Abstract
Fatty acid binding proteins (FABPs) are key regulators of lipid metabolism, energy homeostasis and inflammation. They participate in fatty acid metabolism by regulating their uptake, transport and availability of ligands to nuclear receptors. In the adult brain, FABP7 is especially abundant in astrocytes that are rich in cytoplasmic granules originated from damaged mitochondria. Mitochondrial dysfunction and oxidative stress have been implicated in the neurodegenerative process observed in amyotrophic lateral sclerosis (ALS), either as a primary cause or as a secondary component of the pathogenic process. Here we investigated the expression of FABP7 in animal models of human superoxide dismutase 1 (hSOD1)-linked ALS. In the spinal cord of symptomatic mutant hSOD1-expressing mice, FABP7 is up-regulated in gray matter astrocytes. Using a co-culture model, we examined the effect of increased FABP7 expression in astrocyte-motor neuron interaction. Our data shows that FABP7 over-expression directly promotes an NF-κB-driven pro-inflammatory response in non-transgenic astrocytes that ultimately is detrimental for motor neuron survival. Addition of trophic factors, capable of supporting motor neuron survival in pure cultures, did not prevent motor neuron loss in co-cultures with FABP7 over-expressing astrocytes. In addition, astrocyte cultures obtained from symptomatic hSOD1-expressing mice display up-regulated FABP7 expression. Silencing endogenous FABP7 in these cultures decreases the expression of inflammatory markers and their toxicity towards co-cultured motor neurons. Our results identify a key role of FABP7 in the regulation of the inflammatory response in astrocytes and identify FABP7 as a potential therapeutic target to prevent astrocyte-mediated motor neuron toxicity in ALS.
Keywords: amyotrophic lateral sclerosis, inflammation, astrocytes, motor neurons, NF-κB
Introduction
Fatty acids function both as an energy source and as signaling molecules that affect many cellular functions. Fatty acid dependent enzymatic and transcriptional networks induce gene expression changes that ultimately modulate cell growth, metabolism and inflammatory responses (Hotamisligil, 2017; Hotamisligil & Erbay, 2008). Fatty acid binding proteins (FABPs) exhibit high affinity reversible binding of saturated and unsaturated long-chain fatty acids. FABPs were once considered biologically silent chaperones of fatty acids, but it has now become clear that these proteins are key regulators of lipid metabolism, energy homeostasis and inflammation (Storch & Thumser, 2010; Thumser, Moore, & Plant, 2014). For example, due to its role in intracellular trafficking of long-chain fatty acids and some of their active metabolites, FABPs modulate peroxisome proliferator-activated receptors (PPARs) ligand-dependent activation (Tan et al., 2002; Wolfrum, Borrmann, Borchers, & Spener, 2001).
The mammalian genome codes for at least 10 different FABPs. Many FABPs are prominently expressed in a single tissue or cell type, but in several cell types more than one FABP is expressed, suggesting that these proteins have specialized functions (Smathers & Petersen, 2011; Storch & Thumser, 2010; Thumser et al., 2014). Cells in the central nervous system (CNS) express FABP3, FABP5 and FABP7 (also known as brain lipid binding protein) (Furuhashi & Hotamisligil, 2008). These three FABPs show differential expression during development and in the adult brain (Ju Lee et al., 2017; Y. Liu, Longo, & De Leon, 2000; Y. Liu, Molina, Welcher, Longo, & De Leon, 1997; Owada, Yoshimoto, & Kondo, 1996; Sellner, Chu, Glatz, & Berman, 1995). FABP7 expression is observed in neural stem cells throughout development and its expression decreases and becomes restricted to radial glia-like cells and astrocytes in the adulthood (Kurtz et al., 1994; Owada et al., 1996; Yun et al., 2012). In the adult brain, FABP7 is especially abundant in astrocytes that are rich in cytoplasmic granules that are believed to be originated from damaged mitochondria (Young, Baker, & Muller, 1996).
Amyotrophic lateral sclerosis is characterized by the progressive loss of motor neurons in the motor cortex, brainstem and spinal cord. No cure or effective treatment is currently available, although two drugs (riluzole and edaravone) appear to slightly slow disease progression (Bensimon, Lacomblez, & Meininger, 1994; Sawada, 2017). Approximately 10% of the ALS cases present with a familial history of the disease (familial ALS, FALS) and are most frequently linked to dominant mutations. The rest of the cases do not have a familial history (sporadic ALS, SALS) and may result from yet unidentified interaction between environmental and genetic risk factors (Brown & Al-Chalabi, 2017). Astrocytes play a key role determining motor neuron fate in ALS models and, in co-culture, astrocytes from diverse models of ALS induce motor neuron death (Haidet-Phillips et al., 2011; Kia, McAvoy, Krishnamurthy, Trotti, & Pasinelli, 2018; Nagai et al., 2007; Vargas, Pehar, Cassina, Beckman, & Barbeito, 2006). Many of the different hypotheses proposed to explain motor neuron degeneration in ALS implicate mitochondrial dysfunction and oxidative stress, either as a primary cause or as a secondary component of the pathogenic process (Cozzolino, Ferri, Valle, & Carri, 2013; Pehar, Harlan, Killoy, & Vargas, 2017; Shi, Gal, Kwinter, Liu, & Zhu, 2010). Mitochondrial dysfunction in astrocytes is associated with elevations of cytoplasmic lipids and lipid-binding proteins (Bailey et al., 2015; L. Liu, MacKenzie, Putluri, Maletic-Savatic, & Bellen, 2017; L. Liu et al., 2015).
Here we investigated the expression of FABP7 in the spinal cord of mice expressing ALS-linked mutant superoxide dismutase 1 (SOD1) and the effect of modulating FABP7 expression in ALS-astrocytes. Up-regulation of FABP7 in non-transgenic astrocytes induces a pro-inflammatory phenotype that renders these cells toxic to motor neurons in co-culture. Moreover, decreasing FABP7 expression in ALS-astrocytes partially reverts their neurotoxic phenotype. Taken together, our data indicates that FABP7 expression levels markedly alter the biology of astrocytes and the way these cells interact with neurons.
Methods.
Reagents-
All chemicals and reagents were from Sigma-Aldrich unless otherwise specified. Culture media and supplements were from Invitrogen unless otherwise specified. Primers were obtained from Integrated DNA Technologies (Supplemental table 1).
Animals-
B6.Cg-Tg(SOD1*G93A)1Gur/J, B6.Cg-Tg(SOD1)2Gur/J (Gurney et al., 1994) and B6N.Cg-Tg(Prnp-TARDBP*Q331K)103Dwc/J mice (Arnold et al., 2013) were obtained from The Jackson Laboratory and maintained as hemizygous animals in a C57BL/6J background. B6.Cg-Tg(SOD1)2Gur/J mice express human wild-type SOD1 at similar levels to the mutant human SOD1 in B6.Cg-Tg(SOD1*G93A)1Gur/J mice. hSOD1H46R/H48Q mice were provided by Dr. David Borchelt (J. Wang et al., 2002) and have been backcrossed into C57BL/6J pure background for more than 10 generations.
