Abstract
Metabolic Syndrome (MetS) has detrimental effects on the bladder, including detrusor underactivity. The progression and mechanism of disease are poorly understood. A swine model for diabetic bladder dysfunction (DBD) was established because of the pig's human-sized bladder and its ability to develop MetS by dietary modification alone. The hypothesis of this study is that this swine model will demonstrate oxidative stress associated with MetS, which contributes to both bladder fibrosis and detrusor underactivity (DU). Ossabaw pigs underwent dietary modification consisting of a hypercaloric, atherogenic diet for 10 mo to induce MetS, and were compared with a group of control (lean) pigs. Urodynamic studies were performed in both groups to confirm DU. Thiobarbituric acid reactive substances (TBARS) detected in the urine were used to measure oxidative stress activity in the urinary tract, and urinary IL17a was used to detect profibrotic activity. MetS was confirmed by assessing body weight, blood pressure, glucose tolerance, total cholesterol, and triglycerides. The MetS group exhibited an increase in the relative levels of urinary TBARS and IL17a. Bladder pressures at capacity were lower in the MetS group, suggesting DU. Histologic analysis of a cohort of control (lean) and MetS pigs revealed that as compared with the control pigs, the MetS pigs had significantly more collagen in the muscularis layer, but not in the submucosa or mucosa layer. In conclusion, the Ossabaw pig model for diet-induced MetS is associated with oxidative stress and profibrotic activity in the bladder, which results in DU. This has previously been shown in mice and rats, but never in pigs. This novel model will better represent human MetS and DBD because the mechanism and size of the pig bladder more closely resemble that of a human, resulting in a more valid model and facilitating further study into the signaling mechanisms responsible for this impairment.
Abbreviations: DU, detrusor underactivity; LUTS, IL17a, Interleukin –17a; Lower Urinary Tract Symptoms; NHANES II, National Health and Nutrition Examination Survey II; MetS, Metabolic syndrome; mRNA, Messenger RNA; ROS, Reactive Oxygen Species; SEM, Standard Error of the Mean; TBARS, Thiobarbituric acid reactive substances; T2D, Type 2 Diabetes Mellitus
Metabolic syndrome (MetS) is one of the most significant health epidemics in the United States and has many detrimental effects on health-related quality of life. One widely cited study noted a 22.9% prevalence of metabolic syndrome in the United States.3 Although much has been published on the cardiac effects of MetS, comparatively little has been published about the effects of MetS on the bladder. However, the bladder arguably presents much more commonly with MetS-related comorbidity than does the heart.9 MetS has been associated with significant Lower Urinary Tract Symptoms (LUTS) and decreased quality of life for people affected.32 The largest population-based study to address this, NHANES II, reported a significantly higher prevalence of incontinence (8.7% for those reporting type 2 Diabetes (T2D) compared with 5.3% for those without), but incontinence is only one component of LUTS.10 According to one longitudinal population-based investigation, LUTS are seen in up to 93% of diabetic women.6 Rabbit bladder models, currently used for the study of MetS, have demonstrated increased fibrosis and low-grade inflammation that can be reversed by the administration of testosterone.22 Although the signaling mechanisms responsible for these changes are poorly understood, the study demonstrates that, if detected early, the damage associated with MetS may be reversible.
