Abstract
Epstein-Barr Virus (EBV) is a γ-herpesvirus which infects over 90% of the adult human population. Most notably, this virus causes infectious mononucleosis but it is also associated with cancers such as Hodgkin and Burkitt lymphoma. EBV is a species-specific virus and has been studied in many animal models, including nonhuman primates, guinea pigs, humanized mice, and tree shrews. However, none of these animal models are considered the “gold standard” for EBV research. Recently, rabbits have emerged as a viable alternative model, as they are susceptible to EBV infection. In addition, the EBV infection progresses after immune suppression with cyclosporine A (CsA), modeling the reactivation of EBV after latency. We sought to refine this model for acute or active EBV infections by performing antibody-mediated depletion of certain immune subsets in rabbits. Fourteen 16 to 20-wk old, NZW rabbits were intravenously inoculated with EBV and concurrently treated with either anti-CD4 T-cell antibody, anti-pan-T-cell antibody (anti CD45), CSA, or, as a control, anti-HPV antibody. Rabbits that received the depleting antibodies were treated with CsA 3 times at a dose of 15 mg/kg SC once per day for 4 d starting at the time of EBV inoculation then the dose was increased to 20 mg/kg SC twice weekly for 2 wk. Weights, temperatures, and clinical signs were monitored, and rabbits were anesthetized once weekly for blood collection. When compared with the control group, anti-CD4- treated rabbits had fewer clinical signs and displayed higher levels of viral DNA via qPCR in splenocytes; however, flow cytometry results showed only a partial depletion of CD4 T-cells. Treatment with anti-pan-T-cell antibody did not result in noticeable T-cell depletion. These data suggest the EBV-infected rabbit is a promising model for testing antiviral medications and prophylactic vaccines for EBV.
Abbreviations: CsA, Cyclosporine A; EBER, Epstein-Barr virus-encoded small RNAs; EBV, Epstein-Barr virus; HPV, human papilloma virus; Mab, monoclonal antibody; PALS, peri-arterial lymphatic sheath; VCA, viral capsid antigen
Epstein-Barr Virus (EBV) is a γ-herpesvirus (Human herpesvirus 4) that infects over 90% of the adult human population. It is the main etiologic agent of infectious mononucleosis (IM), and is associated with precursors to cancers such as Hodgkin lymphoma (HL) and Burkitt lymphoma (BL).4 Despite its prevalence, most people are asymptomatic or have nonspecific symptoms, particularly if infected at a young age.4,23 EBV primarily infects B-cells and epithelial cells,5 and then can establish latency in memory B-cells that persists for the lifetime of the host. EBV is mainly transmitted via oropharyngeal secretions during periods when the latent virus is reactivated. Often, susceptible individuals are restricted to a subset of people in early adolescence.35 IM occurs when the host's immune system over-reacts to the presence of EBV.35 Occasionally, events that disrupt T-cell function can lead to a primary infection that leads to reactivation of EBV. An example of this is patients experiencing immunosuppression, whether for organ transplantation or chemotherapy treatments. According to one group, “Persons at greatest risk are transplant recipients undergoing primary EBV infection while receiving an immunosuppressive drug regimen”.30 If these patients harbor latent EBV, or are primarily infected during the period of immunosuppression, a form of B-cell proliferation called post-transplantation lymphoproliferative disorder (PTLD) can occur and may transform into non-Hodgkin lymphoma.5 Due to EBV's wide distribution and its potential to cause serious disease, the need for preclinical models to test prevention and treatments is a high public health priority.
EBV is highly species-specific and does not naturally infect animals other than humans. Animals that survive inoculation with EBV and the potential lymphoproliferative disease it can induce do not become persistently infected.26 Many reports in the literature claim that nonhuman primates (NHP) can be endemically infected with EBV-like viruses. Rhesus lymphocryptovirus4 and Herpesvirus papio (also known as baboon herpesvirus)18 are effective models for EBV in NHPs. New World primates, like owl monkeys (Aotus sp.), cotton-top tamarins (Saguinus oedipus), and marmosets (Callithrix jacchus), can develop B-cell lymphomas when infected with EBV.19 Unlike tamarins and owl monkeys, marmosets can display a humoral response similar to humans when infected with EBV in a laboratory setting.19 However, many barriers preclude using NHPs to study EBV, chief among them being cost and regulatory burden associated with housing NHPs in a research facility, difficulties associated with acquiring these animals, and concerns about zoonotic disease. Nonprimate species used to study EBV include humanized SCID mice,27,36,37 guinea pigs (Cavia porcellus),8 and tree shrews (Tupaia belangeri chinensis).39 Humanized SCID mice can become infected with EBV, but using them may be cost-prohibitive. Moreover, their response to the virus differs from that of natural human infections, making them unsuitable for studying all aspects of EBV infection in humans.22 Thus, a reliable and reproducible animal model to study EBV.
Recently, several laboratories have been successful in infecting rabbits with a variant of EBV that can cause viremia and clinical signs similar to those seen in human patients. These groups have infected rabbits, orally, intravenously, or intranasally.12,13,22,23,28,32,36 One group also demonstrated that immunosuppression of rabbits infected with EBV led to amplified viral genome copies in lymphoid tissues.23
The objective of this study was to assess the rabbit model of EBV infection and to establish the utility of this model for analyzing the role of host immunity in the control of the early stages of infection. In particular, we planned a series of experiments to (1) determine the impact of EBV infection in various strains and sources of laboratory rabbits using outbred rabbits from different venders and an inhouse HLA-A2.1 transgenic rabbit line;16,29 (2) assess the ability of various molecular and antibody probes to detect EBV viral proteins, nucleic acids, and viral RNA; (3) assess the impact of delayed, compared with concurrent, CsA treatments in the outcome of latent compared with acute EBV infections; (4) monitor the core temperature of EBV-infected rabbits via implanted temperature probes; (5) assay serological responses to infection using a commercial kit detecting EBV viral capsid protein; and (6) assess the impact of in vivo immune subset depletion using select mouse monoclonal antibodies to a pan T-cell marker (CD45) and a T-cell subset (CD4) on the EBV life cycle. We hypothesized that early immune suppression of NZW rabbits would enhance systemic EBV viremia and create a more reliable model for EBV research. We also hypothesized that the more specific form of immune suppression (antibody-mediated depletion) would be a more predictable method of inducing EBV disease in rabbits, compared with CsA treatments. These studies were designed to obtain a better understanding of host immune control of EBV infection in rabbits and to further validate rabbits as a useful preclinical model for testing future EBV interventions, such as vaccines and antiviral treatments.
Materials and Methods
Study Design.