Primary cultures-
Neonatal primary astrocyte cultures were prepared from cortex and spinal cord of 1-day-old mice as previously described (Vargas et al., 2006). To prepare primary spinal cord cultures from symptomatic hSOD1G93A, hSOD1H46R/H48Q and age-matched non-transgenic mice, the spinal cords from adult mice were harvested, the meninges were removed and the spinal cord was cut into small pieces with a surgical blade. Individual cords were incubated in 2 mL of a solution containing 1.1 mM EDTA, 5.5 mM L-cysteine, 0.067 mM β-mercaptoethanol, 20 U/mL papain, 1 U/mL Dispase in HBSS without Ca2+ and Mg2+. The incubation was performed at a 37°C for 30 minutes with periodic shaking. After this incubation, the digestion solution was removed and the tissue was mechanically dissociated in culture media (10% FBS + 1% P/S + 0.3% N2 supplement in DMEM-F12) with DNase (0.05 mg/ml) by aspirating the tissue 7-10 times through a wide-bore pipette tip. This step was repeated 3 times, allowing the undissociated tissue to settle at the bottom of the tube and transferring the supernatant to a new tube each time. The supernatant was passed through a 70 μm strainer. Astrocytes were pleated in poly-L-ornithine (1.5 μg/mL)-laminin (3 μg/mL) coated plates. After two weeks in culture, astrocytes were re-plated and all experiments were conducted one week following re-plating.
Motor neuron cultures were prepared from 12.5-embryonic-day mouse spinal cords as previously described (Vargas, Johnson, Sirkis, Messing, & Johnson, 2008). For co-culture experiments with neonatal spinal cord astrocytes, motor neurons were plated on mouse astrocyte monolayers at a density of 300 cells/cm2 and maintained in supplemented L15 medium (Vargas et al., 2006). For co-culture experiments with spinal cord astrocytes from symptomatic animals, motor neurons were plated on mouse astrocyte monolayers at a density of 600 cells/cm2 and maintained in supplemented Neurobasal medium (Kim et al., 2018). Motor neurons were identified by immunostaining with an anti-β III Tubulin antibody (Millipore, 05-559, lot:2757108) and survival was determined by counting all cells displaying intact neurites longer than 4 cell bodies in diameter. Counts were performed over an area of 0.90 cm2 in 24-well plates.
Immunofluorescence-
Antigen retrieval and staining in paraffin embedded tissues was performed as previously described (Harlan et al., 2020). Mice lumbar spinal cord sections were stained with anti-GFAP (Novus, NBP2-29415, lot 2670-1P180807) and anti-FABP7 (Thermo Fisher, Cat#: PA5-24949, lot TL2690549A) antibodies. Nuclei were counterstained with DAPI (4’,6-Diamidino-2-phenylindole dihydrochloride).
Real-time PCR, western blot and chromatin immunoprecipitation (ChIP) analysis-
RNA extraction, RNA retrotranscription, real-time PCR and western blot analysis were performed as previously described (Harlan, Pehar, Killoy, & Vargas, 2019). Membranes were incubated overnight with one of the following antibodies: FABP7 (Thermo Fisher, Cat#: PA5-24949, TL2690549A), Actin (Sigma, A5441, lot: 061M4808) or GADPH (Sigma, SAB2100894, lot: QC9353). Image acquisition was performed in a chemiluminescent western blot scanner (Li-Cor) or exposed on Kodak BioMax Light film. Quantifications were performed using the Image Studio Software (Li-Cor) or the ImageJ Software (NIH). ChIP was performed with the SimpleChIP enzymatic chromatin IP kit (Cell Signaling) using an NF-κB p65 antibody (Cell Signaling, 8242, lot: 13) and analyzed by real-time PCR.
Cell treatment and transfections-
Adenovirus expressing Gfp, wild-type Fabp7 or a mutant Fabp7 [(FABP7(3A)] under a CMV promoter were custom produced by VectorBuilder. The substitution of three critical residues in the ligand binding pocket of FABP7, (substitutions R107A, R127A, Y129A) renders the mutant FABP7(3A) unable to bind fatty acids (Mita, Beaulieu, Field, & Godbout, 2010). Adenovirus-mediated transductions were performed at a multiplicity of infection of 50 and astrocytes were used 48 hours post-transduction. siRNA transfections with an Fabp7-siRNA SMART pool (Dharmacon) were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Astrocytes were transfected with 25 nM of an Fabp7-siRNA or a negative control (NC) siRNA. After 72 hours a second transfection with half the amount of siRNA was performed and 72 hours later cells were subsequently analyzed or used in co-culture experiments.
NF-κB reporter assay-
Adenovirus expressing a firefly luciferase gene under the control of a synthetic promoter that contains direct repeats of the NF-κB binding site (Ad-NFkb-Luc) or a Renilla luciferase under a constitutive promoter (Ad-pRL-Luc) were obtained from Vector Biolabs. Adenovirus-mediated transductions were performed at a multiplicity of infection of 10 and 3 respectively. 72 hours later, cultures were transduced with adenovirus expressing Gfp, Fabp7 or Fabp7(3A) as described above, and firefly and Renilla luciferase activity were consecutively assayed with the Dual-Glo luciferase system (Promega).
ELISA and nitrate/nitrite assay-
CXCL10 levels in astrocyte-conditioned media were determined using a mouse CXCL10/IP-10/CRG-2 ELISA kit (R&D Systems). Total nitrate/nitrite concentration was determined in astrocyte-conditioned media using a Nitrate/Nitrite Fluorometric Assay Kit (Cayman).
Statistical analysis-
Groups of 3-4 animals were used for biochemical analysis. For cell culture, each experiment was repeated in at least three independent primary culture preparations and values from each independent experiment were combined for data reporting. All data are reported as mean ± SD. Comparisons between two groups were performed with an unpaired t-test. Multiple group comparison was performed by one-way ANOVA with Tukey’s post-test. When comparing the effect of genotype and treatments, two-way ANOVA was used followed by Tukey’s post-test. Differences were declared statistically significant if p≤0.05. All statistical computations were performed using Prism 6.0 (GraphPad Software).
Results
To examine whether FABP7 expression may be altered in ALS, we analyzed the expression of FABP7 in two different transgenic mouse models that develop an ALS-like phenotype due to the over-expression of mutant hSOD1. When compared to age-matched non-transgenic animals, FABP7 is significantly up-regulated in the spinal cord of symptomatic hSOD1G93A and hSOD1H46R/H48Q mice. FABP7 up-regulation occurs at both protein and mRNA levels in the spinal cord of ALS mice. In the spinal cord of transgenic mice that over-express human wild-type hSOD1, which do not develop overt motor neuron degeneration, FABP7 levels remain unchanged (Fig. 1). Up-regulation of FABP7 occurs in GFAP-positive cells in the ventral horn of the spinal cord of mutant hSOD1-linked ALS mouse models (Fig. 2), suggesting that reactive astrocytes surrounding motor neurons up-regulate FABP7 expression during the course of the disease. In addition, FABP7 up-regulation is also observed in the spinal cord of 11-month old TDP-43Q331K mice (Fig. 3), a model of mutant TDP-43-linked ALS with age-related motor neuron degeneration (Arnold et al., 2013).
Figure 1.

Up-regulation of FABP7 expression in mutant hSOD1-linked mouse models. A, D and G) Western blot analysis of FABP7 expression in the lumbar spinal cord of age-matched non-transgenic (NonTG) and symptomatic hSOD1G93A (G93A, about 140 days old) (A), or symptomatic hSOD1H46R/H48Q (H46R/H48Q, about 210 days old) (D) or age-matched control hSOD1WT mice (about 210 days old) (G). B, E and H) Quantification of FABP7 protein levels shown in A, D and G, respectively. FABP7 expression was quantified, normalized by the levels of a loading control and expressed as percentage of age-matched NonTG mice (each lane corresponds to a different animal, mean±S.D.). C, F and I) Fabp7 mRNA levels in the lumbar spinal cord of age-matched NonTG and symptomatic G93A (C), or symptomatic H46R/H48Q (F), or age-matched hSOD1WT (I) mice. Fabp7 mRNA levels were determined by real-time PCR and corrected by Actin mRNA levels (n=3-4 mice, mean±S.D.).
