Abstract
Background
Bacteria belonging to the Salmonella genus are major concern for health, as they are widely reported in many cases of food poisoning. The use of antibiotics remains a main stream control strategy for avian salmonellosis as well as typhoid and paratyphoid fevers in humans. Due to the growing awareness about drug resistance and toxicities, the use of antibiotics is being discouraged in many countries whilst advocating potent benign alternatives such as phyto-based medicine. The objective of this work was to isolate, characterise the bioactive compounds of Canarium schweinfurthii; and evaluate their anti-salmonellal activity.
Methods
The hydro-ethanolic extract of Canarium schweinfurthii was fractionated and tested for their anti-salmonellal activity. The most active fractions (i.e. chloroform and ethyl acetate partition fractions) were then explored for their phytochemical constituents. Fractionation on normal phase silica gel column chromatography and size exclusion chromatography on Sephadex LH-20 led to the isolation of four compounds (maniladiol, scopoletin, ethyl gallate and gallic acid) reported for the first time in Canarium schweinfurthii.
Results
Result indicated that scopoletin and gallic acid had greater activity than the crude extracts and partition fractions. Among the isolated compounds, scopoletin showed the highest inhibitory activity with a MIC of 16 μg/ml against Salmonella Typhimurium and Salmonella Enteritidis.
Conclusions
The overall results of this study indicates that the hydro-ethanolic extract as well as some of isolated compounds have interesting anti-salmonellal activities that could be further explored for the development of potent therapy for salmonellosis. Furthermore, the study adds credence to the folkloric applications of the plant.
Keywords: Ethnomedicine, Salmonellosis, Canarium schweinfurthii, Natural substances
Background
Salmonella is a major source of food-borne illness in humans and a major cause of morbidity, mortality and economic loss both in the poultry and human health sectors. The disease caused by bacteria belonging to Salmonella genus is often called salmonellosis. This pathology remains one of the limiting factors in the development of poultry farming especially in developing countries of Asia and Africa [1] because it causes huge direct and indirect losses [2]. The genus Salmonella is very diverse and today it is composed of more than 2500 serotypes, many of which cause enteric diseases in humans and animals. Many serotypes of Salmonella can infect chickens and some serotypes are well adapted although, Salmonella Gallinarum and Salmonella Pullorum cannot be transmitted to human. However, some serotypes can infect both poultry and human and among these serotypes Salmonella Enteritidis and Salmonella Typhimurium are more prevalent in chickens and notable in human disease outbreaks. These serotypes are most commonly implicated in the human Salmonella infections [3, 4]. The poultry is considered one of the main sources of Salmonella human infection usually through poorly cooked foods [5–9] and foodstuffs of avian origin [10]. Salmonella infection represents a considerable burden in both developing and developed countries. Ubiquitous non-typhoidal Salmonella (NTS) which includes Salmonella Enteritidis and Salmonella Typhimurium annually cause more than 93.8 million illnesses and 155,000 deaths each year [11]. Salmonella Enteritidis and Salmonella Typhimurium, both NTS are the most frequently occurring serotypes from poultry causing infection in human [3]. Similarly, each year worldwide, typhoidal serotypes among which Salmonella Typhi and Salmonella Paratyphi, cause approximately 22 million cases of typhoid and 216,500 deaths [12].
Resistance of Salmonella to commonly used antimicrobial agents is increasing both in the veterinary and public health sectors and has emerged as a global health challenge. Several Salmonella serotypes are multidrug resistant, and there is evidence of the spread of these strains from animals to humans. Antimicrobial resistance in NTS is considered one of the major public health threats related with food-animal production, as well as the poultry production chain and poultry meat, which is an additional concern in the management of salmonellosis [13]. Many authors [14–17] have reported that several strains of Salmonella isolated from chicken have shown resistance to many antibiotics commonly used in human medicine and some of these strains have been found in humans [14]. Moreover, antibiotic residues in poultry products intended for consumption may lead to hypersensitivity or poisoning in consumers. Due to the growing awareness of resistance issues, the use of antibiotics is strongly discouraged in many countries whilst encouraging the use of plants as a better alternative due to their diverse nature of bioactive principles [18–20]. The large majority of salmonellosis in humans is carried by foodstuffs; mainly those of avian origin [10, 20, 21], therefore controlling avian salmonellosis by using plant could significantly reduce the prevalence of human gastroenteritis [20]. Several studies have focused on medicinal plants as new control strategies for human salmonellosis [22, 23] or avian salmonellosis [24–28]. But, to our knowledge, no phytomedicine has yet been formulated to control avian salmonellosis. Canarium schweinfurthii Engl. (Burseraceae), is a tree with a cylindrical bole, native to tropical West Africa and grows to about 50 m high [29]. This plant is mainly found in equatorial forest regions from Cameroon, Central African Republic, Gabon to Congo [30] and is used in folk medicine for the treatment of various diseases including malaria, diarrhea and Typhoid fever [31, 32]. Previous studies of Sokoudjou et al. [20, 28] showed that the hydroethanolic extracts of Canarium schweinfurthii were active both in vitro and in vivo against several serotypes of Salmonella. The objective of this work was to isolate, characterise the bioactive compounds of Canarium schweinfurthii; and evaluate their anti-salmonellal activity.
Methods
General experiment
Reagents which include ammonium cerium sulphate, were of analytical grade. Solvents were distilled before being used (St Louis, MO, USA). Thin Layer Chromatography (TLC) was performed on pre-coated silica gel with thickness 0.20 mm 60 F254 plates (MerckKGaA, Germany) and viewed under the UV light (254 and 365 nm). NMR analyses which included 1H NMR, 13C NMR, DEPT 90, DEPT 135, 2D NMR (COSY, HSQC), NOESY and ROESY were performed using deuterated solvents (Acétone-d6, CD3OD and/or CDCl3) on 400 MHz NMR (Ascend™ 400, Bruker) with TMS as internal reference. ESI-MS spectra of the compounds were recorded on a Bruker-Ion Trap MS (MicroTOF-Q mass spectrometer, Bruker) using the positive mode.
Plant collection, identification and extraction
Canarium schweinfurthii stem bark was harvested in West region of Cameroon and identified at the National Herbarium at Yaoundé-Cameroon, where a voucher specimen was deposited under the reference Number 16929/SRF/Cam. The air-dried plant material (3 Kg) was powdered and macerated at room temperature with 12 L of ethanol-water system (50/50, v/v). After 48 h, the mixture was filtrated using Whatman №1 filter paper. The filtrate was evaporated using a Rotary evaporator (Büchi R200) at reduced pressure to afford the crude extract (265 g, 8.8%).
