L, Gotti G, et al. Dose/dense metronomic chemotherapy with fractioned cisplatin and oral daily etoposide enhances the anti-angiogenic effects of bevacizumab and has strong antitumor activity in advanced non-small-cell-lung cancer patients. Cancer Biol Ther. 2010;9:685–693. doi: 10.4161/cbt.9.9.11441. [DOI] [PubMed] [Google Scholar]
- 47.Correale P, Botta C, Basile A, Pagliuchi M, Licchetta A, Martellucci I, Bestoso E, Apollinari S, Addeo R, Misso G, et al. Phase II trial of bevacizumab and dose/dense chemotherapy with cisplatin and metronomic daily oral etoposide in advanced non-small-cell-lung cancer patients. Cancer Biol Ther. 2011;12:112–118. doi: 10.4161/cbt.12.2.15722. [DOI] [PubMed] [Google Scholar]
- 48.Marquette CL, Grant SC, DeShazo M, Reddy V, Cantor A, MileyMary Jerome D, Robert F. Phase II study of metronomic chemotherapy (MC) with bevacizumab (B) in patients (Pts) with advanced (Adv) nonsquamous non-small cell lung cancer (NS-NSCLC) J Clin Oncol. 2013;31(15 Suppl):S8057. doi: 10.1200/jco.2013.31.15_suppl.8057. [DOI] [Google Scholar]
- 49.Revannasiddaiah S, Susheela SP. Chemically enhanced radiotherapy: Visions for the future. Ann Transl Med. 2016;4:52. doi: 10.3978/j.issn.2305-5839.2015.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Revannasiddaiah S, Joshi SC, Pandey KC, Rastogi M, Sharma M, Gupta M. The results with the addition of metronomic cyclophosphamide to palliative radiotherapy for the treatment of non-small cell lung carcinoma. Ann Transl Med. 2015;3:305. doi: 10.3978/j.issn.2305-5839.2015.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pastina P, Nardone V, Botta C, Croci S, Tini P, Battaglia G, Ricci V, Cusi MG, Gandolfo C, Misso G, et al. Radiotherapy prolongs the survival of advanced non-small-cell lung cancer patients undergone to an immune-modulating treatment with dose-fractioned cisplatin and metronomic etoposide and bevacizumab (mPEBev) Oncotarget. 2017;8:75904–75913. doi: 10.18632/oncotarget.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moserle L, Amadori A, Indraccolo S. The angiogenic Switch: Implications in the regulation of tumor dormancy. Curr Mol Med. 2009;9:935–941. doi: 10.2174/156652409789712800. [DOI] [PubMed] [Google Scholar]
- 53.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 54.Correale P, Remondo C, Carbone SF, Ricci V, Migali C, Martellucci I, Licchetta A, Addeo R, Volterrani L, Gotti G, et al. Dose/dense metronomic chemotherapy with fractioned cisplatin and oral daily etoposide enhances the anti-angiogenic effects of bevacizumab and has strong antitumor activity in advanced non-small-cell-lung cancer patients. Cancer Biol Ther. 2010;9:685–693. doi: 10.4161/cbt.9.9.11441. [DOI] [PubMed] [Google Scholar]
- 55.Shaikh AJ, Masood N. Acute lymphoblastic leukemia subsequent to temozolomide use in a 26-year-old man: A case report. J Med Case Rep. 2010;4:274. doi: 10.1186/1752-1947-4-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Deley MC, Leblanc T, Shamsaldin A, Raquin MA, Lacour B, Sommelet D, Chompret A, Cayuela JM, Bayle C, Bernheim A, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: A case-control study by the Societe Francaise d'Oncologie Pediatrique. J Clin Oncol. 2003;21:1074–1081. doi: 10.1200/JCO.2003.04.100. [DOI] [PubMed] [Google Scholar]
- 57.Vergnenegre A, Monnet I, Bizieux A, Bernardi M, Chiapa AM, Léna H, Chouaïd C, Robinet G. Open-label Phase II trial to evaluate safety and efficacy of second-line metronomic oral vinorelbine-atezolizumab combination for stage-IV non-small-cell lung cancer-VinMetAtezo trial, (GFPC‡ 04–2017) Future Oncol. 2020;16:5–10. doi: 10.2217/fon-2019-0730. [DOI] [PubMed] [Google Scholar]
- 58.Platania M, Pasini F, Porcu L, Boeri M, Verderame F, Modena Y, Del Conte A, Nichetti F, Garassino MC, Martinetti A, et al. Oral maintenance metronomic vinorelbine versus best supportive care in advanced non-small-cell lung cancer after platinum-based chemotherapy: The MA.NI.LA. multicenter, randomized, controlled, phase II trial. Lung Cancer. 2019;132:17–23. doi: 10.1016/j.lungcan.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Munzone E, Colleoni M. Clinical overview of metronomic chemotherapy in breast cancer. Nat Rev Clin Oncol. 2015;12:631–644. doi: 10.1038/nrclinonc.2015.131. [DOI] [PubMed] [Google Scholar]
