Abstract
Introduction
Chronic kidney disease (CKD) increases the risk of mortality during coronavirus disease 2019 (COVID-19) episodes, and some reports have underlined the high incidence and severity of this infection in dialysis patients. Information on COVID-19 in nondialysis CKD patients is not available yet.
Case Reports
Here we present 7 patients with grade 4–5 CKD who developed symptomatic COVID-19; they comprise 2.6% of our 267 advanced CKD patients. The estimated GFR was between 12 and 20 mL/min during the month prior to COVID-19. The 3 major symptoms were fever, cough, and dyspnea, and 5 patients showed bilateral pneumonia. Hydroxychloroquine, azithromycin, ceftriaxone, and steroids were the most frequently prescribed drugs. Two patients needed noninvasive mechanical ventilation. All patients showed minimal to moderate kidney function deterioration during admission, with an eGFR decline below 5 mL/min in 6 cases. No patient required acute dialysis. Six patients were discharged alive and remained dialysis free athe t the time of reporting, and one 76-year-old patient died.
Conclusions
COVID-19 affects grade 4–5 CKD patients, but prognosis may be acceptable if prompt supportive measures are applied. These findings should be confirmed in larger cohorts, and further observations will be needed to understand the full spectrum of clinical features and the optimal approach to COVID-19 in patients with advanced CKD.
Keywords: SARS-CoV-2, COVID-19, Chronic kidney disease, Pneumonia
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected over 233,000 people in Spain since early March 2020 [1]. The reported fatality rate in the general population with COVID-19 admitted to a large tertiary Spanish hospital was 20.7%, with 34% in the subgroup aged 70–79 years [2]. Chronic kidney disease (CKD) patients have several conditions that make them a target population for the virus, i.e., an older age, comorbidities, and a frail immunological system. There is scarce information regarding the impact of COVID-19 on renal patients. In descriptive studies in Spain that have included more than 20,000 people, CKD in COVID-19 patients has been found to be directly related to mortality [3]. Patients on hemodialysis attending treatment centers have a high risk of acquiring the infection [4, 5] and our group early designed a protocol to limit the spread of the infection in dialysis facilities [6].
Distinctively, outpatient advanced grade 4–5 CKD recipients may have a high risk of acquiring the infection since they have more contact with hospital facilities. Information regarding the impact of COVID-19 in an advanced CKD program is not available yet.
Here we present the incidence of COVID-19 in our prevalently grade 4–5 CKD patients, controlled at our outpatient clinic, as well as the characteristics of the first 7 symptomatic COVID-19 patients and their outcomes until death or a minimum of 1 month after the start of symptoms.
Case Reports
All patients from our cohort with advanced grade 4–5 CKD (eGFR <30 mL/min) studied at our institution between March 11 and April 20, 2020, for suspected COVID-19 infection were included. Clinical data were prospectively obtained through review of medical records. Each patient was followed until death or at least for 1 month after dialysis. Confirmed COVID-19 corresponds to a patient with a positive reverse transcription-polymerase chain reaction (RT-PCR) SARS-CoV2 assay of a specimen collected on a nasopharyngeal swab or bronchoalveolar lavage. Suspected but nonconfirmed COVID-19 correspond to a patient with a negative RT-PCR.
As of April 20, a total of 10 patients had been admitted with suspected COVID-19; 7 of those cases were confirmed. This led to a prevalence of 2.6% among our 267 ACKD patients oriented to future dialysis.
Demographic factors, comorbidities, baseline treatments, clinical characteristics, laboratory and chest X-ray findings, specific treatments, and outcomes in the 7 COVID-19 confirmed cases are shown in Table 1. The estimated GFR was between 12 and 20 mL/min during the month prior to infection; 2 patients were grade 5 CKD and 5 were grade 4 CKD. The 3 major symptoms observed in COVID-19-confirmed patients were fever, cough, and dyspnea. Other common symptoms included asthenia, myalgia, diarrhea, and headache. Five patients showed bilateral pneumonia on chest X-ray and 1 had unilateral pneumonia (Fig. 1). Two patients (No. 5 and 7) did not present respiratory symptoms but showed a gastrointestinal pattern, with nausea and diarrhea as the presenting features. All of the patients were admitted to the hospital ward, except for patient No. 5; this patient did not have respiratory syndrome or pneumonia and was monitored at home.