Biomarkers for diabetic bladder dysfunction are unknown, and detection currently depends on the MetS patient complaining of LUTS to the clinician, at which point the damage may already be permanent. Urinary biomarkers may aid in early detection. Diabetes mellitus (DM) is closely related to metabolic syndrome, and although the detrimental effects of DM on the human bladder are widely recognized, the reported clinical symptoms vary significantly, highlighting the need for a better understanding of the pathogenesis of this condition. DM-associated LUTS, also known as diabetic cystopathy or diabetic bladder dysfunction (DBD), includes urinary incontinence,10 impaired bladder sensation,22 and most notably, detrusor under-activity, which can lead to urinary retention.5,18 The mechanism by which these changes occur remains unclear, but is likely multifactorial. Animal models have demonstrated that oxidative stress plays a major role and may lead to bladder fibrosis, as well as autonomic neuropathy.5,18 The focus of study has therefore been on the urothelium,15,16 and to a lesser degree, the detrusor muscle of the bladder,26 and more recently, suburothelial myofibroblasts.26
To our knowledge, the Ossabaw miniature pig (Sus scrofa domesticus, Ossabaw Island Hog) is the first large animal model for diet-induced bladder complications of MetS and may more closely model the human bladder dysfunction caused by MetS. Models for diabetic cystopathy consist mostly of small animals such as mice and rats, but these lack some advantages of large animal models. Large animal models for MetS more closely approximate human urinary physiology in terms of volume of urine voided and urinary frequency, as well as bladder pressure and bladder wall thickness, but have not been as widely pursued due to cost and growth time.9 Human urodynamic equipment is not small enough to be used in mice and rats, so studies done on rats and mice are typically done with catheters sewn into the bladder directly, using a wide variety of pressure-measuring equipment. Both factors diminish consistency across models and the need to pierce the bladder to insert a catheter likely alters its natural response. The human-sized bladder of swine allows the use of standard, clinical urodynamic equipment, so pigs represent a more appropriate model for human bladder dysfunction such as fibrosis. Bladder fibrosis has been shown to be reversible if detected early in small animal models, but never in a longitudinal, progressive model.7,22
Metabolic syndrome (MetS) causes systemic oxidative stress and has detrimental effects on the bladder, including underactivity of the detrusor muscle in the bladder, which is involved in urinary release and retention. The progression and mechanism of this condition are incompletely understood. The Ossabaw model for diabetic bladder dysfunction was developed to explore the hypothesis that oxidative stress present in MetS is associated with bladder fibrosis, as well as detrusor underactivity (DU). Some animal models have shown destruction of the urothelium layer in DM/MetS, as well as vasculopathy, but the correlation between actual fibrosis of detrusor smooth muscle (DSM) in MetS in association with functional urodynamic changes has not been conclusively demonstrated.12,13 Identifying biomarkers for fibrosis and oxidative stress in the urine in this model as MetS progresses may lead to early detection of diabetic bladder dysfunction at a stage before permanent replacement of the DSM with collagen.
Our hypothesis is that the Ossabaw miniature pig will develop MetS by dietary modification alone, and MetS pigs will demonstrate urodynamic findings consistent with DU, as well as histologic evidence of DSM fibrosis, validating the model for the study of DU and diabetic bladder dysfunction. Biomarkers for oxidative stress and urinary tract fibrosis may be identified in the urine and in turn may provide clues to the etiology of the condition.
Materials and Methods
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee (Protocol no.10523) at Indiana University School of Medicine, according to recommendations outlined by the National Research Council and the American Veterinary Medical Association Panel on Euthanasia.1,23 All animal care was compliant with AAALAC national standards, as established in the United States of America. Pigs were obtained from a closed, SPF breeding colony at Purdue University (West Lafayette, IN). This breeding colony has historically tested negative for Brucella spp., pseudorabies, vesicular stomatitis virus serovars Indiana and New Jersey, Mycoplasma hyopneumoniae, porcine reproductive and respiratory syndrome virus, porcine parvovirus, swine influenza virus serotypes H1N1 and H3N2, antibodies to transmissible gastroenteritis virus, and Leptospira interrogans serovars (canicola, grippotyphosa, hardjo, icterohaemorrhagiae, pomona, and bratislava).
All animals were housed singly in pens grouped in the same room for community under a 12:12-h light:dark cycle. Temperature was maintained at 20 to 22 °C throughout the study, and humidity was not controlled. Blood glucose and body weight were measured weekly. Pigs are sling trained with food rewards from an early age to facilitate blood draws and the measurement of body weight and blood pressure, minimizing stress to the animal and thus, any confounding effects it may have on the study data.24,25 Lipids and other serum blood tests were measured at multiple times (Antech Diagnostics, Fishers, IN), but only the results taken at the time of the terminal surgery were compared. Water was provided without restriction.
MetS Conditioning.
Ossabaw female miniature pigs underwent dietary modification consisting of a hypercaloric, atherogenic diet that contained of 43.1% calories from fat, 40.4% from carbohydrates, and 16.4% from protein (TestDiet Ossabaw High Fat and High Fructose Diet 5B4L, www.testdiet.com) for 10 mo to induce MetS. These animals were compared with a matched group of control pigs fed a lean diet consisting of 10.5% calories from fat, 71.0% from carbohydrates, and 18.5% from protein (LabDiet Minipig Grower HF 5L80, https://www.labdiet.com). Animals were fed once daily to encourage gorging behavior. This method also makes it more likely that the daily allotment of food is consumed completely.
Anesthesia, Urodynamic Measurement, Tissue Harvest, Euthanasia.