The study design of these experiments is shown in Figure 1. Thirteen rabbits were used to compare delayed (n = 6, 2 female, 4 male) (Figure 1 A) and concurrent (n = 7, 4 female, 3 male) treatment (Figure 1 B) with CsA in association with EBV inoculation. The rabbits included HLA-A2.1 transgenic NZW rabbits16,29 bred on-site, chosen at random (n = 10) and outbred NZW rabbits purchased from Charles River (Charles River Labs, Canada) (n = 3). All rabbits used to study the effects of delayed CsA treatment were HLA transgenic rabbits; of the 7 rabbits used to study concurrent CSA treatment, 4 were HLA transgenic rabbits and the remaining 3 were wild type animals purchased from Charles River. These studies were designed to confirm other investigators’ findings with the rabbit EBV model,12,13,22,23,28,32,36 to model latent compared with primary EBV disease, and to demonstrate the reproducibility of the model in different strains/stocks of rabbits. These initial studies also created the foundation to proceed with the antibody-mediated study. For the antibody-mediated depletion experiment (Figure 1 C), 14 16 to 20 wk-old, outbred NZW rabbits (n = 7 male, 7 female), weighing on average 3.31 kg (range 2.98 to 3.62 kg), were purchased from Robinson Services (RSI, Mocksville, NC). Previous studies indicated that CsA treatment without EBV inoculation did not elicit abnormal clinical signs, so additional study groups were not added.3,15
Figure 1.
Timelines for studies. (A) CsA treatment delayed 5-6 wk post-EBV inoculation; (B) Concurrent CsA treatment and EBV inoculation; (C) Concurrent antibody-mediated depletion treatment and EBV inoculation.
Health Status.
Rabbits purchased from Charles River (Charles River Labs, Canada) were determined SPF (SPF) per the supplier for: Reovirus, LCMV, PIV-1, PIV-5 (PIV-2), Rotavirus, RHDV, Bordetella. bronchiseptica, Helicobacter spp., Lawsonia spp., Pasteurella multocida, P. aeruginosa, Salmonella spp., Treponema, Tyzzer Disease (Clostridium piliforme), CAR Bacillus, C. parasitovorax, L. gibbus, Ps. cuniculi, P. ambiguous, Eimeria spp., E. stiedae, E. cuniculi, and other ectoparasites, helminths, and protozoa. The rabbits purchased from Charles River were approximately 3 mo old and weighed on average 2.88 kg (range 2.86 to 2.96 kg) at the start of the study.
The rabbits that were housed on-site or derived from the inhouse transgenic colony underwent annual health monitoring and were determined negative for Bordetella bronchiseptica, Pasteurella multocida, Encephalitozoon cuniculi, Treponema, Clostridium piliforme, and Rotavirus via serologic testing and nasal swabs. The transgenic colony was considered enzootic for CAR bacillus via serology. Transgenic rabbits used for these studies were approximately 1 y old and weighed on average 3.55 kg (range 3.08 to 4.08 kg) at the start of the study.
Rabbits purchased from RSI (Mocksville, NC) were considered negative for Clostridium piliforme, CAR Bacillus, Rotavirus, Treponema, Encephalitozoon cuniculi, Bordetella bronchiseptica, Pasteurella multocida, Salmonella spp., helminth ova, coccidial oocysts, fur mites, mesostigmatid mites, and lice, according to health reports from RSI. Due to short term housing and low foot traffic, inhouse diagnostics, other than fecal tests, were not performed on these rabbits. Rabbits purchased from RSI in the past had a history of coccidia positivity on fecal exams so these rabbits underwent a 2-wk quarantine and ponazuril treatments (60 mg/kg PO once a day for 3 d, compounded by Wedgewood Pharmacy, Swedesboro, NJ) before beginning the study.
Rabbit Husbandry.
Rabbits were singly housed in metal, lattice-bottom caging in cubicles in an ABSL-2 suite. PPE requirements for entering the ABSL-2 suite included hair bonnet, disposable lab coat, gloves (nitrile or latex), shoe covers, and a facemask. Personnel handling infected rabbits were also required to wear a face shield that was disinfected with MB-10 (Quip Laboratories, Wilmington, DE) between uses. PPE was donned in a clean-side anteroom prior to entering the ABSL-2 suite and doffed in a dirty-side anteroom upon exit. Personnel received training on proper handling, common healthcare issues, and possible side effects for the rabbits in the study. Once the rabbits were inoculated with EBV, biohazard stickers were placed on their cage cards for easy identification. The ABSL-2 suite consisted of negatively-pressurized cubicles that were fogged with a HaloFogger LS (Quip Laboratories, Wilmington, DE) between animal uses.
Caging was arranged so that rabbits could see conspecifics, either in the cubicle across from them or via transparent panel portions in the cages themselves. Rabbits were quarantined and acclimated for 2 wk in the study space. After acclimation, rabbits were identified with ear tags and corresponding cage cards, and were injected with a temperature transponder (Bio Medic Data Systems, Seaford, DE) subcutaneously between the shoulder blades. Rabbits were offered one cup of 2031 High Fiber Rabbit Diet daily (Envigo RMS, Indianapolis, IN), water ad libitum via an automatic watering system, one handful of timothy hay daily (Oxbow Animal Health, Omaha, NE), and timothy hay cubes and fruity gems as treats (BioServ, Flemington, NJ). The housing room was kept on a 12:12h light: dark cycle (lights on at 0700 h and off at 1900 h) and maintained at 69 to 73 °F, because mice and rabbits were cohoused in the ABSL-2 suite in negatively-pressurized cubicles, with humidity between 30% to 70%, and 21 air changes per hour. All rabbits received a rotating schedule of sanitized enrichment devices.
Daily observations of the rabbits were recorded, and temperature data was obtained via the temperature transponders. If rabbits showed signs of illness, such as inappetence, decreased fecal output, lethargy, weight loss, or pyrexia (temperature +2 or +3 standard deviations (SD) from the average baseline temperature before inoculation with EBV),6,9 they were examined by a licensed veterinarian and supportive care was initiated. Humane endpoints were considered to be a combination of the subjective clinical signs listed (inappetence, decreased fecal output, lethargy) plus the objective clinical signs of greater than or equal to 20% body weight loss from baseline, and/or pyrexia. Supportive care involved nutritional support such as DietGel (ClearH2O, Portland, ME), Critical Care (Oxbow Animal Health, Omaha, NE), kale, water bowl, extra timothy hay, or subcutaneous Lactated Ringer solution (LRS) fluids.
This study was approved by the Penn State College of Medicine's IACUC and reviewed by the Penn State College of Medicine's Institutional Biosafety Committee (biosafety approval #NDC17-02-02). All animal use was performed in accordance with the Guide for the Care and Use of Laboratory Animals17 and the Animal Welfare Regulations.1 The Penn State College of Medicine is an AAALAC-accredited program.
EBV Source.
B95-8 cells (EBV producer line) were used to prepare stocks of infectious virions for these studies.31 B95-8 cells harboring EBV were grown in roller bottles at 37 °C for 10 d. The cells were removed by centrifugation, and the supernatant fraction containing the virus was concentrated by tangential flow filtration, then aliquoted and frozen at -140 °C. Viral titer in these preparations was achieved using qPCR standardization using a purified EBV viral genome as the standard curve.