We needed no permission to collect the sample since Canarium schweinfurthii is not a protected species in Cameroon.
Fractionation and isolation of bioactive compounds of Canarium schweinfurthii
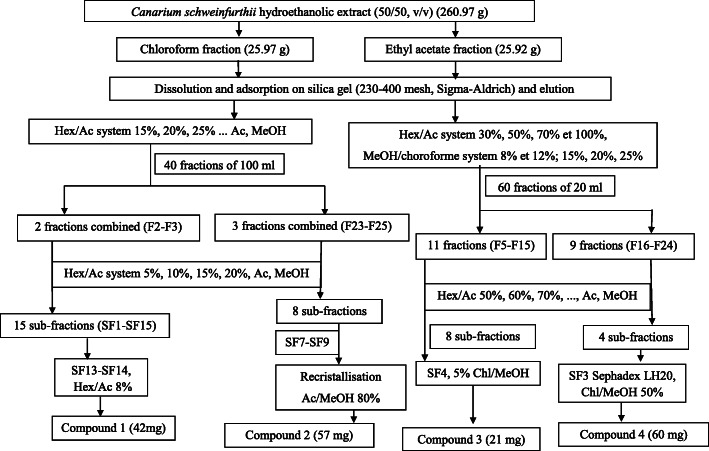
The profiling of the hydro-ethanolic extract of Canarium schweinfurthii on TLC plates with several solvent systems showed no promising separation. In order to facilitate isolation, 260 g of extract was dissolved in distilled water (700 mL) and successively extracted with hexane (500 mL × 2), chloroform (500 mL × 2), ethyl acetate (500 mL × 2) and n-butanol (500 mL × 2) yielding respectively 5.56 g, 25.97 g, 25.92 g and 90.89 g of fractions after evaporation to dryness. These partition fractions were explored for their antibacterial activity and only the most active fractions were selected for the isolation of bioactive principles. Figure 1 below shows the protocol for isolating the bioactive principles of Canarium schweinfurthii.
Fig. 1.
Flow chart for the isolation of compounds from the hydroethanolic extract of Canarium schweinfurthii
Part of Chloroform fraction (23 g) was subjected to silica gel column chromatography using n-hexane-EtOAc (85:15 → 00:100) and MeOH, gradient elution. 40 sub-fractions of 100 mL each were collected and combined on the basis of their TLC profiles to give 5 fractions: A (1–3), B (4–12), C (13–22), D (23–25) and E (25–40). Sub-fraction A (4.5 g) was purified on silica gel column chromatography eluted with n-hexane-EtOAc (95:5 → 80:20) to give compound 1 (42 mg). The purification of sub-fraction D (4 g) on silica gel column chromatography using n-hexane-EtOAc (70:30 → 20:80) afforded compound 2 (57 mg) which was recrystallized in EtOAc-MeOH (20:80).
Part of EtOAc fraction (23 g) was also subjected to silica gel column chromatography eluted with a gradient of n-hexane-EtOAc (70:30 → 00:100) and chloroform-MeOH (92:5 → 75:25) to afford 60 sub-fractions of 20 mL which were combined to four sub-fractions: F (1–4), G (5–15) H (16–24), I (25–60) on the basis of their TLC profile. Sub-fraction G (3.5 g) was purified on silica gel column chromatography using n-hexane-EtOAc (50:50 → 00:100) to yield compound 3 (21 mg) while purification of sub-fraction H (2.6 g) on sephadex LH-20 column eluted with chloroform-methanol (50:50) afforded compound 4 (60 mg). The structures of the isolated compounds were elucidated by combining various techniques comprising 1D Nuclear Magnetic Resonance (NMR):1H NMR, 13C-NMR, DEPT 90, DEPT 135 and 2D NMR (COSY, HSQC), NOESY and ROESY as well as Mass Spectrometry analysis (TOF-ESI-MS). The data of the established structures were compared with those existing in literature.
Anti-salmonellal assay
Chemicals for anti-salmonellal assay
Ciprofloxacin (BDH Chemicals, England) and oxytetracyclin (BDH Chemicals, England) were used as reference antibiotics. P-iodonitrotetrazolium chloride (Sigma-Aldrich, Germany) was used as microbial growth indicator.
Test bacteria and culture media
Three clinical isolates (Salmonella Typhi, Salmonella Enteritidis and Salmonella Typhimurium from Pasteur Center, Yaoundé-Cameroon) and one bacterial strain (Salmonella Typhi ATCC6539 from American Type Culture Collection) were used for antimicrobial evaluation. The culture media used were Salmonella-Shigella Agar (SSA from HiMedia Laboratories, India) and Mueller Hinton Broth (MHB from HiMedia Laboratories, India).
Determination of minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs)
The MIC values of the fractions obtained from partition and compounds from Canarium schweinfurthii were determined in 96-wells microplates using rapid INT colorimetric assay [33, 34]. Briefly, each sample was dissolved in 5% Dimethyl-sulfoxide (DMSO)/MHB. The obtained solution was then added to 100 μL of MHB, and followed by two-fold serial dilution. Then 100 μL of inoculum (1.5 × 106 CFU/mL) prepared in MHB were added to each well except the negative control wells. The plates were covered with a sterile plate sealer and incubated at 37 °C for 18 h. The wells containing either MHB or MHB and 100 μL of inoculum served as control. After the incubation, 40 μL of INT (0.2 mg/mL) was added to each well and plates were re-incubated at 37 °C for 30 min, and the MIC of each sample was recorded. MIC was defined as the lowest concentration of the sample that prevented change in colour and exhibited complete inhibition of microbial growth. The MBC was determined by adding 50 μL aliquots of the preparations, which did not show any growth after incubation during MIC assays, to 150 μL of MHB. These preparations were then incubated at 37 °C for 48 h. The MBC was recorded as the lowest concentration of test sample which did not produce a colour change after addition of INT as previously described. The tests were performed in triplicates.
Results
The yield and physical appearance of each partition fraction of Canarium schweinfurthii extract are as shown below (Table 1).
Table 1.