Figure 2.
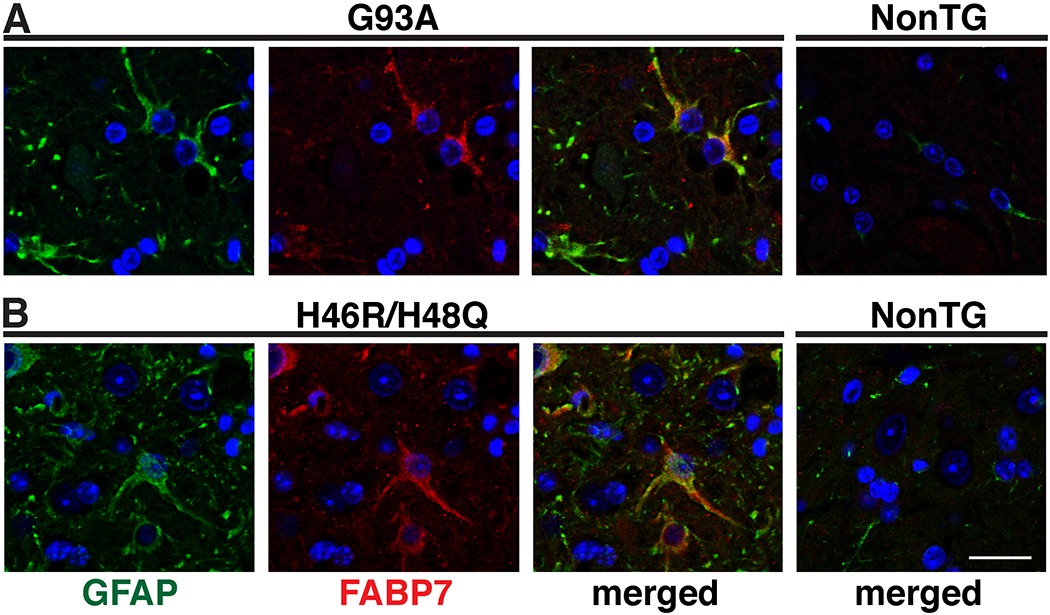
FABP7 up-regulation in ventral horn spinal cord astrocytes in SOD1-linked ALS mouse models. GFAP (green) and FABP7 (red) immunostaining in the ventral horn of the spinal cord of age-matched non-transgenic (NonTG), symptomatic hSOD1G93A (G93A) (A) and symptomatic hSOD1H46R/H48Q (H46R/H48Q) (B) mice. Only the merged image is shown for NonTG mice. Nuclei were counterstained with DAPI (blue). Scale bar: 20μm.
Figure 3.

Up-regulation of FABP7 expression in a TDP-43-linked ALS mouse model. A) FABP7 expression in the lumbar spinal cord of 11-month old TDP-43Q331K mice and age-matched non-transgenic (NonTG) controls. B) FABP7 expression was quantified, corrected by actin and expressed as percentage of NonTG mice (each lane represents a different animal, mean±S.D). C) GFAP (green) and FABP7 (red) immunostaining in the ventral horn of the spinal cord of 11-month old NonTG and TDP-43Q331K mice. Only the merged image is shown for NonTG mice. Scale bar: 20μm.
However, FABP7 upregulation is not observed in pre-symptomatic mice (Fig. 4A–C). Accordingly, primary spinal cord astrocytes obtained from neonatal non-transgenic and hSOD1G93A mice display similar levels of FABP7 expression (Fig. 4D, E). In order to study a potential effect of FABP7 up-regulation in the biology of astrocytes, we first transduced neonatal astrocytes with an adenovirus coding for FABP7 (Fig. 5A–C). No significant changes in GFAP expression were observed in FABP7-overexpressing astrocytes (Fig. 5C). Changes in FABPs levels have been shown to modulate the expression of proinflammatory genes in different cell types (Furuhashi et al., 2008; Makowski, Brittingham, Reynolds, Suttles, & Hotamisligil, 2005; Reynolds et al., 2007). Thus, we examined if FABP7 over-expression could alter the expression of pro-inflammatory genes in astrocytes. FABP7 over-expression in non-transgenic astrocytes stimulates the expression of several pro-inflammatory markers at mRNA level (Fig. 5D). ELISA analysis confirmed the presence of increased levels of CXCL10 in the conditioned media of FABP7-overexpressing astrocytes (Fig. 5E). Moreover, increased levels of nitrites (NO2−) and nitrates (NO3−) in the conditioned media reflected an increase in the activity of inducible nitric oxide synthase (NOS2) (Fig. 5F). In addition, ChIP-qPCR analysis confirmed that following FABP7 over-expression, promoter occupancy by NF-κB increases in the promoters of Cxcl10 and Nos2 genes (Fig. 5G, H).
Figure 4.

FABP7 upregulation is not observed in pre-symptomatic hSOD1G93A mice. A) Fabp7 mRNA levels in the lumbar spinal cord of 30 days old (asymptomatic) or 110 days old (around symptoms onset) hSOD1G93A (G93A) mice and age-matched non-transgenic (NonTG) controls. Fabp7 mRNA levels were determined by real-time PCR and corrected by Actin mRNA levels (n=3-4 mice, mean±S.D.). B) Western blot analysis of FABP7 expression in the lumbar spinal cord of 110 days old G93A mice and age-matched NonTG controls. Each lane corresponds to a different animal. C) Quantification of FABP7 protein levels shown in B. FABP7 expression was quantified, normalized by ACTIN levels and expressed as percentage of age-matched NonTG mice (mean±S.D.). D, E) Western blot analysis of FABP7 expression in neonatal spinal cord astrocyte cultures from NonTG and G93A mice. Quantification of FABP7 levels is shown in E. FABP7 expression was quantified, normalized by the levels of ACTIN, and expressed as percentage of NonTG cultures (mean±S.D.).
Figure 5.