Urodynamic studies were performed to determine the degree of any differences between the groups after 10 mo of dietary treatment. MetS was confirmed by assessing body weight, blood pressure, serum glucose, total cholesterol, and triglycerides (Antech Diagnostics, Fishers, IN). Anesthesia/euthanasia was induced by administering an IM injection of 2.2 mg/kg xylazine and 5.5 mg/kg tiletamine/zolazepam (Fort Dodge Animal Health, Fort Dodge, IA). IV lines were placed, including a central line that was used for a cardiac related protocol. Pigs were then intubated, allowing the administration of 5% isoflurane in 100% oxygen; anesthesia was maintained on 2% to 4% isoflurane in 100% oxygen. The unconscious pig was then transferred to the operating room table and placed in supine position, prepped, and draped in the standard fashion for terminal surgery. Urodynamic bladder pressures at capacity were compared between the MetS group and the lean (control) group of pigs while under anesthesia at the time of terminal surgery (Laborie Medical Technologies, Ontario, Canada). Urodynamic findings were compared between lean (control) and MetS (experimental) groups at the end of the study period. At the conclusion of surgery, pigs were euthanized via cardiectomy.
Detrusor Muscle Processing and Analysis.
Bladder muscle specimens were taken from the dome of the bladder immediately after euthanasia. Specimens were then snap frozen in liquid nitrogen. They were processed and stained with Masson Trichrome and photographed at 10× power using an automated scanning microscope with a motorized stage powered by BioQuant image analysis software (Bioquant.com, Nashville, TN). A technician who was blind to the treatment of the pigs created all masks to demarcate the border between bladder mucosa, submucosa, and muscularis. The software was programmed to count the spectrum consistent with collagen stained with Masson Trichrome for each zone and compare with the non-collagenous tissue.
Urinary Biomarker Processing and Analysis.
A separate cohort of 8 identically treated MetS diet pigs that did not undergo urodynamics had their urine sampled regularly. During the initial 24 wk of dietary treatment, urine samples were taken monthly and flash frozen. At the end of the study period, assays for TBARS and IL17a were performed. A final measurement was taken at 56 wk, together with serum analysis and the measurement of vital signs. MetS related parameters were assessed using body weight, fasting serum glucose, blood pressure, total cholesterol, and triglycerides.
Thiobarbituric acid reactive substances (TBARS -lipid peroxidation byproducts) were measured in the urine because they are stable and can be detected even after most reactive oxygen species have disappeared, allowing samples to be frozen and analyzed at one time, with the same batch of reagent.28 The technique for measuring TBARS consisted of a colorimetric assay using the TBARS Parameter Assay Kit (KGE 013, R and D Systems, Minneapolis, MN). IL17a was measured in the urine using the Porcine IL17A ELISA kit (RAB0870 Sigma–Aldrich, St Louis, MO).
Statistics.
Statistical analysis was performed using Graphpad prism 5.0 (GraphPad, San Diego, CA). A 2-tailed student t test was used for continuous parametric variables and the Mann–Whitney test was used for discrete variables. Data are represented as mean ± SEM. P value < 0.05 was considered statistically significant.
Results
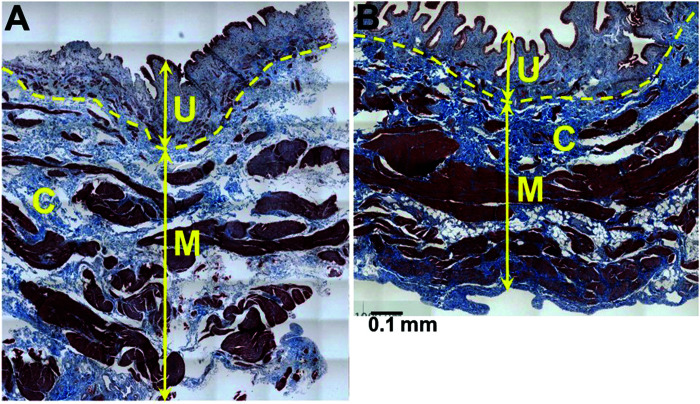
Eleven female Ossabaw pigs completed the MetS protocol and were assessed over 10 mo of dietary treatment. These animals were compared with 5 lean female control pigs. MetS was confirmed by assessing body weight, blood pressure, fasting blood glucose, total cholesterol, and triglycerides at the end of the 10 mo study period (Table 1). To correlate changes in bladder function with the development of MetS, bladder pressures were measured at the end of the 10 mo treatment period. Empty bladder pressure was 0.3 ± 0.3 cm H2O. Full bladder pressure at a mean capacity of 998 ± 252 cc was 22.3 ± 5.3 cm H2O. Compliance was noted to be 51.2 ± 19.2 mL/cm H2O. A representative urodynamic tracing of a MetS pig and a lean pig is shown in Figure 1. Bladder pressures of MetS and LEAN pigs were compared, with results shown in Table 2. The 2 groups showed a statistically significant difference in bladder pressure at capacity (22.3 ± 5.3 compared with 45.6 ± 6.1, P = 0.04). Histologic analysis of a cohort of lean and MetS pigs revealed significantly more collagen in the muscularis layer but not in the submucosa or mucosa layer (22.8% compared with 14.1%, P ≤ 0.01, Table 3, Figure 2) of the bladders of the pigs fed the MetS diet. The groups showed no significant difference in the thickness of the muscularis layer or in the total area analyzed (Table 4). Original hematoxylin and eosin (HE) and Masson Trichrome (TX) stained photomicrographs have been assembled as an electronic supplement to this for the interested reader Figure S1 through Figure S4.
