Abstract
The outbreak of rapidly spreading COVID-19 pandemic in December 2019 has witnessed a major transformation in the health care system worldwide. This has led to the re-organization of the specialty services for the effective utilization of available resources and ensuring the safety of patients and healthcare workers. Suspension of oncology services will have major implications on cancer care due to delayed diagnosis and treatment leading to irreversible adverse consequences. Therefore various oncology organizations have called for a continuation of cancer care during this crisis with diligence. The COVID-19 pandemic has forced the clinicians to transform the components of care from screening to outpatient care and primary management. The purpose of this article is to establish guidelines and recommendations for ocular oncology in the management of ocular tumors set by a multidisciplinary team of experts including ocular, medical and radiation oncologists, and pathologists. As the pandemic is evolving fast, it will require constant updates and reformation of health strategies and guidelines for safe and quality health care.
Keywords: COVID-19, COVID-19 guidelines, ocular malignancies, ocular oncology, ocular tumors
Ocular oncology is a branch that deals with tumors of the eye including eyelid, ocular surface, intraocular structures, and orbit. It has always remained as a specialty with a limited number of trained ocular oncologists and ancillary staff in India and worldwide, as compared to other ophthalmology subspecialties. Ocular oncology functions as a multidisciplinary team and requires a coordinated approach for an optimal outcome. The primary goal in the management of ocular malignancies is life salvage, followed by eye and vision salvage. We relentlessly aim at reducing the mortality and morbidity in ocular oncology practice by time-bound and protocol-based management, which makes it a challenging and engaging specialty. The impact on the outcome can be adverse if the management is neglected or delayed in cancer patients.[1,2] There is a growing concern that coronavirus disease 2019 (COVID-19)/severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) can impact the management of cancer patients by overwhelming the medical care systems and overshadowing the rest of the clinical care. There is an international consensus that any patient with a potentially malignant tumor diagnosed during the COVID-19 pandemic should undergo a thorough clinical assessment, appropriate investigations, and possibly a biopsy to confirm the diagnosis and protocol-based management as appropriate. Ocular oncology is not an exception. Thereby, ocular oncology service continues to function irrespective of the situation, war, or wrath. We recognize the careful balance between prioritized patient care and the risk of inadvertent exposure of healthcare workers and patients to COVID-19. In this report, we aim to propose the basic guidelines and strategies for the continuation of ocular oncology services during the COVID-19 pandemic. These guidelines remain dynamic and may evolve as the nature of disease transmission changes in the future. Individual decisions may have regional influence depending on the COVID-19 burden, medical, logistical, and organizational considerations.
Methods
In May 2020 all the authors including a multidisciplinary team of ocular oncologists, medical oncologists, pediatric oncologists, radiation oncologists, and pathologists coordinated and communicated through email to put forward the disease-specific guidelines in ocular oncology. The guidelines are based on the collective opinion of the experts in the specialty. Some of the authors (FPM, SGH) were also part of the practice guidelines survey conducted by the ocular oncology group for the American Academy of Ophthalmology that has been published.[3]
Guidelines for Ocular Oncology during COVID-19/SARS-CoV-2 Pandemic
I. Basic screening and triage of patients
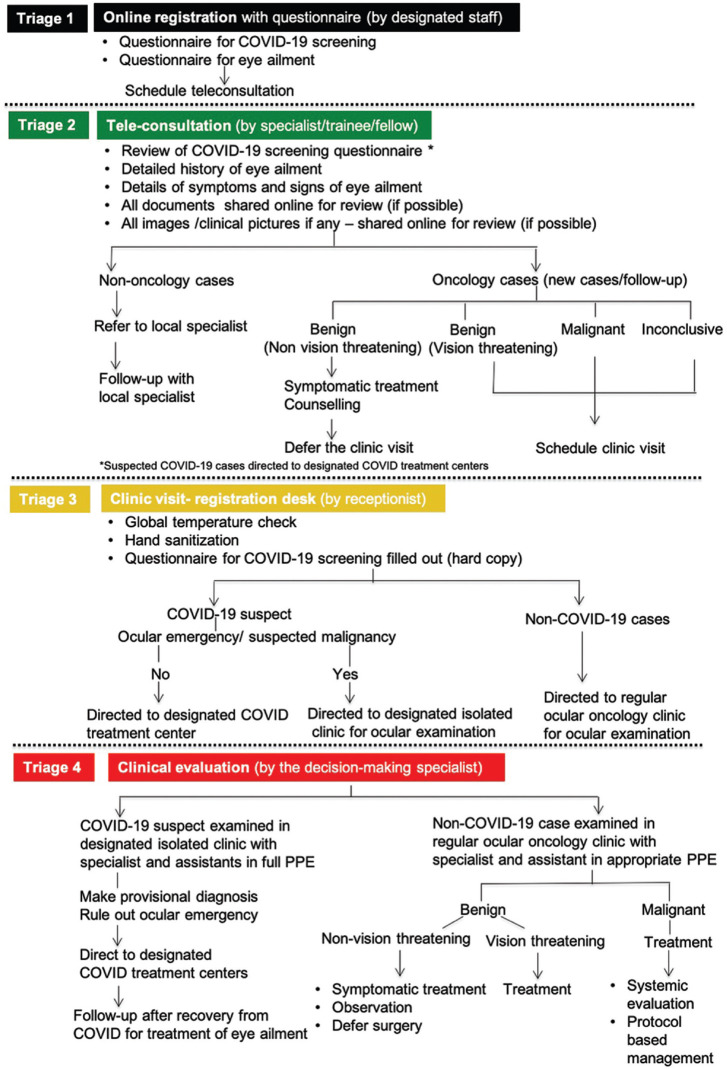
This includes the universal guidelines and triage of patients depending on the symptoms and travel history.[4] Triage of patients at four different levels (Quad-triage) is recommended that includes online registration, teleconsultation with specialist/trainee, onsite physical screening, and clinical evaluation by decision-making specialist [Fig. 1].
Figure 1.