Table 1.
Demographics and baseline clinical characteristics
| Patient No.: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| Age, years | 54 | 47 | 62 | 76 | 75 | 79 | 83 | |
| Sex | Female | Female | Male | Female | Female | Male | Female | |
| Known COVID-19 contact | Yes | Yes | No | Yes | No | No | No | |
| Flu vaccination | Yes | No | No | Yes | Yes | Yes | No | |
| Comorbidities and baseline kidney function | ||||||||
| Charlson index | 4 | 4 | 7 | 4 | 10 | 10 | 13 | |
| Do-not-resuscitate order | No | No | No | No | No | No | Yes | |
| Diabetes mellitus | No | No | Yes | No | Yes | Yes | Yes | |
| Arterial hypertension | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Smoker | No | No | No | No | No | No | Past | |
| Dyslipidemia | Yes | Yes | Yes | No | Yes | Yes | Yes | |
| Obesity | Yes | Yes | No | No | Yes | Yes | No | |
| Heart disease | No | No | No | Yes | No | Yes | Yes | |
| Lung disease | No | No | No | No | No | Yes | No | |
| Previous cancer | No | No | No | No | Yes | Yes | Yes | |
| ACEI/ARB treatment | Yes | No | Yes | Yes | No | Yes | Yes | |
| Estimated GFR in the previous month, mL/min | 12 | 20 | 18 | 16 | 14 | 18 | 18 | |
| SCr, mg/dl | 3.98 | 2.56 | 3.37 | 2.87 | 3.18 | 3.32 | 2.51 | |
| PCOR, mg/g | 534 | 2,200 | 930 | 2,089 | 745 | 1,539 | 640 | |
| Symptoms and signs | ||||||||
| Fever | Yes | Yes | Yes | Yes | No | Yes | No | |
| Cough | Yes | Yes | Yes | No | No | Yes | No | |
| Dyspnea | No | Yes | Yes | Yes | No | Yes | No | |
| Fatigue | Yes | Yes | Yes | No | No | Yes | Yes | |
| Myalgia | Yes | Yes | Yes | No | No | Yes | No | |
| Headache | No | Yes | Yes | No | No | No | No | |
| Ageusia/anosmia | No | No | No | Yes | No | No | No | |
| Gastrointestinal symptoms | No | No | No | No | Yes | No | Yes | |
| Time from symptoms to hospital admission and PCR, days | 3 | 9 | 3 | 5 | 5 | 7 | 4 | |
| Bilateral pneumonia | Yes | Yes | Yes | Yes | No | No | Yes | |
| Respiratory insufficiency | Yes | No | Yes | Yes | No | Yes | No | |
| Laboratory tests at admission | ||||||||
| Hemoglobin, g/dL | 10.9 | 11.7 | 9.9 | 10.7 | 9.6 | 11.3 | 12.9 | |
| Leucocytes, n × 103/µL | 5.14 | 5.41 | 7.99 | 14.6 | 4.09 | 6.8 | 5.6 | |
| Lymphocytes, n × 103/µL | 3.8 | 3.62 | 0.49 | 0.46 | 0.64 | 0.47 | 1 | |