Yield and physical appearance of each partition fraction of Canarium schweinfurthii stem barks extracts
| Partitioned fractions | Yields (%) | Physical characteristics | |
|---|---|---|---|
| Color | Physical appearance | ||
| Hexane fraction | 2 | Green | Oily |
| Chloroform fraction | 10 | Dark brown | Oily |
| Ethylacetate fraction | 10 | Brown | Solid |
| n-butanol fraction | 34 | Blackish | Cristalline powder |
| Residual fraction | 38 | Blackish | Sticky semi-solid (Syrup) |
Characterization of isolated compounds
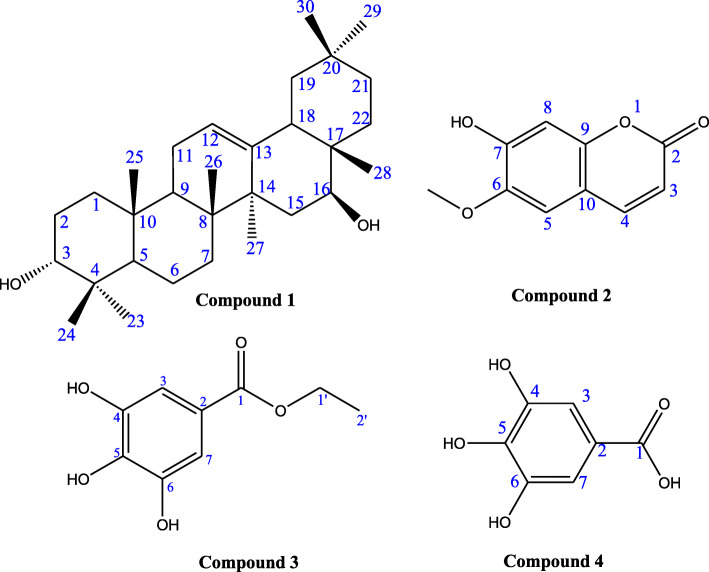
The four compounds isolated and characterized from the stem bark extract of Canarium schweinfurthii are as depicted in Fig. 2.
Fig. 2.
Chemical structures of isolated compounds from Canarium schweinfurthii stem barks extract
Compound 1: Maniladiol (42 mg) white solid, soluble in methanol, with molecular weight 442 calculated for C30H50O2 (ESI-MS: m/z 465.1 [M + Na]).
Compound 2: Scopoletin (57 mg) yellowish crystals, soluble in acetone, with molecular weight 192 calculated for C10H8O4 (ESI-MS: m/z 214.9 [M + Na]).
Compound 3: Ethyl gallate (21 mg) white solid, soluble in methanol, with molecular weight 198 calculated for C9H10O5 (ESI-MS: m/z 221.0 [M + Na]).
Compound 4: Gallic acid (60 mg) white solid, soluble in methanol, with molecular weight 170 calculated for C7H6O5 (ESI-MS: m/z 193.1 [M + Na]).
The 1H-NMR and 13C-NMR data of isolated compounds are presented in the Tables 2, 3, 4 and 5.
Table 2.
1H-NMR and 13C-NMR of compound 1
| Compound 1 | Maniladiol, Quijano et al. [35] | |||
|---|---|---|---|---|
| Positions |
δc (CD3OD+ CDCl3, 100 MHz) |
δH (mult; J) (CD3OD+ CDCl3, 400 MHz) |
δc (CD3Cl, 125 MHz) |
δH (mult; J) (CD3Cl, 500 MHz) |
| 1 | 32.9 |
1.40 (1H;m) 1.12 (1H; m) |
38.5 |
1.64 (1H; m) 0.98 (1H; m) |
| 2 | 24.5 |
1.99 (1H; m) 1.52 (1H; m) |
27.1 |
1.62 (1H; m) 1.58 (1H; m) |
| 3 | 75.4 | 3.35 (1H;dd; 11.9; 4.8) | 78.9 | 3.22 (1H; dd; 11.5; 4.5) |
| 4 | 37.1 | – | 38.7 | – |
| 5 | 48.8 | 1.30 (1H;m) | 55.1 | 0.74 (1H; dd; 11.5; 1.5) |
| 6 | 18.0 |
1.45 (1H; m) 1.44 (1H; m) |
18.3 |
1.58 (1H; t; 3.6) 1.41 (1H; dd; 15.5; 12.0) |
| 7 | 32.4 |
1.62 (1H; m) 1.38 (1H; m) |
32.6 |
1.54 (1H; t; 3.5) 1.33 (1H; t; 3.6) |
| 8 | 39.9 | – | 39.8 | – |
| 9 | 46.5 | 1.06 (1H; m) | 46.8 | 1.51 (1H; dd; 11.0; 6.5) |
| 10 | 36.6 | – | 37.3 | – |
| 11 | 23.4 | 1.91 (2H; m) | 23.5 |
1.92 (1H; ddd; 18.5; 11.0; 3.5) 1.86 (1H; ddd; 18.5; 7.0; 4.0) |
| 12 | 122.3 | 5.26 (1H; t; 3.4) | 122.3 | 5.25 (1H; t; 3.5) |
| 13 | 143.7 | – | 143.5 | – |
| 14 | 43.5 | – | 43.7 | – |
| 15 | 34.9 |
1.71 (1H; m) 1.26 (1H; m) |
35.5 |
1.67 (1H; d; 13.0) 1.31 (1H; dd; 13.0; 5.0) |
| 16 | 65.0 | 4.16 (1H; dd; 11.5; 4.9) | 66.0 | 4.20 (1H; dd; 11.5; 5.0) |
| 17 | 37.0 | – | 36.8 | – |
| 18 | 49.2 | 2.16 (1H; dd; 11.5; 4.9) | 49.0 | 2.15 (1H; dd; 14.0; 4.5) |
| 19 | 46.5 |
1.71 (1H; m) 1.06 (1H; m) |
46.5 |
1.68 (1H; t; 14.0) 1.06 (1H; ddd; 13.5; 4.5; 2.5) |
| 20 | 30.4 | – | 30.9 | – |
| 21 | 34.0 |
1.41 (1H; m) 1.13 (1H; m) |
34.1 |
1.36 (1H; t; 3.7) 1.15 (1H; t; 3.6) |
| 22 | 30.5 |
1.91 (1H; m) 1.88 (1H; m) |
30.5 |
1.83(1H; t; 3.4) 1.20(1H; t; 3.5) |
| 23 | 27.8 | 0.95 (3H; s) | 28.0 | 1.00 (3H; s) |
| 24 | 21.7 | 0.86 (3H; s) | 15.6 | 0.79 (3H; s) |
| 25 | 14.8 | 0.99 (3H; s) | 15.5 | 0.94 (3H; s) |
| 26 | 16.24 | 1.03 (3H; s) | 16.8 | 0.99 (3H; s) |
| 27 | 26.4 | 1.27 (3H; s) | 27.1 | 1.22(3H; s) |
| 28 | 21.4 | 0.80 (3H; s) | 21.4 | 0.80 (3H; s) |
| 29 | 32.6 | 0.90 (3H; s) | 33.2 | 0.89 (3H; s) |
| 30 | 23.2 | 0.92 (3H; s) | 23.9 | 0.90 (3H; s) |
Table 3.