Flow chart showing the triage- 'Quad triage' at 4 levels, for the ocular oncology services during the COVID-19 pandemic
Online appointments and registration can avoid onsite crowding. COVID-19 questionnaires to identify laboratory-confirmed, and suspect case as outlined and defined by the Ministry of Health and Family Welfare (MoHFW) is taken.[5]
If it is a COVID-19 suspect or laboratory-confirmed case, the patient gets directly referred for investigation, and further management at the government designated COVID-19 care center. If associated with emergency symptoms such as intractable eye pain, vomiting, headache, visual loss, and signs associated with increased intraocular pressure and/or suspected malignancy, the patient is given appointment and examination planned in the pre-designated area by the decision-making specialist with full personal protection equipment (PPE) and offered symptomatic primary treatment before referring to the COVID-19 center. If the symptoms are mild and tolerable, the ocular examination is delayed until the patient is tested negative and complete recovery from COVID-19.
Second level triage by teleconsultation with specialists/fellows before clinic visits are encouraged for detailed history and documentation that can reduce the consultation duration in the clinic with the specialist. Teleophthalmology with video consultation in the diagnosis of ocular tumors has major pitfalls especially in new intraocular and orbital cases that may require detailed evaluation. However, eyelid and conjunctival lesions are less challenging.[6] Follow-up cases are scheduled according to the stability of the disease.
Onsite triage is done during the scheduled clinic visit according to the published guidelines with thermal screening and questionnaires.[4] Patients on chemotherapy are isolated from the rest of the patients in the waiting area considering the risk of acquiring infection due to immunosuppression. Clinical triage is recommended wherein a decision-making specialist attends to the patients to differentiate benign from malignant tumors, vision-threatening tumors, and plan management accordingly. Follow-up cases are scheduled according to the stability of the disease.
II. Healthcare worker safety
Every patient is to be considered as a potential COVID-19 case and universal precautions should be taken. Of the 3,387 healthcare workers with COVID-19 in China, 23 died, and 8 were surgeons including 3 ophthalmologists.[7] The healthcare workers are the most valuable resource of the country worldwide. While we are in the process of resuming the clinical and surgical practices, the protection of healthcare workers is of paramount importance.[7,8] Adherence to handwashing, antiseptic foaming, and appropriate patient protection with masks to cover their mouth and nose before entering the clinic must be strictly enforced. The healthcare worker protective measures with PPE depends on the risk of exposure to disease transmission, and regional disease burden. It should be used appropriately and not wastefully. The new guidelines for the rationale use of PPE for non-COVID-19 hospitals were recently released by MoHFW.[9]
Personal protection equipment (PPE) can be
Full PPE: long-sleeved whole body covering impermeable garment (hazardous material suit), shoe cover, N95/3-ply impermeable mask, goggles, full-face shield or visor, headgear/hood, and double gloves. The plastic apron should be worn over permeable garments
Essential PPE: full-face shield or visor, goggles, N95/3-ply impermeable mask, gloves, and +/- surgeon's gown.
Donning and doffing of PPE should be followed as per the standard guidelines.
Teleconsultation before the clinical visit for details regarding the disease as mentioned in the triage system can minimize the time spent in the outpatient clinic [Fig. 1]. Previous medical documents preferably should be submitted online by the patient before the clinical visit, so that the patient details are reviewed by the specialist in advance.[10] No attendants are to be allowed, except in the case of children and senior citizens where only one attendant should be allowed during a consultation. Avoid conversation with the patient while examination. The slit-lamp examination should be performed as mandated with protection. Indirect ophthalmoscopy is performed with a mounted face shield. If at all scleral depression is required, a cotton-tipped applicator is preferred over a scleral depressor. In the case of infants and children, when examined under restraint are likely to increase the aerosol levels due to crying; examiner and assistant should be extremely cautious. Gloves to be discarded/disinfected after every patient. Counseling to be performed in the clinic immediately following the examination, maintaining a safe distance of 1–2 m. Patients and/or families should be given an option of counseling over a video conference for a detailed explanation, and decision making.
III. Surgical safety measures
It is the aerosol generating procedures (AGPs) that carries a high risk of virus transmission. These include intubation, extubation, manual ventilation, open suctioning of the respiratory tract, high flow nasal oxygen, administration of humified oxygen, surgeries involving high-speed drilling, and mucosal exposure.[11,12,13,14] Local anesthesia (LA) is preferred over general anesthesia (GA). Surgical procedures under GA should have an interval of 20–30 min between cases for aerosol clearance. Electrosurgery with diathermy cauterization plays a major role in oncosurgeries for hemostasis, but there is evidence that it can lead to particle aerosolization.[15] Their use should be minimized, and bipolar cautery should be used at the lowest power setting instead of the monopolar device whenever possible to minimize plumes and thus aerosol production.[16] It is preferable to use a high vacuum and high flow suction devices whenever available. This may allow the rapid suction of potentially dangerous aerosols. Hypotensive anesthesia should be preferred for reducing intraoperative bleeding.
The endoscopic surgical approach for orbitotomy and high-speed drilling must be avoided. If the oncosurgery involves sinus exposure as in exenteration or orbitotomy, it is preferable for the surgeons and assistants to use full PPE.[14] Patients presenting with cough and or fever on the day of surgery or EUA must be postponed and systematic screening for COVID-19 should be considered. In case a healthcare worker has been infected, two PCR-negative swabs are required for rejoining the work.
