Abstract
With a worldwide prevalence of 15%, chronic constipation is one of the most frequent gastrointestinal diagnoses made in ambulatory medicine clinics, and is a common source cause for referrals to gastroenterologists and colorectal surgeons in the United States. Symptoms vary among patients; straining, incomplete evacuation, and a sense of anorectal blockage are just as important as decreased stool frequency. Chronic constipation is either a primary disorder (such as normal transit, slow transit, or defecatory disorders) or a secondary one (due to medications or, in rare cases, anatomic alterations). Colonic sensorimotor disturbances and pelvic floor dysfunction (such as defecatory disorders) are the most widely recognized pathogenic mechanisms. Guided by efficacy and cost, management of constipation should begin with dietary fiber supplementation and stimulant and/or osmotic laxatives, as appropriate, followed, if necessary, by intestinal secretagogues and/or prokinetic agents. Peripherally acting μ-opiate antagonists are another option for opioid-induced constipation. Anorectal tests to evaluate for defecatory disorders should be performed in patients who do not respond to over-the-counter agents. Colonic transit, followed if necessary with assessment of colonic motility with manometry and/or a barostat, can identify colonic dysmotility. Defecatory disorders often respond to biofeedback therapy. For specific patients, slow-transit constipation may necessitate a colectomy. No studies have compared inexpensive laxatives with newer drugs with different mechanisms. We review the mechanisms, evaluation, and management of chronic constipation. We discuss the importance of meticulous analyses of patient history and physical examination, advantages and disadvantages of diagnostic testing, guidance for individualized treatment, and management of medically refractory patients.
The prevalence of chronic constipation (CC) among adults is approximately 15%, making it the sixth most common gastrointestinal symptom. CC often results in visits to ambulatory clinics and gastroenterology referrals.1–3 Although the prevalence is greater in non-Caucasians than Caucasians, in women (median female to male ratio of 1.5:1), and in institutionalized rather than community-living elderly persons, symptoms can affect all ages, races, socioeconomic groups, and nationalities.
Definition and Classification
Chronic constipation is either primary or secondary (attributed to another disease), determined from patient history and results from examinations and laboratory tests (Table 1).4 The Rome IV criteria for primary constipation are based on results from anorectal tests and categorize patients as having functional constipation (FC), constipation-predominant irritable bowel syndrome (IBS-C), or defecatory disorders (DDs) (Supplementary Figure 1).5 FC and IBS-C are primarily defined by symptoms alone (Table 2). DDs are defined by symptoms (such as FC or IBS-C) and results from anorectal tests that indicate impaired rectal evacuation. Prior American Gastroenterological Association reviews and this update classify patients with constipation based on assessments of colonic transit and anorectal function; the classifications are normal transit constipation (NTC), slow transit constipation (STC), and pelvic floor dysfunction or DDs (Supplementary Figure 1).4,6
Table 1.
Common Medical Conditions Associated With Constipation
| Cause | Comments |
|---|---|
| Drug effects | See Supplementary Table 1 |
| Mechanical obstruction: colon cancer, external compression from malignant lesion, strictures (diverticular or post ischemic), rectocele (if large), megacolon, anal fissure | Often associated with alarm clinical features or laboratory tests, apparent on digital rectal examination (fissure) or x-ray image of the abdomen, or preceded by the primary event (diverticulitis) |
| Metabolic conditions: diabetes mellitus, hypothyroidism, hypercalcemia, hypokalemia, hypomagnesemia, uremia, heavy metal poisoning, uremia, heavy metal poisoning | All are associated with/can be diagnosed by abnormal results from laboratory tests, which should be performed only when there is a high index of suspicion (such as in patients on diuretics) |
| Myopathies: amyloidosis, scleroderma | Typically associated with other clinical features of these conditions |
| Neuropathies: Parkinson’s disease, spinal cord injury or tumor, cerebrovascular disease, and multiple sclerosis | Constipation, either due to slow colon transit and/or DD, is common in patients with these disorders, which have many other features |
| Other conditions: depression, degenerative joint disease, autonomic neuropathy, cognitive impairment, immobility, cardiac disease | The disorder and/or medications may contribute to constipation |
Table 2.
Definitions of Constipation
| Rome IV criteria |
Criteria used in studies of pharmacologic agents |
||
|---|---|---|---|
| FC | IBS-C | FC169 | IBS-C170 |
| Symptoms for ≥6 mo and 2 or more of the following symptoms for >25% of defecations during past 3 mo: ∘ Straining ∘ Lumpy or hard stools ∘ Sensation of incomplete evacuation ∘ Sensation of anorectal obstruction/blockade ∘ Manual maneuvers to facilitate defecations; ∘ <3 defecations/wk ∘ Loose stools are not present, and there are insufficient criteria for IBS |
Recurrent abdominal pain at least 1 d/wk in the past 3 mo associated with 2 or more of the following: ∘ Related to defecation ∘ Onset associated with change in frequency of stool ∘ Onset associated with change in form (appearance) of stool ∘ >25% of bowel movements were hard and <25% were loose stools |
<3 SBMs/wk and 1 or more of the following symptoms for at least 12 wk during the preceding 12 mo ∘ Straining in >25% of defecations ∘ Lumpy or hard stools in >25% of defecations ∘ Sensation of incomplete evacuation in >25% of defecations ∘ No loose or watery SBMs (Bristol Stool Form Scale scores of 6 or 7) |
Abdominal pain associated with 2 or more of the following: ∘ Related to defecation ∘ Onset associated with change in frequency of stool ∘ Onset associated with change in form and (appearance) of stool ∘ <3 SBMs and <3 CSBMs/wk; and at least 1 additional symptom ∘ Straining in ≥25% of defecations ∘ Lumpy or hard stools in ≥25% of defecations ∘ Sensation of incomplete evacuation in ≥25% of defecations |
CSBM, complete spontaneous bowel movements; SBM, spontaneous bowel movement.
Patients with constipation have infrequent stools (fewer than 3 bowel movements per week) and, more importantly, straining at stool, a feeling of incomplete evacuation, a need for digital assistance to evacuate stool, bloating, and hard or lumpy stools.7 The Rome IV criteria are predominantly symptom-based and as such require that patients with a diagnosis of FC have 2 or more of these symptoms, which affect >25% of bowel movements for at least 6 months and active symptoms for the past 3 months (Table 1). By contrast, IBS-C is defined by abdominal pain that is associated with 2 of 3 features: altered stool form, altered stool frequency, or relief of abdominal pain with defecation. Although patients with CC also have abdominal pain, the pain is not, in contrast to the definition for IBS-C, associated with the symptoms mentioned. In (real-world) clinical practice, it is more useful to conceptualize FC and IBS-C along a spectrum; it is sometimes difficult to distinguish FC from IBS-C and to determine which patients are true medication responders using the definitions used in clinical trials (see Table 2). IBS-C patients are more likely to predominantly have abdominal pain, heightened rectal sensation,8 upper gastrointestinal symptoms (eg, heartburn, dyspepsia), anxiety and depression, and urinary symptoms.9,10 However, blurring the distinction between FC and IBS-C, 1 study found that approximately 90% of patients with IBS-C also met criteria for FC and 44% of the FC patients also met criteria for IBS-C.11 In approximately one-third of patients, symptoms shift over time between FC and IBS-C.11 In individual patients, a diagnosis of either FC or IBS-C is possible only because the Rome criteria specify that patients with symptoms of IBS-C and FC be designated as IBS-C not FC.
This limitation can be overcome by classifying constipated patients, based on the presence or absence of moderate to severe abdominal pain, into 1 of 2 categories, such as painful or painless constipation (Supplementary Figure 2).9,10 In contrast to the Rome IV criteria for IBS-C, these definitions do not specify the temporal relationship, or lack thereof, between abdominal pain and bowel habits. Similar to the differences for FC and IBS-C, compared to mild pain constipation, patients with painful constipation have more prominent bowel, upper gastrointestinal (such as dyspepsia), anorectal, urinary and sexual symptoms, anxiety and depression, and slower rectosigmoid transit. The widespread symptoms in painful constipation could partly reflect increased perception of visceral sensations. Symptom-based criteria for discriminating between painful and mild-pain constipation have been proposed but require finalization.
Physiology of Colonic Motor Functions
The right colon is a reservoir that mixes and stores contents.12–14 The left colon functions primarily as a conduit. The rectum and anal canal enable defecation and maintain fecal continence. Our understanding of motor activity, which is derived mostly from studies with non-high-resolution manometry catheters in which sensors were separated by 7.5 cm or more,13 suggest that most colonic motor activity is irregular and nonpropagated and serves to segment and mix intraluminal contents. By comparison, newer high-resolution catheters have sensors separated by 1–2.5 cm and are more accurate for detecting propagated motor events (Supplementary Figure 3).15 Colonic motor patterns are diverse, and include individual or rhythmic events, which may be simultaneous or propagated (antegrade or retrograde), and have low or high amplitude.13 Of these patterns, the gastrocolonic response to a meal and high-amplitude propagated contractions are arguably the most physiologically important. The gastrocolonic response begins shortly, often within a few seconds, after eating and may last for up to 2½ hours.16 Although a 1000-kcal meal invariably elicits a response, 600 kcal is probably equivalent.17 Propagated contractions, categorized as low (5–40 mmHg) or high-amplitude propagated contractions (HAPCs, >75 mmHg), occur an average of 6 times per day, originate predominantly in the cecum or ascending colon, cause mass movement of colon contents, and often precede defecation.18 HAPCs occur more frequently after awakening and after meals and can account for the urge to defecate in healthy subjects and in patients with IBS. HAPCs occur spontaneously, occasionally in response to luminal distention, or can be induced by glycerol, bisacodyl, oleic acid, and the cholinesterase inhibitor neostigmine.
Pathophysiology
Colonic sensorimotor disturbances and pelvic floor dysfunction are the most widely recognized causes. Other factors, such as reduced caloric intake, disturbances of the microbiome, anatomical issues, or medications, can also contribute.
Colonic Sensorimotor Dysfunctions and the Microbiome
Isolated slow-transit constipation (eg, no DD) is used as a marker of colonic motor dysfunction(s), perhaps due to reductions in colonic intrinsic nerves and interstitial cells of Cajal.19,20 Manometry can reveal colonic motor disturbances, such as reduced propagated and nonpropagated activity and reduced phasic contractile responses to a meal and/or to bisacodyl or neostigmine, in patients with STC.21–23 Manometry catheters only measure phasic pressure activity. A barostat balloon device also records colonic tone; fasting tone and tonic contractile responses to a meal and/or neostigmine are reduced in STC (Figure 1).21–23 Colonic inertia, which represents profound motor dysfunction, can only be identified by manometry or a barostat and is defined by reduced or absent contractile response to a meal and to pharmacologic stimuli (such as bisacodyl or neostigmine).23,24
Figure 1.