1H-NMR and 13C-NMR of compound 2
| Compound 2 | Scopoletin, Mogana et al. [36] | |||
|---|---|---|---|---|
| Positions |
δC (acétone-d6, 100 MHz) |
δH (mult; J) (acétone-d6, 400 MHz) |
δc (CD3Cl, 100 MHz) |
δH (mult; J) (CD3Cl, 400 MHz) |
| 1 | – | – | – | – |
| 2 | 160.4 | – | 161.6 | – |
| 3 | 112.5 | 6.20 (1H; d; 9.5) | 111.6 | 6.30 (1H; d; 9.5) |
| 4 | 143.6 | 7.86 (1H; d; 9.5) | 143.3 | 7.63 (1H; d; 9.5) |
| 5 | 102.8 | 6.81 (1H; s) | 103.2 | 6.87 (1H; s) |
| 6 | 144.9 | – | 144.6 | – |
| 7 | 150.8 | – | 150.2 | – |
| 8 | 108.9 | 7.20 (1H; s) | 107.4 | 6.95 (1H; s) |
| 9 | 150.0 | – | 149.7 | – |
| 10 | 112.1 | – | 113.5 | – |
| 6-OCH3 | 55.9 | 3.92 (3H; s) | 56.4 | 3.98 (3H; s) |
| 7-OH | – | 8.78 (1H; s) | – | – |
Table 4.
1H-NMR and 13C-NMR of compound 3
| Compound 3 | Ethyl gallate, Ooshiro et al. [37] | |||
|---|---|---|---|---|
| Positions |
δc (CD3OD, 100 MHz) |
δH (mult; J) (CD3OD, 400 MHz) |
δc (CD3OD, 150 MHz) |
δH (mult; J) (CD3OD, 600 MHz) |
| 1 | 168.8 | – | 168.5 | – |
| 2 | 121.7 | – | 121.7 | – |
| 3/7 | 110.0 | 7.07 (2H; s) | 110.0 | 7.04 (2H; s) |
| 4/6 | 146.2 | – | 146.4 | – |
| 5 | 139.6 | – | 139.7 | – |
| 1’ | 61.6 | 4.28 (2H; q; 7.1) | 61.6 | 4.28 (2H; q; 7.3) |
| 2’ | 14.7 | 1.35 (3H; t; 7.1) | 14.6 | 1.33 (3H; t; 7.3) |
Table 5.
1H-NMR and 13C-NMR of compound 4
| Compound 4 | Gallic acid, Chanwitheesuk et al. [38] | |||
|---|---|---|---|---|
| Positions |
δc (CD3OD, 100 MHz) |
δH (mult; J) (CD3OD, 400 MHz) |
δc (acétone-d6, 100 MHz) |
δH (mult; J) (acétone-d6, 400 MHz) |
| 1 | 168.8 | – | 167.3 | – |
| 2 | 120.7 | – | 120.8 | – |
| 3/7 | 108.0 | 7.08 (2H; s) | 109.1 | 7.15 (2H; s) |
| 4/6 | 145.0 | – | 144.9 | |
| 5 | 138.1 | – | 137.7 | |
Anti-salmonellal activity of partition fractions and isolated compounds from stem barks extract of Canarium schweinfurthii
Table 6 shows the inhibition parameters (MIC, MBC, MBC/MIC ratio) of the crude extract, partition fractions and isolated compounds of Canarium schweinfurthii against pathogenic Salmonella. The isolated compounds have variable activity (16 ≤ MIC≤1024 μg/mL) on the tested Salmonella serotypes. It appears that the activity of isolated compounds is greater than those of the crude extract and partitions. Among the partition fractions, chloroform and ethyl acetate fractions showed the best anti-salmonellal activity while among the isolated compounds, scopoletin showed the highest inhibitory activity with a MIC of 16 μg/mL against Salmonella Typhimurium and Salmonella Enteritidis. MIC values of other compounds and extract ranged between 128 and 1024 μg/mL, while hexane and residual fractions are the less active substances with MICs of 512 or 1024 μg/mL.
Table 6.
Inhibition parameters (MIC, MBC) of partition fractions and isolated compounds from Canarium schweinfurthii against different test microorganisms
| Tested samples | Studied parameters (μg/mL) | Strain/isolates | |||
|---|---|---|---|---|---|
| ST | STs | STM | SE | ||
| HEE 50/50 | MIC | 256 | 128 | 64 | 128 |
| MBC | 512 | 512 | 256 | 512 | |
| MBC/MIC | 2 | 4 | 4 | 4 | |
| Hexane partition | MIC | 1024 | 1024 | 512 | > 1024 |
| MBC | > 1024 | > 1024 | > 1024 | > 1024 | |
| MBC/MIC | – | – | – | – | |
| Chloroform partition | MIC | 512 | 1024 | 256 | 1024 |
| MBC | 1024 | > 1024 | > 1024 | > 1024 | |
| MBC/MIC | 2 | – | – | – | |
| Ethyle acetate partition | MIC | 256 | 256 | 128 | 32 |
| MBC | > 1024 | 1024 | > 1024 | 128 | |
| MBC/MIC | – | 4 | – | 4 | |
| n-butanol partition | MIC | > 1024 | 1024 | 512 | > 1024 |
| MBC | > 1024 | > 1024 | > 1024 | > 1024 | |
| MBC/MIC | – | – | – | – | |
| Residual partition | MIC | > 1024 | > 1024 | > 1024 | 1024 |
| MBC | > 1024 | 512 | 256 | > 1024 | |
| MBC/MIC | – | – | – | – | |
|
Compound 1 Maniladiol |
MIC | 512 | 512 | 32 | 64 |
| MBC | > 1024 | > 1024 | 128 | 256 | |
| MBC/MIC | – | – | 4 | 4 | |
|
Compound 2 Scopoletin |
MIC | 32 | 32 | 16 | 16 |
| MBC | 64 | 128 | 32 | 64 | |
| MBC/MIC | 2 | 4 | 2 | 4 | |
|
Compound 3 Ethyl gallate |
MIC | 128 | 1024 | 64 | 1024 |
| MBC | > 1024 | > 1024 | > 1024 | > 1024 | |
| MBC/MIC | – | – | – | – | |
|
Compound 4 Gallic acid |
MIC | 32 | 32 | 64 | 128 |
| MBC | 32 | 32 | 128 | 256 | |
| MBC/MIC | 1 | 1 | 2 | 2 | |
| Oxytetracycline | MIC | 8 | 8 | 4 | 2 |
| MBC | 32 | 64 | 32 | 16 | |
| MBC/MIC | 4 | 8 | 8 | 8 | |
| Ciprofloxacine | MIC | 0,5 | 1 | 4 | 4 |
| MBC | 2 | 2 | 8 | 8 | |
| MBC/MIC | 4 | 2 | 2 | 2 | |
ST Salmonella Typhi, STs Salmonella Typhi ATCC6539, STM Salmonella Typhimurium, SE Salmonella Enteritidis, MIC Minimum inhibitory concentration, MBC Minimum bactericidal concentration.