IV. Disease-specific guidelines in ocular oncology
1. Retinoblastoma
Retinoblastoma is considered a priority where new and active cases are attended to and treated as usual.[17] It is challenging as it involves examination under anesthesia (EUA) for new patients and follow-up cases. Unlike adult ocular tumors EUA is performed in all case scenarios for grouping and staging before commencing the treatment in children. It is preferable to perform bone marrow biopsy and or cerebrospinal fluid cytology during the initial EUA for suspected malignancy in children to avoid repeated anesthesia if facilities are available. EUA carries a moderate risk of disease transmission. During EUA, the anesthetist and assistant are expected to be in full PPE with proper precaution during intubation and extubation. The examiner and assistant must stay outside the operation theatre during the same. Considering the need for fundus evaluation and visualization without compromising on personal protection, the examiner is expected to be protected with a 3-ply mask/N95 mask, protective glasses, gloves, and a gown. Instillation of 5% Povidone-iodine in the conjunctival cul-de-sac 5 min before examination is known to have a virucidal effect.[17,18] Indirect ophthalmoscope and lens should be disinfected after every examination as per the standard guidelines by the vitreoretinal society.[4]
Regarding the primary management protocols and indications, it remains unchanged be it systemic intravenous chemotherapy, interventional intra-arterial chemotherapy, or enucleation.[19,20,21] During treatment, ideally every child undergoes EUA before the chemotherapy cycles every 3–4 week. After the initial EUA, during chemotherapy cycles, in order to reduce the repeated risk of aerosol transmission and infection, subsequent EUAs and focal therapy may be performed after 2–3 cycles or alternative cycles unless the clinical situation demands more frequent intervention. Focal therapy including laser, cryotherapy, local injections may be planned as appropriate [Table 1].
Table 1.
Intraocular tumors-disease specific guidelines for ocular oncology services during SARS-Cov-2/COVID-19 pandemic
| Ocular Tumors | Standard of Care | COVID-19 guidelines | Challenges | Comments |
|---|---|---|---|---|
| 1. Retinoblastoma | ||||
| Diagnosis | Grouping/staging EUA Ultrasonography MRI/CT-scan BM biopsy/Lumbar puncture |
No change in diagnostic modalities of newly diagnosed cases for grouping and staging | Risks of COVID-19 transmission Accessibility of care |
Patient and health care worker protection |
| Primary management | Systemic chemotherapy Intra-arterial chemotherapy Enucleation |
Not delayed Indications of primary management remains same In unilateral RB, advanced group D/E eyes, enucleation can be considered |
High risk for COVID-19 Immunosuppression Monthly hospital visits |
Defer EUA during initial 2-3 cycles of chemotherapy Professionally monitored personal protection Out-patient chemo suits Transfer of pediatric oncology care locally |
| Focal adjuvant treatment | Cryotherapy Laser Intravitreal chemotherapy |
Local therapy as indicated | High risk for COVID-19 | Patient and health care worker protection |
| Follow-up | EUA Clinical evaluation in older children |
Screening in familial RB not to be delayed Active cases not to be delayed for more than 4 to 6 weeks Inactive for <3 months, not to be delayed for more than 4 to 6 weeks Inactive for >1 year can be delayed for 3 months |
Risks of disease transmission Accessibility of care Recurrence |
Strict follow-up |
| Radiological imaging | Surveillance MRI | Can be delayed for 3 to 4 months | Pinealoblastoma screening Postenucleation | If symptomatic, consider urgent MRI Can be postponed. |
| Genetic testing | Gene analysis of patient and parent | Defer until after pandemic | Visit to geneticist | Will not influence initial management |
| 2. Uveal Melanoma | ||||
| Diagnosis | Ultrasonography/OCT Systemic evaluation |
No change/delay in diagnostic and systemic screening protocols Investigations performed according to guidelines |
Patients often>65 years and high risk for COVID-19 | Basic work-up can be done locally before referral for primary management |
| Primary Treatment |
Plaque brachytherapy Enucleation Transpupillary thermotherapy |
No delay in management Treatment as indicated according to the tumor size and extent as per protocols |
Surgery done under general anaesthesia. | Patient and health care worker protection as per guidelines in the clinic and operation theatre Radiation safety guidelines strictly followed |
| Follow-up | Clinical evaluation | Postenucleation follow-up locally Routine visits delayed Teleophthalmology Systemic screening reports to be submitted online and avoid hospital visits Intravitreal anti-VEGF/steroids post-radiation delayed for 2 to 3 months |
Frequent hospital visits and risks involved | Postenucleation, observation in patients >65 years Post-plaque evaluation delayed to 6 weeks |
| Genetic testing | Gene analysis during primary management. | Performed during primary management with enucleation/plaque brachytherpay | FNAB specimens sent in sealed double pack container | Inform the pathologist prior |
| 3. Primary Intravitreal Lymphoma | ||||
| Diagnosis | Serological investigations MRI CNS evaluation-LP Vitreous biopsy |
No change/delay in diagnostics or systemic evaluation In the presence of CNS involvement clinically, LP to be considered for diagnosis rather than vitreous biopsy |
Patients on chemotherapy is at high risk for multiple hospital visits | Patient and health care worker protection as per guidelines in the clinic and operation theatre Patients on chemotherapy to be isolated from other patients in OPD to reduce risk of infection |
| Primary management | Intravitreal injections Stereotactic radiotherapy Systemic chemotherapy |
Intravitreal injection involves frequent hospital visits Hypofractionated stereotactic radiotherapy reduces treatment burden Systemic chemotherapy as indicated and without delay |
Multiple injections required Avoid frequent hospital visits Risk of infection secondary to immunosuppression |
Treatment should be strategically planned |
| Follow up | Clinical evaluation | If disease stable>6 months posttreatment, visits delayed for 3 months Intravitreal injection will require 4 weekly follow-up |
Detection of recurrence Risks of COVID-19 transmission |
Regular follow up locally Telementoring |
| 4. Benign Intraocular Tumors | ||||
| Diagnosis | USGB scan/OCT FFA/ICG |
Avoid FFA/ICG | Risks associated with the procedure | |
| Treatment | Laser Intraocular injections Cryotherapy Observation |
Delayed if no vision threatening symptoms. Consult with local ocular oncologist/retina specialist with regular follow up |
Disease progression and complications | Patient counselling regarding alarming symptoms for urgent care Transfer of care to ocular oncologist/retina specialist locally and review |
EUA – Examination under anesthesia; MRI – Magnetic resonance imaging; CT – Computerized tomography; BM – Bone marrow; OCT – Optical coherence tomography; FNAB – Fine needle aspiration biopsy; LP – Lumbar puncture; OPD – Out patient department; FNAB – Fine needle aspiration biopsy; LA – Local anesthesia
Transfer of care to local pediatric/medical oncologists may be considered to avoid the risk of COVID-19 transmission during travel. Considering the risk of community-acquired infection in children undergoing chemotherapy, the private transport vehicle is preferable to commute to and fro from the hospital. Local support groups and NGO may be approached for aid and assistance. In patients who are under remission for < 6 months, follow-up EUA's should not be delayed for > 4–6 weeks. For those who have been deemed stable for more than a year after the last treatment for disease activity, EUA may be deferred by 3 months. Retinoblastoma screening in children with family history including sibling screening should not be delayed due to the known risk that can have adverse consequences. Screening of the fellow eye in unilateral retinoblastoma can be postponed depending on the risks involved (germline/familial and age of the child). However, older children can be seen in the clinic and fundus findings documented with ultra-widefield fundus imaging (Octopus/Clarus) if available. Radiological imaging is performed in all new cases with bilateral and unilateral retinoblastoma as a part of evaluation and staging. Scheduled radiological surveillance may be deferred for 3 months.