Table 1.
Demographic parameters after 10 mo MetS inducing diet modification (n = 11 MetS, n = 5 Lean).
| Metabolic syndrome | Lean | |||
| Body Weight (kg) | 105.6 ± 2.3 | 81.4 ± 8.5 | P = <0.001 | |
| BP-Systolic (mm Hg) | 163.6 ± 6.8 | 124.8 ± 4.4 | P = 0.001 | |
| BP-Diastolic | 93.0 ± 5.1 | 75.8 ± 2.5 | P = 0.014 | |
| Fasting Blood Glucose (mg/dL) | 81.4 ± 2.3 | 69.2 ± 2.3 | P = 0.003 | |
| Total Cholesterol (mg/dL) | 481.1 ± 68.5 | 73.6 ± 7.9 | P = <0.001 | |
| Triglycerides (mg/dL) | 49.2 ± 5.9 | 37.5 ± 9.2 | P = 0.136 |
Figure 1.
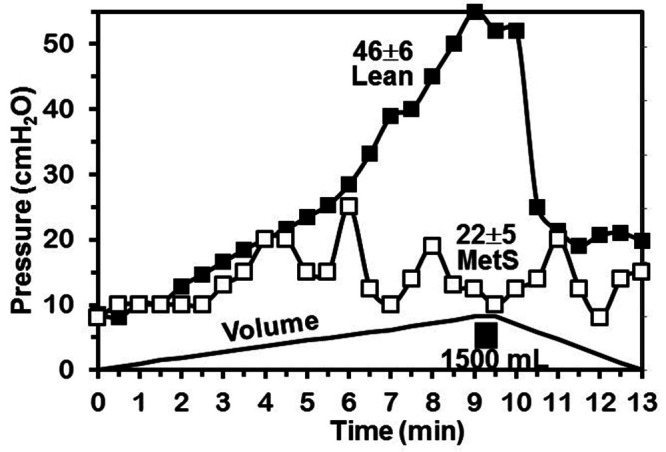
Urodynamic tracing of metabolic syndrome (MetS) Ossabaw pig (open squares) and Lean pig (closed squares) filled to capacity of 1500 mL.
Table 2.
Urodynamic measurements of bladder tone in MetS diet pig at 10 mo (n = 11 female) compared with lean diet pig (n = 5 female)
| Pves empty | Pves full | Compliance | Age (mo) | |||||||
| After 10 mo MetS diet | 0.3 ± 0.3 | cmH2O | 22.3 ± 5.3 | cmH2O | 51.2 ± 19.2 | cmH2O | 36.2 ± 0.03 | |||
| Lean diet | 7.6 ± 2.2 | cmH2O | 45.6 ± 6.1 | cmH2O | 28.3 ± 3.1 | cmH2O | 59.1 ± 19.4 | |||
| P = 0.05 | P = 0.04 | P = 0.20 | P = 0.41 | |||||||
Table 3.
Percent collagen by tissue layer, analyzed by colorimetric software (Masson Trichrome, ± SEM, 2 tail T-test).
| MetS Pigs n = 5 | Lean Pigs n = 5 | ||||
| Muscularis | 22.8 ± 1.7 | 14.1 ± 1.8 | P ≤ 0.01 | ||
| SubMucosa | 35.6 ± 3.6 | 21.6 ± 10.3 | P = 0.24 | ||
| Mucosa | 19.4 ± 4.1 | 14.0 ± 8.0 | P = 0.57 |
Figure 2.
Increased fibrosis / collagen (C, blue) in detrusor smooth muscle (DSM) layer in (A) Metabolic Syndrome (MetS) compared with (B) Lean pigs. U = urothelium, M= muscularis (Masson Trichrome).
Table 4.