Antibody Production for Immune Subset Depletion.
Hybridomas for antibody production were purchased from ATCC (American Type Culture Collection, Manassas, VA) (anti-Pan T-cell), from colleagues (anti-LMP-1), or made inhouse (antirabbit CD4, H16.5A [anti-HPV16L1 VLP]). Hybridomas were adapted to serum-free culture conditions, and batches of concentrated antibody were prepared using CELLine culture devices (BD Biosciences, San Jose, CA).
The antibodies were isotyped using the Pierce Rapid ELISA Mouse mAb Isotyping Kit by Thermo Fisher Scientific (Waltham, MA), and were purified using the Protein A/G Spin Kit by Thermo Fisher Scientific, as described by the manufacturer. Purified Mabs were prepared in physiologic saline for infusion into rabbits (mouse antirabbit anti-CD4, anti-Pan T-cell, and mouse anti-HPV 16, H16.5A) or used in immunohistochemistry (anti-LMP-1). H16.5A was used as an isotype negative control Mab for the antirabbit CD4 and Pan T-cell antibodies used in the in vitro treatments.
Immunosuppression Concurrent with EBV Infection.
Rabbits were randomly sorted into groups with even numbers of each sex per group. Each group received either anti-CD4 T-cell antibody (n = 4), anti-Pan T-cell antibody (n = 4), negative control (received anti-HPV antibody, n = 4), or treated with CsA (n = 2). On day 0 of the study, the rabbits were weighed and anesthetized with to 40 mg/kg ketamine (Virbac, Fort Worth, TX) and 5 mg/kg xylazine (BiMeda, Oakbrook Terrace, IL), injected into the epaxial muscles and anesthesia confirmed by a lack of withdrawal or ear pinna reflex. Respiratory rate, mucous membrane color, and body temperature were monitored during anesthesia, and ophthalmic ointment (Paralube, Dechra, Portland, ME) was placed on both eyes to prevent desiccation of the corneas. Approximately 7 mL of blood was collected from the central ear artery and divided between serum and EDTA-containing vacuum phlebotomy tube tubes as the baseline prior to inoculation. Rabbits were then inoculated with 0.6-0.9 mL EBV preparation in the caudal auricular vein (viral inoculum per rabbit ranged from 2 × 108 to 8 × 108 genome copies as measured by qPCR of viral stock solution). Rabbits received the first dose of antibody via the caudal auricular vein. This inoculum is comparable to published studies, where EBV copy number ranged between 2 × 107 and 2 × 108.21,26 Antibody doses were as follows: 2 mg anti-CD4 T-cell antibody per treatment, 320 µg anti-Pan T-cell antibody per treatment, or 1 mg anti-HPV antibody per treatment, per rabbit. Rabbits received one dose per week for 3 wk (total of 3 doses), each on the same day of the week.
The CsA treatment was as follows: rabbits received 15 mg/kg SC of CsA (Sandimmune, Novartis Pharmaceuticals Corporation, East Hanover, NJ) once daily for 5 d, then 20 mg/kg SC twice a week for 2 wk.15,23
Each week, weights and blood samples were obtained for all rabbits until the experimental endpoint at 4 wk after inoculation. Blood collections were performed in a Class II, Type A2 biosafety cabinet (NuAire, Plymouth, MN) lined with paper for reasons of personnel and animal safety, and because of reduced space in the ABSL-2 suite for working with rabbits. Each blood sample, a total of 7 mL of blood, was collected with a 21g vacuum phlebotomy tube needle system from the central auricular artery and divided between serum and EDTA vacuum phlebotomy tube tubes. Hemostasis was achieved with 4 × 4 nonsterile gauze and pressure over the venipuncture site. After sample collection, rabbits were injected with 0.2 mg/kg Yohimbine (Akorn, Lake Forest, IL) in the epaxial musculature to reverse the effects of xylazine. All rabbits were left to recover in their home cages and were observed every 30 min until able to maintain an upright posture.
Tissue Collection.
All rabbits were anesthetized as described above prior to euthanasia. A terminal blood sample was collected via cardiac puncture using a 1.5 inch 21 gauge needle vacuum phlebotomy tube system, and the sample was divided between serum and EDTA vacuum phlebotomy tube tubes. The rabbits were euthanized with 1.5 mL Euthasol, administered (Virbac, Fort Worth, TX) in the caudal auricular ear vein with a butterfly catheter. Death was confirmed by lack of heartbeat and subsequent removal of vital organs. Carcasses were necropsied immediately before disposal as biohazardous waste. Samples of the liver, tongue, mesenteric lymph nodes, and spleen were collected, and tissue fragments and cell suspensions were preserved in 10% neutral buffered formalin (NBF), DMSO-containing freezing medium, or hexane/methanol mixture for histology, liquid nitrogen storage, and other future studies, respectively. A small sample of spleen was also placed in PBS for splenocyte isolation, which was performed by methodically squeezing the spleen lengthwise into a sterile culture dish until only cells remain. After fixation, the liver, spleen, and lymph nodes were processed for histology and stained with a standard hematoxylin and eosin (H and E) stain. H and E stained histology slides were assessed for major histologic features by a veterinary pathologist who was blind to the treatment groups.
Immunohistochemistry.
Frozen tissue sections of spleen and liver from initial study rabbits were cut on a Microm Cryostat model HM 505 N (Waldorf, Germany) and then fixed in acetone for 30 min, followed by treatment with Bloxall (Vector Laboratories, Burlingame, CA) for 5 min to minimize non-specific binding. The samples were blocked by incubation in 2.5% normal horse serum for 15 min, then incubated with the primary antibody for 2 h. Antibody targets were latent membrane protein-1 (LMP-1, 1:50 dilution20), and EBV viral capsid antigen (VCA, 1:10 dilution). After washing, an antimouse HRP-conjugated secondary antibody was added for 40 min, and HRP activity was detected using the NovaRED Peroxidase Substrate Kit (Vector Laboratories, Burlingame, CA). Next, the samples were stained with Mayer hematoxylin for 2 min, a cover-slip was applied, and the slides imaged for analysis.
EBER (Epstein-Barr virus-encoded small RNAs) were detected using an EBV Kit VP-Y177 (Vector Laboratories, Burlingame, CA) according to the manufacturer's specifications.
PBMC Isolation.
After blood collection, the purple top EDTA tubes were placed on ice and processed immediately. The blood was mixed with either serum-free media or PBS at a 1:1 ratio. Lympholyte-Mammal (CedarLane, Burlington, NC) was added at a 1:0.5 ratio of blood/PBS to Lympholyte slowly to the bottom of the tube. The tubes were then centrifuged at 1500rpm for 30 min. The PBMC fraction was separated into a 50cc conical tube and PBS was added until a full 50cc was obtained per tube. The 50cc tubes were centrifuged at 378 x g for 10 min. The excess liquid was removed and discarded. The cells were resuspended in 2 mL of freezing medium (90% FBS + 10% DMSO) in a cryotube and stored in liquid nitrogen until needed.