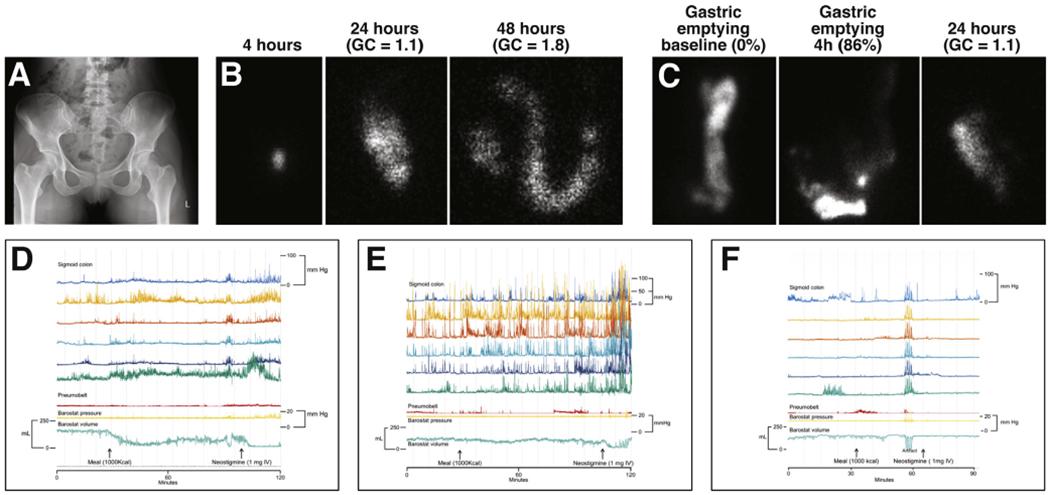
Patterns of colonic motor dysfunctions in patients with CC. The pronounced reduction in sigmoid colonic balloon volume indicates a normal tonic response to a meal (D) in a patient with excess colonic stool burden (A). Anorectal tests (not shown) identified a DD. During scintigraphy, colonic transit is usually measured using an isotope111 coated with a pH-sensitive methacrylate that dissolves in the terminal ileum. In (B), the isotope is in an intact capsule (left) observed in the ascending colon at 24 hours (center panel) and then in the transverse colon at 48 hours (right panel). The geometric center (GC), which is the weighted distribution of the isotope throughout the colon, indicates slow colon transit; normal values are 1.4–3.6 at 24 hours and 2.1–4.9 at 48 hours. In this patient, the colonic manometry (E) depicts considerable phasic pressure activity during the fasting period, increased phasic activity after a meal, and more so after intravenous neostigmine. However, the tonic contractile response to the meal was reduced. (C) shows a patient with delayed colonic transit with normal gastric emptying. In this patient, the colonic manometry (F) reveals sparse phasic pressure activity and tonic or phasic contractile responses to a meal.
Unfortunately, NTC and STC are imperfect markers of normal and impaired colonic motor function, respectively. For example, fasting and/or postprandial colonic tone and/or compliance were reduced in 40% of patients with NTC, 47% in patients with STC, 53% in patients with DD and normal transit, and 42% in patients with DD and slow transit.23 Similarly, 43% of patients with STC had normal fasting colonic motility and motor responses to a meal and bisacodyl.25 Patients with NTC might have symptoms of FC or IBS-C; 23% of patients with FC or IBS-C had delayed colonic transit.26,27 Some patients have increased perception of rapid distention and reduced perception of slow distention.28 Increased rectal sensitivity is associated with abdominal pain and bloating, suggestive of IBS.29–31
Germ-free mice colonized with the fecal microbiome from patients with constipation developed slow colonic transit.32,33 Slow colon transit correlates inversely with colonic serotonin content, associates with a decreased relative abundance of Firmicutes and increased Bacteroidetes, and associates with altered fecal content of short-chain fatty acids and bile acids.32 In humans, CC is associated with alterations in colonic mucosal microbiota, especially more plentiful phylum Bacteroidetes, resulting from a greater abundance of Flavobacterium. 34 Adjusted for colonic transit, the colonic mucosal microbiome discriminated patients with constipation from controls with 94% accuracy, even after adjusting for diet and colonic transit. By contrast, fecal microbiomes were associated with colonic transit and increased methane in breath samples, but not with constipation.
Defecatory Disorders
DDs (also called functional outlet obstruction, anorectal dyssynergia, or pelvic floor dysfunction) are caused by reduced rectal propulsive forces and/or increased resistance to evacuation (Figure 2).35 Increased resistance results from high anal resting pressure (anismus) or paradoxical contraction or incomplete relaxation36 of the pelvic floor and external anal sphincters (dyssynergia).37 These patterns are not associated with specific clinical patterns or the response to pelvic floor retraining.38 DDs primarily develop via maladaptive pelvic floor contraction during defecation.39 Other abnormalities, especially reduced rectal sensation and structural deformities (such as rectoceles and excessive perineal descent), can coexist and be primary or secondary to constipation.14,40–44 Reduced rectal sensation could reduce desire to defecate40,45; as many as 50% of patients with constipation have delayed colonic transit.23,37,46 Beside colonic motor dysfunction unrelated to DD,22 retained stool can physically obstruct passage of contents or evoke rectocolonic inhibitory reflexes.47 Over time, excessive straining can weaken the pelvic floor, increasing risk for excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy. Pudendal neuropathy can weaken anal sphincters, increasing risk for fecal incontinence.45,48–50
Figure 2.
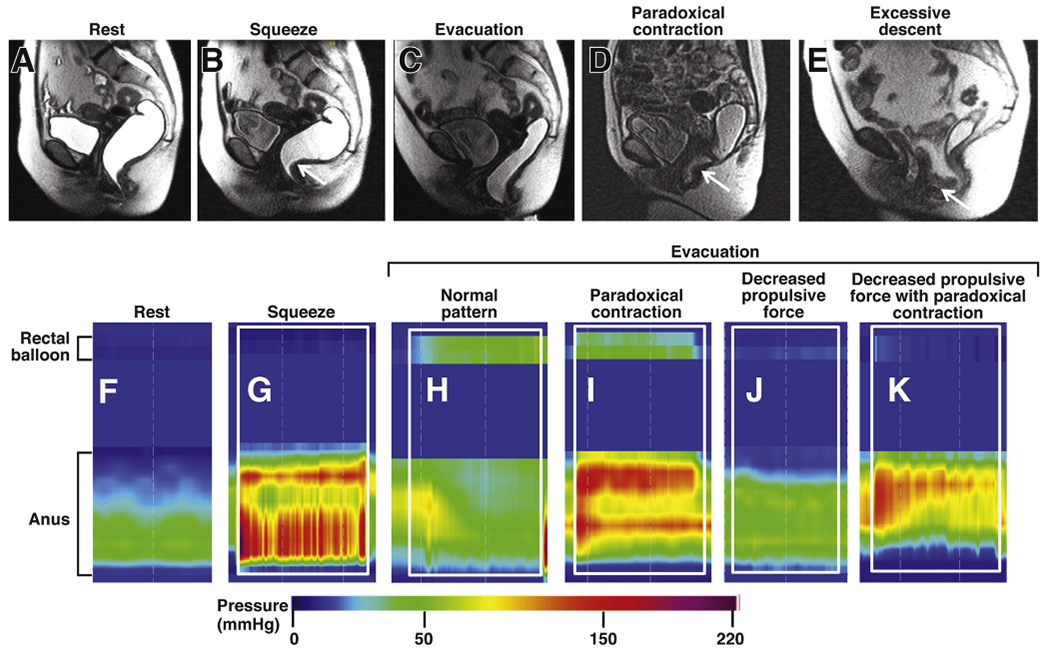
Normal and abnormal anorectal evacuation. Evacuation was recorded by MR imaging (top row) and high-resolution manometry (bottom row). MR imaging shows anorectum filled with gel at rest (A), increased puborectalis indentation during squeeze (B, arrow) and normal relaxation of the puborectalis, perineal descent, opening of the anal canal, and evacuation of ultrasound gel during evacuation (C). In patients with constipation, during evacuation, there is paradoxical contraction of the puborectalis (D, arrow) and exaggerated perineal descent with an enterocele (E, arrow). High-resolution manometry shows anal pressure at rest (F) and increased anal pressure during squeeze (G) compared to rest (F). The white rectangle demarcates the duration of squeeze (G) and evacuation (H–K). Note the increased rectal pressure with anal relaxation during evacuation in a healthy person (H). By contrast during evacuation in constipated patients, note increased rectal pressure with paradoxical anal contraction (I), no change in rectal pressure vs rest (J), and no change in rectal pressure with paradoxical anal contraction (K). Reproduced from Bharucha and Wald,72 with permission.
The precise contribution of dyssynergia to impaired evacuation is unclear because dyssynergia has been reported in asymptomatic people as well as patients with rectal pain.38,51,52 This might be because it is a challenge to simulate defecation in the laboratory. When dyssynergia and structural abnormalities (such as large rectoceles) overlap, it is difficult to determine the contributions of each to the symptoms (Figure 2). Some features (such as delayed colonic transit) are consequences of DD and improve after biofeedback therapy.53 Other factors, particularly stool form, affect development of symptoms in patients with DD.54,55 The pathogenesis of DD is unclear. DDs are believed to result from maladaptive learning of sphincter contraction, possibly initiated by avoidance of pain, or trauma,56 or even neglecting the call to defecate. One-third of children with constipation continued to have severe symptoms beyond puberty.57 There is no evidence for an association between obstetric trauma and DD.58
Clinical Evaluation
The bowel symptom questionnaire is a quick and effective tool for evaluating symptoms of constipation (Table 1). Questionnaires provide a snapshot of symptoms, whereas a 2-week bowel diary provides a more refined assessment of day-to-day variations and the relationship between stool form and other symptoms.54 Analyses of bowel diaries recorded by patients when they are off laxatives can help determine contribution of laxatives to symptoms (such as bloating). It is also important to collect information on prior bowel habits, when bowel habits changed, and what patients consider as normal, because perceptions, influenced by societal and cultural norms, influence symptom reporting. Some patients report constipation because they do not, perhaps in contrast to a spouse, pass a daily bowel movement. Other patients, in retrospect, have had mild and/or intermittent symptoms for longer than initially acknowledged (such as since childhood). Inadvertent withholding, perhaps secondary to an aversion to using public toilets, or constipation after recent surgery, medication changes, or coexistent urinary symptoms, are not uncommon. Addressing the most bothersome symptoms of constipation can increase patients’ quality of life.59
Colonic transit affects fecal form, which is assessed using the Bristol Stool Form Scale and ranges from liquid, to semi-formed, to pellet-like stools.60–62 Abdominal pain and its relationship to bowel movements can differentiate patients with CC from patients with IBS-C. Co-existing gastrointestinal symptoms should be identified; constipated patients frequently have bloating, which can be due to the underlying disorder and/or medications—especially fiber and osmotic laxatives. The presence of multiple other gastrointestinal symptoms (such as dyspepsia), especially in a younger patient without warning signs, supports a functional cause of symptoms.63 However, distinct mechanisms can cause dyspepsia (such as impaired gastric accommodation) and constipation in the same patients.64 In constipated patients, fecal impaction, perhaps compounded by laxative-induced overflow diarrhea, increases the risk for fecal incontinence.65 It is important to ask patients about diet (for adequate fiber intake, intake of calories, and poorly absorbed carbohydrates that contribute to bloating),66 lifestyle (such as level of activity), toileting habits, medical conditions, obstetric history, and surgery (Table 1). At the appropriate time, patients should be asked whether they have a history of abuse, which is common in patients with DD.67 Medications and supplements should be reviewed (Supplementary Table 1). Warning signs, such as unintentional weight loss >10% of body weight, anemia, rectal bleeding, a family history of colorectal cancer, and polyposis syndromes should be identified, which have low predictive values in patients with CC.68
A meticulous and directed physical examination is essential for several reasons. Considered in isolation, symptoms (such as straining vs infrequent bowel movements) do not discriminate between DD and other causes of CC.38,69 Examinations can identify an organic cause for constipation, such as an abdominal mass, whereas dry skin is associated with hypothyroidism. Examinations also reassure patients that their concerns are taken seriously.