Discussion
The antimicrobial effects of some plants and their extracts are well known today [39, 40]; the diversity of plant species is a valuable source for the search for new classes of antibiotics. These plants may proffer valuable alternative to address certain human and veterinary health challenges. It is in this perspective that the hydro-ethanolic extract of Canarium schweinfurthii has been explored for its anti-salmonellal activity and its bioactive compounds. Several plants are traditionally used against human salmonellosis [41–46] and avian salmonellosis [24–26, 47]. Plants with high anti-salmonellal potential that show promise for the control of avian salmonellosis include Aloe secundiflora [47], Thymus vulgaris [48], Curcuma longa and Scutellaria baicalensis [25] and Erica mannii [27]. Plant extracts as well as traditionally improved drugs are one of the promising ways to combat human salmonellosis [23, 47, 49]. Several authors [22, 23, 28, 50–53] have shown that plant extracts depending on their concentrations are active both in vitro and in vivo against several Salmonella serotypes. Most of these extracts treat salmonellosis in the same range of time as conventional medicines. These findings corroborate our results which showed that the hydroethanolic extract of Canarium schweinfurthii is active against Salmonella serotypes with MIC range from 64 to 128 μg/ml, moreover this extract have previously demonstrated an in vivo anti-salmonellal activity [20], curing avian salmonellosis on day 9 and with the doses 19 and 75 mg/kg bw of the extract. In addition to the therapeutic efficacy of the hydroethanolic extract of Canarium schweinfurthii, the antibacterial activity of its partitions was evaluated. Among the partitions, chloroform and ethyl acetate fractions showed the best anti-salmonellal activity. It also appears that the activity of isolated compounds is greater than those of the crude extract and partitions. This could be due to the low concentration of these compounds in the plant extract or to the antagonism effect of other compounds present in the same extract. The anti-salmonellal activity of plants is linked to the diversity and complexity of their secondary metabolites. The in vitro anti-salmonellal effect of hydroethanolic extract of Canarium schweinfurthii found in this study and its therapeutic efficacy [20] can be linked to a combined action of its secondary metabolites. Indeed, at the molecular level, compounds such as gallic acid and scopoletin found in plants belonging to Canarium genus [54] could act synergistically and could be partly responsible for the anti-infectious activity of Canarium schweinfurthii. In order to verify this possibility and to have a clear idea on the active principles of this plant, the fractionation of its stem bark extract was performed.
Gallic acid, ethyl gallate, scopoletin and maniladiol were isolated from the Canarium schweinfurthii stem bark extract, these compounds were reported for the first time in this medicinal plant species and belong to the classes of polyphenols, triperpenes and coumarins. From the previous reports [54], only gallic acid and scopoletin have been isolated from other plants belonging to the same genus as Canarium schweinfurthii and these compounds were reported to have antibacterial and antioxidant properties. The isolated compounds have variable activities (16 ≤ MIC≤1024 μg/mL) against the tested Salmonella serotypes. Among the pure isolated compounds, scopoletin showed the highest inhibitory activity with a MIC of 16 μg/mL against Salmonella Typhimurium and Salmonella Enteritidis. The activity of most of the isolated compounds was less than those of oxyphylline B (10 μg/mL) isolated from Zizyphus oxyphylla Edgew against Salmonella Typhi [55] and lespedin (12.25 μg/ml) isolated from Brillanta isialamium against Salmonella Typhi [56]. However the anti-salmonellal activity of gallic acid and scopoletin against Salmonella Typhi (32 μg/mL) was better than those of Bafoudiosbulbins A and Bafoudiosbulbins B isolated from Dioscorea bulbifera L. var. sativa [57]. These results corroborate the finding of Lunga et al. [44] who showed that the anti-salmonellal activity of isolated compounds from Paullinia pinnata Linn ranged from 0.781 to 100 μg/mL. According to the Kuete’s classification scale [39], the antibacterial activity of a compound is significant when the MIC< 10 μg/mL; moderate when 10 < MIC≤100 μg/mL and low when MIC> 100 μg/ml. With regard to this scale, the anti-salmonellal activities of the isolated compound from Canarium schweinfurthii are moderate (10 < MIC≤100 μg/mL). Scopoletin and gallic acid are significantly active against Salmonella Typhi, Salmonella Typhi ATCC6539 and Salmonella Typhimurium. These results corroborate those of Okoli et al. [58] who showed that 3β-hydroxylolean-12,18-diene isolated from Canarium schweinfurthii was active on Salmonella with a MIC of 12.5 μg/ml against Salmonella Typhi. It has been shown that in addition to its immunomodulatory effect [59], scopoletin reduces the intracellular survival of Salmonella Typhi within U937 human macrophage cell line [60]. Gallic acid has in addition to its in vitro and in vivo antibacterial effect against Salmonella Typhimurium [61, 62], an antioxidant activity. These compounds related properties corroborate the findings of Sokoudjou et al. [20] who reported that the ability of the extract of Canarium schweinfurthii to cure salmonellosis in broilers could be explained by its ability to directly kill Salmonella and/or boost the immune system of the host. The dosage of the compounds isolated from this plant can be used to normalize the extract during the phytomedicine evaluation and preparation.