ELISA.
ELISA (enzyme-linked immunosorbent assay) was performed on serum samples from study rabbits. An Epstein-Barr virus VCA (viral capsid antigen) IgG ELISA Kit was purchased from Abnova (Taiwan, China) to detect IgG in rabbit serum. Directions were followed for the qualitative/semiquantitative antibody determination, except for the following. Serum samples were diluted 1:50 with Dilution buffer and added to the plate. The secondary antibody was goat antirabbit IgG AP (ImmunoPure, Thermo Fisher Scientific, Waltham, MA) diluted at 1:2000 in PBS and 5% milk powder protein. Signals were detected using 1 mg/mL 4-Nitrophenyl phosphatase disodium salt hexahydrate (Sigma, St Louis, MO) followed by colorimetric analysis at 405 nm. Standard signals for the positive control were detected at 450 nm per kit directions. All samples were read at 45 min after substrate addition.
qPCR.
qPCR was completed on PBMC samples from various time points and spleen suspension samples. The DNA was extracted according to the DNeasy Blood and Tissue Kit directions (Qiagen, Germantown, MD). DNA was eluted with either 50 µL or 100 µL of elution buffer instead of 200 µL. Primers for the EBV polymerase gene BALF5 were purchased from Integrated DNA Technologies (Coralville, IA). The forward primer was: 5′ AGTCCTTCTTGGCTAGTCTGTTGAC 3′. The reverse primer was: 5′ CTTTGGCGCGGATCCTC 3′. The Dual-labeled fluorogenic probe (TaqMan) was: 5′ (FAM) CATCAAGAAGCTGCTGGCGGCCT (TAMARA) 3′.
Most of the PCR reactions consisted of 7.8 µL of ultrapure water, 10 µL of Brilliant III qPCR Master Mix (Agilent Technologies, Santa Clara, CA), 5uM of the BALF5 probe, 20uM each of forward and reverse primers, and 1 µL of DNA template. DNA was quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA). PCR conditions were: initial denaturation at 95 °C for 3 min, followed by 40 cycles at 95 °C for 5 seconds and incubation at 60 °C for 10 seconds, run on an Agilent AriaMx (Agilent Technologies, Santa Clara, CA). All samples were tested in duplicates.
Results were read by AgilentAria q3 software (Agilent Technologies, Santa Clara, CA). 10-fold serial dilutions (108-102) of the recombinant EBV Akata BAC (gift from Teru Kanda) were used to develop a standard curve. The number of copies for each sample was determined using Cq and the standard curve.
Flow Cytometry.
PBMCs were prepared as described above for flow cytometry analyses. Antirabbit CD4 FITC and antirabbit T-cell FITC antibodies were obtained from Antigenix America (Huntington Station, NY). Rabbit lymphocyte samples were incubated in the appropriate antibody for 45 min- 1 h in the dark and on ice. (1:10 dilution of antibody). Cells were washed with 200 µL of 2% FBS/PBS and centrifuged at 329 x g for 2 min, 3 times. After the final wash the cells were resuspended in 200 µL of 2% PFA/PBS. Samples were counted using 1-color flow cytometry (FACS Calibur, BD Biosciences, San Jose, CA) and the populations of CD4 T-cells or Pan T-cells were calculated. Samples were analyzed in triplicate.
Statistics.
Paired Student t tests and one-way ANOVA tests were used to evaluate the significance of weight changes, spleen and liver weights at necropsy, ELISA data, qPCR results, and flow cytometry results. Results of the statistical tests are reported as appropriate in each section of the Results or in the figure legend. P values less than 0.05 were considered significant. Results were graphed using SigmaPlot 11.0 software (Systat Software, San Jose, CA).
Results
Initial studies
A series of experiments were first conducted to determine whether we could reproduce results of previously published studies using NZW rabbits inoculated with EBV. We were able to confirm that NZW rabbits, including our HLA-A2.1 transgenic rabbits,16,29 were susceptible to EBV infection, and that rabbits inoculated with CsA showed higher levels of viral DNA. In addition, we compared delayed and concurrent, CsA treatment as a method to model both acute and latent EBV infections. Rabbits treated with delayed CsA had a comparable number of viral genomes in their PBMCs (Figure 2 A) as did rabbits treated with concurrent CsA (Figure 2 B) (data shown for only 7 of 13 of the rabbits in the initial study, as not all samples were analyzed and PBMCs were not collected from all rabbits). We detected viral genomes and proteins in PBMCs and in spleen cell suspensions (Figure 3). All rabbits that received CsA showed a trend of weight loss and displayed clinical signs including hyporexia, lethargy, and reduced fecal output. The rabbits obtained from Charles River were younger and still growing and therefore did not lose weight as readily as the older, transgenic rabbits, despite CsA treatment (data not shown). A novel observation was the detection of more lytic components of the viral life cycle in the spleen of one rabbit in which VCA (viral capsid antigen) was detected by immunostaining (Figure 3). Collectively, these data were deemed sufficient to proceed with the next phase involving antibody-mediated depletion of immune cell subsets as an alternative strategy to assess the role of host immune cells in the control of EBV infections.
Figure 2.
(A) qPCR data of PBMCs where the rabbits were inoculated with EBV at time 0 then CsA treatment was delayed until 42 d (6 wk) postinoculation. (*) indicates significance of last time point to first time point for each rabbit via one-way ANOVA (P = 0.004). (B) qPCR data of PMBCs from a study where the rabbits were concurrently inoculated with EBV and began CsA treatment on day 0. (↓) indicates CsA treatment start. Each symbol represents an individual rabbit within these studies. A paired t test between the last timepoint data from panel A and the data on panel B (approximately 14 d post-CsA start) is not considered significant (P = 0.152). For all rabbits in this study, blood collected prior to EBV inoculation was negative for EBV DNA by qPCR evaluation.
Figure 3.
Immunohistochemistry (IHC) and in situ hybridization (ISH) confirmation of Epstein-Barr virus (EBV) infection in spleen from initial study rabbits. (A) Periarteriolar lymphoid sheaths (PALS) in an EBV-infected rabbit exhibited mild lymphoid depletion. H and E, 100×. (B) In addition, lymphocytes remaining in the PALS were diffusely and strongly nuclear-positive (arrows) for EBV-encoded small RNAs (EBER) with ISH analysis, ALP substrate, eosin and Mayer hematoxylin counterstain, 200×. (C) Up to 30% of lymphocytes in the PALS from the infected rabbit were strongly cytoplasmic positive (arrows) for latent membrane protein-1 (LMP-1) and (D) moderately and diffusely cytoplasmic positive (arrows) for EBV viral capsid antigen (VCA). IHC 1:10 dilution and 1:50 dilution respectively, NovaRed substrate, Mayer hematoxylin counterstain, 200×. (E) The spleen from an uninfected rabbit was negative after anti-VCA antibody IHC staining, 1:10 dilution, NovaRed substrate, Mayer hematoxylin counterstain, 200×.