Digital rectal examinations (DREs) identify not only structural disorders (such as anal fissures, hemorrhoids, fecal impactions, descending perineum syndrome, or anorectal cancer), but also pelvic floor dyssynergia, which is treated differently.70 Although DREs are recommended by many different societies, many providers do not perform them for constipated patients.71 DREs identified patients with dyssynergia with 75% sensitivity and 87% specificity when manometry was used as the reference standard.70 Compared with the rectal balloon expulsion test (BET), the sensitivity and specificity of a DRE were 80% and 56%, respectively. For some people with normal pelvic floor function, it is a challenge to simulate defecation during a DRE. This might account for the lower specificity compared with a BET. A normal result from a DRE is more useful than an abnormal result from an examination.70 Nonetheless, patients with persistent symptoms and a normal finding from DRE should be referred for anorectal testing to exclude DDs.
Thereafter, in the absence of warning signs, patients should receive a diagnosis of CC. A complete blood count is helpful in making a diagnosis, but other laboratory studies are unnecessary.4,5,72 Colonoscopies are only necessary for patients with alarm symptoms, or as required for age-appropriate screening. Physicians and their patients should discuss the benign nature of CCs, treatment options, and their efficacy, safety, and cost; questions about special tests should be answered.
Diagnostic Tests
Anorectal testing with manometry and BET are recommended for patients who have not responded to a high-fiber diet and/or simple laxatives.4,73,74 Because access to anorectal testing is not universal, some practitioners treat patients empirically with prescription laxatives before anorectal testing. However, <25% of patients with IBS-C report being very satisfied with prescription laxatives, perhaps partly because they have a DD. This observation supports the need for anorectal testing.75
During anorectal tests, maintenance of privacy will facilitate cooperation and reduce embarrassment, which can account for false-positive results from tests in asymptomatic and constipated patients.52 Results should be interpreted along with clinical features. There is no criterion standard diagnostic test, or symptom, for a diagnosis of DD. Although different tests provide different information about individual patients,14,44 overall, the results of anorectal high-resolution anorectal manometry (HRM), BET, and magnetic resonance (MR) imaging are significantly correlated with each other, which substantiates the criterion validity of these tests,44 even though these tests are performed in different positions, with or without rectal filling. Therefore, the Rome IV criteria propose that a diagnosis of DD be confirmed by abnormal findings from 2 of 4 tests (such as manometry, rectal BET, surface electromyography, or barium or MR defecography). Taking all factors (such as diagnostic utility, usability, availability, risk, and expense) into consideration, the rectal BET is the most useful test to make a diagnosis of DD.
Balloon Expulsion Test
The BET is a simple procedure generally performed in conjunction with anorectal manometry to evaluate the time required to evacuate a 50-mL water-filled balloon (warm tap water) in the seated position. The normal values depend on the type of rectal balloon used for the test.76–78 Most centers use a party or commercial balloon, for which the upper limit of normal is 1 minute. For a Foley catheter inflated to 50 mL, which is above the manufacturer-recommended limit of 30 mL, the upper limit of normal is 2 minutes.76 Even with the 2-minute cutoff, 25% of healthy people would be misclassified as abnormal because they require more than 2 minutes.79
Among 106 patients with FC and 24 patients with DD, the BET identified those with DD, documented with defecography, with 88% sensitivity and 89% specificity; positive and negative predictive values were 64% and 97% for diagnosis of DD, respectively.80 However, this uncontrolled study excluded patients with secondary (such as medication-induced) CC. The rectal balloon was inflated not to a fixed volume but until patients experienced the desire to defecate, averaging 183 mL, which may compensate for reduced rectal sensation identified in some patients with DD.41
Anorectal Manometry
Conventional catheters that incorporate water-perfused, air-charged, or solid-state sensors, HRM, or high-definition manometry can be used.81–83 HRM and high-definition anorectal manometry catheters have more closely spaced sensors that straddle the entire anal canal. They provide better spatial resolution, and allow pressures to be assessed without a pull-through maneuver (Figure 2). Although conventional and HRM catheters measure anal pressures with comparable levels of precision, pressures are much higher with HRM or high-definition anorectal manometry than conventional catheters.84 Anorectal pressures vary among techniques, so they must be compared with normal values measured by the same technique. Compared with healthy individuals, patients with DD have lower rectoanal pressure gradients (rectal–anal pressure) during evacuation. The rectoanal pressure pattern can also indicate causes of DD, such as decreased propulsive force, paradoxical contraction, or both.69 However, the rectoanal gradient measured by HRM is negative in many asymptomatic people, which limits the utility of this parameter for making a diagnosis of DD.52,82,85 Anorectal manometry is best performed in a seated rather than a left lateral, recumbent position.86 Other features of DD include a higher anal resting pressure (anismus) and/or reduced voluntary augmentation during pelvic floor contraction.
Barium and Magnetic Resonance Defecography
Some practitioners prefer defecography to the BET. Others use defecography as a backup method, when the other anorectal tests produce results that are inconsistent with clinical findings, to identify anatomic abnormalities, or for patients with persistent symptoms after biofeedback therapy.87–90 Abnormalities include inadequate (such as a spastic disorder) or excessive (such as in descending perineum syndrome) widening of the anorectal angle and/or perineal descent during defecation. Internal intussusception, solitary rectal ulcers, rectoceles, and rectal prolapse are also observed.91 Enteroceles, bladder, and uterovaginal prolapse can be visualized when the vagina and small intestine are opacified.
The techniques for barium defecography are incompletely standardized; some radiologists have limited enthusiasm for the test.88 Asymptomatic subjects have features of DD. Methodological limitations to barium defecography (such as limited reproducibility of anorectal angle measurements) can be minimized by using standardized techniques.14,92 In contrast to barium defecography, MR defecography is performed in the supine position but avoids radiation exposure and can is better for visualizing pelvic organ prolapse and the bony landmarks, which are necessary to measure pelvic floor motion.40,74,93,94 MR defecography is especially useful for patients who are believed to have DDs with a normal BET; this group includes >90% of patients with a large rectocele, enterocele, peritoneocele, and/or peritoneocele (Figure 2).40,44 Average anal electromyographic activity is recorded by electrodes mounted on an acrylic anal plug or taped to the perianal skin, and is used to identify dyssynergia,88 and to provide biofeedback training for DD.95,96 Reduction of ≥20% in anal electromyographic activity during evacuation is considered normal. By contrast, impaired reduction is considered abnormal and is correlated with rectoanal dyssynergia, identified by manometry and abnormal results from a BET.76
Colon Transit and Intraluminal Measurements of Motor Activity
As many as 50% of patients with DD have slow colon transit, so slow transit does not preclude the need for anorectal testing. The algorithm recommends assessment of anorectal functions before colonic transit (Figure 3). DDs are treated with pelvic biofeedback regardless of colonic transit, so colonic transit should be assessed, using radiopaque markers, colon scintigraphy, colonic manometry, or the wireless motility capsule, only when indicated after anorectal testing, and after discontinuing medications that can affect colonic transit (Figures 2 and 3). Stool frequency is not, although stool form is, modestly correlated with colonic transit.62 Radiopaque marker studies (Sitz-Mark, Konsyl Pharmaceuticals, Fort Worth, TX) are inexpensive, readily available, easy to perform, and entail modest radiation exposure.97
Figure 3.
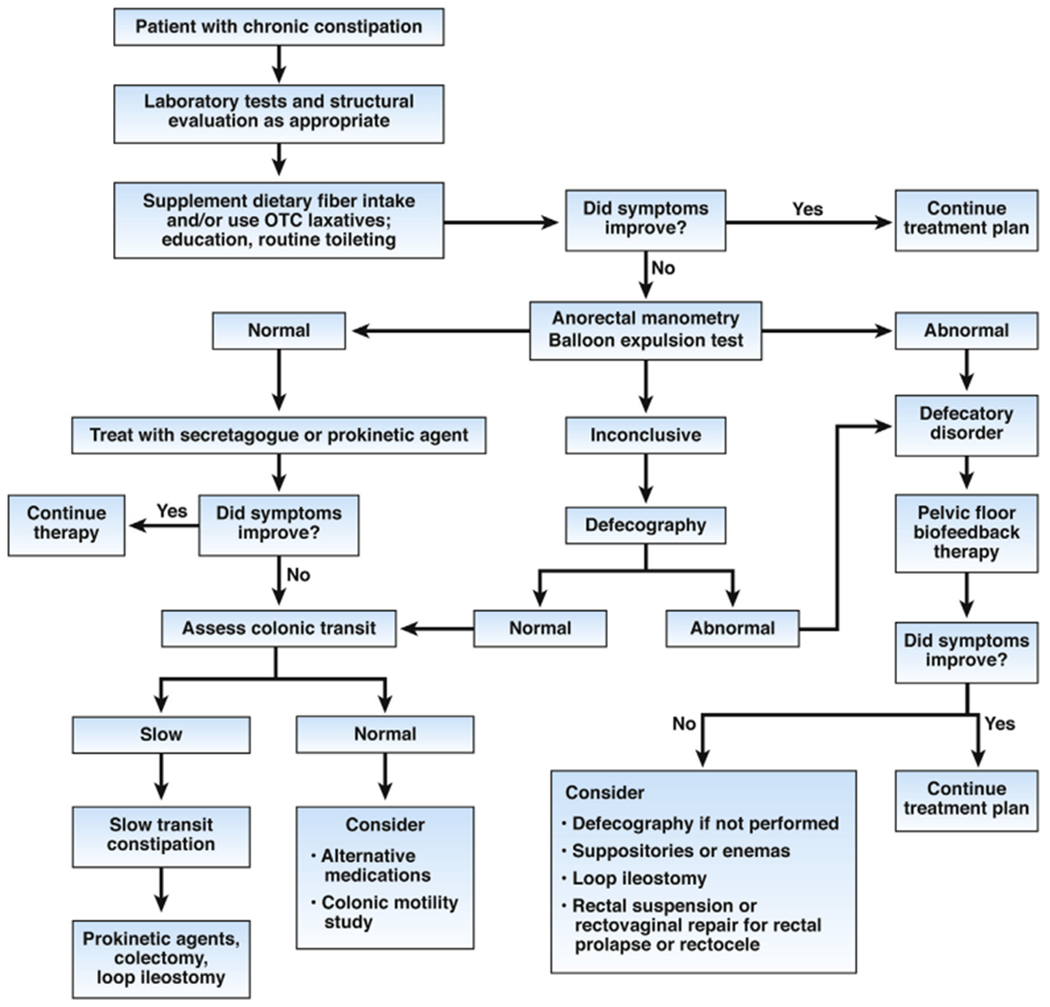
Treatment algorithm for CC.
Of the techniques for assessing colonic transit, the simplest entails checking an abdominal x-ray image at 120 hours after 20 or 24 markers are ingested. Colonic transit is normal if >80% of markers have been passed. However, the actual transit time is not estimated. With the Metcalf method, a plain x-ray image is obtained on day 4 after ingesting 24 markers on each of 3 days; the maximum detectable colonic transit time is 72 hours. The number of markers provides the total colonic transit time; <62 hours is probably normal (personal communication, Michel Bouchoucha). The number of markers in each segment (right, left, and rectosigmoid colon) provides the segmental transit time. In patients with delayed colonic transit, the actual transit time can be more accurately quantified from a single x-ray image on day 7 after ingestion of 12 markers on days 1–6; the maximum detectable colonic transit time is 144 hours.97
Assessments of colonic transit are reproducible in patients with simple constipation,61 but less so in patients with DD or STC.98 Colonic transit should therefore be reevaluated to confirm the diagnosis of STC before surgery. Contrary to older studies, a more recent multicenter study observed that the distribution of markers in the rectosigmoid colon is not associated with DD.99 However, rectal gas volume, measured with computed tomography, is greater in patients with vs without a rectal evacuation disorder.100
Less widely used and more expensive approaches include radionuclide gamma scintigraphy 26,101 or a wireless pH-pressure capsule, which can concurrently measure gastric emptying and small intestinal transit.102,103 Scintigraphy, performed only at a few select academic centers, requires 24 or 48 hours of measurement compared to 5–7 days for radiopaque marker tests (Figure 1).104 Colonic transit measured using radiopaque markers correlates well with scintigraphy 61 and the wireless motility-pH capsule.102 The capsule also measures colonic motor activity.103 Bowel cleansing shortens colonic transit but is unnecessary because it does not affect the characterization of patients as having normal or slow colon transit.105
Although all 3 techniques are equally accurate for measuring colonic transit, only radio-opaque markers and scintigraphy assess regional colonic transit. Of these techniques, the radio-opaque marker method is the simplest and least expensive, and is therefore the best way to measure colonic transit. Scintigraphy or the motility pH capsule should be used when it is also necessary to measure gastric emptying and/or small intestinal transit. If patients can only discontinue medications for a few days, scintigraphy is preferred. The ability to distinguish normal from slow transit can aid in determining the need for colectomy.
Colonic phasic motor activity recorded with manometry varies even in healthy people. Few centers offer to assess colonic tone and phasic pressure activity with a barostat manometry assembly, for which extensive normal values are available.23 For some patients with medically refractory STC, it is useful to evaluate colonic motility with colonic manometry or barostat manometry and document colonic motor dysfunction; findings can support the decision to proceed with subtotal colectomies for patients who do not have pelvic floor dysfunction (Figure 1).21 This approach is more widely used in children than adults with STC.
Putting It All Together
At the conclusion of the initial clinical evaluation, it should be possible to tentatively classify patients with symptoms of constipation as NTC, STC when pelvic floor function is normal, DDs (anismus/dyssynergia-failure of relaxation; or descending perineal syndrome and other flaccid disorders), or a combination of these (see Figure 3). Patients could also have organic constipation (mechanical obstruction or drug side effect; see Table 1 and Supplementary Table 1). The severity of pain can be used to characterize patients as mild pain/painless constipation or painful constipation (see Supplementary Figure 1).
Management
Management options for patients differ in ease of use, availability, cost, mechanism, efficacy, and safety (Table 3). Some, but not all, have been studied in large, randomized, controlled trials. Others, although commonly used, have not been carefully scrutinized (such as cascara). The aim of treatment is to correct bowel disturbances in patients with CC or IBS-C and reduce abdominal pain in patients with IBS-C. A treatment algorithm has been developed, based on factors that include efficacy, availability, ease of use, and cost, but not validated. Each patient requires an individual treatment plan; it is important to remember many patients in gastroenterology clinics have already been failed by over-the-counter agents, so prescription medications might be the logical first choice after DDs are excluded (for specific measures for opioid-induced constipation, see Luthra et al106).
Table 3.
Medical Management of Chronic Constipation
| Treatment and frequency | Dose | Number needed to treat for CC and IBS-C | Cost/mo, US$ (2019) | Comments |
|---|---|---|---|---|
| Bulking agents, psyllium, daily | CC: Variable dose IBS-C: Variable dose |
CC: 2 (95% CI, 1–3)79 IBS-C: 10 (95% CI, 6–33)79 |
8.34 | Start with low dose and increase gradually. |
| PEG, daily | CC: 17 g IBS-C: 17 g |
CC: 3 (95% CI, 2–4)2 IBS-C: NA |
30.90 | More evidence from studies of CC than IBS-C. Improved bowel symptoms but not abdominal pain in patients with IBS-C.80 |
| Lactulose, daily | 20 g | NA | 11.20 | Can produce bloating and distension. |
| Bisacodyl, daily | CC: 10 mg IBS-C: 10 mg |
CC: 4 (95% CI, NA)81 IBS-C: NA |
5.17 | Available as suppository, preferably administered 30 min after breakfast. |
| Senna, daily | 17.2–34.4 mg | — | 5.90 | Widely used anthraquinone laxative |
| Prucalopride, daily | CC: 2 mg IBS-C: NA |
CC: 6 (95% CI, 5–9)2 IBS-C: NA |
395.67 | Approved in the United States; available in Mexico, Canada, and Europe. |
| Linaclotide, daily | CC: 72 μg IBS-C: 290 μg |
CC: 72 μg, 12 (95% CI, 6–29); 145 μg, 10 (95% CI, 6–19)78 IBS-C: 290 μg, 6 (95% CI, 4–16) |
395.41 | Reduces abdominal pain, bloating, and overall symptoms of IBS in patients with IBS-C. |
| Lubiprostone, twice daily | CC: 24 μg IBS-C: 8 μg |
CC: 24 μg, 4 (95% CI, 3–6)79 IBS-C: 8 μg, 12 (95% CI, 8–25) |
342.92 | Also reduces abdominal bloating, discomfort, and severity of constipation in patients with opioid-induced constipation.82 |
| Plecanatide, daily | CC: 3 mg or 6 mg IBS-C: 6 mga |
CC: 3 mg, 11 (95% CI, 8–19) 6 mg, 12 (95% CI, 8–23)78 IBS-C: 3 mg, 9 (95% CI, 6–16) 6 mg, 9 (95% CI, 6–17) |
384.36 | Similar to linaclotide. |
| Tenapanor, twice daily | CC: NA IBS-C: 50 mg |
IBS-C: NA | NA | Only studied in patients with IBS-C and only approved in the United States. Diarrhea is the most frequent side effect. Safety analysis underway in 2 phase 3 trials |
NOTE. Modified from Bharucha and Wald,72 with permission.
CI, confidence interval; NA, not available.
FDA-approved dose for CC and IBS-C is 3 mg daily.
It is important to educate patients about CC and its progression; patients’ questions, concerns, and goals should all be addressed. Caloric intake should be estimated because caloric restriction might cause constipation.107 Patients should be encouraged to consume 25–30 g fiber daily as fresh fruit, fresh vegetables, legumes, and whole grains, because fiber adds bulk to stool and stimulates intestinal transit.108 Although exercise is generally beneficial, its effects on constipation are unclear.109,110 Taking advantage of the gastrocolic reflex, bathroom time should be scheduled after meals. There is no evidence that drinking more water is beneficial for constipation.109
Fiber
Soluble (such as psyllium or ispaghula) but not insoluble dietary fiber (such as wheat bran) supplements improve symptoms in patients with CC108 or IBS-C.111 Of 4 trials of fiber, the largest enrolled 201 patients with CC112 and 3 gave patients psyllium, but only for 4 weeks.108 In a meta-analysis of 17 trials, soluble fiber improved symptoms in IBS-C but had variable effects on abdominal pain.111 In a randomized trial of 275 primary care patients with IBS-C given psyllium (10 g, twice daily) or bran, only psyllium was more effective than placebo after 1 month.113 At 3 months, bran was more effective placebo. More than 60% of patients given bran or psyllium reported adverse effects, primarily constipation or diarrhea. However, 29% and 40% of patients dropped out by 2 and 3 months of follow-up.
The potential therapeutic effects, low cost, safety profile, and other potential health benefits of dietary fiber justify increasing dietary fiber intake, either as a standardized supplement or with foods, as a first step for patients with CC—particularly those in primary care. In contrast to NTC, patients with drug-induced or STC are unlikely to respond to fiber supplementation.114 Patients should be instructed to begin with 5 g fiber daily, divided in 2 doses, to be taken with fluids and/or meals and gradually increase at 1- to 2-week intervals up to 10–15 g daily. Unlike a purgative, the response may take several weeks to be evident. Fiber supplements may increase gaseousness but the symptoms often decrease after several days. Sometimes gaseousness can be reduced by switching to another fiber supplement.
Osmotic and Stimulant Laxatives
Osmotic agents include polyethylene glycol (PEG)-based solutions, magnesium citrate–based products, sodium phosphate–based products, and nonabsorbable carbohydrates. Through osmosis, these hypertonic products extract fluid into the intestinal lumen to soften stools and accelerate colon transit. By contrast, the PEG and electrolyte lavage solution used for colonic cleansing, typically not for CC, is iso-osmotic with plasma; bowel evacuation is by high-volume lavage.
The osmotic laxatives are consistently better than placebo for improving the symptoms of CC.115 The strongest evidence exists for PEG, which has been evaluated in 19 trials, mostly compared to placebo, followed by lactulose and tegaserod.115,116 In the largest study (304 patients treated for 6 months), patients given PEG had more bowel movements per week than patients given placebo.117 One controlled trial with PEG was of 6 months’ duration.115 In a retrospective series study, its efficacy was maintained for up to 24 months, which is longer than the 7-day recommended trial.117,118 Patients prefer PEG preparations without electrolyte supplements,119 which are necessary when a large volume is used.120
Although magnesium hydroxide and other salts are sparingly absorbed and generally safe, these have not been tested in randomized controlled trials. Severe hypermagnesemia may occur in patients with renal impairment.121 Sodium phosphate-based bowel cleansing preparations should be avoided because they are associated with hyperphosphatemia, hypocalcemia, and hypokalemia, and in fewer than 1 in 1000 individuals, with acute phosphate nephropathy.121,122 A Cochrane review of 10 randomized trials concluded that PEG was superior to lactulose for improving stool frequency, stool consistency, and abdominal pain.123 Among nonabsorbable carbohydrates, lactulose and sorbitol had similar laxative effects, but lactulose was associated with more nausea in a randomized crossover study of 30 men124; sorbitol is less sweet than lactulose and accelerates proximal colonic emptying.125,126 Dosing of prescription laxatives varies from patient to patient and from agent to agent. The general goal is to improve predominant symptoms reported by patients. Bacterial metabolism of unabsorbed carbohydrate leads to gas production and abdominal cramping, which can limit long-term use. There have been no large studies of lactitol, mannitol, or sorbitol for treating constipation.
Stimulant Laxatives
Stimulant laxatives, which include diphenylmethane derivatives (bisacodyl and sodium picosulfate) and anthraquinone derivatives (senna, aloe, cascara sagrada), are often used on a rescue basis, such as for patients who have not defecated for 2–3 days. Bisacodyl and sodium picosulfate are converted, respectively, by mucosa deacetylase enzymes and desulfatases of the colonic microflora to the same active metabolite, bis-(p-hydroxyphenyl)-pyridyl-2-methane, which has anti-absorptive secretory effects and induces colonic HAPCs.18,127 The anthraquinones also increase colonic motility, perhaps following epithelial damage, and alter colonic absorption and secretion.128 Two 4-week double-blind, randomized controlled trials demonstrated that sodium picosulfate (10 mg/d) or bisacodyl (10 mg/d) were more effective than placebo in 367 and 247 patients, respectively, with FC.129,130 However, there have been no long-term studies or comparisons with other drugs. Use is often limited by side effects of abdominal pain and diarrhea.
An ionic surfactant, docusate sodium, decreases the surface tension at the stool oil–water interface, allowing water to penetrate the stool. Although it is frequently recommended, there are few data to support its use. In a double-blind, randomized, controlled trial of 170 patients with constipation, docusate sodium (100 mg twice daily) was less effective than psyllium.131
Secretagogues
The US Food and Drug Administration (FDA) and the European Medicines Evaluation Agency approved lubiprostone, linaclotide, and plecanatide for treatment of CC and IBS-C. The US FDA also approved tenapanor for treatment of IBS-C in September 2019. These secretagogues stimulate net efflux of ions and water into the intestinal lumen, likely accelerate transit, and facilitate ease of defecation.
By activating channels on the apical (luminal) enterocyte surface, these secretagogues increase intestinal chloride secretion. Other ion channels and transporters secrete sodium into the intestine to maintain electroneutrality; water secretion follows. Lubiprostone is a bicyclic fatty acid derived from prostaglandin E1 that activates type 2 chloride channels on the apical membrane of epithelial cells. In a 4-week, randomized trial, lubiprostone was more effective than placebo in improving bowel symptoms in patients with CC.132 Nausea, typically mild, was the most common adverse event. Linaclotide and plecanatide are guanylate cyclase C activators that induce fluid secretion into the gastrointestinal tract via an increase in cyclic guanosine monophosphate and subsequent activation of the cystic fibrosis transmembrane regulator. These drugs are similar to endogenous peptides, primarily expressed in the small (uroguanylin) or large (guanylin) intestine, that also activate guanylate cyclase C.133 Fasting and/or postprandial levels of prouroguanylin and uroguanylin are reduced in patients with CC and IBS-C, which might impair fluid and electrolyte secretion to cause constipation. Increased cyclic guanosine monophosphate improved abdominal pain in an animal model of visceral pain.134
In two 12-week, double-blind, randomized controlled trials, comprising 1276 patients with CC, linaclotide (290 μg or 145 μg, once daily) reduced symptoms more effectively than placebo.135 A lower dose (72 μg, once daily) was also more effective than placebo and has been approved by the FDA for treatment of CC.135,136 Plecanatide (3- and 6-mg doses) was more effective than placebo in 2 phase 3 randomized trials comprising 2804 adults with CC; the 3-mg dose has been approved by the FDA.137,138
Diarrhea, the most common adverse event of linaclotide or plecanatide therapy, necessitated discontinuation of treatment in <5% of patients.135–138 Linaclotide and plecanatide are of similar efficacy and tolerability for treatment of CC.139 Tenapanor, a small-molecule inhibitor of the gastrointestinal sodium-hydrogen exchanger-3, was superior to placebo in improving constipation symptoms in a phase 2 study140,141 and in 2 phase 3 trials. In these trials, 12 weeks (610 patients) and 26 weeks (593 patients) of tenapanor (50 mg, twice daily) were more effective than placebo in patients with IBS-C.142
Prokinetic and Other Agents
Prucalopride, a dihyrodrobenzofurancarboxamide derivative, is a selective agonist of serotonin 5-HT4 receptors, which are found throughout the gastrointestinal tract, including the colon.143 Multicenter, double-blind, randomized trials of prucalopride in patients with CC differed in sample size, study duration (4–24 weeks), and dose (1 mg, 2 mg, or 4 mg vs placebo).143 In 6 of 7 studies, prucalopride was more effective than placebo. The only 24-week study did not find a statistically significant difference between effects of prucalopride and placebo.144 The most frequent, generally transient, side effects were headache, nausea, and diarrhea. There were no significant cardiovascular side effects. The European Medicines Agency and the FDA have approved prucalopride for treatment of CC. Based on 3 pivotal studies in 3199 patients, the 5-HT4 agonist tegaserod was approved for treatment of IBS-C and then voluntarily withdrawn from the market in 2007 after a greater number of cardiovascular events were observed in a database of 18,000 patients given tegaserod (n = 13; 0. 11%) or placebo (n = 1; 0.01%).145 In April 2019, the FDA reviewed extensive supplementary data and approved the use of tegaserod in women younger than 65 years of age who do not have a history of ischemic cardiovascular disease and who have no more than 1 risk factor for cardiovascular disease.
A systematic review found that probiotics (Bifidobacterium lactis DN173010, Lactobacillus casei Shirota, Lactobacillus casei YIT) can increase mean stool frequency.146 However, the assessment was limited by a high risk of bias in these studies. Prunes (approximately 6 twice daily) might reduce symptoms of CC via their combination of fiber and fructose.147 Hemp seed extract might also be effective in some patients.148
Comparison of Agents
In the absence of rigorous, direct comparisons of drugs,117,149 the relative efficacy of these agents can be assessed by comparing the number needed to treat among trials or through network meta-analyses of CC 150,151 and IBS-C.141 Although these comparisons are useful, they are limited by differences in patient populations, definitions of end points and adverse events, and durations of treatment. Nonetheless, lubiprostone, linaclotide, and prucalopride are all more effective than placebo and of comparable efficacy, with estimated numbers needed to treat of 4, 6, and 6, respectively, based on end points of complete spontaneous bowel movements and increases in complete spontaneous bowel movements compared with baseline.150
Plecanatide had not been rigorously evaluated when this analysis was performed. More recently, a network meta-analysis by Luthra et al151 found that stimulant diphenyl methane laxatives were most effective at 4 weeks, although prucalopride (2 and 4 mg) and linaclotide (290 μg) had similar efficacies at 12 weeks. There have been no direct comparisons of the secretagogues or prucalopride vs over-the-counter laxatives. Little is known about the incremental effects, if any, of these agents vs more inexpensive laxatives.
Management of Defecatory Disorders
DDs should be managed with biofeedback-aided pelvic floor retraining.152 Through visual or auditory feedback of anorectal and pelvic floor muscle activity, which is recorded with surface electromyographic sensors or manometry, patients learn to increase intra-abdominal pressure and relax the pelvic floor muscles during defecation. Subsequently, patients practice by expelling an air-filled balloon. Sensory retraining, in which patients learn to recognize weaker sensations of rectal filling, may also be provided to patients with reduced rectal sensation. Although therapy may also include Kegel exercises, the emphasis is on appropriately coordinating abdominal and pelvic floor motion during evacuation.
Regrettably, biofeedback therapy is underused, perhaps because its benefits are not widely recognized and the expertise is not widely available. Contrary to findings from a previous study,153 pelvic floor retraining was found to be more effective for DD than in isolated STC.53 Colonic transit normalized after biofeedback therapy in 65% of patients with DD, but in only 8% of patients with STC, indicating that delayed colonic transit could be secondary to pelvic floor dysfunction.53 In controlled studies that provided 5–6 training sessions lasting 30–60 minutes at 2-week intervals, biofeedback therapy was more effective than PEG,95 sham feedback,154 or diazepam.96 Alternatively, daily sessions can be provided. The skill and experience of therapists and patients’ motivation affect responses to biofeedback therapy. Dietitians and behavioral psychologists should also be involved, as necessary. Third-party coverage for biofeedback therapy in DD has improved but is suboptimal.
Management of Chronic Constipation Refractive to Medical Therapy
Refractory symptoms, practically defined by a 4-week trial of pharmacologic therapy to each drug or a 3-month trial of pelvic floor behavioral therapy, are not uncommon.155 A systematic review of colectomy for CC found that most studies were of ileorectal anastomosis.156 Although the studies were limited by inconsistent reporting and heterogeneity, an average of 86% of patients benefited at 1 year or more after surgery. When the Cleveland Clinic Constipation scoring system was used, patients’ scores decreased from a preoperative mean of 20 points or more, indicating severe constipation, to a low to normal range of 2–3. However, 18.2% of patients had persistent constipation after a colectomy. Others had diarrhea and fecal incontinence (5%–15% of patients), abdominal pain (30%–50% of patients), or bloating (10%–40%). When abdominal pain and/or bloating are predominant symptoms, the decision to perform a colectomy should be made carefully. If a colectomy is to be performed, it might be preceded by temporary loop ileostomy to determine whether symptoms emanate from the small intestine or colon. Outcomes after colectomy are poorer in patients with generalized intestinal, rather than isolated colonic, dysmotility and in some but not all patients with DD.
Patients with DD should therefore undergo biofeedback therapy before colectomy. Guided by regional measurements of colonic transit, segmental resection (such as left hemicolectomy) has been proposed but does not perform better than an ileorectal anastomosis. Approximately 20%–30% of patients have perioperative complications, primarily ileus and early or late small bowel obstruction. In some studies, these complications were less frequent after laparoscopic vs open surgery.
Therapeutic options for patients with DD refractory to biofeedback therapy are limited. Although enemas or suppositories are frequently used by patients, there have been no controlled studies of their utility or safety in patients with DD. The adrenergic α1 receptor antagonist alfuzosin, which probably relaxes the internal anal sphincter, reduced anal pressures in healthy people and patients with DD, but did not improve symptoms of DD.55,157 Sacral nerve stimulation increases pancolonic antegrade propagating sequence frequency in patients with STC.158 Contrary to findings from uncontrolled studies,159 findings from more rigorous studies160–162 and from a double-blind study of 45 patients with constipation are less encouraging.163 Sacral nerve stimulation is therefore not recommended for treating refractory constipation. Poor-quality studies found that rectal suspension procedures, a form of rectopexy, might improve symptoms and also be accompanied by healing of prolapse-associated solitary rectal ulcer syndrome in patients with refractory constipation and rectal intussusception.164 A systematic review of rectovaginal reinforcement procedures in patients with constipation and a large rectocele found limited evidence to support the efficacy of these procedures; most evidence was derived from observational studies and comparisons.165
Percutaneous endoscopic cecostomy is used for colonic irrigation in patients with sigmoid volvulus, chronic intestinal pseudo-obstruction, or CC.155 Performed under local anesthesia and conscious sedation, it is a less-invasive alternative to surgery. Among 12 reports, the procedure was successful in 122 of 127 patients with medically refractory constipation, of which 56% had procedure-related complications (chronic pain [15%], granulation tissue at stoma site [12%], and local-site infections [10%]); only 1 patient developed peritonitis. Assessments of efficacy, albeit suboptimal, found symptoms to improve in >50% of patients. Further studies are necessary before this procedure can be recommended.
During transanal irrigation, a device is used to lavage the colon with water (volumes of 70–1000 mL). In 7 uncontrolled, mostly retrospective reports of transanal irrigation (comprising 254 patients with constipation), 30%–65% of patients reported a positive outcome.166 A separate retrospective study analyzed outcome questionnaires given to 102 of 148 consecutive patients who used transanal irrigation for an average duration of 60.5 weeks.167 Overall satisfaction was increased in 67% of patients; 39% reported being moderately better and 28% reported being very much better. Prospective controlled studies are underway168 to evaluate different methods of irrigation and the cost-effectiveness of transanal irrigation.
Summary and Future Directions
An algorithm is useful for managing CC. Identifying the predominant symptom and a meticulous DRE are essential. Aside from a complete blood count, no tests are necessary in the absence of alarm symptoms; age-appropriate colonic structural evaluations should be performed. If feasible, medications that can cause constipation, especially opioids, should be discontinued. Thereafter, an empiric trial of fiber with osmotic and/or stimulant laxatives is advisable. Many patients obtain symptom relief with these measures, which are safe for long-term use. For patients who do not respond, anorectal testing is necessary; biofeedback therapy is the cornerstone for managing DD. Assessment of colonic transit will identify STC, which should be treated initially with laxatives and, if necessary, with secretagogues or prokinetic agents. Patients who do not respond to these treatments should be considered for surgery or other less-invasive options, although few will qualify after careful physiologic evaluation. Psychological conditions should be considered as contributing factors. Future trials of patients with IBS-C or FC should assess for and exclude patients with DD, compare newer therapeutic agents with over-the-counter laxatives, and evaluate the clinical effectiveness and cost-effectiveness of the management algorithm presented.
Supplementary Material
Acknowledgments
Author contributions: Drs Bharucha and Lacy jointly drafted the manuscript and approved the final version. 2019 Mayo Foundation for Medical Education and Research.
Funding
This work was supported in part by US Public Health Service grant R01 DK78924.
Abbreviations used in this paper:
- BET
balloon expulsion test
- CC
chronic constipation
- DD
defecatory disorder
- DRE
digital rectal examination
- FC
functional constipation
- FDA
US Food and Drug Administration
- HAPC
high-amplitude propagated contraction
- HRM
high-resolution anorectal manometry
- IBS
irritable bowel syndrome
- IBS-C
constipation-predominant irritable bowel syndrome
- MR
magnetic resonance
- NTC
normal transit constipation
- PEG
polyethylene glycol
- STC
slow transit constipation
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dxdoi.org/10.1053/j.gastro.2019.12.034.
Conflicts of interest
The authors disclose the following: Adil E. Bharucha: Intellectual property and royalties (Medspira, Medtronic). Brian Lacy: Scientific advisory boards (Salix, Ironwood, Takeda), consultant (Viver).
References
- 1.Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol 2011;25:3–18. [DOI] [PubMed] [Google Scholar]
- 2.Lacy BE, Levenick JM. Epidemiology of constipation In: Talley NJ, Locke GR, Moayyedi P, et al. , eds. Gl Epidemiology: Diseases and Clinical Methodology. Hoboken, NJ: Blackwell, 2014:235–248. [Google Scholar]
- 3.Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 2019; 156:254–272.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Pemberton JH, Locke GR 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology 2013;144:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016;18:18. [DOI] [PubMed] [Google Scholar]
- 6.Locke GR 3rd, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterology 2000; 119:1766–1778. [DOI] [PubMed] [Google Scholar]
- 7.Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol 2001;96:3130–3137. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead WE, Palsson OS, Simren M. Biomarkers to distinguish functional constipation from irritable bowel syndrome with constipation. Neurogastroenterol Motil 2016;28:783–792. [DOI] [PubMed] [Google Scholar]
- 9.Bharucha AE, Sharma M. Painful and painless constipation: all roads lead to (a change in) Rome. Dig Dis Sci 2018;63:1671–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchoucha M, Devroede G, Mary F, et al. Painful or mild-pain constipation? A clinically useful alternative to classification as irritable bowel syndrome with constipation versus functional constipation. Dig Dis Sci 2018;63:1763–1773. [DOI] [PubMed] [Google Scholar]
- 11.Wong RK, Palsson OS, Turner MJ, et al. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am J Gastroenterol 2010;105:2228–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharucha AE, Camilleri M. Physiology of the colon and its measurement In: Yeo CJ, DeMeester SR, McFadden DW, et al. , eds. Shackelford’s Surgery of the Alimentary Tract. Volume 2, The Colon. 8th ed. Philadelphia: Elsevier, 2018:1728–1739. [Google Scholar]
- 13.Corsetti M, Costa M, Bassotti G, et al. First “translational” consensus on terminology and definition of colonic motility as studied in humans and animals by means of manometric and non-manometric techniques. Nat Clin Pract Gastroenterol Hepatol 2019;16:559–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palit S, Thin N, Knowles CH, et al. Diagnostic disagreement between tests of evacuatory function: a prospective study of 100 constipated patients. Neurogastroenterol Motil 2016;28:1589–1598. [DOI] [PubMed] [Google Scholar]
- 15.Dinning PG, Wiklendt L, Gibbins I, et al. Low-resolution colonic manometry leads to a gross misinterpretation of the frequency and polarity of propagating sequences: initial results from fiber-optic high-resolution manometry studies. Neurogastroenterol Motil 2013;25:e640–e649. [DOI] [PubMed] [Google Scholar]
- 16.Narducci F, Bassotti G, Granata MT, et al. Colonic motility and gastric emptying in patients with irritable bowel syndrome. Effect of pretreatment with octylonium bromide. Dig Dis Sci 1986;31:241–246. [DOI] [PubMed] [Google Scholar]
- 17.Scott SM. Manometric techniques for the evaluation of colonic motor activity: current status. Neurogastroenterol Motil 2003;15:483–513. [DOI] [PubMed] [Google Scholar]
- 18.Bharucha AE. High amplitude propagated contractions. Neurogastroenterol Motil 2012;24:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He CL, Burgart L, Wang L, et al. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology 2000;118:14–21. [DOI] [PubMed] [Google Scholar]
- 20.Farrugia G Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil 2008;20(Suppl 1):54–63. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Bharucha AE, di Lorenzo C, et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil 2008;20:1269–1282. [DOI] [PubMed] [Google Scholar]
- 22.Dinning PG, Smith TK, Scott SM. Pathophysiology of colonic causes of chronic constipation. Neurogastroenterol Motil 2009;21(Suppl 2):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravi K, Bharucha AE, Camilleri M, et al. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology 2010;138:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassotti G If it is inert, why does it move? Neurogastroenterol Motil 2004;16:395–396. [DOI] [PubMed] [Google Scholar]
- 25.Herve S, Savoye G, Behbahani A, et al. Results of 24-h manometric recording of colonic motor activity with endoluminal instillation of bisacodyl in patients with severe chronic slow transit constipation. [see comment]. Neurogastroenterol Motil 2004;16:397–402. [DOI] [PubMed] [Google Scholar]
- 26.Manabe N, Wong BS, Camilleri M, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil 2010;22:293.e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadley SJ, Walsh BT. Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Curr Drug Targets CNS Neurol Disord 2003;2:1–9. [DOI] [PubMed] [Google Scholar]
- 28.Mertz H, Naliboff B, Mayer E. Physiology of refractory chronic constipation. Am J Gastroenterol 1999;94:609–615. [DOI] [PubMed] [Google Scholar]
- 29.Lanfranchi GA, Bazzocchi G, Brignola C, et al. Different patterns of intestinal transit time and anorectal motility in painful and painless chronic constipation. Gut 1984; 25:1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posserud I, Syrous A, Lindstrom L, et al. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology 2007;133:1113–1123. [DOI] [PubMed] [Google Scholar]
- 31.Mertz H, Naliboff B, Mayer EA. Symptoms and physiology in severe chronic constipation. Am J Gastroenterol 1999;94:131–138. [DOI] [PubMed] [Google Scholar]
- 32.Cao H, Liu X, An Y, et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep 2017; 7:10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge X, Zhao W, Ding C, et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci Rep 2017; 7:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parthasarathy G, Chen J, Chen X, et al. Relationship between colonic vs fecal microbiota and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 2016; 150:367–379.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao SS, Bharucha AE, Chiarioni G, et al. Functional anorectal disorders. Gastroenterology 2016;25:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preston DM, Lennard-Jones JE. Anismus in chronic constipation. Dig Dis Sci 1985;30:413–418. [DOI] [PubMed] [Google Scholar]
- 37.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol 1998;93:1042–1050. [DOI] [PubMed] [Google Scholar]
- 38.Rao SS, Mudipalli RS, Stessman M, et al. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (anismus). Neurogastroenterol Motil 2004;16:589–596. [DOI] [PubMed] [Google Scholar]
- 39.Palit S, Lunniss PJ, Scott SM. The physiology of human defecation. Dig Dis Sci 2012;57:1445–1464. [DOI] [PubMed] [Google Scholar]
- 40.Bharucha AE, Fletcher JG, Seide B, et al. Phenotypic variation in functional disorders of defecation. Gastroenterology 2005;128:1199–1210. [DOI] [PubMed] [Google Scholar]
- 41.Gladman MA, Lunniss PJ, Scott SM, et al. Rectal hyposensitivity. Am J Gastroenterol 2006;101:1140–1151. [DOI] [PubMed] [Google Scholar]
- 42.Burgell RE, Lelic D, Carrington EV, et al. Assessment of rectal afferent neuronal function and brain activity in patients with constipation and rectal hyposensitivity. Neurogastroenterol Motil 2013;25:260–267; e167–e168. [DOI] [PubMed] [Google Scholar]
- 43.Palit S, Bhan C, Lunniss PJ, et al. Evacuation proctography: a reappraisal of normal variability. Colorectal Dis 2014;16:538–546. [DOI] [PubMed] [Google Scholar]
- 44.Prichard DO, Lee T, Parthasarathy G, et al. High-resolution anorectal manometry for identifying defecatory disorders and rectal structural abnormalities in women. Clin Gastroenterol Hepatol 2017;15:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henry MM, Parks AG, Swash M. The pelvic floor musculature in the descending perineum syndrome. Br J Surg 1982;69:470–472. [DOI] [PubMed] [Google Scholar]
- 46.Preston DM, Lennard-Jones JE. Severe chronic constipation of young women: "idiopathic slow transit constipation.". Gut 1986;27:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law NM, Bharucha AE, Zinsmeister AR. Rectal and colonic distension elicit viscerovisceral reflexes in humans. Am J Physiol Gastrointest Liver Physiol 2002; 283:G384–G389. [DOI] [PubMed] [Google Scholar]
- 48.Schweiger M, Alexander-Williams J. Solitary-ulcer syndrome of the rectum: its association with occult rectal prolapse. Lancet 1977;309:170–171. [DOI] [PubMed] [Google Scholar]
- 49.Parks AG, Swash M, Urich H. Sphincter denervation in anorectal incontinence and rectal prolapse. Gut 1977; 18:656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartram CI, Turnbull GK, Lennard-Jones JE. Evacuation proctography: an investigation of rectal expulsion in 20 subjects without defecatory disturbance. Gastrointest Radiol 1988;13:72–80. [DOI] [PubMed] [Google Scholar]
- 51.Chiarioni G, Nardo A, Vantini I, et al. Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. Gastroenterology 2010;138:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oblizajek NR, Gandhi S, Sharma M, et al. Anorectal pressures measured with high-resolution manometry in healthy people—normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterol Motil 2019;0: e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129:86–97. [DOI] [PubMed] [Google Scholar]
- 54.Bharucha AE, Seide BM, Zinsmeister AR, et al. Insights into normal and disordered bowel habits from bowel diaries. Am J Gastroenterol 2008;103:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakraborty S, Feuerhak K, Muthyala A, et al. Effects of alfuzosin, an a1 adrenergic antagonist, on anal pressures and bowel habits, in women with and without defecatory disorders. Clin Gastroenterol Hepatol 2019;17:1138–1147.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitehead WE, di Lorenzo C, Leroi AM, et al. Conservative and behavioural management of constipation. Neurogastroenterol Motil 2009;21(Suppl 2):55–61. [DOI] [PubMed] [Google Scholar]
- 57.van Ginkel R, Reitsma JB, Buller HA, et al. Childhood constipation: longitudinal follow-up beyond puberty. Gastroenterology 2003;125:357–363. [DOI] [PubMed] [Google Scholar]
- 58.Klingele CJ, Bharucha AE, Fletcher JG, et al. Pelvic organ prolapse in defecatory disorders. Obstet Gynecol 2005;106:315–320. [DOI] [PubMed] [Google Scholar]
- 59.Wald A, Scarpignato C, Kamm MA, et al. The burden of constipation on quality of life: results of a multinational survey. Aliment Pharmacol Ther 2007;26:227–236. [DOI] [PubMed] [Google Scholar]
- 60.Barnett JL, Hasler WL, Camilleri M. American Gastroenterological Association Medical position statement on anorectal testing techniques. Gastroenterology 1999; 116:732–760. [DOI] [PubMed] [Google Scholar]
- 61.Degen LP, Phillips SF. How well does stool form reflect colonic transit? Gut 1996;39:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saad RJ, Rao SSC, Koch KL, et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol 2010; 105:403–411. [DOI] [PubMed] [Google Scholar]
- 63.Locke GR 3rd, Zinsmeister AR, Fett SL, et al. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil 2005;17:29–34. [DOI] [PubMed] [Google Scholar]
- 64.Bharucha AE, Chakraborty S, Sletten CD. Common functional gastroenterological disorders associated with abdominal pain. Mayo Clin Proc 2016;91:1118–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cauley CE, Savitt LR, Weinstein M, et al. A quality-of-life comparison of two fecal incontinence phenotypes: isolated fecal incontinence versus concurrent fecal incontinence with constipation. Dis Colon Rectum 2019; 62:63–70. [DOI] [PubMed] [Google Scholar]
- 66.Skoog SM, Bharucha AE. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol 2004; 99:2046–2050. [DOI] [PubMed] [Google Scholar]
- 67.Noelting J, Eaton JE, Choung RS, et al. The incidence rate and characteristics of clinically diagnosed defecatory disorders in the community. Neurogastroenterol Motil 2016;28:1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adelstein B-A, Macaskill P, Chan SF, et al. Most bowel cancer symptoms do not indicate colorectal cancer and polyps: a systematic review. BMC Gastroenterol 2011; 11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ratuapli SK, Bharucha AE, Noelting J, et al. Phenotypic identification and classification of functional defecatory disorders using high resolution anorectal manometry. Gastroenterology 2013;144:314–322.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tantiphlachiva K, Rao P, Attaluri A, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol 2010; 8:955–960. [DOI] [PubMed] [Google Scholar]
- 71.Wong RK, Drossman DA, Bharucha AE, et al. The digital rectal examination: a multicenter survey of physicians’ and students’ perceptions and practice patterns. Am J Gastroenterol 2012;107:1157–1163. [DOI] [PubMed] [Google Scholar]
- 72.Bharucha AE, Wald A. Chronic constipation. Mayo Clin Proc 2019;28:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wald A Constipation: advances in diagnosis and treatment. JAMA 2016;315:185–191. [DOI] [PubMed] [Google Scholar]
- 74.Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guidelines: management of benign anorectal disorders. Am J Gastroenterol 2014;109:1141–1157. [DOI] [PubMed] [Google Scholar]
- 75.Rangan V, Patel R, Nee J, et al. Medication utilization and satisfaction in irritable bowel syndrome: insight from the IBS in America Survey. Gastroenterology 2019; 156:S189–S190. [Google Scholar]
- 76.Chiarioni G, Kim SM, Vantini I, et al. Validation ofthe balloon evacuation test: reproducibility and agreement with findings from anorectal manometry and electromyography. Clin Gastroenterol Hepatol 2014;12:2049–2054. [DOI] [PubMed] [Google Scholar]
- 77.Rao SS, Hatfield R, Softer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol 1999;94:773–783. [DOI] [PubMed] [Google Scholar]
- 78.Ratuapli S, Bharucha AE, Harvey D, et al. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterol Moti 2013;25:e813–e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazor Y, Prott G, Jones M, et al. Anorectal physiology in health: a randomized trial to determine the optimum catheter for the balloon expulsion test. Neurogastroenterol Motil 2019;31:e13552. [DOI] [PubMed] [Google Scholar]
- 80.Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology 2004;126:57–62. [DOI] [PubMed] [Google Scholar]
- 81.Bharucha AE, Stroetz R, Feuerhak K, et al. A novel technique for bedside anorectal manometry in humans. Neurogastroenterol Motil 2015:1504–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee TH, Bharucha AE. How to perform and interpret a high-resolution anorectal manometry test. J Neurogastroenterol Motil 2016;22:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basilisco G, Bharucha AE. High-resolution anorectal manometry: an expensive hobby or worth every penny? Neurogastroenterol Motil 2017;29(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gosling J, Plumb A, Taylor SA, et al. High-resolution anal manometry: repeatability, validation, and comparison with conventional manometry. Neurogastroenterol Motil 2019;31:e13591. [DOI] [PubMed] [Google Scholar]
- 85.Grossi U, Carrington EV, Bharucha AE, et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut 2016; 65:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu GJ, Xu F, Lin L, et al. Anorectal manometry: should it be performed in a seated position? Neurogastroenterol Motil 2017;29(5). [DOI] [PubMed] [Google Scholar]
- 87.Shorvon PJ, McHugh S, Diamant NE, et al. Defecography in normal volunteers: results and implications. Gut 1989;30:1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diamant NE, Kamm MA, Wald A, et al. American Gastroenterological Association medical position statement on anorectal testing techniques. Gastroenterology 1999;116:732–760. [DOI] [PubMed] [Google Scholar]
- 89.Bharucha AE, Wald A, Enck P, et al. Functional anorectal disorders. Gastroenterology 2006;130:1510–1518. [DOI] [PubMed] [Google Scholar]
- 90.Bharucha AE. Update of tests of colon and rectal structure and function. J Clin Gastroenterol 2006; 40:96–103. [DOI] [PubMed] [Google Scholar]
- 91.Agachan F, Pfeifer J, Wexner SD. Defecography and proctography. Results of 744 patients. Dis Colon Rectum 1996;39:899–905. [DOI] [PubMed] [Google Scholar]
- 92.Noelting J, Bharucha AE, Lake DS, et al. Semi-automated vectorial analysis of anorectal motion by magnetic resonance defecography in healthy subjects and fecal incontinence. Neurogastroenterol Motil 2012; 24:e467–e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reiner CS, Tutuian R, Solopova AE, et al. MR defecography in patients with dyssynergic defecation: spectrum of imaging findings and diagnostic value. Br J Radiol 2011;84:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tirumanisetty P, Prichard D, Fletcher JG, et al. Normal values for assessment of anal sphincter morphology, anorectal motion, and pelvic organ prolapse with MRI in healthy women. Neurogastroenterol Motil 2018; 30:e13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006; 130:657–664. [DOI] [PubMed] [Google Scholar]
- 96.Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum 2007; 50:428–441. [DOI] [PubMed] [Google Scholar]
- 97.Bharucha AE, Anderson B, Bouchoucha M. More movement with evaluating colonic transit in humans. Neurogastroenterol Motil 2019;31:e13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nam YS, Pikarsky AJ, Wexner SD, et al. Reproducibility of colonic transit study in patients with chronic constipation. Dis Colon Rectum 2001;44:86–92. [DOI] [PubMed] [Google Scholar]
- 99.Staller K, Barshop K, Ananthakrishnan AN, et al. Rectosigmoid localization of radiopaque markers does not correlate with prolonged balloon expulsion in chronic constipation: results from a multicenter cohort. Am J Gastroenterol 2015;110:1049–1055. [DOI] [PubMed] [Google Scholar]
- 100.Park SY, Khemani D, Nelson AD, et al. Rectal gas volume measured by computerized tomography identifies evacuation disorders in patients with constipation. Clin Gastroenterol Hepatol 2017;15:543–552.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burton DD, Camilleri M, Mullan BP, et al. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med 1997;38:1807–1810. [PubMed] [Google Scholar]
- 102.Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole gut transit with wireless motility capsule and radioopaque markers in constipation. Clin Gastroenterol Hepatol 2009;7:537–544. [DOI] [PubMed] [Google Scholar]
- 103.Hasler WL, Saad RJ, Rao SS, et al. Heightened colon motor activity measured by a wireless capsule in patients with constipation: relation to colon transit and IBS. Am J Physiol Gastrointest Liver Physiol 2009; 297:G1107–G1114. [DOI] [PubMed] [Google Scholar]
- 104.Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology 1987;92:40–47. [DOI] [PubMed] [Google Scholar]
- 105.Sloots CEJ, Felt-Bersma RJF. Effect of bowel cleansing on colonic transit in constipation due to slow transit or evacuation disorder. Neurogastroenterol Motil 2002; 14:55–61. [DOI] [PubMed] [Google Scholar]
- 106.Luthra P, Burr NE, Brenner DM, et al. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and network meta-analysis. Gut 2018;5(5). [DOI] [PubMed] [Google Scholar]
- 107.Chun AB, Sokol MS, Kaye WH, et al. Colonic and anorectal function in constipated patients with anorexia nervosa. Am J Gastroenterol 1997;92:1879–1883. [PubMed] [Google Scholar]
- 108.Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacolo Ther 2011;33:895–901. [DOI] [PubMed] [Google Scholar]
- 109.Muller-Lissner SA, Kamm MA, Scarpignato C, et al. Myths and misconceptions about chronic constipation. Am J Gastroenterol 2005;100:232–242. [DOI] [PubMed] [Google Scholar]
- 110.Tantawy SA, Kamel DM, Abdelbasset WK, et al. Effects of a proposed physical activity and diet control to manage constipation in middle-aged obese women. Diabetes Metab Syndr Obes 2017;10:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bijkerk CJ, Muris JWM, Knottnerus JA, et al. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004;19:245–251. [DOI] [PubMed] [Google Scholar]