Conclusion
Gallic acid, ethyl gallate, scopoletin and maniladiol were isolated from the Canarium schweinfurthii stem bark extract. These compounds were reported for the first time in this plant species. The four isolated compounds showed in vitro anti-salmonellal activity against Salmonella serotypes and particularly scopoletin was the most active and highly selective against both non-typhoidal Salmonella and typhoidal Salmonella with MIC of 16 or 32 μg/mL. The anti-salmonellal activity of the compounds isolated from Canarium schweinfurthii justifies the use of this plant in traditional medicine and confirms the anti-salmonellal effect of the hydroethanolic extract thus adding credence to its use in the treatment of avian salmonellosis. Further studies will be necessary to verify the in vivo activity of these compounds and to elucidate their mechanisms of action.
Supplementary information
Acknowledgements
Authors thank the researchers of the Natural Products Chemistry Laboratory of CUI, Abbottabad Campus, Pakistan for their useful suggestions.
Authors’ contributions
All the authors contributed to carry out this study. JBS was the principal investigator, OA and GSSN contributed to evaluate the anti-salmonellal activities. CNT, ANB and NK contributed to the fractionation purification and structural elucidation of isolated compounds. NK revised the manuscript, AK and DG co-supervised the work. All authors read and approved the final manuscript.
Funding
This research work was supported in part by The Academy of Sciences for the Developing World (TWAS) in collaboration with COMSATS University Islamabad (CUI) under grant FR number 3240299471 (TWAS-CIIT Postgraduate fellowship). The obtained fund was used for compound isolation and characterization.
Availability of data and materials
They are available as Supporting information.
Ethics approval and consent to participate
Not applicable in this section.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
Authors have declared that no competing interests exist.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12906-020-03100-5.
References
- 1.Rajagopal R, Mini M. Outbreaks of salmonellosis in three different poultry farms of Kerala, India. Asian Pac J Trop Biomed. 2013;3(6):496–500. doi: 10.1016/S2221-1691(13)60103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awa DN, Achukwi MD. Livestock pathology in the central African region: some epidemiological considerations and control strategies. Anim Health Res Rev. 2010;11:235–244. doi: 10.1017/S1466252309990077. [DOI] [PubMed] [Google Scholar]
- 3.Ali MZ, Sultana S. Avian salmonellosis, Newcastle disease and aspergillosis. Tech Rep. 2012;25. 10.13140/RG.2.1.1981.0404.
- 4.Threlfall EJ, Wain J, Peters T, Lane C, De Pinna E, Little CL, et al. Egg-borne infections of humans with Salmonella: not only an S. Enteritidis problem. Worlds Poult Sci J. 2014;70:15–26. doi: 10.1017/S0043933914000026. [DOI] [Google Scholar]
- 5.Carli KT, Unal CB, Caner V, Eyigor A. Detection of Salmonellae in chicken feces by a combination of tetrathionate broth enrichment, capillary PCR, and capillary gel electrophoresis. J Clin Microbiol. 2001;39:1871–1876. doi: 10.1128/JCM.39.5.1871-1876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crilly J, Power EP, Cowman HJ, Cryan B, Buckley JF. Epidemiology of Salmonella infection in the south of Ireland. Ir J OfAgri and Food Resea. 2001;40:215–226. [Google Scholar]
- 7.INFOSAN (Réseau international des autorités de sécurité sanitaire des aliments). Lutte contre les salmonelles à la source. Note d'information INFOSAN n° 03/2007– Salmonelles. 2007;4.
- 8.Danan C, Granier S, Bohnert M, Piquet C, Lalande F, Fremy S, et al. Surveillance active de la résistance aux antibiotiques des Salmonella isolées de la filière «poulet de chair » à différentes étapes de la chaine alimentaire (données 2008-2009) Bull Épidémiol Sante Anim Aliment. 2008;44:13–17. [Google Scholar]
- 9.Batz MB, Hoffmann S, Morris JG. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J Food Prot. 2012;75:1278–1291. doi: 10.4315/0362-028X.JFP-11-418. [DOI] [PubMed] [Google Scholar]
- 10.Mercado M, Ávila J, Rey M, Montoya M, Gamboa A, Carrascal AK, et al. BrotesporSalmonella spp., Staphylococcus aureus y Listeria monocytogenesasociados al consumo de pollo. Biomédica. 2012;32(3):375–385. doi: 10.7705/biomedica.v32i3.697. [DOI] [PubMed] [Google Scholar]
- 11.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kira M, O’Brien SJ, et al. The global burden of non typhoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 12.WHO (World Health Organisation). Fièvre typhoïde et utilisation des Vaccins en Asie du Sud-Est et la région de l’ouest du pacifique occidental, 2009-2013. Relevé Epidémiol Hebd 2014;89(40):429–440.
- 13.Antunes P, Mourão J, Campos J, Peixe L. Salmonellosis: the role of poultrymeat. Clin Microbiol Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Cardinale E, Perrier JD, Aidara A, Tall F, Coudert C, Gueye IL, et al. Identification d’une nouvelle salmonelle multirésistante dans Une viande de poulet de chair au Sénégal. Revue Élev Méd vét Pays trop. 2000;53(1):5–8. [Google Scholar]
- 15.Ngoune TL, Tanedjeu KS, Mbofung CMF. Impact de l’utilisation des antibiotiques Sur la sensibilité des bactéries pathogènes de poules dans la ville de Ngaoundéré. Cameroon J Exp Biol. 2009;5(2):52–61. [Google Scholar]
- 16.Chen MH, Hwang WZ, Wang SW, Shih YC, Tsen HY. Pulsed field gel electrophoresis (PFGE) analysis for multidrug resistant SalmonellaentericaserovarSchwarzengrund isolates collected in six years (2000-2005) from retail chicken meat in Taiwan. Food Microbiol. 2011;28(3):399–405. doi: 10.1016/j.fm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Lamas A, Fernandez-No IC, Miranda JM, Vazquez B, Cepeda A, Franco CM. Prevalence, molecular characterization and antimicrobial resistance of Salmonella serovars isolated from northwestern Spanish broiler flocks (2011–2015) Poult Sci. 2016;95:2097–2105. doi: 10.3382/ps/pew150. [DOI] [PubMed] [Google Scholar]
- 18.Bada-Alambedji R, Cardinal E, Biagui C, Akakpo AJ. Recherche de résidus de substances à activité antibactérienne dans la chair de poulet consommée dans la région de Dakar (Sénégal) Bull Acad Vet Fr. 2004;157(2):67–70. [Google Scholar]
- 19.Dahan M, Hanotaux P, Durand F, Liebert F. Encadrement des pratiques commerciales pouvant influencer la prescription des antibiotiques vétérinaires. RAPPORT N°RM2013-078P/IGF2013-M-006-02/CGAAER N°13014, 119. 2013;123.