In situ Hybridization and Immunohistochemistry.
ISH and IHC performed on spleen (Figure 3) and liver (data not shown) in select rabbits from the initial studies confirmed that EBV infection in rabbits was similar to that of humans. ISH assays show EBV-infected rabbits had diffusely and strongly nuclear positive EBER lymphocytes within splenic peri-arterial lymphatic sheath (PALS) (Figure 3 B). The cytoplasm of PALS lymphocytes in EBV-infected rabbits was also strongly positive for LMP-1 (Figure 3 C) and EBV VCA (Figure 3 D). Tissue from uninfected rabbits was also processed by IHC to detect VCA and was diffusely negative (Figure 3 E). The results of the IHC show that we were able to successfully detect viral EBER (Epstein-Barr virus-encoded small RNAs) and proteins in spleen tissue, confirming that rabbits were able to support productive infection by EBV.
Clinical Results.
At baseline and throughout the study, rabbits were bright, alert, and responsive, showing no abnormal clinical signs, with the exception of the rabbits indicated in Table 1. The clinically ill rabbits presented with lethargy, hyporexia/anorexia, and reduced fecal output. They were treated with subcutaneous Lactated Ringers solution (LRS; 60 mL twice a day) and nutritional support (extra water bowl, kale, extra hay, DietGel, and/or Critical Care). The CsA-treated rabbits and one of the anti-CD4 treated rabbits reached humane endpoints (body weight loss, inappetence, decreased fecal output, lethargy, pyrexia) 3 d before the experimental endpoint and were euthanized. Table 1 provides a complete assessment of the clinical outcome for these rabbits, ante- and postmortem.
Table 1.
Clinical parameters, interventions, and postmortem analysis results
| Treatment Group | ||||
| CsA | Anti-CD4 | Control group | Anti-Pan T-cell | |
| Clinical Parameters | ||||
| BW loss | 2/2 | 2/4 | 0/4 | 0/4 |
| Inappetence (hyporexia/anorexia) | 2/2 | 1/4 | 0/4 | 0/4 |
| Decreased fecal output | 2/2 | 1/4 | 0/4 | 0/4 |
| Lethargy | 2/2 | 1/4 | 0/4 | 0/4 |
| Max Temperature (+2 SD) | 2/2 | 2/4 | 2/4 | 2/4 |
| Max Temperature (+3 SD) | 2/2 | 2/4 | 1/4 | 2/4 |
| Interventions | ||||
| LRS SC fluids | 2/2 | 0/4 | 0/4 | 0/4 |
| Kale | 2/2 | 1/4 | 0/4 | 0/4 |
| Critical care/Diet Gel supplementation | 2/2 | 0/4 | 0/4 | 0/4 |
| Euthanized early (met humane endpoints) | 2/2 | 1/4 | 0/4 | 0/4 |
| Necropsy Parametersc | ||||
| Spleen weighta | 2.5g ± 0.9 | 2.7g ± 0.33 | 2.1g ± 0.5 | 2.75g ± 0.43 |
| Spleen weight, % of body weighta | 0.07% ± 0.025 | 0.06% ± 0.006 | 0.05% ± 0.009 | 0.07% ± 0.1 |
| Liver weighta | 122.55g ± 10.45 | 104.2g ± 10.6 | 104.5g ± 6.4 | 114.4g ± 10 |
| Liver weight, % of body weighta | 3.3% ± 0.23 | 2.6% ± 0.33 | 2.5% ± 0.15 | 2.7% ± 0.16 |
| Histology Parameters | ||||
| Liver | ||||
| Peri-portal inflammatory infiltrate | 2/2 | 3/4 | 3/4 | 4/4 |
| Spleen | ||||
| PALS depletion | 0/2 | 1/4 | 1/4 | 0/4 |
| Paramyloid in PALS | 0/2 | 1/4 | 2/4 | 2/4 |
| Mesenteric Lymph Node | ||||
| Lymphoid depletion | 1/2 | 2/3b | 2/4 | 1/4 |
| Follicle development | 1/2 | 1/3b | 2/4 | 2/4 |
BW, body weight; SD, standard deviation; SEM, standard error of the mean;
Values represent the mean ± SEM;
No lymph node identified for the fourth rabbit in this group;
Necropsy parameters are not considered significantly different via either one way ANOVA or paired t tests (P values range from 0.193–0.983).
Temperature Results.
Temperature transponders were used to detect the rabbits’ body temperatures throughout the study. Generally, during any anesthetic event, the rabbits’ temperatures would decrease and then in the recovery period after anesthesia, temperatures returned to baseline for each rabbit. Sporadically some rabbits showed periods of increased temperature which was occasionally but inconsistently correlated with clinical signs (Table 1).
Pathology/Histology.
The majority of the rabbits infected with EBV had a minimal to mild, peri-portal infiltration consisting of lymphocytes, plasmacytes, histiocytes, and/or heterophils (12/14, 85.7%) (Figures 4 A and 4 B, Table 1). Other histologic findings included minimal-to-mild peri-arterial lymphatic sheath (PALS) depletion (3/14, 21.4%) (Figure 4 C) and minimal-to-moderate hyaline material deposition in the PALS (paramyloid)40 (5/14, 35.7%) (Figure 4 D) in the spleen. Lymph nodes occasionally exhibited minimal-to-mild lymphoid depletion (6/14, 42.9%), or minimal-to-mild follicle development (6/14, 42.9%). No significant differences or trends were noted in the histologic features among treatment groups.
Figure 4.
Histology images from various antibody-treated rabbits, H and E stain. (A) Liver, multifocal areas of minimal to mild, peri-portal lymphoplasmacytic-infiltration, 100×; (B) Liver, portal triad with minimal-to-mild lymphoplasmacytic infiltration, 400×; (C) Spleen, PALS area showing increased cellularity and germinal center formation, 200×; (D) Spleen, multifocal, mild-to-moderate, eosinophilic hyaline material (paramyloid) in the marginal zone and decreased peri-arteriolar lymphocytes, 200×.
Body Weight Results.
No significant difference in body weight occurred from time 0 to day 2 after EBV inoculation (Figure 5). After day 2, the CsA-treated rabbits displayed a greater loss of weight than did compared with the other groups, which were respectively 6% or 15% of baseline body weight at the termination of the study. The mean weight difference between the CsA-treated rabbits and all other treatment groups are considered significantly different at day 7 (P = 0.004) and day 14 (P = 0.002) via paired t-tests. Generally, the rabbits in the anti-CD4, control, and anti-Pan T-cell treated groups maintained or gained weight, with only a few instances of minor weight loss (Figure 5, Table 1).