2. Uveal melanoma
All newly diagnosed cases are treated as appropriately without delay due to its deadly nature.[22] However, a delay of 2–3 weeks may not affect the outcome. Detailed evaluation including indirect ophthalmoscopy and documentation followed by ultrasonography are to be performed following established guidelines. Systemic screening with laboratory investigations and radiological evaluation for staging must be done. Management options and indication of brachytherapy versus enucleation remain unchanged.[22] The surgical procedure is done under general anesthesia following all the COVID-19 safety recommendations. Long-term follow-up patients post-brachytherapy > 1 year, and post-enucleation > 3 months can be deferred by 3 months. Teleophthalmology with a coordinated approach with ocular oncologist/retina specialists locally and transfer of care for follow-up should be considered. Intravitreal anti-VEGF/steroid injections post-radiation for retinopathy and maculopathy can be delayed by 1–2 months.[23]
3. Vitreoretinal lymphoma
Primary vitreoretinal lymphoma that has a central nervous sytem (CNS) involvement is often initially diagnosed by an ophthalmologist. Initial evaluation includes serological tests to rule out inflammatory mimics and systemic evaluation. The disease being lethal will mandate immediate attention and treatment.[24] In the presence of a CNS involvement with radiological evidence, a diagnostic lumbar puncture can help avoid vitrectomy.[24,25] Therefore, radiological assessment is done before surgical intervention in suspected vitreoretinal lymphoma. Currently accepted management modalities involve stereotactic radiotherapy or intravitreal injections. Intravitreal injections will require frequent hospital visits and risky for the patient especially if they are on concurrent chemotherapy and immunocompromised. Stereotactic radiotherapy as a primary management modality can avoid such associated risks [Table 1].[25]
4. Benign intraocular tumors
The most common benign intraocular tumor is circumscribed choroidal hemangioma. Vascular tumors of retina and choroid can cause significant visual compromise due to exudation and sub-foveal fluid accumulation.[26] This may require early intervention. They are often diagnosed clinically, and ancillary procedures such as fundus fluorescein angiography (FFA) and indocyanine green angiography (ICG) aid in the diagnosis. It is preferable to avoid angiographic procedures unless absolutely indicated. In the presence of progressive visual loss, treatment cannot be delayed. Laser therapy may be performed as per the existing protocols. Photodynamic therapy (PDT) must be deferred for the meanwhile considering the associated medical risks. This can be substituted with anti-VEGF injections in the interim period [Table 1].[27]
5. Orbital tumors
Malignant orbital tumors that have aggressive lethal behavior that may lead to death or visual compromise and permanent disability require immediate attention. These include rhabdomyosarcoma, primary neuroectodermal tumors (PNET), sarcoma, and optic nerve glioma in children.[28] Similarly, lacrimal gland adenoid cystic carcinoma, adenocarcinoma, orbital metastasis, lymphoma, and other high-grade malignancy mandates urgent attention in adults.[29] Orbitotomy and biopsy be it incision or excision should not be delayed. Orbital exenteration if indicated may involve exposure of sinus mucosa that is known to carry a high risk for disease transmission. Care must be taken not to breach the bony orbital wall especially the thin medial wall to enter the ethmoid sinus. In cases with expected sinus mucosal exposure due to local invasion into the paranasal sinus and/or lacrimal apparatus, surgeons are advised to wear a full PPE as discussed earlier. Management is according to the standard protocols be it systemic chemotherapy or radiotherapy. Benign tumors except those that can cause a potential threat to vision and permanent disability can be delayed until after the peak of the pandemic are seen [Table 2].
Table 2.