Detail on bladder thickness by tissue layer in MetS compared with Lean pigs. Full thickness from mucosa to serosa, including all muscle but not perivesical fat.
| Thickness (µm) | ||||||||
| MetS pigs n = 6 | Lean pigs n = 5 | |||||||
| Muscularis | 533 ± 69.6 | 947 ± 550.4 | P = 0.22 | |||||
| SubMucosa | 77 ± 14.6 | 251 ± 158.8 | P = 0.15 | |||||
| Mucosa | 86 ± 14.2 | 161 ± 81.4 | P = 0.20 | |||||
| Total area of tissue analyzed by layer MetS compared lean pigs | ||||||||
| Total area (pixels) | ||||||||
| Muscularis | 4.9E + 05 ± 5.8E +04 | 3.9E + 06 ± 3.52E + 06 | P = 0.31 | |||||
| SubMucosa | 8.3E + 04 ± 1.3E +04 | 1.1E + 06 ± 9.77E + 05 | P = 0.35 | |||||
| Mucosa | 7.1E + 04 ± 1.3E +04 | 3.5E + 05 ± 2.94E + 05 | P = 0.37 | |||||
Total area analyzed was included for comparison (Masson Trichrome, ± SEM, 2 tail T-test). Tissue was analyzed at 10 ×.
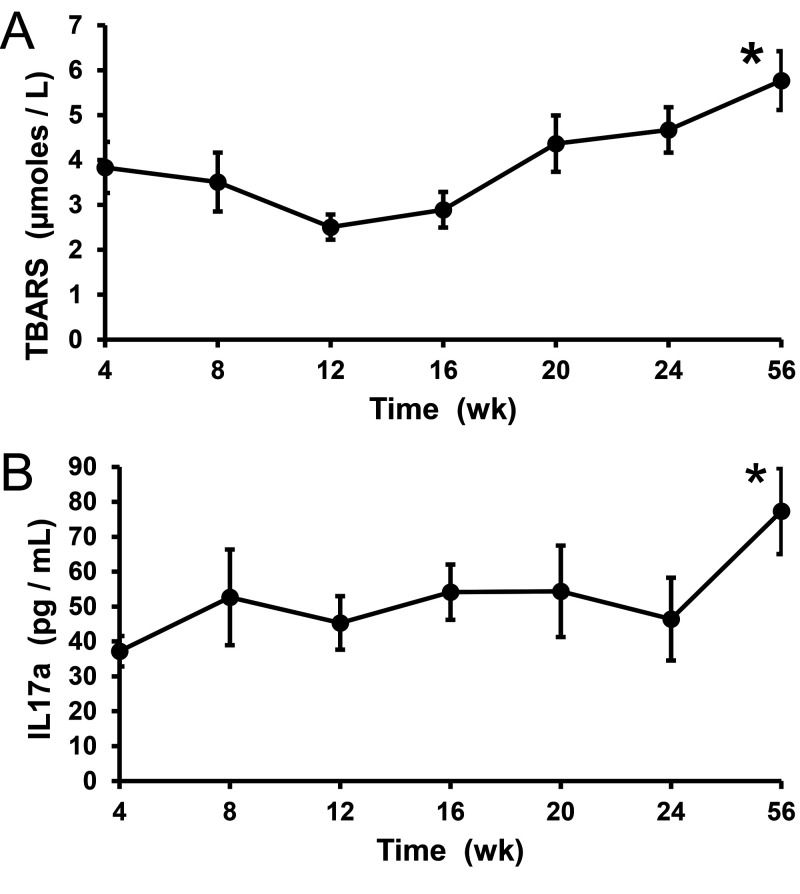
A separate cohort of 8 MetS animals exhibited a 1.4 fold increase in the relative levels of urinary TBARS, from a value of 3.9 µmoles/L to 5.5 µmoles/L over a 56 wk observation period (Figure 3, P = 0.04). The level of IL17a also increased from 37 pg/mL to 77 pg/mL (relative increase of 2.1 fold) during the study period (Figure 3, P = 0.008).
Figure 3.
Levels of urinary thiobarbituric acid reactive substances (TBARS) and interleukin-17a (IL17a) as a marker for oxidative stress and urinary tract fibrosis, respectively (n = 8 MetS pigs). Units are µm. Error bars are ± SEM (SEM).
Discussion
This study is the first in which a large animal has been used to demonstrate in vivo DU associated with MetS. Studies in small animals have been performed previously using detrusor muscle strips in-vitro or in mice and rats with catheters sewn into the bladder. This manipulation alters bladder anatomy, affecting function and reducing the translatability of research data. All pigs were female, avoiding gender-based variability seen in LUTS and MetS.