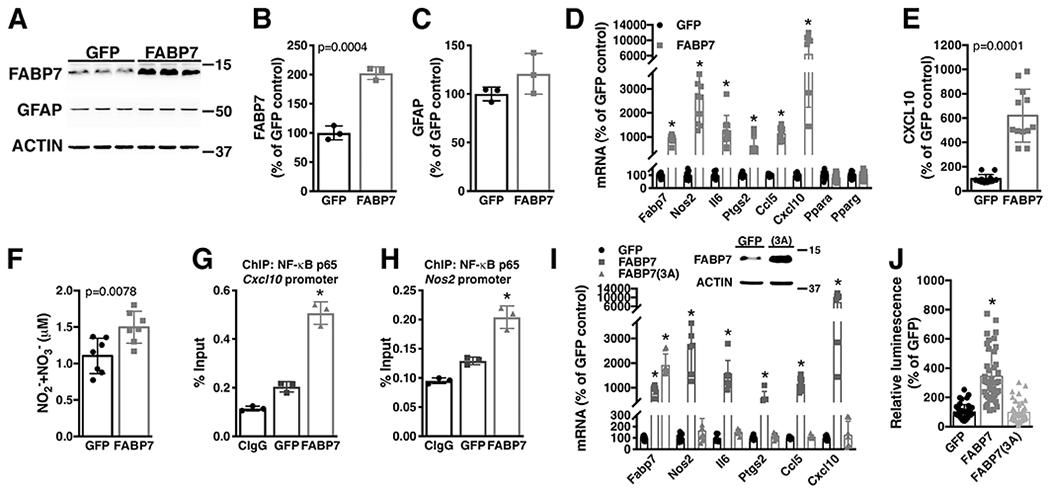
Over-expression of FABP7 confers upon non-transgenic astrocytes a proinflammatory phenotype. A) Primary confluent spinal cord astrocyte cultures obtained from neonatal NonTG mice were transduced with adenovirus expressing GFP or FABP7. 48hs later, FABP7 and GFAP protein levels were determined and corrected by actin levels. B, C) Quantification of the images shown in A. D) Fabp7, Nos2, Il6, Ptgs2, Ccl5, Cxcl10, Ppara and Pparg mRNA levels in astrocytes treated as in A. mRNA levels were determined by real-time PCR and corrected by Actin mRNA levels. Data is expressed as percentage of GFP control cells. E) CXCL10 levels in conditioned media (48hs) from GFP or FABP7 over-expressing NonTG astrocytes. Data is expressed as percentage of GFP control cells. F) Nitrite (NO2−) and nitrate (NO3−) levels in conditioned media (48hs) from GFP or FABP7 over-expressing NonTG astrocytes. G, H) Astrocytes were treated as in A and 48hs later chromatin immunoprecipitation was performed with a pre-immune IgG (CIgG) or an anti-NF-κB p65 subunit antibody. Purified DNA was analyzed by real-time PCR with specific primers flanking an NF-κB binding site in the Cxcl10 (G) or Nos2 (H) promoter. I) Real-time PCR analysis of Fabp7, Nos2, Il6, Ptgs2, Ccl5, Cxcl10 mRNA expression levels 48hs after FABP7 or FABP7(3A) over-expression in neonatal NonTG astrocytes. mRNA levels were corrected by Actin levels and expressed as percentage of GFP control cells. The data points for GFP and FABP7 in panel K were also included in panel F. Panel F includes additional biological replicates performed independently of the experiment described in panel K. J) Relative luminescence produced by firefly luciferase expressed under an NF-κB-driven promoter 48hs after FABP7 or FABP7(3A) over-expression in neonatal NonTG astrocytes. Relative firefly luciferase luminescence was corrected by the amount of Renilla luciferase activity controlled by a constitutive promoter and expressed as percentage of GFP control cells. For all graph panels, data are expressed as mean±S.D. (*p<0.05).
To investigate if the inflammatory phenotype that FABP7 over-expression confers to astrocytes depends on its ligand biding capabilities, we over-expressed a ligand-binding impaired FABP7 [FABP7(3A)] that is unable to bind fatty acids (Mita et al., 2010). In contrast to the wild-type FABP7, over-expression of the ligand-binding impaired FABP7(3A) did not up-regulated the expression of inflammatory genes nor stimulated the expression from a NF-κB-driven promoter in astrocytes (Fig. 5I, J).
To examine the effect that FABP7 up-regulation could have in the way astrocytes interact with neighboring neurons, we tested the survival of motor neurons co-cultured with astrocytes over-expressing FABP7. Over-expression of wild-type or mutant FABP7 does not alter the number of motor neurons that attach to the astrocyte monolayer, since a similar number of motor neurons was observed after 16 hours in co-culture (Fig. 6A). However, after three days, a decrease in motor neuron survival was observed in co-cultures with FABP7 over-expressing astrocytes (Fig. 6B). Astrocytes over-expressing FABP7(3A) did not induce the death of co-cultured motor neurons, indicating that the neurotoxic phenotype depends on the capacity of FABP7 to bind ligands. Moreover, the addition of exogenous trophic factors (GDNF 10 ng/ml and BDNF 0.1 ng/ml), capable of supporting motor neuron survival in pure cultures, did not prevent motor neuron loss in co-cultures with FABP7 over-expressing astrocytes (Fig. 6C).
Figure 6.
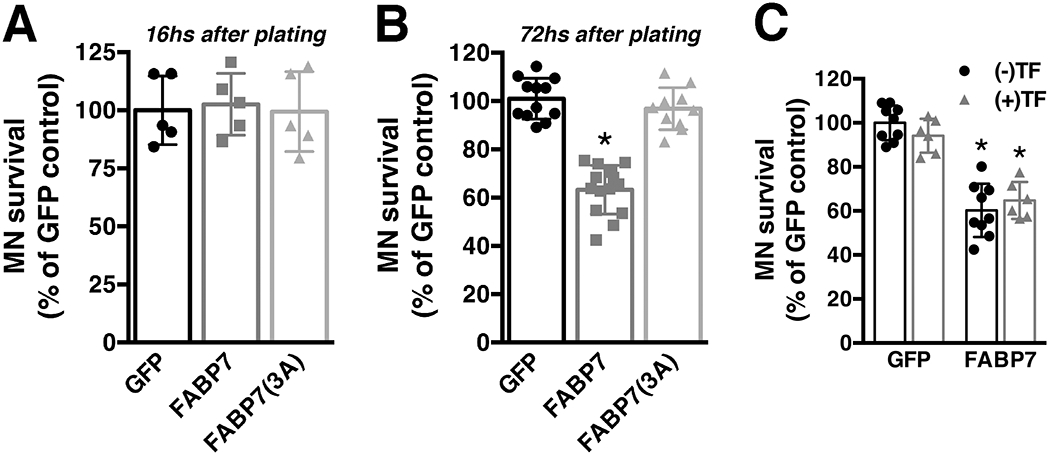
Over-expression of FABP7 confers upon non-transgenic astrocytes a neurotoxic phenotype. A) Spinal cord non-transgenic (NonTG) astrocytes were transduced with adenovirus expressing GFP, FABP7 or FABP7(3A). 48hs later embryonic motor neurons were plated on top. Motor neuron (MN) survival was determined 16hs later (data obtained from two independent co-culture experiments, 2-3 replicas per co-culture). B) The same co-culture setup as in A but motor neuron survival was determined 72hs later. C) Addition of trophic factors (TF: GDNF 10ng/ml and BDNF 0.1ng/ml) to the co-culture does not prevent motor neuron loss induced by astrocytes over-expressing FABP7. Co-cultures were treated at the time of motor neuron plating with (+TF) or without (−TF) trophic factors and motor neuron survival was determined 72hs later. For B and C data was obtained from at least three independent co-culture experiments, 2-3 replicas per co-culture. For all panels data are expressed as mean±S.D. (*p<0.05).
While primary astrocytes isolated from neonatal ALS mice did not show increased FABP7 expression; astrocyte cultures obtained from the spinal cord of symptomatic hSOD1G93A displayed up-regulated FABP7 expression when compared to non-transgenic astrocytes isolated from age-matched mice (Fig. 7A–C). A similar result was obtained in astrocytes isolated from symptomatic hSOD1H46R/H48Q mice (Fig. 7D–E). Since it has been previously shown that astrocytes isolated from the spinal cord of symptomatic ALS animals are toxic to motor neurons in co-culture (Diaz-Amarilla et al., 2011), we tested the effect of silencing FABP7 in these cells. Silencing FABP7 expression in astrocyte cultures isolated from symptomatic ALS-mice (which endogenously express elevated levels of the protein) significantly decreased the expression of pro-inflammatory genes (Fig. 8A–C). In addition, this strategy partially reverted the toxicity of these astrocytes towards motor neurons in co-culture (Fig. 8D), and suggests that the increase in astrocyte FABP7 expression observed in the animal models of ALS may have a similar detrimental effect.
Figure 7.

Spinal cord astrocytes cultures obtained from symptomatic ALS mice display up-regulated FABP7 expression. A, B) Western blot analysis of FABP7 expression in astrocyte cultures obtained from age-matched non-transgenic (NonTG) and symptomatic hSOD1G93A (G93A) mice. Quantification of FABP7 levels is shown in B. FABP7 expression was quantified, normalized by ACTIN levels and expressed as percentage of NonTG cultures (each lane corresponds to a different primary culture, mean±S.D.). C) Representative microphotographs of astrocyte cultures from age-matched NonTG and symptomatic G93A mice immunostained for GFAP (green). Nuclei were counterstained with DAPI (blue). Scale bar: 50μm. D, E) Western blot analysis of FABP7 expression in astrocyte cultures from age-matched NonTG and symptomatic hSOD1H46R/H48Q (H46R/H48Q) mice. Quantification of FABP7 levels is shown in E. FABP7 expression was quantified, normalized by ACTIN levels and expressed as percentage of NonTG cultures (each lane corresponds to a different primary culture, mean±S.D.).
Figure 8.

Decreasing FABP7 expression in ALS-astrocytes confers protection to co-cultured motor neurons. A, B) Primary spinal cord astrocyte cultures from symptomatic hSOD1H46R/H48Q (H46R/H48Q) (A), and symptomatic hSOD1G93A (G93A) (B) mice were transfected with a negative control (NC) or an Fabp7-siRNA. Six days after transfection Fabp7, Nos2, Il6, Ptgs2, Ccl5 and Cxcl10 mRNA levels were determined by real-time PCR and corrected by Actin mRNA levels. Data is expressed as percentage of NC-siRNA treated cells (*p<0.05, different from NC-siRNA). C) Representative image of Western blot analysis of FABP7 expression in primary spinal cord astrocytes from symptomatic G93A mice 6 days after transfection with a negative control (NC) or an Fabp7-siRNA. D) Primary spinal cord astrocyte cultures from age-matched NonTG and symptomatic G93A mice were treated as in A. Six days after transfection embryonic motor neurons were plated on top. Motor neuron (MN) survival was determined 72hs later. (*p<0.05, different from NC-siRNA treated NonTG, #p<0.05, different from NC-siRNA treated G93A). For all panels, data are expressed as mean±S.D.
Discussion
Astrocytes play a key role shaping motor neuron degeneration in ALS models (Chen et al., 2015; Ditsworth et al., 2017; Haidet-Phillips et al., 2011; Kia et al., 2018; Nagai et al., 2007; Pehar et al., 2017; Qian et al., 2017; Vargas et al., 2006; L. Wang, Gutmann, & Roos, 2011; Yamanaka et al., 2008), and therapeutic strategies that abrogate the neurotoxic phenotype of ALS-astrocytes in vitro, have been shown to increase motor neuron survival when translated into ALS mouse models (de Boer et al., 2014; Harlan et al., 2020; Miquel et al., 2014; Song et al., 2016; Vargas et al., 2008). Here we show that the changes that occur in FABP7 expression in mutant hSOD1-expressing ALS mouse models could critically alter the biology of astrocytes, as well as the way these cells interact with motor neurons.
Lipid peroxidation and mitochondrial dysfunction are observed in all neurodegenerative diseases, including ALS (Pedersen et al., 1998; Simpson, Henry, Henkel, Smith, & Appel, 2004; Smith, Henry, Mattson, & Appel, 1998). Elevated oxidative stress and mitochondrial dysfunction in neurons induce the transfer of lipids to neighboring astrocytes and the accumulation of lipid droplets in glial cells. In turn, accumulation of peroxidated lipids and oxidative stress can lead to mitochondrial dysfunction in glial cells and contribute to the neurodegenerative process (Bailey et al., 2015; L. Liu et al., 2017; L. Liu et al., 2015). Gomori-positive astrocytes represent a subset of astrocytes that display unusual cytoplasmic inclusions (Schipper, 1991). Gomori-positive inclusions originate from autophagosomes that contain damaged mitochondria (Brawer, Reichard, Small, & Schipper, 1994; Brawer, Stein, Small, Cisse, & Schipper, 1994). The cause of this seemingly selective damage to astrocyte mitochondria is not yet fully characterized but could be linked to altered fatty acid metabolism observed in this cell type (Ioannou et al., 2019; Schipper, Song, Tavitian, & Cressatti, 2019). These data raise the possibility that FABP7 up-regulation in ALS astrocytes may result from unusual lipid metabolism or dysfunctional mitochondria. The observation that the up-regulation of FABP7 is not observed in neonatal ALS-astrocytes but occurs in astrocytes from symptomatic ALS mice, suggests that this up-regulation is the result of a change in astrocyte biology occurring in response to the ongoing neurodegenerative process. The aging process could also be contributing to this observation. However, a previous report observed a decrease in brain FABP7 expression in mice during aging (Pu et al., 1999). It remains to be determined whether this observation is region-specific or if FABP7 expression is upregulated in the context of other age-linked neurodegenerative process.
Activation of the canonical NF-κB pathway is a prominent feature of activated astrocytes in ALS patients and animal models (Haidet-Phillips et al., 2011; Sun et al., 2015; Swarup et al., 2011). Accordingly, many of the inflammatory makers found to be up-regulated in ALS astrocytes are under direct control of the transcription factor NF-κB (Barbeito et al., 2004; Philips & Robberecht, 2011; Vargas & Johnson, 2010). Moreover, in vivo cell-type specific translational profiling of astrocytes from hSOD1G37R-over-expressing mice identified up-regulation of inflammatory genes and down-regulation of metabolic genes as the earlier changes observed (Sun et al., 2015). The differentially expressed genes belong mainly to pathways controlled by PPARs and the liver X receptor (Sun et al., 2015). Since these receptors are normally activated by long-chain fatty acids and cholesterol derivatives, these data suggest an early dysfunction in lipid-mediated signaling in ALS astrocytes.
PPARs are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily. Besides their role in metabolic regulation, PPARs also contribute to the inactivation of proinflammatory genes. In particular, PPARγ agonism has been shown to limit neuroinflammation following CNS injury (Cai et al., 2017; Sauer, 2015). PPARγ transcriptional regulation involves ligand-independent repression, ligand-dependent transactivation and ligand-dependent transrepression (Cai et al., 2017; Sauer, 2015). Ligand-dependent transrepression involves PPARγ-dependent repression of genes in the absence of direct binding of PPARγ to the DNA. Ligand-dependent transrepression of NF-κB-driven genes appears to be the predominant way by which PPARγ promotes anti-inflammatory effects by antagonizing NF-κB activation (Daynes & Jones, 2002; Li, Pascual, & Glass, 2000; Pascual et al., 2005; Ricote, Li, Willson, Kelly, & Glass, 1998; Welch, Ricote, Akiyama, Gonzalez, & Glass, 2003).
A diverse group of endogenous metabolites have been described as ligands for PPARs, including long-chain fatty acids and some of their active metabolites. In fact, the spectrum of ligands that bind to PPARs is reminiscent to that of FABPs (Schupp & Lazar, 2010). Due to its role in the intracellular trafficking of these metabolites, FABPs modulate ligand-dependent PPARs transcriptional regulation (Tan et al., 2002; Wolfrum et al., 2001). The binding of an activating ligand serves to stabilize a conformation that promotes the translocation of FABPs to the nucleus and subsequent interaction with the associated receptor. The resulting complex, channels the ligand from the FABP to the receptor (Armstrong, Goswami, Griffin, Noy, & Ortlund, 2014; Gillilan, Ayers, & Noy, 2007; Hostetler et al., 2009; Huang et al., 2004; Huang, Starodub, McIntosh, Kier, & Schroeder, 2002; Schug, Berry, Shaw, Travis, & Noy, 2007). While FABPs selectively cooperate with PPARs to regulate transcription (Kannan-Thulasiraman, Seachrist, Mahabeleshwar, Jain, & Noy, 2010; Levi, Wang, Doud, Hazen, & Noy, 2015; Tan et al., 2002; Wolfrum et al., 2001; Yu, Levi, Casadesus, Kunos, & Noy, 2014), in some instances FABPs expression can also limit the activity of all three PPAR isotypes (Furuhashi et al., 2007; Garin-Shkolnik, Rudich, Hotamisligil, & Rubinstein, 2014; Helledie et al., 2000; Makowski et al., 2001; Makowski et al., 2005).
To effectively transport and donate bound ligands, FABPs display affinities in the same range or slightly weaker than those exhibited by PPARs (Ellinghaus, Wolfrum, Assmann, Spener, & Seedorf, 1999; Schroeder et al., 2008; Wolfrum, Borchers, Sacchettini, & Spener, 2000). Thus, up-regulation of FABPs expression can create a “sink effect” that limits the availability of endogenous ligands for PPARs (i.e., increased intracellular levels of FABPs will result in decreased PPARs activation). This, in turn, will limit the capacity of PPARs to antagonize NF-κB activation. This idea is supported by our in vitro data showing that over-expression of a ligand-impaired mutant FABP7 does not increases NF-κB-driven gene expression, expression of inflammatory markers nor confers upon non-transgenic astrocytes a neurotoxic phenotype. These results suggest that the up-regulation of FABP7 expression in reactive astrocytes in ALS models could contribute to the altered lipid-mediated signaling and aberrant NF-κB activation previously described in these cells (Sun et al., 2015). Interestingly, FABP7 is involved in the proliferation of glioblastoma cells in culture and the rate of survival of glioblastoma patients is inversely correlated with the level of FABP7 expression (De Rosa et al., 2012; Liang et al., 2005; Mita et al., 2010). An observation that appears to be related to an increase in inflammatory markers and a down-regulation of PPARγ signaling in FABP7(+)-glioblastomas (Elsherbiny, Emara, & Godbout, 2013).
Overall, our data suggest that FABP7 up-regulation directly promotes an NF-κB-driven pro-inflammatory response in astrocytes that ultimately is detrimental for motor neuron survival. Thus, modulating FABP7 expression could be a potential therapeutic target to prevent astrocyte-mediated motor neuron toxicity.
Supplementary Material
Main points.
FABP7 up-regulation promotes a pro-inflammatory response in astrocytes.
FABP7 up-regulation in astrocytes is detrimental for co-cultured motor neurons.
Silencing FABP7 expression in ALS-astrocytes decreases astrocyte-mediated motor neuron toxicity.
Acknowledgements:
This study was funded by NIH grants R21NS102599 and R01NS089640.
Footnotes
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest: The authors declare no competing financial interests.
References
- Armstrong EH, Goswami D, Griffin PR, Noy N, & Ortlund EA (2014). Structural basis for ligand regulation of the fatty acid-binding protein 5, peroxisome proliferator-activated receptor beta/delta (FABP5-PPARbeta/delta) signaling pathway. J Biol Chem, 289(21), 14941–14954. doi: 10.1074/jbc.M113.514646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D, … Cleveland DW (2013). ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci U S A, 110(8), E736–745. doi: 10.1073/pnas.1222809110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AP, Koster G, Guillermier C, Hirst EM, MacRae JI, Lechene CP, … Gould AP (2015). Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell, 163(2), 340–353. doi: 10.1016/j.cell.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, … Beckman JS (2004). A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev, 47(1-3), 263–274. doi: 10.1016/j.brainresrev.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, & Meininger V (1994). A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med, 330(9), 585–591. doi: 10.1056/NEJM199403033300901 [DOI] [PubMed] [Google Scholar]
- Brawer JR, Reichard G, Small L, & Schipper HM (1994). The origin and composition of peroxidase-positive granules in cysteamine-treated astrocytes in culture. Brain Res, 633(1-2), 9–20. doi: 10.1016/0006-8993(94)91516-4 [DOI] [PubMed] [Google Scholar]
- Brawer JR, Stein R, Small L, Cisse S, & Schipper HM (1994). Composition of Gomori-positive inclusions in astrocytes of the hypothalamic arcuate nucleus. Anat Rec, 240(3), 407–415. doi: 10.1002/ar.1092400313 [DOI] [PubMed] [Google Scholar]
- Brown RH, & Al-Chalabi A (2017). Amyotrophic Lateral Sclerosis. N Engl J Med, 377(2), 162–172. doi: 10.1056/NEJMra1603471 [DOI] [PubMed] [Google Scholar]
- Cai W, Yang T, Liu H, Han L, Zhang K, Hu X, … Chen J (2017). Peroxisome proliferator-activated receptor gamma (PPARgamma): A master gatekeeper in CNS injury and repair. Prog Neurobiol. doi: 10.1016/j.pneurobio.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Qian K, Chen W, Hu B, Blackbourn L. W. t., Du Z, … Zhang SC (2015). Human-derived neural progenitors functionally replace astrocytes in adult mice. J Clin Invest, 125(3), 1033–1042. doi: 10.1172/JCI69097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino M, Ferri A, Valle C, & Carri MT (2013). Mitochondria and ALS: implications from novel genes and pathways. Mol Cell Neurosci, 55, 44–49. doi: 10.1016/j.mcn.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Daynes RA, & Jones DC (2002). Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol, 2(10), 748–759. doi: 10.1038/nri912 [DOI] [PubMed] [Google Scholar]
- de Boer AS, Koszka K, Kiskinis E, Suzuki N, Davis-Dusenbery BN, & Eggan K (2014). Genetic validation of a therapeutic target in a mouse model of ALS. Sci Transl Med, 6(248), 248ra104. doi: 10.1126/scitranslmed.3009351 [DOI] [PubMed] [Google Scholar]
- De Rosa A, Pellegatta S, Rossi M, Tunici P, Magnoni L, Speranza MC, … Bakker A (2012). A radial glia gene marker, fatty acid binding protein 7 (FABP7), is involved in proliferation and invasion of glioblastoma cells. PLoS One, 7(12), e52113. doi: 10.1371/journal.pone.0052113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Amarilla P, Olivera-Bravo S, Trias E, Cragnolini A, Martinez-Palma L, Cassina P, … Barbeito L (2011). Phenotypically aberrant astrocytes that promote motoneuron damage in a model of inherited amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A, 108(44), 18126–18131. doi: 10.1073/pnas.1110689108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditsworth D, Maldonado M, McAlonis-Downes M, Sun S, Seelman A, Drenner K, … Da Cruz S (2017). Mutant TDP-43 within motor neurons drives disease onset but not progression in amyotrophic lateral sclerosis. Acta Neuropathol, 133(6), 907–922. doi: 10.1007/s00401-017-1698-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus P, Wolfrum C, Assmann G, Spener F, & Seedorf U (1999). Phytanic acid activates the peroxisome proliferator-activated receptor alpha (PPARalpha) in sterol carrier protein 2-/ sterol carrier protein x-deficient mice. J Biol Chem, 274(5), 2766–2772. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9915808 [DOI] [PubMed] [Google Scholar]
- Elsherbiny ME, Emara M, & Godbout R (2013). Interaction of brain fatty acid-binding protein with the polyunsaturated fatty acid environment as a potential determinant of poor prognosis in malignant glioma. Prog Lipid Res, 52(4), 562–570. doi: 10.1016/j.plipres.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, & Hotamisligil GS (2008). Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest, 118(7), 2640–2650. doi: 10.1172/JCI34750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, & Hotamisligil GS (2008). Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov, 7(6), 489–503. doi: 10.1038/nrd2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, … Hotamisligil GS (2007). Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature, 447(7147), 959–965. doi: 10.1038/nature05844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin-Shkolnik T, Rudich A, Hotamisligil GS, & Rubinstein M (2014). FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes, 63(3), 900–911. doi: 10.2337/db13-0436 [DOI] [PubMed] [Google Scholar]
- Gillilan RE, Ayers SD, & Noy N (2007). Structural basis for activation of fatty acid-binding protein 4. J Mol Biol, 372(5), 1246–1260. doi: 10.1016/j.jmb.2007.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, … et al. (1994). Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science, 264(5166), 1772–1775. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8209258 [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, … Kaspar BK (2011). Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol, 29(9), 824–828. doi: 10.1038/nbt.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan BA, Killoy KM, Pehar M, Liu L, Auwerx J, & Vargas MR (2020). Evaluation of the NAD(+) biosynthetic pathway in ALS patients and effect of modulating NAD(+) levels in hSOD1-linked ALS mouse models. Exp Neurol, 327, 113219. doi: 10.1016/j.expneurol.2020.113219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan BA, Pehar M, Killoy KM, & Vargas MR (2019). Enhanced SIRT6 activity abrogates the neurotoxic phenotype of astrocytes expressing ALS-linked mutant SOD1. FASEB J, 33(6), 7084–7091. doi: 10.1096/fj.201802752R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helledie T, Antonius M, Sorensen RV, Hertzel AV, Bernlohr DA, Kolvraa S, … Mandrup S (2000). Lipid-binding proteins modulate ligand-dependent transactivation by peroxisome proliferator-activated receptors and localize to the nucleus as well as the cytoplasm. J Lipid Res, 41(11), 1740–1751. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11060343 [PubMed] [Google Scholar]
- Hostetler HA, McIntosh AL, Atshaves BP, Storey SM, Payne HR, Kier AB, & Schroeder F (2009). L-FABP directly interacts with PPARalpha in cultured primary hepatocytes. J Lipid Res, 50(8), 1663–1675. doi: 10.1194/jlr.M900058-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2017). Inflammation, metaflammation and immunometabolic disorders. Nature, 542(7640), 177–185. doi: 10.1038/nature21363 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, & Erbay E (2008). Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol, 8(12), 923–934. doi: 10.1038/nri2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Starodub O, McIntosh A, Atshaves BP, Woldegiorgis G, Kier AB, & Schroeder F (2004). Liver fatty acid-binding protein colocalizes with peroxisome proliferator activated receptor alpha and enhances ligand distribution to nuclei of living cells. Biochemistry, 43(9), 2484–2500. doi: 10.1021/bi0352318 [DOI] [PubMed] [Google Scholar]
- Huang H, Starodub O, McIntosh A, Kier AB, & Schroeder F (2002). Liver fatty acid-binding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells. J Biol Chem, 277(32), 29139–29151. doi: 10.1074/jbc.M202923200 [DOI] [PubMed] [Google Scholar]
- Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, … Liu Z (2019). Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell, 177(6), 1522–1535 e1514. doi: 10.1016/j.cell.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Ju Lee H, Bartsch D, Xiao C, Guerrero S, Ahuja G, Schindler C, … Vilchez D (2017). A post-transcriptional program coordinated by CSDE1 prevents intrinsic neural differentiation of human embryonic stem cells. Nat Commun, 8(1), 1456. doi: 10.1038/s41467-017-01744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan-Thulasiraman P, Seachrist DD, Mahabeleshwar GH, Jain MK, & Noy N (2010). Fatty acid-binding protein 5 and PPARbeta/delta are critical mediators of epidermal growth factor receptor-induced carcinoma cell growth. J Biol Chem, 285(25), 19106–19115. doi: 10.1074/jbc.M109.099770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia A, McAvoy K, Krishnamurthy K, Trotti D, & Pasinelli P (2018). Astrocytes expressing ALS-linked mutant FUS induce motor neuron death through release of tumor necrosis factor-alpha. Glia, 66(5), 1016–1033. doi: 10.1002/glia.23298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Vargas MR, Harlan BA, Killoy KM, Ball LE, Comte-Walters S, … Pehar M (2018). Nitration and Glycation Turn Mature NGF into a Toxic Factor for Motor Neurons: A Role for p75(NTR) and RAGE Signaling in ALS. Antioxid Redox Signal, 28(18), 1587–1602. doi: 10.1089/ars.2016.6966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, & Muller T (1994). The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development, 120(9), 2637–2649. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7956838 [DOI] [PubMed] [Google Scholar]
- Levi L, Wang Z, Doud MK, Hazen SL, & Noy N (2015). Saturated fatty acids regulate retinoic acid signalling and suppress tumorigenesis by targeting fatty acid-binding protein 5. Nat Commun, 6, 8794. doi: 10.1038/ncomms9794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Pascual G, & Glass CK (2000). Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol, 20(13), 4699–4707. doi: 10.1128/mcb.20.13.4699-4707.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Diehn M, Watson N, Bollen AW, Aldape KD, Nicholas MK, … Israel MA (2005). Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A, 102(16), 5814–5819. doi: 10.1073/pnas.0402870102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, MacKenzie KR, Putluri N, Maletic-Savatic M, & Bellen HJ (2017). The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab, 26(5), 719–737 e716. doi: 10.1016/j.cmet.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, … Bellen HJ (2015). Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell, 160(1-2), 177–190. doi: 10.1016/j.cell.2014.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Longo LD, & De Leon M (2000). In situ and immunocytochemical localization of E-FABP mRNA and protein during neuronal migration and differentiation in the rat brain. Brain Res, 852(1), 16–27. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10661491 [DOI] [PubMed] [Google Scholar]
- Liu Y, Molina CA, Welcher AA, Longo LD, & De Leon M (1997). Expression of DA11, a neuronal-injury-induced fatty acid binding protein, coincides with axon growth and neuronal differentiation during central nervous system development. J Neurosci Res, 48(6), 551–562. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9210525 [DOI] [PubMed] [Google Scholar]
- Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, … Linton MF (2001). Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med, 7(6), 699–705. doi: 10.1038/89076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski L, Brittingham KC, Reynolds JM, Suttles J, & Hotamisligil GS (2005). The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem, 280(13), 12888–12895. doi: 10.1074/jbc.M413788200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel E, Cassina A, Martinez-Palma L, Souza JM, Bolatto C, Rodriguez-Bottero S, … Cassina P (2014). Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic Biol Med, 70, 204–213. doi: 10.1016/j.freeradbiomed.2014.02.019 [DOI] [PubMed] [Google Scholar]
- Mita R, Beaulieu MJ, Field C, & Godbout R (2010). Brain fatty acid-binding protein and omega-3/omega-6 fatty acids: mechanistic insight into malignant glioma cell migration. J Biol Chem, 285(47), 37005–37015. doi: 10.1074/jbc.M110.170076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, & Przedborski S (2007). Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci, 10(5), 615–622. doi: 10.1038/nn1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owada Y, Yoshimoto T, & Kondo H (1996). Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J Chem Neuroanat, 12(2), 113–122. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9115666 [DOI] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, … Glass CK (2005). A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature, 437(7059), 759–763. doi: 10.1038/nature03988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WA, Fu W, Keller JN, Markesbery WR, Appel S, Smith RG, … Mattson MP (1998). Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann Neurol, 44(5), 819–824. doi: 10.1002/ana.410440518 [DOI] [PubMed] [Google Scholar]
- Pehar M, Harlan BA, Killoy KM, & Vargas MR (2017). Role and Therapeutic Potential of Astrocytes in Amyotrophic Lateral Sclerosis. Curr Pharm Des, 23(33), 5010–5021. doi: 10.2174/1381612823666170622095802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, & Robberecht W (2011). Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol, 10(3), 253–263. doi: 10.1016/S1474-4422(11)70015-1 [DOI] [PubMed] [Google Scholar]
- Pu L, Igbavboa U, Wood WG, Roths JB, Kier AB, Spener F, & Schroeder F (1999). Expression of fatty acid binding proteins is altered in aged mouse brain. Mol Cell Biochem, 198(1-2), 69–78. doi: 10.1023/a:1006946027619 [DOI] [PubMed] [Google Scholar]
- Qian K, Huang H, Peterson A, Hu B, Maragakis NJ, Ming GL, … Zhang SC (2017). Sporadic ALS Astrocytes Induce Neuronal Degeneration In Vivo. Stem Cell Reports, 8(4), 843–855. doi: 10.1016/j.stemcr.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JM, Liu Q, Brittingham KC, Liu Y, Gruenthal M, Gorgun CZ, … Suttles J (2007). Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J Immunol, 179(1), 313–321. doi: 10.4049/jimmunol.179.1.313 [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, & Glass CK (1998). The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature, 391(6662), 79–82. doi: 10.1038/34178 [DOI] [PubMed] [Google Scholar]
- Sauer S (2015). Ligands for the Nuclear Peroxisome Proliferator-Activated Receptor Gamma. Trends Pharmacol Sci, 36(10), 688–704. doi: 10.1016/j.tips.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Sawada H (2017). Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expert Opin Pharmacother, 18(7), 735–738. doi: 10.1080/14656566.2017.1319937 [DOI] [PubMed] [Google Scholar]
- Schipper HM (1991). Gomori-positive astrocytes: biological properties and implications for neurologic and neuroendocrine disorders. Glia, 4(4), 365–377. doi: 10.1002/glia.440040404 [DOI] [PubMed] [Google Scholar]
- Schipper HM, Song W, Tavitian A, & Cressatti M (2019). The sinister face of heme oxygenase-1 in brain aging and disease. Prog Neurobiol, 172, 40–70. doi: 10.1016/j.pneurobio.2018.06.008 [DOI] [PubMed] [Google Scholar]
- Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, … Kier AB (2008). Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids, 43(1), 1–17. doi: 10.1007/s11745-007-3111-z [DOI] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Shaw NS, Travis SN, & Noy N (2007). Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell, 129(4), 723–733. doi: 10.1016/j.cell.2007.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp M, & Lazar MA (2010). Endogenous ligands for nuclear receptors: digging deeper. J Biol Chem, 285(52), 40409–40415. doi: 10.1074/jbc.R110.182451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellner PA, Chu W, Glatz JF, & Berman NE (1995). Developmental role of fatty acid-binding proteins in mouse brain. Brain Res Dev Brain Res, 89(1), 33–46. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8575091 [DOI] [PubMed] [Google Scholar]
- Shi P, Gal J, Kwinter DM, Liu X, & Zhu H (2010). Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim Biophys Acta, 1802(1), 45–51. doi: 10.1016/j.bbadis.2009.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EP, Henry YK, Henkel JS, Smith RG, & Appel SH (2004). Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology, 62(10), 1758–1765. doi: 10.1212/wnl.62.10.1758 [DOI] [PubMed] [Google Scholar]
- Smathers RL, & Petersen DR (2011). The human fatty acid-binding protein family: evolutionary divergences and functions. Hum Genomics, 5(3), 170–191. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21504868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Henry YK, Mattson MP, & Appel SH (1998). Presence of 4-hydroxynonenal in cerebrospinal fluid of patients with sporadic amyotrophic lateral sclerosis. Ann Neurol, 44(4), 696–699. doi: 10.1002/ana.410440419 [DOI] [PubMed] [Google Scholar]
- Song S, Miranda CJ, Braun L, Meyer K, Frakes AE, Ferraiuolo L, … Kaspar BK (2016). Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis. Nat Med, 22(4), 397–403. doi: 10.1038/nm.4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch J, & Thumser AE (2010). Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem, 285(43), 32679–32683. doi: 10.1074/jbc.R110.135210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Sun Y, Ling SC, Ferraiuolo L, McAlonis-Downes M, Zou Y, … Cleveland DW (2015). Translational profiling identifies a cascade of damage initiated in motor neurons and spreading to glia in mutant SOD1-mediated ALS. Proc Natl Acad Sci U S A, 112(50), E6993–7002. doi: 10.1073/pnas.1520639112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup V, Phaneuf D, Dupre N, Petri S, Strong M, Kriz J, & Julien JP (2011). Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor kappaB-mediated pathogenic pathways. J Exp Med, 208(12), 2429–2447. doi: 10.1084/jem.20111313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, … Noy N (2002). Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol, 22(14), 5114–5127. doi: 10.1128/mcb.22.14.5114-5127.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumser AE, Moore JB, & Plant NJ (2014). Fatty acid binding proteins: tissue-specific functions in health and disease. Curr Opin Clin Nutr Metab Care, 17(2), 124–129. doi: 10.1097/MCO.0000000000000031 [DOI] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, & Johnson JA (2008). Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci, 28(50), 13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, & Johnson JA (2010). Astrogliosis in amyotrophic lateral sclerosis: role and therapeutic potential of astrocytes. Neurotherapeutics, 7(4), 471–481. doi: 10.1016/j.nurt.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Beckman JS, & Barbeito L (2006). Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem, 97(3), 687–696. doi: 10.1111/j.1471-4159.2006.03742.x [DOI] [PubMed] [Google Scholar]
- Wang J, Xu G, Gonzales V, Coonfield M, Fromholt D, Copeland NG, … Borchelt DR (2002). Fibrillar inclusions and motor neuron degeneration in transgenic mice expressing superoxide dismutase 1 with a disrupted copper-binding site. Neurobiol Dis, 10(2), 128–138. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12127151 [DOI] [PubMed] [Google Scholar]
- Wang L, Gutmann DH, & Roos RP (2011). Astrocyte loss of mutant SOD1 delays ALS disease onset and progression in G85R transgenic mice. Hum Mol Genet, 20(2), 286–293. doi: 10.1093/hmg/ddq463 [DOI] [PubMed] [Google Scholar]
- Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, & Glass CK (2003). PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci U S A, 100(11), 6712–6717. doi: 10.1073/pnas.1031789100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum C, Borchers T, Sacchettini JC, & Spener F (2000). Binding of fatty acids and peroxisome proliferators to orthologous fatty acid binding proteins from human, murine, and bovine liver. Biochemistry, 39(6), 1469–1474. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10684629 [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Borrmann CM, Borchers T, & Spener F (2001). Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha - and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc Natl Acad Sci U S A, 98(5), 2323–2328. doi: 10.1073/pnas.051619898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, … Cleveland DW (2008). Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci, 11(3), 251–253. doi: 10.1038/nn2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Baker JH, & Muller T (1996). Immunoreactivity for brain-fatty acid binding protein in gomori-positive astrocytes. Glia, 16(3), 218–226. doi: [DOI] [PubMed] [Google Scholar]
- Yu S, Levi L, Casadesus G, Kunos G, & Noy N (2014). Fatty acid-binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) in the brain. J Biol Chem, 289(18), 12748–12758. doi: 10.1074/jbc.M114.559062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SW, Leong C, Zhai D, Tan YL, Lim L, Bi X, … Chang YT (2012). Neural stem cell specific fluorescent chemical probe binding to FABP7. Proc Natl Acad Sci U S A, 109(26), 10214–10217. doi: 10.1073/pnas.1200817109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