Orbital tumors-disease specific guidelines for ocular oncology services during SARS-Cov-2/COVID-19 pandemic
| Ocular Tumors | Standard of Care | COVID-19 guidelines | Challenges | Comments |
|---|---|---|---|---|
| 1. Malignant Orbital Tumors | ||||
| Diagnosis | CT-scan/MRI Orbital biopsy Systemic evaluation PETscan |
No delay in diagnosis and evaluation | Risks of COVID-19 transmission | Patient and health care worker protection as per guidelines in the clinic and operation theatre |
| Primary management | Orbitotomy, excision biopsy | Not delayed and performed as indicated Avoid/minimize use of electrosurgery (diathermy cautery) during orbitotomy Avoid endoscopic approach Hisopathology specimens in leak proof double layered container |
Surgical aerosol generation Long duration of surgery Fresh specimen for frozen section to be transported carefully |
Operation theatre safety guidelines strictly followed Pathologist informed prior if frozen section/FNAC |
| Stereotactic radiotherapy | Hypofractionated stereotactic radiotherapy | Frequent hospital visits, less when compared to conventional radiotherapy | Lesser treatment duration | |
| Chemotherapy | No change in standard protocols | Associated risk in older patients and children Immunosuppression |
Professionally monitored personal protection Out-patient chemo suits |
|
| Follow-up | Clinical evaluation | Posttreatment can be delayed by 4 to 6 weeks. Postradiation to be seen after 3 months |
Postsurgical complications | Not delayed if vision threatening |
| Radiological assessment | Delayed for 3 months if clinically stable | Do not delay in aggressive high grade malignancy | Screening for recurrence | |
| 2. Benign Orbital Tumors | ||||
| Diagnosis | Clinical evaluation Visual fields +/- CTscan/MRI |
Evaluation of vision and risks of visual compromise Radiological image review and discussion with radiologist |
Orbital lymphoma, even high grade can initially present as benign or inflammatory condition | Thorough assessment of history, symptoms and vision |
| Primary management | Orbitotomy and excision biopsy | Delayed if no vision threatening symptoms secondary to optic nerve compression, diplopia, and exposure keratopathy | Regular follow up | Patient counselling regarding alarming symptoms for urgent care |
CT- Computerised tomography; MRI - Magnetic resonance imaging; PET- Positron emission tomography
6. Eyelid tumors
Malignant eyelid tumors, especially the lethal ones including sebaceous gland carcinoma, malignant melanoma, and Merkle cell carcinoma require timely attention and cure. Delay in the management of basal cell carcinoma and squamous cell carcinoma can lead to progression of growth, often leading to invasion and difficulty in complete excision. Primary management is surgical excision with frozen section margin clearance followed by reconstruction.[30] Fresh tissues for the frozen section has to be handled with utmost precaution while transporting to the pathologist. Staff responsible should be wearing PPE including, masks, protective glasses, and gloves. The pathologist should be informed in advance regarding the procedure and time of tissue retrieval so that the laboratory is prepared. Chemoradiation in indicated cases is not delayed. Benign tumors of the eyelid can be observed, and surgery delayed. Postoperative follow-up duration of malignant tumors will remain the same as per the protocol. Teleophthalmology could be utilized for routine follow-ups [Table 3].[10]
Table 3.
Eyelid tumors-disease specific guidelines for ocular oncology services during SARS-Cov-2/COVID-19 pandemic
| Ocular Tumors | Standard of Care | COVID-19 guidelines | Challenges | Comments |
|---|---|---|---|---|
| 1. Malignant Eyelid Tumors | ||||
| Diagnosis | Clinical evaluation CTscan/MRI if indicated |
No delay in diagnosis | Risks of COVID-19 transmission | Patient and health care worker protection as per guidelines in the clinic and operation theatre |
| Primary Treatment |
Surgical excision with margin clearance | No delay in the management of malignant eyelid lid tumorsAvoid 2 staged eyelid reconstructive procedure | Surgical exposure to aerosols LA preferredAvoid electrosurgery (diathermy cautery) Fresh specimen for frozen section to be transported carefully |
Operation theatre safety guidelines strictly followed. Pathologist informed prior if frozen section/FNAC |
| Chemoradiation | No delay if indicated | Associated risk in older patients | Professionally monitored personal protection. Out-patient chemo suits |
|
| Sentinal lymph node biopsy | Delayed by 4 to 6 weeks | Procedure associated risks | Performed by head and neck surgeon | |
| Follow-up | Clinical evaluation Teleophthalmology |
Post-operative delayed for 4 to 6 weeks If stable can be delayed by 3 months in the first 1 year If stable after 1 year can be delayed by 6 months |
Post-surgical complications Early detection of recurrence |
Transfer of care locally |
| 2. Benign Eyelid Tumors | ||||
| Diagnosis | Clinical examination/Teleophthalmology | Confirm the diagnosis | Malignant lesions can mimic benign or inflammatory eyelid lesion Avoid misdiagnosis especially in teleophthalmology |
Look for alarming signs Review with ophthalmologists locally |
| Primary management | Observation Surgical excision |
Surgery delayed until and after the pandemic | Cosmetic concern of patient | Patient counseling regarding risks vs benefits |
CT – Computerized tomography; MRI – Magnetic resonance imaging
7. Conjunctival tumors
Ocular surface tumors that are potentially benign can be observed and management delayed. Malignant tumors of conjunctiva often do not exhibit rapid progression. The gold standard in the management of conjunctival tumors including conjunctival melanoma and ocular surface squamous neoplasia (OSSN) is surgical excision. Although topical therapy in OSSN is an established primary modality, in the current situation to avoid/minimize regular hospital and pharmacy visits, primary surgical excision under local anesthesia may be preferred.[31] The decision to choose surgery versus topical treatment may be guided by local logistics. In a diagnosed COVID-19 patient, topical therapy is preferred over surgical excision. Drugs can be dispensed for 1 month and review planned after patient testing negative for COVID-19. Teleophthalmology and telementoring in conjunctival tumors is an option for diagnosis and management in collaboration with the primary ophthalmologist as it is not difficult to get high-quality ocular surface images. Plaque brachytherapy if indicated as an adjuvant procedure is to be performed 4 weeks post-surgical excision that may aid in buying time, or even delayed to a maximum of 6 weeks [Table 4].
Table 4.