Figure 5.
The average amount of changes in weight (in kilograms) from baseline weight plotted against time in days post-EBV inoculation (Day 0). Groups represented on the graph are: anti-CD4 treated rabbits (●); anti-Pan T-cell treated rabbits (○); control rabbits (▼); and CsA treated rabbits (Δ). Mean weight difference at day 7 (P = 0.004) and day 14 (P = 0.002) via paired t tests between CsA-treated and control groups are significantly different (*).
ELISA.
We analyzed rabbit serum for IgG against EBV VCA (Figure 6). IgG increased over time in rabbits treated with anti-CD4 and Pan T-cell antibodies as compared with the control and CsA-treated groups. IgG levels at day 28 post-inoculation are considered significantly different between all of the groups (P = 0.027) via one-way ANOVA, for the anti-pan T-cell treated group compared to the control group (P = 0.028) via a paired t-test, and for the anti-CD4 treated group compared to the control group (P = 0.012) via a paired t-test (Figure 6). The control group was considered to be positive, but weaker compared with the anti-CD4 and Pan T-cell treated groups. The CsA-treated group was considered to be negative for the detection of IgG.
Figure 6.
Plot of serological responses against time measuring IgG against EBV VCA for rabbits treated with (●) anti-CD4 antibody, (▼) anti-Pan T-cell antibody, (○) anti-HPV antibody (control), and (Δ) CsA. (↓) indicates anesthesia; (±) indicates inoculation with EBV. A few of the time 0 or baseline time point samples were increased above a true negative or low value due to mild hemolysis. Day 28 after inoculation is considered significantly different (*) between all of the groups (P = 0.027) via one-way ANOVA, for the anti-Pan T-cell treated group compared with the control group (P = 0.028) via a paired t test, and for the anti-CD4 treated group compared with the control group (P = 0.012) via a paired t test. The anti-Pan T-cell treated group is considered significantly different from the control group (α) via paired t tests at day 7 (P = 0.041) and day 22 (P = 0.022) after inoculation.
EBV DNA qPCR in PBMCs and Spleen Cells.
EBV DNA was consistently not detected over the course of the study using qPCR of PBMCs from all treatment groups (data not shown). In contrast, qPCR analyses of splenocytes showed positive signals for EBV DNA (Figure 7). The rabbits from the anti-CD4 treated group had the highest average copies of EBV DNA per microgram of splenocyte DNA, as compared with the other treatment groups (71,000, 150,000, 175,000, and 400,000 copies per microgram of splenocyte DNA). A paired t-test performed between anti-CD4 treated and CsA-treated rabbit data was considered significant (P = 0.025). A oneway ANOVA performed for all of the information was not considered significant (P = 0.054) but indicated a trend (P < 0.1) that the control group had low to negative EBV DNA in the spleen, much like the PBMCs (PBMC data not shown). Two rabbits in the anti-Pan T-cell treated group had detectable EBV DNA in spleen cells (49,000 and 190,000 copies per microgram of DNA) and 2 rabbits in that group had undetectable levels. Figure 7 incorporates data from all rabbits in these studies treated with CsA concurrent with EBV-inoculation (total of 9 rabbits); their qPCR results ranged from undetectable to 100,000 copies per microgram of splenocyte DNA.
Figure 7.
Average PCR data from spleen cell suspensions per study group in copies per microgram of DNA. Groups represented on the graph are: anti-CD4 treated rabbits (n = 4) (●); anti-Pan T-cell treated rabbits (n = 4) (○); control rabbits (n = 4) (▼); and CsA treated rabbits (n = 9) (Δ). A paired t test performed between anti-CD4 treated and CsA-treated rabbit data was considered significant (P = 0.025), notated by (*). A one-way ANOVA performed for all of the information was not considered significant (P = 0.054). Paired t tests performed on the remainder of the data were not considered significant (P values 0.057-0.812).
We calculated an average of 2 copies of EBV DNA per cell genome, which is in the range reported for human lymphoblastoid cell lines (absolute range between 1 and 350 copies per cell).14
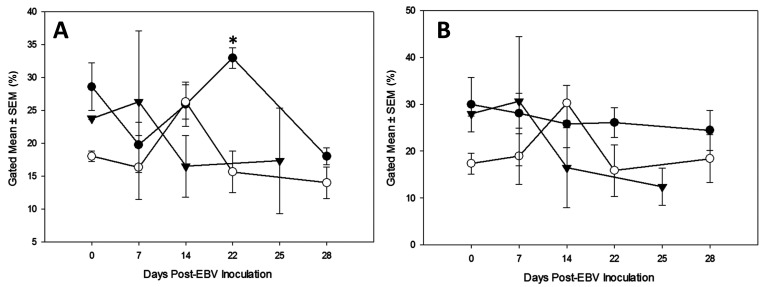
Flow Cytometry.
The results of flow cytometric analysis of PBMCs demonstrated that an overall partial, but not complete, reduction in T-cell subsets (Figure 8). In rabbits treated with antibody against a subset of immune cells, some rabbits showed a decline in T-cell populations, whereas other rabbits showed an increase in T-cells. In the control group, the T-cell populations peaked at day 14 after inoculation and then returned to baseline. The CsA-treated group generally showed a pattern of mild increase from baseline, followed by a reduction for the remainder of the study.
Figure 8.
Results of flow cytometry presented as percent gated mean ± SEM (SEM) for each group plotted against days post-EBV inoculation. (A) Flow cytometry detecting CD4 T-cells for the following groups: anti-CD4 treated rabbits (●), control rabbits (○), and CsA-treated rabbits (▼). Day 22 the data points are considered significant via a paired t test (P = 0.007) (*). One-way ANOVAs run for days 7 and 14 were not significant (P = 0.525, P = 0.208, respectively). A paired t test run for day 28 was not significant (P = 0.229). (B) Flow cytometry detecting Pan T-cells for the following groups: anti-Pan T-cell treated rabbits (●), control rabbits (○), and CsA-treated rabbits (▼). One-way ANOVAs run for days 7 and 14 were not significant (P = 0.467, p = 0.304, respectively). Paired t tests run for days 22 and 28 were not significant (P = 0.157, P = 0.399, respectively).
Discussion
The studies presented here describe a novel rabbit model of EBV infection that mimics the acute or early events of EBV disease in a laboratory setting. We used both CsA administration and in vivo depletion of CD4 T-cells to modulate host immune responses to the EBV infection and monitored a variety of viral and host parameters to assess disease status. The data presented provide new information on the role of the rabbit immune system in the control of EBV infection and further establishes the rabbit as an appropriate laboratory animal model for EBV studies.