| Neutrophils, n × 103/µL | 1.2 | 1.4 | 7.39 | 13.68 | 2.78 | 5.65 | 4 | |
| Platelets, n × 103/µL | 165 | 277 | 204 | 200 | 161 | 235 | 131 | |
| D-dimer, µg/L | 2,110 | 670 | 1040 | 650 | 2620 | 590 | ND | |
| Fibrinogen, mg/dL | ND | >500 | >500 | >500 | >500 | >500 | >500 | |
| GOT, UI/L at 37° C | 30 | 127 | 66 | 37 | 17 | 17 | 21 | |
| LDH, UI/L at 37° C | 269 | 406 | 97 | 259 | 196 | 175 | 349 | |
| Troponin T, ng/L | ND | ND | ND | ND | ND | 98.7 | 38.5 | |
| CK, UI/L at 37° C | ND | 71 | 558 | ND | 32 | 68 | ND | |
| Albumin, g/dL | 4.2 | 3.1 | 3.3 | 3.4 | 3.9 | 3.4 | 3.1 | |
| 1L-6, pg/mL | ND | 11.4 | 3.2 | 98.6 | ND | 174 | ND | |
| C-reactive protein, mg/dL | 11.2 | 5.2 | 4.3 | 0.7 | 0.3 | 15.5 | 2.2 | |
| Ferritin, ng/mL | 2,800 | 990 | 944 | 1450 | 1,152 | 477 | 3,662 | |
| 25-OH vitamin D3, ng/mL | 10 | 13 | 16 | 9 | 5 | 16 | 6 | |
| PTH, pg/mL | 198 | 61 | 170 | 145 | 213 | 252 | 152 | |
| COVID-19 treatment | ||||||||
| Hydroxychloroquine | No | Yes | Yes | Yes | No | Yes | Yes | |
| Azithromycin | No | Yes | Yes | Yes | No | Yes | Yes | |
| Ceftriaxone | No | No | Yes | Yes | No | Yes | No | |
| Corticoids | Yes | No | Yes | Yes | No | No | Yes | |
| Lopinovir/ritonavir | Yes | No | No | No | No | No | No | |
| Tocilizumab | Yes | No | No | Yes | No | No | No | |
| Enoxaparin | No | Yes | Yes | Yes | No | Yes | Yes | |
| Vitamin D | No | Yes | Yes | No | No | Yes | No | |
| Other specific treatment | Igs | No | No | No | No | No | No | |
| Evolution and outcome | ||||||||
| Hospital admission/ICU admission | Yes/no | Yes/no | Yes/no | Yes/no | No/no | Yes/no | Yes/no | |
| Noninvasive mechanical ventilation | Yes | No | Yes | No | No | No | No | |
| Peak SCr, mg/dL | 4.94 | 2.96 | 6.78 | 3.55 | 3.80 | 3.34 | 2.60 | |
| Nadir estimated GFR, ml/min | 11 | 17 | 8 | 12 | 11 | 17 | 15 | |
| Acute estimated GFR decrease during COVID-19 vs. the previous month, mL/min | −1 | −3 | −10 | −4 | −3 | −1 | −3 | |
| Need for dialysis | No | No | No | No | No | No | No | |
| Time from symptoms to resolution, discharge, or death, days | 11 | 18 | 10 | 9 | 27 | 21 | 9 | |
| Resolution and discharge | Yes | Yes | Yes | No (dead) | Yes | Yes | Yes | |
ND, not done; Igs, immunoglobulins because of associated Guillain-Barré syndrome.
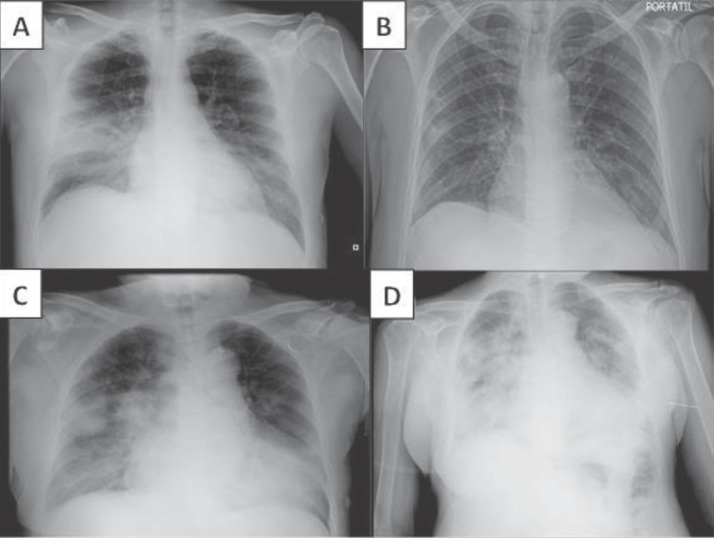
Fig. 1.