- 60.de Castro G, Puglisi F, de Azambuja E, El Saghir NS, Awada A. Angiogenesis and cancer: A cross-talk between basic science and clinical trials (the ‘do ut des’ paradigm) Crit Rev Oncol Hemat. 2006;59:40–50. doi: 10.1016/j.critrevonc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Kerbel RS. A decade of experience in developing preclinical models of advanced- or Early-stage spontaneous metastasis to study antiangiogenic drugs, metronomic chemotherapy, and the tumor microenvironment. Cancer J. 2015;21:274–283. doi: 10.1097/PPO.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 62.Maiti R. Metronomic chemotherapy. J Pharmacol Pharmacother. 2014;5:186–192. doi: 10.4103/0976-500X.136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938–6943. [PubMed] [Google Scholar]
- 64.Grant DS, Williams TL, Zahaczewsky M, Dicker AP. Comparison of antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere) Int J Cancer. 2003;104:121–129. doi: 10.1002/ijc.10907. [DOI] [PubMed] [Google Scholar]
- 65.Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- 66.Mpekris F, Baish JW, Stylianopoulos T, Jain RK. Role of vascular normalization in benefit from metronomic chemotherapy. Proc Natl Acad Sci USA. 2017;114:1994–1999. doi: 10.1073/pnas.1700340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarmiento R, Gasparini G. Antiangiogenic metronomic chemotherapy. Onkologie. 2008;31:161–162. doi: 10.1159/000119925. [DOI] [PubMed] [Google Scholar]
- 68.Laquente B, Vinals F, Germa JR. Metronomic chemotherapy: An antiangiogenic scheduling. Clin Transl Oncol. 2007;9:93–98. doi: 10.1007/s12094-007-0018-3. [DOI] [PubMed] [Google Scholar]
- 69.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: New rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 70.Andre N, Carre M, Pasquier E. Metronomics: Towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11:413–431. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 71.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaked Y, Pham E, Hariharan S, Magidey K, Beyar-Katz O, Xu P, Man S, Wu FT, Miller V, Andrews D, Kerbel RS. Evidence Implicating immunological host effects in the efficacy of metronomic low-dose chemotherapy. Cancer Res. 2016;76:5983–5993. doi: 10.1158/0008-5472.CAN-16-0136. [DOI] [PubMed] [Google Scholar]
- 73.Kosmaczewska A, Ciszak L, Potoczek S, Frydecka I. The significance of Treg cells in defective tumor immunity. Arch Immunol Ther Exp (Warsz) 2008;56:181–191. doi: 10.1007/s00005-008-0018-1. [DOI] [PubMed] [Google Scholar]
- 74.Hao YB, Yi SY, Ruan J, Zhao L, Nan KJ. New insights into metronomic chemotherapy-induced immunoregulation. Cancer Lett. 2014;354:220–226. doi: 10.1016/j.canlet.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 75.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58:1627–1634. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghiringhelli F, Ménard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: Relevance during tumor progression. Immunol Rev. 2006;214:229–238. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 77.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: Suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, Shurin GV, Shurin MR, Umansky V. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol. 2013;190:2464–2471. doi: 10.4049/jimmunol.1202781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 80.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: An attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015;358:100–106. doi: 10.1016/j.canlet.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin-Padura I, Marighetti P, Agliano A, Colombo F, Larzabal L, Redrado M, Bleau AM, Prior C, Bertolini F, Calvo A. Residual dormant cancer stem-cell foci are responsible for tumor relapse after antiangiogenic metronomic therapy in hepatocellular carcinoma xenografts. Lab Invest. 2012;92:952–966. doi: 10.1038/labinvest.2012.65. [DOI] [PubMed] [Google Scholar]