Conjunctival Tumors-Disease specific guidelines for ocular oncology services during SARS-Cov-2/COVID-19 pandemic
| Ocular Tumors | Standard of Care | COVID-19 guidelines | Challenges | Comments |
|---|---|---|---|---|
| 1. Malignant Conjunctival Tumors | ||||
| Diagnosis | Clinical evaluation AS-OCT UBM if indicated Systemic evaluation |
No delay in investigations or evaluation UBM avoided if possible |
Risks of COVID-19 transmission Common in patients >65 years Immunocompromised |
Patient and health care worker protection as per guidelines in the clinics |
| Primary management | Surgical excision + Cryotherapy |
Excision biopsy planned as per indications Plan according to local logistics. | Avoids frequent hospital/pharmacy visits Surgical exposure to aerosols LA preferred. Avoid electrosurgery (diathermy cautery) Careful handling of specimen |
Operation theatre safety guidelines strictly followed Pathologist informed prior |
| Topical therapy chemotherapy/immunotherapy | Topical treatment in COVID-19 suspect | Frequent hospital/pharmacy visits Risk of disease transmission |
Patient safety | |
| Plaque brachytherapy | No delay in primary plaque brachytherapy Secondary plaque is planned 4 to 6 weeks postsurgical excision |
Surgical exposure to aerosols LA preferred |
Radiation safety guidelines followed | |
| Sentinal lymph node biopsy | Delayed by 4 to 6 weeks | Procedure associated risks | Performed by head and neck surgeon | |
| Follow up | Clinical evaluation Teleophthalmology |
Posttreatment delayed by 4 to 6 weeks. If stable can be delayed by 3 months in the first 1 year If stable for 1 year can be delayed by 6 months |
Subtle recurrences can go unnoticed with images during teleophthalmology | Ensure high quality images Suspicious signs require clinical evaluation Transfer of care to ophthalmologist locally |
| 2. Benign Conjunctival Tumors | ||||
| Diagnosis | Clinical evaluation Teleophthalmology |
Confirm the diagnosis | Differentiate benign from malignant lesions Avoid misdiagnosis especially in teleophthalmology | Look for alarming signs Review with ophthalmologists locally |
| Primary management | Observation Surgical excision | Surgery delayed until and after the pandemic. Symptomatic treatment for discomfort or irritation |
Cosmetic concern of patient | Patient counseling regarding risks vs benefits |
AS-OCT - Anterior segment optical coherence tomography; UBM - Ultrasound biomicroscopy
V. Ocular pathology guidelines
COVID-19 pandemic poses risk to ocular pathologists and technicians and other personnel involved in the storage and transportation of specimens, as it can be a source of disease transmission.[14] The protocols if followed strictly will help minimize the associated risks.[32]
1. General measures and laboratory procedures
All the specimens should be considered potentially contaminated. Utmost precautions, immediate clean-up of spillage, and general cleanliness should be the rule. Work should be confined to a limited area. Personnel handling tissues should wear gloves, N95 masks, face masks with shields (or protective glasses), aprons or fluid-impervious gowns. Areas, where tissues are handled, must be used by authorized personnel only.
2. Tissue handling/Transportation (Routine HPE Tissue)
All specimens are to be placed in well-constructed containers with a secure lid to avoid leaking during transportation. Care should be taken to avoid contamination of the outside of the container while collection. The specimen should be kept moist in proper fixative. Tissues to be kept in fixative for 24–48 h depending on the size.
3. Frozen section/intra-operative cytology tissue
The risk of disease transmission is high during the frozen section with fresh unfixed tissues. Frozen section for ocular pathology specimens that are of the highest risk is conjunctiva, nasolacrimal specimens, nasal, and sinus biopsies. There should be communication between the clinicians and pathologists to identify COVID-19 suspect/laboratory confirmed cases prior to the frozen section procedure. On case–to-case basis the value of frozen section should be evaluated by the clinicians and pathologists. It is advisable to defer the procedure if the report will not contribute to a high-value intraoperative intervention, and alter patient outcome. Recommended PPE is to be worn while handling tissues. While performing frozen sections confine contamination to a smaller area. Do not contaminate reusable items like mounting media containers. Precautions should be taken so that aerosols are not created in the cryostat. The frozen section slides should be immersed in alcohol immediately. Following this, the staining solution must be discarded into appropriate disinfectant (e.g., 10% bleach/sodium hypochlorite). While disinfecting the cryostat it is mandatory to wear protective personnel equipment. Defrost and decontaminate with appropriate, hospital-approved disinfectant (1% Sodium hypochlorite solution). The blades must be removed, and discarded in a 1% sodium hypochlorite solution. The entire cryostat must be wiped from inside with a 1% sodium hypochlorite solution. Immediate transfer of tissue to 10% neutral buffered formalin is advised as soon as the frozen section procedure is complete. The work area should be cleaned with a % hypochlorite solution wearing a fresh pair of gloves.
4. Tissue disposal, cleaning, and disinfection
All tissues should be labeled “Hazardous Infectious Waste” double-lined with red bag and sent to off-site for incineration. Work surfaces and instruments should be cleaned and disinfected with 1% sodium hypochlorite. Needles, blades, glass slides, unbroken glassware, and sharp plastic items are to be disposed of in puncture-proof containers with 1% sodium hypochlorite.
5. Digital pathology
Telepathology/digital pathology has allowed us to capture high-resolution images of biopsy specimens and has enabled us to continue operations during the COVID-19 pandemic. These digital images can be shared and viewed anywhere. The images are sent through a microscope with a camera. The slides moved around by a biotechnician and images are beamed live through team viewer. Also, the digital scanner captures images from different areas of the slides and sends it to the reporting ocular pathologist. The digital scanner requires someone to load the images and this can sometimes be a limitation.
VI. Medical/pediatric oncology guidelines
The rapidly spreading COVID-19 poses a greater challenge in patients with cancer undergoing treatment especially considering their immune status. Basic guidelines of handwashing and use of personal protection equipment by patients and staff are professionally monitored on entering and leaving the hospital premises. Patients undergoing treatment must be under strict physical isolation with home confinement during cycles and single room admission in hospitals. For the patients to survive cancer and this pandemic, it is important to support the families to access the care for treatment and, ensure the availability of trained oncology teams.
The initial reports from Wuhan were alarming but also depends on regional cancer epidemiology, heterogeneity of cancer types, stage of presentation, and age of the patient.[33] The guidelines are set for continuing multidisciplinary care during SARS-CoV-2/COVID-19 pandemic around the index cancers that is more common. The WHO Global Initiative for Childhood Cancer (GICC) has identified 6 common pediatric cancers as index cancers that have a good prognosis, and curative using established standard of care and regimens.[19,34,35] This fortunately also includes retinoblastoma. There should not be any delay in the diagnosis and timely management of cancer patients during any infectious disease pandemic with the available resources. Any patient newly diagnosed with cancer should be thoroughly investigated without delay for accurate diagnosis, staging, and risk stratification for planning the treatment during and beyond the pandemic. The indications of ancillary diagnostic procedures like bone marrow biopsy and lumbar puncture for staging remains the same. Systemic chemotherapy is commenced as indicated with the standard protocols. The primary or secondary growth factor can be considered to abbreviate periods of neutropenia and hospitalization. Despite the known risk of COVID-19 infection in cancer patients due to the immunocompromised status, there is still no evidence or published guidelines for prophylactic therapy with antivirals. In the presence of concurrent COVID-19 infection in a patient, the safest approach will be to commence disease-oriented therapy after recovery from the viral infection.[36] Telemedicine in oncology can aid in remote (local) chemotherapy supervision, review of laboratory reports, symptomatic management, and follow-up of inactive/stable cases. Tumor board meetings can be conducted through teleconferencing.