- 20.Sokoudjou JB, Fodouop SPC, Djoueudam FG, Kodjio N, Kana JR, Fowa AB, et al. Antisalmonellal and antioxidant potential of hydroethanolic extract of Canarium schweinfurthii Engl. (Burseraceae) in Salmonella enterica serovar Typhimurium-infected chicks. Asian Pac J Trop Biomed. 2019;9(11):474–483. doi: 10.4103/2221-1691.270980. [DOI] [Google Scholar]
- 21.Patrick ME, Adcock PM, Gomez TM, Altekruse SF, Holland BH, Tauxe RV, et al. Salmonella Enteritidis infections, United States, 1985–1999. Emerg Infect Dis. 2004;10:1–7. doi: 10.3201/eid1001.020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tala DS, Gatsing D, Fodouop SPC, Fokunang C, Kengni F, Djimeli MN. In vivo anti-salmonella activity of aqueous extract of Euphorbia prostrate Aiton (Euphorbiaceae) and its toxicological evaluation. Asian Pac J Trop Biomed. 2015;5(4):310–318. doi: 10.1016/S2221-1691(15)30350-6. [DOI] [Google Scholar]
- 23.Kodjio N, Atsafack SS, Njateng GSS, Sokoudjou JB, Kuiate J-R, Gatsing D. Antioxidant effect of aqueous extract of Curcuma longa rhizomes (Zingiberaceae) in the typhoid fever induced in Wistarrats model. J Adv Med Pharm Sci. 2016;7(3):1–13. [Google Scholar]
- 24.Ayachi A, Alloui N, Bennoune O, Yakhlef G, DaasAmiour S, Bouzid W, et al. Antibacterial activity of some fruits; berries and medicinal herb extracts against poultry strains of Salmonella. American-Eurasian J Agric Environ Sci. 2009;6(1):12–15. [Google Scholar]
- 25.Varmuzova K, Matulova ME, Gerzova L, Cejkova D, Gardan-Salmon D, Panhéleux M, et al. Curcuma and Scutellaria plant extracts protect chickens against inflammation and Salmonella Enteritidis infection. Poultry Sci. 2015;94:2049–2058. doi: 10.3382/ps/pev190. [DOI] [PubMed] [Google Scholar]
- 26.Abiala M, Olayiwola J, Babatunde O, Aiyelaagbe O. Akinyemi. Evaluation of therapeutic potentials of plant extracts against poultry bacteria threatening public health. BMC Complement Altern Med. 2016;16(417):1–8. doi: 10.1186/s12906-016-1399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchoua N. Evaluation des activités antisalmonelles et antioxydantes des extraits de Erica mannii chez le poulet de chair. Thèse de Mater, Université de Dshang. Faculté des sciences, école doctorale des Sciences Fondamentales et Technologiques. 2016;97.
- 28.Sokoudjou JB, Njateng GSS, Fodouop SPC, Kodjio N, Atsafack SS, Fowa AB, et al. In vitro antisalmonellal and antioxidant activities of Canarium schweinfurthii stem bark extracts. Acad J Med Plants. 2018;6(10):331–341. [Google Scholar]
- 29.Kamdem RS, Wafo O, Yousuf S, Ali Z, Adhikari A, Rasheed S, Khan IA. Canarene: a triterpenoid with a unique carbon skeleton from Canarium schweinfurthii. Org Lett. 2011;13:5492–5495. doi: 10.1021/ol202217d. [DOI] [PubMed] [Google Scholar]
- 30.Coronel RH. Pili nut Canarium ovatum Engl. Promoting the conservation and use of underutilized and neglected crops, Inst. Plant. Genet. Crop Plant Res. Gaters/International Plant Genet. Resour. Institute, Rome, Italy. 1996;.
- 31.Koudou J, Abena AA, Ngaissona P, Bessiere JM. Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia. 2005;76:700–703. doi: 10.1016/j.fitote.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Yousuf S, Kamdem RS, Wafo P, Ngadjui BT, HoongKun F. A cocrystal of 3α-hydroxy-tirucalla-8,24-dien-21-oic acid and 3β-fluoro-tirucalla-7,24-dien-21-oic acid. Acta Cryst. 2011;E67:1015–1016. [Google Scholar]
- 33.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 34.Mativandlela SPN, Lall N, Meyer JJM. Antibacterial, antifungal and antitubercular activity of Pelargonium reniforme (CURT) and Pelargonium sidoides (DC) (Geraniaceae) root extracts. S Afr J Bot. 2006;72:232–237. doi: 10.1016/j.sajb.2005.08.002. [DOI] [Google Scholar]
- 35.Quijano L, Rios T, Fronczek FR, Fischer NH. The molecular structure of maniladiol from Baccharis salicina. Phytochemistry. 1998;49(7):2065–2068. doi: 10.1016/S0031-9422(98)00363-X. [DOI] [Google Scholar]
- 36.Mogana R, Teng-Jin K, Wiart C. Anti-inflammatory, anticholinesterase, and antioxidant potential of scopoletin isolated from Canarium patentinervium Miq. (Burseraceae Kunth). Evidence-Based Complement. Alt. Med. 2013;2013:1–7. [DOI] [PMC free article] [PubMed]
- 37.Ooshiro A, Hiradate S, Kawano S, Takushi T, Fujii Y, Natsume M, et al. Identification and activity of ethyl gallate as an antimicrobial compound produced by Geranium carolinianum. Weed Biol Manage. 2009;9:169–172. doi: 10.1111/j.1445-6664.2009.00335.x. [DOI] [Google Scholar]
- 38.Chanwitheesuk A, Teerawutgulrag A, Kilburn JD, Rakariyatham N. Antimicrobial gallic acid from Caesalpinia mimosoides Lamk. Food Chem. 2007;100:1044–1048. doi: 10.1016/j.foodchem.2005.11.008. [DOI] [Google Scholar]
- 39.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010;76:1–13. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 40.Kuete V, Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Frontiers in Pharmacol. 2010;1(123):1–19. doi: 10.3389/fphar.2010.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasha C, Sayeed S, Ali MS, Khan MZ. Antisalmonella activity of selected medicinal plants. Turk J Biol. 2009;33:59–64. [Google Scholar]