Chest x-rays of two patients with advanced CKD and COVID-19. Patient No. 3 showed bilateral opacities at admission, predominantly in the right lung median lobe (a), which mostly disappeared at discharge (b). Patient No. 4 showed bilateral pneumonia at admission (c), which intensely worsened until the night before death (d).
Hydroxychloroquine, azithromycin, ceftriaxone, and glucocortocoids were the most frequently prescribed drugs (Table 1). The glucocorticoids were given in different dosages as follows: case 1, three i.v. boluses of 200 methylprednisolone; cases 3 and 7, dexamethasone at 8 mg every 8 h for 4 days, 4 mg every 8 h for 4 days, and 4 mg every 24 h for 4 additional days; and case 4, a single 60-mg i.v. dose of methylprednisolone on the day she died. Tocilizumab was given in a single i.v. 400-mg dose in cases 1 and 4. Prophylactic enoxaparin was prescribed in 5 cases and none of them presented thrombotic events. Two patients did not receive any specific treatment. Treatments with remdesivir or favipiravir were not available at that time. We did not detect any drug-related adverse event.
ICU admission was not indicated for any patient and 2 patients were managed with noninvasive mechanical ventilation. All of the patients showed minimal to moderate kidney function deterioration during admission, but the estimated GFR decline was lower than 5 mL/min in 6 cases and 10 mL/min in the remaining one, and none required acute dialysis. The lowest estimated GFR during the COVID-19 episode was 8 mL/min/m2.
At the end of follow-up, 6 patients were discharged alive (5 of them from the hospital and another one from home hospitalization) and remained dialysis free. One patient died. She was a 73-year-old woman who presented to the emergency room on March 29, 2020, due to a 5-day history of dyspnea, low-grade fever, anosmia, ageusia, and dry cough. She was admitted with a diagnosis of COVID-19 (PCR+) bilateral pneumonia and decompensated heart failure. Her twin sister presented similar symptoms and was admitted to another hospital. Treatment with hydroxychloroquine, azithromycin, and ceftriaxone was started on admission day, when she showed respiratory failure with oxygen requirements of up to 50% FiO2. She had a bad evolution, with desaturation and the need for noninvasive mechanical ventilation, and started treatment with tocilizumab and dexamethasone. Despite an initial improvement (O2 saturation: 98%), on the morning of April 2nd, the patient was found with an absence of vital signs. Her twin sister died the same night in another hospital.
Discussion
We present our 7 patients with grade 4–5 CKD who had COVID-19 during the 40-day period of the epidemic peak in our city. This represents a prevalence of 2.6% of the patients controlled in our advanced CKD clinic, i.e., a lower proportion than expected in a high-risk population in a strongly affected region [7]. Only 1 of those patients died, and the majority were cured and discharged safely. The symptoms upon admission were those currently described for this entity. Fever was the most common, followed by cough, dyspnea, and fatigue. Chest x-ray findings showed pneumonia in 6 cases, with bilateral lung involvement in 5 of them, in agreement with many other studies [2, 7, 8]. Interestingly, as previously noted in hemodialysis patients in a short series [9], 2 patients did not present respiratory symptoms but showed a gastrointestinal pattern, with nausea and diarrhea as the presenting features. These patients showed a favorable evolution. Acute kidney injury superimposed to CKD was frequent but mild in these patients; all patients showed minimal kidney function deterioration during admission, but the estimated GFR decline was lower than 5 ml/min in most cases and none required acute dialysis. As a result, some of these patients showed lymphopenia, and in fact the lowest lymphocyte count was noted in the patient who died. Other laboratory findings such as high levels of C-reactive protein, IL-6, D-dimer, and procalcitonin were seen in some patients but without a clear pattern associated to severity. No effective treatment is available for COVID-19, but most of our patients received combinations of hydroxychloroquine and azithromycin. It is not possible to establish any therapeutic advantage for any of those treatments.