Differences in bladder muscle mRNA expression have been reported previously in the swine model, supporting significant change after 10 mo.21 Our results indicate that 10 mo is an adequate period to develop MetS, which appears to be progressive, as prior work with up to 13 mo treatment found a significant difference between the pigs treated for as little as 10 mo.29 The Ossabaw pig model has a bladder capacity comparable to that of humans, allowing the same urodynamic equipment to be used, cystoscopy to be employed for in vivo muscle biopsy if desired, and the implantation of novel implanted devices, such as the UPLINK wireless bladder pressure monitor.11,17 Much of the prior work related to MetS and diabetic bladder complications has been done in mice and rats, and some investigators have found increased compliance and altered purinergic contraction in streptozotocin-induced models for Type 1 DM.20,30 Impaired contractility of detrusor muscle strips have been noted in rat and rabbit models.19,31 The decreased bladder pressures noted in Ossabaw pigs fed a MetS diet are consistent with those findings. The pig model also resulted in a relative increase in collagen compared with detrusor smooth muscle, which would impair contraction.
Clinical work has largely been based on outcomes gained in retrospective prevalence studies using validated questionnaires, and the results are heterogeneous.14,32 Studies of diabetic bladder complications using urodynamic outcomes are rare, often retrospective in nature, with conflicting findings and significant variability in the stage of disease affecting the subjects.2,6,27 This novel animal model for MetS and diabetic bladder complications allows investigators to standardize the degree of severity of the experimental diabetic bladder complications that are induced. Controlling for the stage and severity of DM may be critical, as others have found that the bladder appears to have a hyper-contractile compensatory response early in DM, and a hypo-contractile response as DM progresses.8 The mechanism of this change likely involves oxidative stress, as reflected by urinary TBARS levels. Loss of contractility due to fibrosis also observed and warrants further investigation.
Some weaknesses to this study should be mentioned. The urinary markers were measured in a separate cohort of pigs under a different protocol. However, all the animals we used were from the same breeding colony and received the same MetS diet and treatment. Not all MetS pigs underwent urodynamic testing due to equipment availability, and the urodynamic testing was done under general anesthesia using isoflurane gas. Isoflurane decreases the frequency of uninhibited contractions and is thought to be less irritating than urethane in the rat model, but was used in both lean and MetS pigs, normalizing the control and MetS groups to the same anesthetic.4
Conclusion
The Ossabaw pig is a novel and more directly analogous model for human DBD/MetS. Our data have demonstrated that MetS causes fibrosis of the DSM in the muscularis layer, with concurrent urodynamic changes suggestive of DU in this model of MetS / diabetic bladder dysfunction. The presence of markers for oxidative stress and fibrosis in the urine suggest the etiology of disease and provide a possible early biomarker to guide clinical intervention before permanent fibrosis can occur. This model has the potential to advance functional and mechanistic understanding of MetS and diabetes beyond current small animal models and represents an advance in animal modeling of diabetic bladder disease.
Supplementary Materials
Lean detrusor muscle stained with Hemotoxylin and Eosin photomicrographs at 10× power, (A) through (E). Lean pig detrusor muscle stained with Masson Trichrome staining photomicrographs at 10× power, (F) though (J).
Metabolic syndrome affected detrusor muscle from pigs treated with hypercaloric, atherogenic diet that contained of 43.1% calories from fat, 40.4% from carbohydrates, and 16.4% from protein stained with Hematoxylin and Eosin photomicrographs at 10× power, (A) through (F).
Metabolic syndrome affected detrusor muscle from pigs treated with hypercaloric, atherogenic diet that contained of 43.1% calories from fat, 40.4% from carbohydrates, and 16.4% from protein stained with Masson Trichrome photographs at 10× power, (A) through (F). There is a relative increase in thickness of the collagen layer of the muscularis compared to the lean group of pigs.
Acknowledgments
All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest.
This project was supported by a Project Development Team within the ICTSI NIH/NCRR Grant Number RR025761 (Powell, PI) and NIDDK DiaComp Pilot & Feasibility project, sub-award DK076169 (Powell, PI) and NIH grant HL062552 (Sturek, PI), NIH UL1 RR025761 (Sturek, CO-I), NIH P30 DK097512 (Sturek Co-I), NIH NHLBI R01HL109288 (Vittal, PI).