- 42.Kengni F, Tala DS, Djimeli MN, Fodouop SPC, Kodjio N, Magnifouet HN, et al. In vitro antimicrobial activity of Harungana madagascriensis and Euphorbia prostrate extracts against some pathogenic Salmonella sp. Int J Biol Chem Sci. 2013;7(3):1106–1118. doi: 10.4314/ijbcs.v7i3.17. [DOI] [Google Scholar]
- 43.Tsobou R, Mapongmetsem PM, Van Damme P. Medicinal plants used against typhoid fever in Bamboutos division, Western Cameroon. Ethnobotany Res Appl. 2013;11:163–174. [Google Scholar]
- 44.Lunga PK, Tamokou JDD, Fodouop SPC, Kuiate JR, Tchoumboue J, Gatsing D. Antityphoid and radical scavenging properties of the methanol extracts and compounds from the aerial part of Paullinia pinnata. Springer Plus. 2014;3(302):1–9. doi: 10.1186/2193-1801-3-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porte SM. Overview of folk medecine used for typhoid in India. Int J Res Ayurveda Pharm. 2014;5(2):219–224. doi: 10.7897/2277-4343.05243. [DOI] [Google Scholar]
- 46.Noghogne LR, Gatsing D, Kodjio N, Sokoudjou JB, Kuiate JR. In vitro antisalmonellal and antioxidant properties of Mangifera indica L. stem bark crude extracts and fractions. Br J Pharm Res. 2015;5(1):29–41. doi: 10.9734/BJPR/2015/13390. [DOI] [Google Scholar]
- 47.Waihenya RK, Mtambo MMA, Nkwengulila G, Minga UM. Efficacy of crude extract of Aloe secundiflora against Salmonella gallinarum in experimentally infected free-range chickens in Tanzania. J Ethnopharmacol. 2002;79(3):317–323. doi: 10.1016/S0378-8741(01)00397-X. [DOI] [PubMed] [Google Scholar]
- 48.Khan RU, Naz S, Nikousefat Z, Tufarelli V, Laudadio V. Thymus vulgaris: alternative to abtibiotics in poultry feed. World Poult Sci J. 2012;68:401–408. doi: 10.1017/S0043933912000517. [DOI] [Google Scholar]
- 49.Atsafack SS, Kodjio N, Njateng GSS. SokoudjouJB, Kuiate J-R, Gatsing D. anti-infectious and in vivo antioxidant activities of albizia gummifera aqueous stem bark extract against salmonella typhi-induced typhoid fever in rats. Int J Pharm. 2016;6(2):20–30. [Google Scholar]
- 50.Iroha IR, Ilang DC, Ayogu TE, Oji AE, Ugbo EC. Screening for anti-typhoid activity of some medicinal plants used in traditional medicine in Ebonyi state, Nigeria. Afr J Pharm Pharm. 2010;4(12):860–864. [Google Scholar]
- 51.Akinyemi OI, Dada EO. In vivo antityphoid activities and proximate analysis of ethanolic leaf extracts of Parquetina nigrescens. J Pharm Biol Sci. 2014;9(5):115–112. [Google Scholar]
- 52.Gul S, Eraj A, Ashraf Z. Glycyrrhiza glabra and Azadirachta indica against Salmonella Typhi: herbal treatment as an alternative therapy for typhoid fever. iMedPub J (Arch Med) 2015;7(6/4):1–5. [Google Scholar]
- 53.Ngoudjou TD, Arfat YM, Njateng GSS, Fokunang C, Nyemb JN, Nighat F, et al. GC/MS analysis, antisalmonellal potential of methanol leaf extracts of Tristemma mauritianum and effects on hematological parameters on Wistar rats infected with Salmonella Typhi. Int J Pharm. 2017;7(2):120–131. [Google Scholar]
- 54.Mogana R, Wiart C. Canarium L.: A phytochemical and pharmacological review. J Pharm Res. 2011;4(8):2482–2489. [Google Scholar]
- 55.Kaleem WA, Nisar M, Qayum M, Zia-Ul-Haq M, Adhikari A, Feo VD. New 14-membered cyclopeptide alkaloids from Zizyphus oxyphylla Edgew. Int J Mol Sci. 2012;13:11520–11529. doi: 10.3390/ijms130911520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamokou JDD, Kuiate JR, Tene M, Nwemeguela TJK, Tane P. The antimicrobial activities of extract and compounds isolated from Brillanta isialamium. Iran J Med Sci. 2011;36(1):24–31. [PMC free article] [PubMed] [Google Scholar]
- 57.Teponno RB, Tapondjou AL, Gatsing D, Djoukeng JD, Abou-Mansour E, Tabacchi R, et al. Bafoudiosbulbins a, and B, two anti-salmonellal clerodane diterpenoids from Dioscorea bulbiferaL. Var sativa. Phytochemistry. 2006;67(17):1957–1963. doi: 10.1016/j.phytochem.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 58.Okoli BJ, Ndukwe GI, Ayo RG, Habila JD. Inhibition of the developmental stages of ascaris suum and antimicrobial activity of 3β-hydroxylolean-12,18-diene isolated from the aerial parts of Canarium schweinfurthii (Engl) American Chem Sci J. 2016;11(3):1–11. doi: 10.9734/ACSJ/2016/21377. [DOI] [Google Scholar]
- 59.Manuele MG, Ferraro G, Arcos MLB, López P, Cremaschi G, Anesini C. Comparative immunomodulatory effect of scopoletin on tumoral and normal lymphocytes. Life Sci. 2006;79:2043–2048. doi: 10.1016/j.lfs.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 60.Acharya D, Bogati B, Risal P. Scopoletin reduces intracellular survival of Salmonella typhi within U937 human macrophage cell line in vitro. Sky J Microbiol Res. 2013;1(6):47–51. [Google Scholar]
- 61.Reyes AWB, Hong TG, Hop HT, Arayan LT, Huy TXN, Min W, et al. The in vitro and in vivo protective effects of tannin derivatives against Salmonella enterica serovar Typhimurium infection. Microb Pathog. 2017;109:86–93. doi: 10.1016/j.micpath.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 62.López-Romero JC, Valenzuela-Melendres M, Juneja VK, García-Dávila J, Camou JP, Peña-Ramos A, et al. Effects and interactions of gallic acid, eugenol and temperature on thermal inactivation of Salmonella spp. in ground chicken. Food Res Int. 2018;103:289–294. doi: 10.1016/j.foodres.2017.10.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
They are available as Supporting information.