- 83.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 84.Vives M, Ginesta MM, Gracova K, Graupera M, Casanovas O, Capellà G, Serrano T, Laquente B, Viñals F. Metronomic chemotherapy following the maximum tolerated dose is an effective anti-tumour therapy affecting angiogenesis, tumour dissemination and cancer stem cells. Int J Cancer. 2013;133:2464–2472. doi: 10.1002/ijc.28259. [DOI] [PubMed] [Google Scholar]
- 85.Yan H, Chen X, Zhang QP, Qin J, Li H, Liu C, Calhoun-Davis T, Coletta LD, Klostergaard J, Fokt I, et al. Drug-tolerant cancer cells show reduced tumor-initiating capacity: Depletion of CD44(+) cells and evidence for epigenetic mechanisms. PLoS One. 2011;6:e24397. doi: 10.1371/journal.pone.0024397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Udagawa T. Tumor dormancy of primary and secondary cancers. Apmis. 2008;116:615–628. doi: 10.1111/j.1600-0463.2008.01077.x. [DOI] [PubMed] [Google Scholar]
- 88.Natale G, Bocci G. Does metronomic chemotherapy induce tumor angiogenic dormancy? A review of available preclinical and clinical data. Cancer Lett. 2018;432:28–37. doi: 10.1016/j.canlet.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Schirrmacher V. T-cell immunity in the induction and maintenance of a tumour dormant state. Semin Cancer Biol. 2001;11:285–295. doi: 10.1006/scbi.2001.0384. [DOI] [PubMed] [Google Scholar]
- 90.Weir G, Hrytsenko O, Stanford M, Karkada M, Berinstein N, Mansour M. Abstract 2508: Multi-modal treatment with peptide vaccine, metronomic cyclophosphamide and anti-PD1 monoclonal antibody provides effective control of tumors in multiple models. Cancer Res. 2015:75. doi: 10.1158/1538-7445.AM2015-2508. [Google Scholar]
- 91.Rajasekaran T, Ng QS, Tan DS, Lim WT, Ang MK, Toh CK, Chowbay B, Kanesvaran R, Tan EH. Metronomic chemotherapy: A relook at its basis and rationale. Cancer Lett. 2017;388:328–333. doi: 10.1016/j.canlet.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 92.Eichhorn ME, Ischenko I, Luedemann S, Strieth S, Papyan A, Werner A, Bohnenkamp H, Guenzi E, Preissler G, Michaelis U, et al. Vascular targeting by EndoTAG-1 enhances therapeutic efficacy of conventional chemotherapy in lung and pancreatic cancer. Int J Cancer. 2010;126:1235–1245. doi: 10.1002/ijc.24846. [DOI] [PubMed] [Google Scholar]
- 93.Panigrahy D, Kaipainen A, Butterfield CE, Chaponis DM, Laforme AM, Folkman J, Kieran MW. Inhibition of tumor angiogenesis by oral etoposide. Exp Ther Med. 2010;1:739–746. doi: 10.3892/etm.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones BS, Jerome MS, Miley D, Jackson BE, DeShazo MR, Reddy VV, Singh KP, Brown OC, Robert F. Pilot phase II study of metronomic chemotherapy in combination with bevacizumab in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer. 2017;106:125–130. doi: 10.1016/j.lungcan.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giorgio CG, Giuffrida D, Pappalardo A, Russo A, Santini D, Salice P, Blanco G, Castorina S, Failla G, Bordonaro R. Oral temozolomide in heavily pre-treated brain metastases from non-small cell lung cancer: Phase II study. Lung Cancer. 2005;50:247–254. doi: 10.1016/j.lungcan.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 96.Banna GL, Camerini A, Bronte G, Anile G, Addeo A, Rundo F, Zanghì G, Lal R, Libra M. Oral metronomic vinorelbine in advanced non-small cell lung cancer patients unfit for chemotherapy. Anticancer Res. 2018;38:3689–3697. doi: 10.21873/anticanres.12647. [DOI] [PubMed] [Google Scholar]
- 97.Bilir C, Durak S, Kizilkaya B, Hacibekiroglu I, Nayir E, Engin H. Efficacy of metronomic vinorelbine in elderly patients with advanced non-small-cell lung cancer and poor performance status. Curr Oncol. 2017;24:e199–e204. doi: 10.3747/co.24.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mencoboni M, Filiberti RA, Taveggia P, Del Corso L, Del Conte A, Covesnon MG, Puccetti C, Donati S, Auriati L, Amoroso D, Camerini A. Safety of first-line chemotherapy with metronomic single-agent oral vinorelbine in elderly patients with NSCLC. Anticancer Res. 2017;37:3189–3194. doi: 10.21873/anticanres.11679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are available in the MEDLINE/PubMed database (http://www.webofknowledge.com/https://pubmed.ncbi.nlm.nih.gov).