VII. Radiation oncology guidelines
The indications of radiotherapy and the practical guidelines during COVID-19 have been discussed by the experts.[36,37] Conventional stereotactic radiotherapy includes 25 fractions extending over 4–5 weeks. Due to COVID-19 situation, there is a push towards hypofractionated protocols across the globe by various international societies like American Society of Therapeutic Radiation Oncology, European Society of Therapeutic Radiation Oncology), European Society of Medical Oncology, and the stereotactic radiosurgery/radiotherapy societies.[38] This minimizes the number of visits to the hospital and thereby minimizes the exposure to COVID-19 atmosphere as it is much faster than conventional stereotactic radiotherapy. Hypofractionated radiosurgery has been tried earlier for large meningiomas.[39] Frameless hypofractionated image-guided volumetric modulated arc (stereotactic radiotherapy) to a dose of 25 Gy in 5 fractions over 1 week can be considered in orbital tumors/intraocular tumors requiring radiotherapy.
Conclusion
In conclusion, the management of ocular malignancy should not be delayed or deferred as compared to benign tumors. It is worrying for cancer patients to contract COVID-19 infection. All the more worrying is patients becoming indirect victims of the pandemic due to late diagnosis and treatment and progression of disease leading to poor prognosis. As we are witnessing the evolution of the COVID-19, it is convincing to believe that the virus is here to stay. Therefore, a careful coexistence, and understanding of the overall impact of the pandemic to health and healthcare is essential.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shinde RS, Naik MD, Shinde SR, Bhandare MS, Chaudhari VA, Shrikhande SV, et al. To do or not to do.-A review of cancer surgery triage guidelines in COVID-19 pandemic?? Indian J Surg Oncol. 2020:1–7. doi: 10.1007/s13193-020-01086-7. doi: 10.1007/s13193-020-01086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Surgeons. COVID-19 guidelines for triage of cancer surgery patients Facsorg. 2020. [Last accessed on 2020 May 25]. Available from: https://wwwfacsorg/covid-19/clinical-guidance/elective-case/cancer-surgery .
- 3.Skalet AH, Allen RC, Shields CL, Wilson MW, Mruthyunjaya P, Gombos DS. Considerations for the management and triage of ocular oncology cases during the COVID-19 pandemic. Ocul Oncol Pathol. 2020;6:219–22. doi: 10.1159/000507734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sengupta S, Honavar SG, Sachdev MS, Sharma N, Kumar A, Ram J, et al. All India Ophthalmological Society-Indian Journal of Ophthalmology consensus statement on preferred practices during the COVID-19 pandemic. Indian J Ophthalmol. 2020;68:711–24. doi: 10.4103/ijo.IJO_871_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidelines for notifying COVID-19 affected persons by private institutions. COVID-19 case definitions Ministry of Health and Family Welfare Government of India. [Last accessed on 2020 May 25]. Available from: https://wwwmohfwgovin/pdf/GuidelinesfornotifyingCOVID-19affectedpersonsby PrivateInstitutionspdf .
- 6.Kang S, Thomas PBM, Sim DA, Parker RT, Daniel C, Uddin JM. Oculoplastic video-based telemedicine consultations: Covid-19 and beyond? Eye (Lond) 2020:1–3. doi: 10.1038/s41433-020-0953-6. doi: 10.1038/s41433-020-0953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan M, Qin Y, Xue X, Zhu S. Death from Covid-19 of 23 health care workers in China. N Engl J Med. 2020 doi: 10.1056/NEJMc2005696. doi: 101056/NEJMc2005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang D, Xu H, Rebaza A, Sharma L, Dela Cruz CS. Protecting health care worker from subclinical coronavirus infection. Lancet Respir Med. 2020;8:e13. doi: 10.1016/S2213-2600(20)30066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novel Coronavirus Disease 2019 (COVID-19): Additonal guidelines on rational use of Personal Protective Equipment (setting approach for Health functionaries working in non-COVID areas) Ministry of Health and Family Welfare Government of India. [Last accessed on 2020 May 25]. Available from https://wwwmohfwgovin/pdf/UpdatedAdditional guidelinesonrationaluseofPersonalProtectiveEquip mentsettingapproachforHealthfunctionariesworkinginnon COVID19areaspdf .