References
- 1.AVMA Panel on Euthanasia. American Veterinary Medical Association. 2001. 2000 Report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc 218:669–696. 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- 2.Bansal R, Agarwal MM, Modi M, Mandal AK, Singh SK. 2011. Urodynamic profile of diabetic patients with lower urinary tract symptoms: association of diabetic cystopathy with autonomic and peripheral neuropathy. Urology 77:699–705. 10.1016/j.urology.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 3.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. 2013. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol 62:697–703. 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang HY, Havton LA. 2008. Differential effects of urethane and isoflurane on external urethral sphincter electromyography and cystometry in rats. Am J Physiol Renal Physiol 295:F1248–F1253. 10.1152/ajprenal.90259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Changolkar AK, Hypolite JA, Disanto M, Oates PJ, Wein AJ, Chacko S. 2005. Diabetes induced decrease in detrusor smooth muscle force is associated with oxidative stress and overactivity of aldose reductase. J Urol 173:309–313. 10.1097/01.ju.0000141583.31183.7a. [DOI] [PubMed] [Google Scholar]
- 6.Changxiao H, Zhengyong Y, Shibing Y, Caiwen W, Yingchuan H, Wei H, Hanhui W, Dong L, Peng H, Jing L, Rui Z, Jia L, Hong S. 2014. Clinical and urodynamic evaluation of women referred with diabetes mellitus. Int Urogynecol J 25:979–983. 10.1007/s00192-014-2354-5. [DOI] [PubMed] [Google Scholar]
- 7.Comeglio P, Morelli A, Cellai I, Vignozzi L, Sarchielli E, Filippi S, Maneschi E, Corcetto F, Corno C, Gacci M, Vannelli GB, Maggi M. 2013. Opposite effects of tamoxifen on metabolic syndrome-induced bladder and prostate alterations: a role for GPR30/GPER? Prostate 74:10–28. 10.1002/pros.22723. [DOI] [PubMed] [Google Scholar]
- 8.Daneshgari F, Huang X, Liu G, Bena J, Saffore L, Powell CT. 2006. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am J Physiol Regul Integr Comp Physiol 290:R1728–R1735. 10.1152/ajpregu.00654.2005. [DOI] [PubMed] [Google Scholar]
- 9.Daneshgari F, Leiter EH, Liu G, Reeder J. 2009. Animal models of diabetic uropathy. J Urol 182 6 Suppl:S8–S13. 10.1016/j.juro.2009.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danforth KN, Townsend MK, Curhan GC, Resnick NM, Grodstein F. 2009. Type 2 diabetes mellitus and risk of stress, urge and mixed urinary incontinence. J Urol 181:193–197. 10.1016/j.juro.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletter PC, Majerus S, Peng C, Damaser MS, Ko WH, Young DJ, Garverick SL. 2009. Wireless micromanometer system for chronic bladder pressure monitoring. Sixth International Conference on Networked Sensing Systems (INSS), Pittsburgh, Pennsylvania, 17–19 June 2009. IEEE. p 1–4. 10.1109/INSS.2009.5409917 [DOI] [Google Scholar]
- 12.Gasbarro G, Lin DL, Vurbic D, Quisno A, Kinley B, Daneshgari F, Damaser MS. 2010. Voiding function in obese and type 2 diabetic female rats. Am J Physiol Renal Physiol 298:F72–F77. 10.1152/ajprenal.00309.2009. [DOI] [PubMed] [Google Scholar]
- 13.Hanna-Mitchell AT, Ruiz GW, Daneshgari F, Liu G, Apodaca G, Birder LA. 2013. Impact of diabetes mellitus on bladder uroepithelial cells. Am J Physiol Regul Integr Comp Physiol 304:R84–R93. 10.1152/ajpregu.00129.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong GS, Shim BS, Chung WS, Yoon H. 2010. Correlation between metabolic syndrome and lower urinary tract symptoms of males and females in the aspect of gender-specific medicine: a single institutional study. Korean J Urol 51:631–635. 10.4111/kju.2010.51.9.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang YH, Kuo HC. 2017. Urothelial barrier deficits, suburothelial inflammation and altered sensory protein expression in detrusor underactivity. J Urol 197:197–203. 10.1016/j.juro.2016.07.071. [DOI] [PubMed] [Google Scholar]
- 16.Klee NS, McCarthy CG, Lewis S, McKenzie JL, Vincent JE, Webb RC. 2018. Urothelial senescence in the pathophysiology of diabetic bladder dysfunction—A novel hypothesis. Front Surg 5:1–13. 10.3389/fsurg.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SS, Kim A, Chitnis G, Powell CR, Ziaie B. 2013. A modular embedded system design for implantable wireless bladder pressure sensing. Presented at the 7th International Conference on microtechnologies in Medicine and Biology. Marina Del Ray, California:10–12 April 2013. MMB and interteq.com.