Through a series of experiments, we compared concurrent and delayed CsA treatments in rabbits inoculated with EBV. The differential time-course treatments with CsA allow comparison of EBV infection and disease progression in an acute and a latent EBV infection model. These studies also investigated antibody-mediated immunosuppression, as treatment with CsA consistently caused clinical signs in rabbits (lethargy, weight loss, inappetence, and others). Treatment with delayed CsA appeared to lead to a higher viral genome load in PBMCs, which is consistent with the literature.23 Comparatively, we detected more viral genomes in spleen cell suspensions from rabbits that received concurrent CsA treatment. However, the concurrent CsA-treated rabbits were short term studies, lasting 4 to 5 wk, whereas the delayed CsA-treated rabbits were longer-term, having been inoculated with EBV and then maintained 5 to 6 wk before initiating CsA treatments. Given these findings, further studies with CsA treatments may benefit from a time-course analysis. Overall, all rabbit strains used in the studies were susceptible to infection with EBV, comparable to the literature.21,26
This study used a small sample size of outbred rabbits. Arguably, outbred rabbits represent the outbred human population better than an inbred strain. Our sample included an outlier rabbit and an expected death. The outlier rabbit was treated with anti-CD4 antibody and did not appear to respond to the antibody treatment (Figure 8 A). At time point day 22, this outlier rabbit showed an increase in CD4 T-cells rather than the expected decrease. This rabbit presented with clinical signs similar to those seen in CsA-treated rabbits and reached a humane endpoint 3 d prior to the rest of the cohort. We hypothesize that this rabbit had a subclinical, underlying infection that caused a rebound in the immune system and overcame the attempts at CD4 T-cell depletion, despite no gross lesions detected at necropsy. This model will benefit from further projects to address potential differences between the strains.
Time-course analysis of anti-VCA antibody production provides an additional immune assessment of acute EBV infection. Overall, rabbits receiving antibody treatments had increasing levels of EBV VCA antibody throughout the study. A possible explanation for this event is that the antibody treatments directly stimulated adaptive immunity, leading to the B-cells producing higher amounts of antibodies to EBV because of insufficient T-cell subset depletion or because they were acting as activating signals. The finding that antibody responses were highest for the rabbits given pan T-cell treatment, despite a lack of subset depletion, suggest that this monoclonal antibody is nondepleting and may have in fact activated T-cells, as has been observed for a number of other monoclonal antibody treatments.7,24,25 We can also conclude that the rabbits were producing antibody as a response to confirmed systemic infection, as compared with a response to inoculation alone, because the antibody titers increased throughout the study, similar to data from previously published reports.39 In contrast, treatment with CsA appears to have suppressed adaptive immunity, with no anti-VCA antibody detected despite higher levels of EBV genomes.
The overall results of the flow cytometry data show that anti-CD4 antibody treatments resulted in partial CD4 T-cell depletion. A pilot test of this Mab in a rabbit showed peripheral depletion of CD4 cells from the PBMCs (data not shown), thus encouraging us to continue with the studies presented in this manuscript. By comparison, the results were less consistent with anti-Pan T-cell antibody (Figure 8) indicating that we used insufficient Mab to achieve depletion, or that this Mab is nondepleting in vivo. The anti-CD4 treated rabbits had higher average levels of detectable EBV DNA in their spleens as compared with the other groups in this study (Figure 7). One of the anti-CD4 treated rabbits also displayed signs of systemic illness and reached a humane endpoint earlier than the rest of the cohort. The clinical illness mimicked the signs observed in rabbits treated with CsA, but was less severe. In contrast, the anti-Pan T-cell treated rabbits were healthy for the duration of the study and had less detectable virus in the spleen as compared with the anti-CD4 treated rabbits. Based on the qPCR and ELISA results, the addition of antibody likely affected virus detection despite relatively low and inconsistent levels of T cell depletion and thus minimal immune modulation of the host.
The clinical features of rabbits infected with EBV show some similarities to humans with IM or an active EBV infection. Human clinical signs include fever, hepatosplenomegaly, lymphadenopathy, pharyngitis, hepatitis manifesting as headache, vomiting, nausea, and anorexia, and possibly skin rashes.11,33 We did not detect lymphadenopathy, pharyngitis, skin-related issues, or hepatosplenomegaly in our rabbits. However, some of them did have a transient fever, based on temperature measurements that were 2 or 3 times the standard deviation above that rabbit's baseline temperature. Occasionally, these temperature increases correlated with clinical signs (Table 1). Rabbits are incapable of vomiting, but they can still feel nausea, so this is a clinical sign that is not directly comparable to humans; similarly, identifying a headache in a rabbit is not clinically feasible. However, the rabbits that showed clinical signs did exhibit lethargy, inappetence, decreased fecal output, and weight loss. These signs are nonspecific in rabbits but do indicate a pathologic process.
In general, the rabbits treated with CsA in this study showed trend of weight loss over time (P = 0.054). This weight loss is expected in the rabbits treated with CsA, as shown in the literature and previous studies.10,23 Body weight loss is considered a common indicator of declining rabbit condition, and we did not detect significant weight loss in antibody-treated groups, suggesting that the antibody treatments did not influence the rabbits’ health. According to the animal use protocol, a body weight loss of greater than or equal to 20% was considered a humane endpoint. None of the rabbits in these experiments reached the 20% body weight loss mark, but a downward trend for only the CsA treated group occurred as compared with the weight changes of both the control and antibody-treated groups (Figure 5).
The use of the temperature transponders throughout this study provided insight into the full clinical picture of the rabbits infected with EBV. The transponders provided a noninvasive method of temperature recording. Placing the transponders is similar to placing a microchip, which requires only normal restraint methods, reducing the risk and potential experimental artifact associated with sedation or anesthesia. When we used anesthesia for drug administration or blood collection, the rabbits did not receive supplemental heat because the fall in temperature is mild, and the rabbits recover rapidly. Rabbits in this study showed this expected transient and mild decrease in body temperature without ill effects. Ketamine/xylazine provides approximately 35 min of anesthesia and most of the rabbits were reversed with Yohimbine, providing a short period of immobilization.
A limitation in the current study was the amount of anti-CD4 T-cell and anti-Pan T-cell antibodies available. Higher concentrations (from 0.6 to 25 mg) of antibodies usually are needed to achieve antibody depletion in other species, including humans.21,34 We believe that the relatively small amounts of antibodies used in this study are the reason that the flow cytometry results did not reveal higher levels of depletion of the CD4 or Pan T-cell populations. A study using cats found that one larger dose of antibody (9 mg/kg), rather than the 3 smaller doses (3 d of 3 mg/kg) we used in rabbits, resulted in more thorough antibody depletion for all of the cell types they targeted.34
We were able to successfully detect EBV in the spleen, possibly due to higherincreased numbers of B-lymphocytes in the spleen compared with peripheral blood. The number of circulating lymphocytes vary in a clinically healthy rabbit,2 with B cells only comprising 16% to 52% of total lymphocytes.38 These relatively small B-cell counts in normal rabbits may obscure finding EBV in PBMCs, especially if only a small infectious dose was used. We also noted that rabbits infected with EBV and then immune-suppressed 5 wk later with CsA had a slightly higher level of EBV in their PBMCs; a finding similar to that observed by others.23 We hypothesize that allowing time for EBV to establish before immune suppression could have created a large, latent population of EBV-infected lymphocytes that was then able to expand upon immune suppression. On the other hand, concurrent EBV and immune suppression creates an acute, active infection likely does not result in development of this population of latently infected cells.