CKD patients have a high risk of symptomatic infection, mainly due to an impaired immune response, chronic inflammation, increased oxidative stress, uremic toxin accumulation, and endothelial dysfunction [10]. Despite the facts that CKD increases the risk of mortality during COVID-19 episodes [2, 11, 12] and some reports have underlined the risk and severity of this infection in dialysis patients [4, 5], information on the incidence and outcome of COVID-19 in nondialysis CKD patients is not available yet.
In summary, we described 7 advanced CKD patients who developed COVID-19. In addition to fever, cough, dyspnea, and fatigue, diarrhea was common. These findings should be confirmed in larger cohorts, and further observations will be needed to understand the full spectrum of clinical features and the optimal diagnostic and treatment approach for COVID-19 in patients with advanced CKD. Our small series confirms that COVID-19 affects grade 4–5 ACKD patients, but the prognosis may be quite good if prompt supportive measures are applied.
Statement of Ethics
This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The authors declare that they obtained consent from each patient reported in this article for publication of the information about him or her that appears within this case report.
Conflict of Interest Statement
The authors have no conflict of interests to declare.
Funding Sources
No funding was received for this work.
Author Contributions
All of the authors contributed to the management of these patients, made substantial contributions to the concept of this work, and approved the final version of this paper. S.C. and M.D.A contributed equally.
Acknowledgement
We thank all of the medical and nursing staff at the Hospital del Mar for their dedicated care of our renal patients during the COVID-19 epidemic.
References
- 1.Centro Nacional de Epidemiologia ISC COVID-19 en Espana. Available from: https://covid19.isciii.es/.
- 2.Borobia AM, Carcas AJ, Arnalich F, Álvarez-Sala R, Montserrat J, Quintana M, et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med. doi: 10.3390/jcm9061733. doi: 10.3390/jcm9061733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivanco-Hidalgo RM, Vela E, Clèries M, Monterde D. Informe sobre les característiques sociodemogràfiques, clíniques i els factors pronòstics dels pacients amb el diagnòstic de COVID-19 a Catalunya: resum executiu. Barcelona: Agència de Qualitat i Avaluació Sanitàries de Catalunya; 2020. [Google Scholar]
- 4.Albalate M, Arribas P, Torres E, Cintra M, Alcázar R, Puerta M, et al. Alta prevalencia de COVID 19 asintomático en hemodiálisis: aprendiendo dia a dia el primer mes de pandemia de COVID19. Nefrologia. 2020. [DOI] [PMC free article] [PubMed]
- 5.Goicoechea M, Sánchez Cámara LA, Macías N, Muñoz de Morales A, González Rojas A, Bascuñana A, et al. COVID-19: clinical course and outcomes of 36 maintenance hemodialysis patients from a single center in Spain. Kidney Int. 2020. [DOI] [PMC free article] [PubMed]
- 6.Arenas MD, Villar J, González C, Cao H, Collado S, Crespo M, et al. [Management of the SARS-CoV-2 (COVID-19) coronavirus epidemic in hemodialysis units] Nefrologia. 2020 May-Jun;40((3)):258–64. doi: 10.1016/j.nefro.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. ISARIC4C investigators Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020 May;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 May;8((5)):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R, Liao C, He H, Hu C, Wei Z, Hong Z, et al. COVID-19 in Hemodialysis Patients: A Report of 5 Cases. Am J Kidney Dis. 2020 Jul;76((1)):141–3. doi: 10.1053/j.ajkd.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syed-Ahmed M, Narayanan M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv Chronic Kidney Dis. 2019 Jan;26((1)):8–15. doi: 10.1053/j.ackd.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020 Jun;52((6)):1193–4. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]