- 10.Saleem SM, Pasquale LR, Sidoti PA, Tsai JC. Virtual ophthalmology: Telemedicine in a Covid-19 era. Am J Ophthalmol. 2020 doi: 10.1016/j.ajo.2020.04.029. doi: 10.1016/j.ajo.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong W, Kong Y, See J, Kan R, Lim M, Chen O, et al. Anaesthetic management of patients with COVID-19: Infection prevention and control measures in the operating theatre. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.04.014. doi: 10.1016/j.bja.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soma M, Jacobson I, Brewer J, Blondin A, Davidson G, Singham S. Operative team checklist for aerosol generating procedures to minimise exposure of healthcare workers to SARS-CoV-2. Int J Pediatr Otorhinolaryngol. 2020 doi: 10.1016/j.ijporl.2020.110075. doi: 10.1016/j.ijporl.2020.11.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healy DW, Cloyd BH, Brenner MJ, Kupfer RA, Anam KS, Schechtman SA. The COVID-19 pandemic: implications for the head and neck anesthesiologist. J Head Neck Anesth. 2020;4:10. [Google Scholar]
- 14.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–4. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalski LP, Sanabria A, Ridge JA, Ng WT, de Bree R, Rinaldo A, et al. COVID-19 pandemic: Effects and evidence-based recommendations for otolaryngology and head and neck surgery practice? Head Neck. 2020;42:1259–67. doi: 10.1002/hed.26164. doi: 10.1002/hed. 26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weld KJ, Dryer S, Ames CD, Cho K, Hogan C, Lee M, et al. Analysis of surgical smoke produced by various energy-based instruments and effects on laproscopic visibility. J Endoeurol. 2007;21:347–351. doi: 10.1089/end.2006.9994. [DOI] [PubMed] [Google Scholar]
- 17.Lu CW, Liu XF, Jia ZF. 2019-nCOV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggers M, Eickmann M, Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA) Infect Dis Ther. 2015;4:491–501. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan M, Bouffet E, Rodriguez-Galindo C, Luna-Fineman S, Khan MS, Kearns P, et al. The COVID-19 pandemic: A rapid global response for children with cancer from SIOP, COG, SIOP-E, SIOP-PODC, IPSO, PROS, CCI, and St Jude Global. Pediatr Blood Cancer. 2020;67:e28409. doi: 10.1002/pbc.28409. doi: 10.1002/pbc.28409. Epub 2020 May 13. PMID: 32400924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimaras H, Corson T, Cobrinik D, White A, Zhao J, Munier FL, et al. Retinoblastoma. Nat Rev Dis Primers. 2015;1:15021. doi: 10.1038/nrdp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manjandavida FP, Stathopoulos C, Zhang J, Honavar SG, Shields CL. Intra-arterial chemotherapy in retinoblastoma-A paradigm change. Indian J Ophthalmol. 2019;67:740–54. doi: 10.4103/ijo.IJO_866_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaliki S, Shields CL. Uveal melanoma: Relatively rare but deadly cancer. Eye (Lond) 2017;31:241–57. doi: 10.1038/eye.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shmueli O, Chowers I, Levy J. Current safety preferences for intravitreal injections during COVID-19 pandemic? Eye (Lond) 2020:1–3. doi: 10.1038/s41433-020-0925-x. doi: 10.1038/s41433-020-0925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grommes C, Rubenstein JL, DeAngelis LM, Ferreri AJM, Batchelor TT. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019;21:296–305. doi: 10.1093/neuonc/noy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulay K, Narula R, Honavar SG. Primary vitreoretinal lymphoma. Indian J Ophthalmol. 2015;63:180–6. doi: 10.4103/0301-4738.156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalvin LA, Lim LS, Chang M, Udyaver S, Mazloumi M, Vichitvejpaisal P, et al. Circumscribed choroidal hemangioma: Clinical features and outcomes by age category in 458 cases. Saudi J Ophthalmol. 2019;33:219–28. doi: 10.1016/j.sjopt.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandal S, Naithani P, Venkatesh P, Garg S. Intravitreal bevacizumab (avastin) for circumscribed choroidal hemangioma. Indian J Ophthalmol. 2011;59:248–51. doi: 10.4103/0301-4738.81051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Dorado Garcia H, Scheer M, Henssen AG. Current and future treatment strategies for rhabdomyosarcoma. Front Oncol. 2019;9:1458. doi: 10.3389/fonc.2019.01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J, Kim YD, Woo KI, Sobti D. Long-term outcomes of eye-sparing surgery for adenoid cystic carcinoma of lacrimal gland. Ophthalmic Plast Reconstr Surg. 2018;34:74–8. doi: 10.1097/IOP.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 30.Sundar G, Manjandavida FP. Excision of Eyelid Tumors: Principles and Techniques. In: Chaugule S, Honavar S G, Finger P T, editors. Surgical Ophthalmic Oncology. New York: Springer; 2019. pp. 15–32. [Google Scholar]
- 31.Honavar SG, Manjandavida FP. Tumors of the ocular surface: A review. Indian J Ophthalmol. 2015;63:187–203. doi: 10.4103/0301-4738.156912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.COVID-19: Safe handling and processing for samples in laboratory. [Last accessed on 2020 May 20]. Available from: https://wwwgovuk/government/publications/wuhan-novel-coronavirus-guidance-for-clinical-diagnostic-laboratories/wuhan-novel-coronavirus-handling-and-processing-of-laboratory-specimens .
- 33.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21:335–7. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen SA, Thompson LA. Coronavirus Disease 2019 and Children: What Pediatric Health Care Clinicians Need to Know. JAMA Pediatr. 2020 Apr 3; doi: 10.1001/jamapediatrics.2020.1224. doi: 101001/jamapediatrics20201224 Epub ahead of print PMID: 32242896. [DOI] [PubMed] [Google Scholar]
- 35.Bouffet E, Challinor J, Sullivan M, Biondi A, Rodriguez-Galindo C, Pritchard-Jones K. Early advice on managing children with cancer during the COVID-19 pandemic and a call for sharing experiences? Pediatr Blood Cancer. 2020;67:e28327. doi: 10.1002/pbc.28327. doi: 10.1002/pbc. 28327. [DOI] [PubMed] [Google Scholar]
- 36.Lee AWM, Xu Z, Lin L, Xu J, Yang J, Lee E, et al. Advocacy to provide good quality oncology services during the COVID-19 pandemic – Actions at 3-levels. Radiother Oncol. 2020;149:25–9. doi: 10.1016/j.radonc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagar H, Formenti SC. Cancer and COVID-19 - potentially deleterious effects of delaying radiotherapy. Nat Rev Clin Oncol. 2020;17:332–4. doi: 10.1038/s41571-020-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filippi AR, Russi E, Magrini SM, Corvo R. Letter from Italy: First practical indications for radiation therapy departments during COVID-19 outbreak. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2020.03.007. pii: S0360-3016(20)30930-5 doi 10.1016/j.ijrobp.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith DE, Ghosh S, O'Leary M, Chu C, Brody D. Hypofractionated radiosurgery for meningiomas—A safer alternative for large tumors? Transl Cancer Res. 2014;3:367–72. [Google Scholar]