In summary, our data show that rabbits can respond to CD4 T-cell depletion using a mouse anti-CD4 monoclonal antibody. Published studies for both human and animal antibody-mediated subset depletion support the need to use higher amounts of antibody than we used in this study. The future goals of this research are to repeat anti-CD4 T-cell antibody-mediated depletion using higher amounts of anti-CD4 antibody. Altogether, our research further defines the utility of rabbits with EBV infection and immune modulation via antibody-mediated lymphocyte depletion as a model of human EBV infection.
Acknowledgments
The authors thank the following sources of funding for this project: The Department of Comparative Medicine endowment funds and the Jake Gittlen Memorial Golf Tournament Fund. We would also like to thank all of the Comparative Medicine staff who provided excellent care for these rabbits and their tissues including care staff, veterinary staff, and the Comparative Histology Laboratory (Ellen Mullady, Gretchen Snavely, and Jessica Wingate). We sincerely appreciate the skill and expertise of Lynn Budgeon for her assistance with IHC.
References
- 1.Animal Welfare Regulations. 2008. 9 CFR § 3129.
- 2.Carpenter JW, Marion CJ. 2018. Exotic animal formulary, 5th ed. St Louis (MO): Elsevier. [Google Scholar]
- 3.Christensen ND, Cladel NM, Reed CA, Han R. 2000. Rabbit oral papillomavirus complete genome sequence and immunity following genital infection. Virology 269:451–461. 10.1006/viro.2000.0237. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JI, Fauci AS, Varmus H, Nabel GJ. 2011. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci Transl Med 3:1–6. 10.1126/scitranslmed.3002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui X, Cao Z, Chen Q, Arjunaraja S, Snow AL, Snapper CM. 2016. Rabbits immunized with Epstein-Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine 34:4050–4055. 10.1016/j.vaccine.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Dawson DG, Bower KA, Burnette CN, Holt RK, Swearengen JR, Dabisch PA, Scorpio A. 2017. Using telemetry data to refine endpoints for New Zealand white rabbits challenged with Bacillus anthracis. J Am Assoc Lab Anim Sci 56:792–801. [PMC free article] [PubMed] [Google Scholar]
- 7.Deligne C, Milcent B, Josseaume N, Teillaud JL, Siberil S. 2017. Impact of depleting therapeutic monoclonal antibodies on the host adaptive immunity: A bonus or a malus? Front Immunol 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowler KW, Mccormick S, Armstrong JA, Hsiung GD. 1984. Lymphoproliferative changes induced by infection with a lymphotropic herpesvirus of guinea-pigs. J Infect Dis 150:105–111. 10.1093/infdis/150.1.105. [DOI] [PubMed] [Google Scholar]
- 9.Fox JG. 2002. Laboratory animal medicine, 2nd ed. New York (NY): Academic Press. [Google Scholar]
- 10.Gratwohl A, Riederer I, Graf E, Speck B. 1986. Cyclosporine toxicity in rabbits. Lab Anim 20:213–220. 10.1258/002367786780865692. [DOI] [PubMed] [Google Scholar]
- 11.Ha SY, Chung CW, Ko YH. 2004. Severe chronic active EBV infection in an adult patient: Case report. J Korean Med Sci 19:453–457. 10.3346/jkms.2004.19.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi K, Akagi T. 2000. An animal model for Epstein-Barr virus (EBV)-associated lymphomagenesis in the human: Malignant lymphoma induction of rabbits by EBV-related herpesvirus from cynomolgus. Pathol Int 50:85–97. 10.1046/j.1440-1827.2000.01018.x. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Jin ZS, Onoda S, Joko H, Teramoto N, Ohara N, Oda W, Tanaka T, Liu YX, Koirala TR, Oka T, Kondo E, Yoshino T, Takahashi K, Akagi T. 2003. Rabbit model for human EBV-associated hemophagocytic syndrome (HPS)—Sequential autopsy analysis and characterization of IL-2 dependent cell lines established from herpesvirus papio-induced fatal rabbit lymphoproliferative diseases with HPS. Am J Pathol 162:1721–1736. 10.1016/S0002-9440(10)64306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houldcroft CJ, Petrova V, Liu JZ, Frampton D, Anderson CA, Gall A, Kellam P. 2014. Host genetic variants and gene expression patterns associated with Epstein-Barr virus copy number in lymphoblastoid cell lines. PLoS One 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J, Peng X, Cladel NM, Pickel MD, Christensen ND. 2005. Large cutaneous rabbit papillomas that persist during cyclosporin A treatment can regress spontaneously after cessation of immunosuppression. J Gen Virol 86:55–63. 10.1099/vir.0.80448-0. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. 2006. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol 177:8037–8045. 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 18.Jenson HB, Ench Y, Gao SJ, Rice K, Carey D, Kennedy RC, Arrand JR, Mackett M. 2000. Epidemiology of herpesvirus papio infection in a large captive baboon colony: Similarities to Epstein-Barr virus infection in humans. J Infect Dis 181:1462–1466. 10.1086/315385. [DOI] [PubMed] [Google Scholar]
- 19.Jenson HB, Ench Y, Zhang YJ, Gao SJ, Arrand JR, Mackett M. 2002. Characterization of an Epstein-Barr virus-related gammaherpesvirus from common marmoset (Callithrix jacchus). The DNA sequence reported in this study has been deposited in GenBank with the accession number AF291653. J Gen Virol 83:1621–1633. 10.1099/0022-1317-83-7-1621. [DOI] [PubMed] [Google Scholar]
- 20.Jiwa NM, Oudejans JJ, Dukers DF, Vos W, Horstman A, van der Valk P, Middledorp JM, Walboomers JM, Meijer CJ. 1995. Immunohistochemical demonstration of different latent membrane protein-1 epitopes of Epstein-Barr virus in lymphoproliferative diseases. J Clin Pathol 48:438–442. 10.1136/jcp.48.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung SR, Suprunenko T, Ashhurst TM, King NJC, Hofer MJ. 2018. Collateral damage: What effect does anti-CD4 and anti-CD8α antibody-mediated depletion have on leukocyte populations? J Immunol 201:2176–2186. 10.4049/jimmunol.1800339. [DOI] [PubMed] [Google Scholar]
- 22.Kanai K, Kato K, Sano H, Nagata K, Okuno K, Kuwamoto S, