Abstract
Xp11.2 Translocation Renal Cell Carcinoma is a very rare subtype of renal neoplasm. The present report describes the first confirmed reported case of percutaneous ablation of this subtype of tumor. The patient presented an aggressive local recurrence 12 months after the procedure, with an infiltrative large mass occupying almost the whole kidney. The patient was submitted to radical nephrectomy. As the use of ablative methods expands, the treatment of rare renal tumor subtypes, which can present unusual clinical outcomes, may become more frequent. It is essential that these uncommon outcomes are promptly recognized, allowing early therapeutic salvage approaches.
Keywords: Renal cell carcinoma, Small renal mass, Radiofrequency ablation, Minimally invasive ablative therapy
Highlights
-
•
Xp11.2 translocation is a relatively new distinct subtype of Renal Cell Carcinoma.
-
•
Reports suggest an aggressive clinical course in adult cases.
-
•
This is the first percutaneous ablation therapy reported on a Xp11.2 translocation Renal Cell Carcinoma.
-
•
The patient presented with an aggressive local recurrence 12 months after the procedure.
-
•
It is essential that these uncommon outcomes are promptly recognized, allowing early therapeutic salvage approaches.
Introduction
As imaging technology is improving and becoming more accessible, the world is facing an increase in the diagnosis of renal cell carcinomas (RCCs). Furthermore, this early detection is shifting the diagnosis toward a remarkable growth on incidental findings and initial tumors, favoring small renal masses and clinical stage T1.
The gold standard treatment for these initial tumors is Nephron Sparing Surgery, with partial nephrectomy (PN) becoming the first therapeutic choice. However, in this scenario, minimally invasive ablative therapies as radiofrequency and cryoablation have emerged as alternative treatments for patients with advanced age, multiple comorbidities, solitary kidneys and baseline renal dysfunction, as well as post surgical remainings of renal masses or patients who are unwilling to undergo a surgical procedure. Those therapies are especially applied to patients with lesions smaller than 3 cm, with greater potential for renal function preservation compared to surgical options.1
The success of the ablation relies on an adequate clinical and radiological follow up, which, in case of failure of the procedure, allows an early diagnosis of the recurrence and adequate management of the tumor, eventually with new ablation sessions or different approaches. Therefore, it is essential that the physicians are aware of the recurrence imaging patterns and the usual tumor behavior.
Ablation zone appears as a non-enhancing area of low attenuation on CT. Nodular enhancement in the ablation zone is considered a sign of residual disease or recurrence.
On immediate post-operative imaging, tumors treated with ablation therapy appear to increase in size, as it is not possible to distinguish the real tumor from the margin of normal adjacent renal parenchyma, which is included in the ablated zone. On subsequent imaging, in the case of successfully treated tumors, the ablation zone usually retracts.
Nevertheless, sometimes the outcome is not as expected and the early recognition of an outlier pattern could allow adequate management.
Case presentation
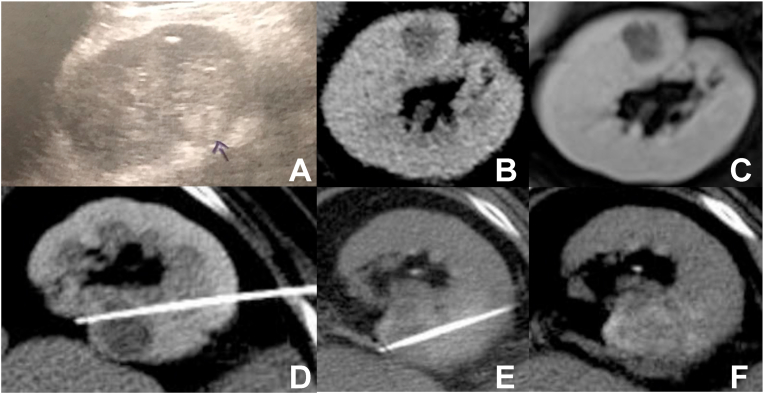
A 70-year-old man with idiopathic chronic liver disease was diagnosed with a cholangiocarcinoma and submitted to a right hepatectomy. During this process, a hyperechoic nodule on the right kidney, measuring 2.0 cm, was detected on ultrasound exam (Fig. 1a). After the hepatic surgery, a computer tomography (CT) was performed (Fig. 1b), showing a hypovascularized solid nodule on the anterior cortex of the medial third of the right kidney, measuring 1.8 cm, suggestive of a RCC, without exophytic component and located 3.0 cm from the renal sinus, that was confirmed by a magnetic resonance imaging (MRI) (Fig. 1c).
Fig. 1.
Ultrasound (A), contrast enhanced CT (B) and contrast enhanced MRI (C) show a small hypovascularized nodule on the anterior portion of the middle third of the right kidney; (D) and (E) with two different positions of the RFA needle, enabling overlapping and adequate ablation area; (F) final immediate aspect of the ablated area, including the whole lesion, with estimated 0.5–1.0 cm of safety margin.
Biopsy of the tumor was executed just before radiofrequency ablation (RFA), in the same procedure setting, and was compatible with clear cell RCC. The RFA was executed with a Cool-Tip Ablation Single Electrode (Covidien, Boulder, CO, USA), with the use of a 17G single needle 15/30. Three cycles were performed (with overlapping areas), being the first and the second of 12 minutes at 60 °C and 63 °C, respectively, and the third one of 6 minutes at 65 °C, using automatic algorithms (Fig. 1d and e).
As the lesion was not well characterized on non enhanced CT, it was opted for intravenous contrast administration at the beginning of the procedure, allowing allocation of the ablation needle in the center of the nodule and the planning of additional overlapping sessions. The ablation zone included the whole lesion, with estimated 0.5–1.0 cm of safety margin (Fig. 1f). However, for preservation of kidney function, new contrast administration was not performed promptly after the procedure. At the end of the procedure, track ablation was performed to prevent tumor seeding. Procedure was performed with general anesthesia without immediate intercurrences.
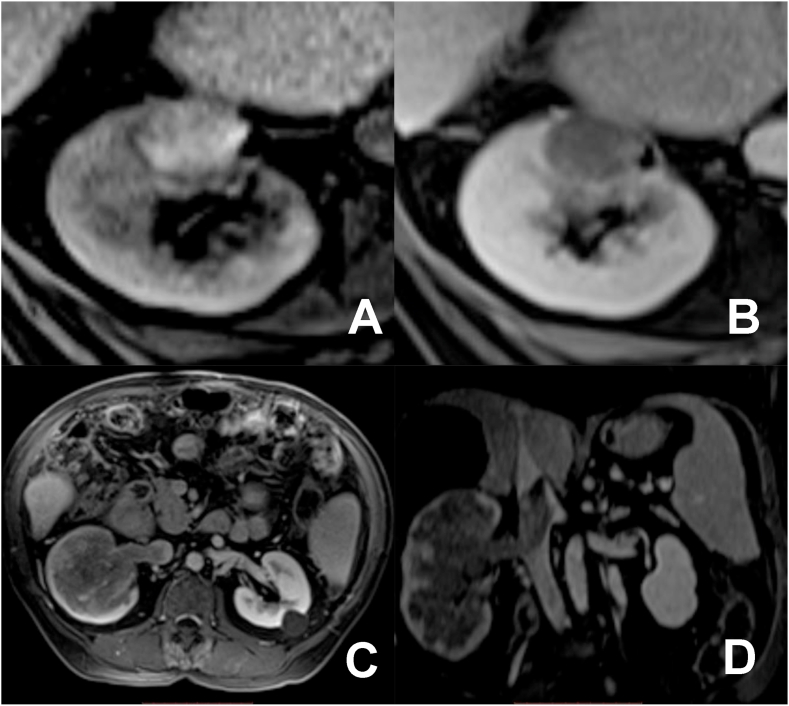
Gadolinium contrasted 6-month follow-up MRI showed spontaneous T1 hyperintense area, with no contrast enhancement, compatible with ablation zone with no signs of local recurrence (Fig. 2a and b).
Fig. 2.
(A) and (B) 6 months follow-up MRI, showing high T1 signal and absence of enhancement after contrast injection, suggesting local tumor control; (C) and (D) contrasted 12 months follow-up MRI presenting an infiltrative mass, infiltrating right renal vein and extending through the inferior vena cava until its retrohepatic portion (tumoral thrombosis).
However, 12-month follow-up MRI showed infiltrative mass affecting almost the entirety of the right kidney, measuring 14.0 cm. It infiltrates the right renal vein and extends through the inferior vena cava until its retrohepatic portion, determining tumoral thrombosis (Fig. 2c and d).
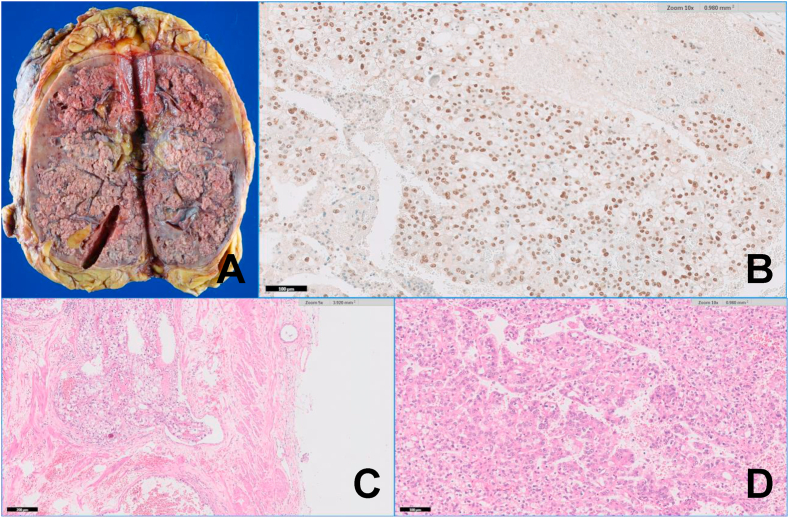
The patient was submitted to a right radical nephrectomy. Anatomopathological and immunohistochemical analysis were compatible with Xp11.2 translocation RCC (XtRCC) (Fig. 3).
Fig. 3.
(A) Gross examination of the radical nephrectomy showing a large tumor, diffusely infiltrative in the renal parenchyma; (B) Immunohistochemistry showing strong and diffuse nuclear positivity for TFE3; (C) TFE3 rearranged carcinoma with papillary and alveolar growth pattern, composed of clear to eosinophilic discohesive pseudostratified cells with voluminous cytoplasm and high grade nuclei; (D) Neoplastic vascular invasion, an histological sign of aggressive behavior.
One year after the surgery, the patient remains under oncological follow-up, asymptomatic and with no evidence of metastatic disease.
Discussion
XtRCC is a relatively new distinct subtype of this neoplasm, first recognized by the World Health Organization in 2004. It is a rare tumor, predominantly reported in pediatric patients, representing around one-third of RCCs in this particular population. Meanwhile, only a few adult cases have been reported.2,3 Although data available is still limited, while infant cases are believed to be more indolent, reports suggest an aggressive clinical course in adult cases.2,4
This new entity is characterized by various translocations involving chromosome Xp11.2, determining translocation of TFE3 (transcription factor E3) gene from this chromosome to another chromosome. These different translocations could make this group of tumors heterogeneous, and may explain part of this unusual sporadic aggressive behavior.
There are no classic imaging findings established for XtRCC. However, some studies suggest that when non typical or heterogeneous features (such as hemorrhage, necrosis, cystic changes and calcification) are identified or when tumor is diagnosed accompanied by metastatic evidence, this diagnostic should be considered.5
To the authors’ knowledge, this is the first percutaneous ablation therapy reported on XtRCC.
Conclusion
In spite of the technical success of the procedure, the patient did not respond well to the interventional treatment, probably due to an intrinsic aggressiveness of the tumor, with a late recurrence and a disruptive progression. Although this is just a single case, not allowing any generalization, it is conceivable that, in the future, the knowledge of the tumor subtype may influence and even determine the management of the case. However, because of its rarity, a pre-RFA screening for XtRCC is probably not justifiable nowadays, but this subtype of RCC may be considered in cases with a poor response to ablative treatment or with an unexpected recurrence.
The lack of knowledge of clinical outcomes of XtRCCs and the unexplored response of these tumors to conventional RCC treatments warrant further studies.
References
- 1.Castro A., Jenkins L.C., Salas N., Lorber G., Leveillee R.J. Ablative therapies for small renal tumours. Nat Rev Urol. 2013;10:284–291. doi: 10.1038/nrurol.2013.68. [DOI] [PubMed] [Google Scholar]
- 2.Meyer P.N., Clark J.I., Flanigan R.C., Picken M.M. Xp11.2 translocation renal cell carcinoma with very aggressive course in five adults. Am J Clin Pathol. 2007;128:70–79. doi: 10.1309/LR5G1VMXPY3G0CUK. [DOI] [PubMed] [Google Scholar]
- 3.Armah H.B., Parwani A.V. Xp11.2 translocation renal cell carcinoma. Arch Pathol Lab Med. 2010;134:124–129. doi: 10.5858/2008-0391-RSR.1. [DOI] [PubMed] [Google Scholar]
- 4.Hora M., Urge T., Trávníček I. MiT translocation renal cell carcinomas: two subgroups of tumours with translocations involving 6p21 [t (6; 11)] and Xp11.2 [t (X;1 or X or 17)] SpringerPlus. 2014;3:245. doi: 10.1186/2193-1801-3-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W., Ding J., Li Y. Magnetic resonance imaging and computed tomography characteristics of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099990. [DOI] [PMC free article] [PubMed] [Google Scholar]