- 112.Fenn GC, Wilkinson PD, Lee CE, et al. A general practice study of the efficacy of Regulan in functional constipation. Br J Clin Pract 1986;40:192–197. [PubMed] [Google Scholar]
- 113.Bijkerk CJ, de Wit NJ, Muris JWM, et al. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009; 339:b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Voderholzer WA, Schatke W, Muhldorfer BE, et al. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol 1997;92:95–98. [PubMed] [Google Scholar]
- 115.Ford A, Suares N. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut 2011;60:209–218. [DOI] [PubMed] [Google Scholar]
- 116.Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol 2014;28:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.DiPalma JA, Cleveland MV, McGowan J, et al. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol 2007; 102:1436–1441. [DOI] [PubMed] [Google Scholar]
- 118.Migeon-Duballet I, Chabin M, Gautier A, et al. Long-term efficacy and cost-effectiveness of polyethylene glycol 3350 plus electrolytes in chronic constipation: a retrospective study in a disabled population. Curr Med Res Opin 2006;22:1227–1235. [DOI] [PubMed] [Google Scholar]
- 119.Szojda MM, Mulder CJJ, Felt-Bersma RJF. Differences in taste between two polyethylene glycol preparations. J Gastrointest Liver Dis 2007;16:379–381. [PubMed] [Google Scholar]
- 120.DiPalma JA, Smith JR, Cleveland Mv. Overnight efficacy of polyethylene glycol laxative. Am J Gastroenterol 2002; 97:1776–1779. [DOI] [PubMed] [Google Scholar]
- 121.Nyberg C, Hendel J, Nielsen OH. The safety of osmotically acting cathartics in colonic cleansing. Nat Rev Gastroenterol Hepatol 2010;7:557–564. [DOI] [PubMed] [Google Scholar]
- 122.Ainley EJ, Winwood PJ, Begley JP. Measurement of serum electrolytes and phosphate after sodium phosphate colonoscopy bowel preparation: an evaluation. Dig Dis Sci 2005;50:1319–1323. [DOI] [PubMed] [Google Scholar]
- 123.Lee-Robichaud H, Thomas K, Morgan J, et al. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst Rev 2010;7:CD007570. [DOI] [PubMed] [Google Scholar]
- 124.Lederle FA, Busch DL, Mattox KM, et al. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med 1990;89:597–601. [DOI] [PubMed] [Google Scholar]
- 125.Wesselius-De Casparis A, Braadbaart S, Bergh-Bohlken GE, et al. Treatment of chronic constipation with lactulose syrup: results of a double-blind study. Gut 1968;9:84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Skoog SM, Bharucha AE, Camilleri M, et al. Effects of an osmotically active agent on colonic transit. Neurogastroenterol Motil 2006;18:300–306. [DOI] [PubMed] [Google Scholar]
- 127.Ewe K, Holker B. [The effect of a diphenolic laxative (Bisacodyl) on water- and electrolyte transport in the human colon (author’s transl)]. Klin Wochenschr 1974; 52:827–833. [DOI] [PubMed] [Google Scholar]
- 128.van Gorkom BA, de Vries EG, Karrenbeld A, et al. Review article: anthranoid laxatives and their potential carcinogenic effects. Aliment Pharmacol Ther 1999;13:443–452. [DOI] [PubMed] [Google Scholar]
- 129.Mueller-Lissner S, Kamm MA, Wald A, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am J Gastroenterol 2010;105:897–903. [DOI] [PubMed] [Google Scholar]
- 130.Kamm MA, Mueller-Lissner S, Wald A, et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol 2011; 9:577–583. [DOI] [PubMed] [Google Scholar]
- 131.McRorie JW, Daggy BP, Morel JG, et al. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther 1998;12:491–497. [DOI] [PubMed] [Google Scholar]
- 132.Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, doseranging study to evaluate efficacy and safety.[see comment]. Aliment Pharmacol Ther 2007;25:1351–1361. [DOI] [PubMed] [Google Scholar]
- 133.Waldman SA, Tenenbaum R, Foehl HC, et al. Blunted evoked prouroguanylin endocrine secretion in chronic constipation. Clin Transl Gastroenterol 2019;10:e00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Castro J, Harrington AM, Hughes PA, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3’,5’-monophosphate. Gastroenterology 2013; 145:1334–1346; e1–e11. [DOI] [PubMed] [Google Scholar]
- 135.Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med 2011;365:527–536. [DOI] [PubMed] [Google Scholar]
- 136.Schoenfeld P, Lacy BE, Chey WD, et al. Low-dose linaclotide (72 μg) for chronic idiopathic constipation: a 12-week, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol 2018;113:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.DeMicco M, Barrow L, Hickey B, et al. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Therap Adv Gastroenterol 2017;10:837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miner PB Jr, Koltun WD, Wiener GJ, et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol 2017;112:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-C agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol 2018;113:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chey WD, Lembo AJ, Rosenbaum DP. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol 2017;112:763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Black CJ, Burr NE, Quigley EMM, et al. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology 2018;155:1753–1763. [DOI] [PubMed] [Google Scholar]
- 142.Ardelyx Receives FDA Approval for IBSRELA® (Tenapanor), an NHE3 sodium transport inhibitor, for the treatment of irritable bowel syndrome with constipation. Available at: https://www.prnewswire.com/news-releases/ardelyx-receives-fda-approval-for-ibsrela-tenapanor-an-nhe3-sodium-transport-inhibitor-for-the-treatment-of-irritable-bowel-syndrome-with-constipation-300917407.html Published September 12, 2019 Accessed February 23, 2020. [Google Scholar]
- 143.Omer A, Quigley EMM. An update on prucalopride in the treatment of chronic constipation. Therap Adv Gastroenterol 2017;10:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Piessevaux H, Corazziari E, Rey E, et al. A randomized, double-blind, placebo-controlled trial to evaluate the efficacy, safety, and tolerability of long-term treatment with prucalopride. Neurogastroenterol Motil 2015; 27:805–815. [DOI] [PubMed] [Google Scholar]
- 145.US Food and Drug Administration, Sloan Pharma. ZELNORM™ (tegaserod maleate): For the treatment of Irritable Bowel Syndrome with Constipation (IBS-C). FDA Joint Meeting of the Gastrointestinal Drugs Advisory Committee and Drug Safety and Risk Management Advisory Committee Briefing Document. Sloan Pharma, US WorldMeds. Available at:https://www.fda.gov/media/119013/download. Published October 17, 2018 Accessed February 23, 2020. [Google Scholar]
- 146.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 2014; 109:1547–1561; quiz 1546, 1562. [DOI] [PubMed] [Google Scholar]
- 147.Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther 2011;33:822–828. [DOI] [PubMed] [Google Scholar]
- 148.Cheng CW, Bian ZX, Zhu LX, et al. Efficacy of a Chinese herbal proprietary medicine (hemp seed pill) for functional constipation. Am J Gastroenterol 2011;106:120–129. [DOI] [PubMed] [Google Scholar]
- 149.Zhong LLD, Cheng CW, Kun W, et al. Efficacy of MaZiRenWan, a Chinese herbal medicine, in patients with functional constipation in a randomized controlled trial. Clin Gastroenterol Hepatol 2019;17:1303–1310.e18. [DOI] [PubMed] [Google Scholar]
- 150.Nelson AD, Camilleri M, Chirapongsathorn S, et al. Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta-analysis. Gut 2017;66:1611–1622. [DOI] [PubMed] [Google Scholar]
- 151.Luthra P, Camilleri M, Burr NE, et al. Efficacy of drugs in chronic idiopathic constipation: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2019;29:29. [DOI] [PubMed] [Google Scholar]
- 152.Narayanan SP, Bharucha AE. A practical guide to biofeedback therapy for pelvic floor disorders. Curr Gastroenterol Rep 2019;21(5):21. [DOI] [PubMed] [Google Scholar]
- 153.Chiotakakou-Faliakou E, Kamm MA, Roy AJ, et al. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut 1998;42:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5:331–338. [DOI] [PubMed] [Google Scholar]
- 155.Wilkinson-Smith V, Bharucha AE, Emmanuel A, et al. When all seems lost: management of refractory constipation—surgery, rectal irrigation, percutaneous endoscopic colostomy, and more. Neurogastroenterol Motil 2018;30:e13352. [DOI] [PubMed] [Google Scholar]
- 156.Knowles CH, Grossi U, Chapman M, et al. Surgery for constipation: systematic review and practice recommendations: results I: colonic resection. Colorectal Dis 2017;19(Suppl 3):17–36. [DOI] [PubMed] [Google Scholar]
- 157.Muthyala A, Feuerhak KJ, Harmsen WS, et al. Effects of psychosensory stimulation on anal pressures: effects of alfuzosin. Neurogastroenterol Motil 2019;31: e13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dinning PG, Fuentealba SE, Kennedy ML, et al. Sacral nerve stimulation induces pan-colonic propagating pressure waves and increases defecation frequency in patients with slow-transit constipation. Colorectal Dis 2007;9:123–132. [DOI] [PubMed] [Google Scholar]
- 159.Pilkington SA, Emmett C, Knowles CH, et al. Surgery for constipation: systematic review and practice recommendations: Results V: sacral nerve stimulation. Colorectal Dis 2017;19(Suppl 3):92–100. [DOI] [PubMed] [Google Scholar]
- 160.Dinning PG, Hunt L, Patton V, et al. Treatment efficacy of sacral nerve stimulation in slow transit constipation: a two-phase, double-blind randomized controlled crossover study. Am J Gastroenterol 2015;110:733–740. [DOI] [PubMed] [Google Scholar]
- 161.Patton V, Stewart P, Lubowski DZ, et al. Sacral nerve stimulation fails to offer long-term benefit in patients with slow-transit constipation. Dis Colon Rectum 2016; 59:878–885. [DOI] [PubMed] [Google Scholar]
- 162.Zerbib F, Siproudhis L, Lehur PA, et al. Randomized clinical trial of sacral nerve stimulation for refractory constipation. Br J Surg 2017;104:205–213. [DOI] [PubMed] [Google Scholar]
- 163.Yiannakou Y, Etherson K, Close H, et al. A randomized double-blinded sham-controlled cross-over trial of tined-lead sacral nerve stimulation testing for chronic constipation. Eur J Gastroenterol Hepatol 2019;31:653–660. [DOI] [PubMed] [Google Scholar]
- 164.Grossi U, Knowles CH, Mason J, et al. Surgery for constipation: systematic review and practice recommendations: results II: hitching procedures for the rectum (rectal suspension). Colorectal Dis 2017;19(Suppl 3):37–48. [DOI] [PubMed] [Google Scholar]
- 165.Grossi U, Horrocks EJ, Mason J, et al. Surgery for constipation: systematic review and practice recommendations: Results IV: recto-vaginal reinforcement procedures. Colorectal Dis 2017;19(Suppl 3):73–91. [DOI] [PubMed] [Google Scholar]
- 166.Emmett CD, Close HJ, Yiannakou Y, et al. Trans-anal irrigation therapy to treat adult chronic functional constipation: systematic review and meta-analysis. BMC Gastroenterol 2015;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Etherson KJ, Minty I, Bain IM, et al. Transanal irrigation for refractory chronic idiopathic constipation: patients perceive a safe and effective therapy. Gastroenterol Res Pract 2017;2017:3826087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Emmett C, Close H, Mason J, et al. Low-volume versus high-volume initiated trans-anal irrigation therapy in adults with chronic constipation: study protocol for a randomised controlled trial. Trials 2017;18:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lembo AJ, Kurtz CB, MacDougall JE, et al. Efficacy of linaclotide for patients with chronic constipation. Gastroenterology 2010;138:886–895. [DOI] [PubMed] [Google Scholar]
- 170.Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012;107:1714–1724; quiz 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