- 18.Lee WC, Wu HP, Tai TY, Yu HJ, Chiang PH. 2009. Investigation of urodynamic characteristics and bladder sensory function in the early stages of diabetic bladder dysfunction in women with type 2 diabetes. J Urol 181:198–203. 10.1016/j.juro.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Li WJ, Oh SJ. 2010. Diabetic cystopathy is associated with PARP/JNK/mitochondrial apoptotic pathway-mediated bladder apoptosis. Neurourol Urodyn 29:1332–1337. 10.1002/nau.20869. [DOI] [PubMed] [Google Scholar]
- 20.Liu G, Daneshgari F. 2005. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol 288:F1220–F1226. 10.1152/ajprenal.00449.2004. [DOI] [PubMed] [Google Scholar]
- 21.Mattern HM, Lloyd PG, Sturek M, Hardin CD. 2007. Gender and genetic differences in bladder smooth muscle PPAR mRNA in a porcine model of the metabolic syndrome. Mol Cell Biochem 302:43–49. 10.1007/s11010-007-9423-8. [DOI] [PubMed] [Google Scholar]
- 22.Morelli A, Comeglio P, Filippi S, Sarchielli E, Cellai I, Vignozzi L, Yehiely-Cohen R, Maneschi E, Gacci M, Carini M, Adorini L, Vannelli GB, Maggi M. 2012. Testosterone and farnesoid X receptor agonist INT-747 counteract high fat diet-induced bladder alterations in a rabbit model of metabolic syndrome. J Steroid Biochem Mol Biol 132:80–92. 10.1016/j.jsbmb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. p 220 Washington (DC): National Academies Press. [Google Scholar]
- 24.Otis CR, Wamhoff BR, Sturek M. 2003. Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine. Comp Med 53:53–64. [PubMed] [Google Scholar]
- 25.Panepinto LM, Phillips RW, Westmoreland NW, Cleek JL. 1982. Influence of genetics and diet on the development of diabetes in Yucatan miniature swine. J Nutr 112:2307–2313. 10.1093/jn/112.12.2307. [DOI] [PubMed] [Google Scholar]
- 26.Philyppov IB, Paduraru ON, Gulak KL, Skryma R, Prevarskaya N, Shuba YM. 2016. TRPA1-dependent regulation of bladder detrusor smooth muscle contractility in normal and type I diabetic rats. J Smooth Muscle Res 52:1–17. 10.1540/jsmr.52.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell CR. 2014. Is the diabetic bladder a neurogenic bladder? Evidence from the literature. Curr Bladder Dysfunct Rep 9: 261–267. 10.1007/s11884-014-0255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pryor WA. 1991. The antioxidant nutrients and disease prevention—what do we know and what do we need to find out? Am J Clin Nutr 53:391S–393S. 10.1093/ajcn/53.1.391S. [DOI] [PubMed] [Google Scholar]
- 29.Roth J, Kim A, Alloosh M, Ziaie B, Sturek M, Powell CR. 2016. Detrusor underactivity is seen in an animal model for metabolic syndrome, Abstract: MP60-12. American Urological Association Annual Meeting; San Diego, California, 6–10 May 2016. J Urology 195:e799. [Google Scholar]
- 30.Steers WD, Mackway AM, Ciambotti J, de Groat WC. 1990. Effects of streptozotocin-induced diabetes on bladder function in the rat. J Urol 143:1032–1036. 10.1016/S0022-5347(17)40177-7. [DOI] [PubMed] [Google Scholar]
- 31.Su X, Changolkar A, Chacko S, Moreland RS. 2004. Diabetes decreases rabbit bladder smooth muscle contraction while increasing levels of myosin light chain phosphorylation. Am J Physiol Renal Physiol 287:F690–F699. 10.1152/ajprenal.00027.2004. [DOI] [PubMed] [Google Scholar]
- 32.Tai HC, Chung SD, Ho CH, Tai TY, Yang WS, Tseng CH, Wu HP, Yu HJ. 2010. Metabolic syndrome components worsen lower urinary tract symptoms in women with type 2 diabetes. J Clin Endocrinol Metab 95:1143–1150. 10.1210/jc.2009-1492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lean detrusor muscle stained with Hemotoxylin and Eosin photomicrographs at 10× power, (A) through (E). Lean pig detrusor muscle stained with Masson Trichrome staining photomicrographs at 10× power, (F) though (J).
Metabolic syndrome affected detrusor muscle from pigs treated with hypercaloric, atherogenic diet that contained of 43.1% calories from fat, 40.4% from carbohydrates, and 16.4% from protein stained with Hematoxylin and Eosin photomicrographs at 10× power, (A) through (F).
Metabolic syndrome affected detrusor muscle from pigs treated with hypercaloric, atherogenic diet that contained of 43.1% calories from fat, 40.4% from carbohydrates, and 16.4% from protein stained with Masson Trichrome photographs at 10× power, (A) through (F). There is a relative increase in thickness of the collagen layer of the muscularis compared to the lean group of pigs.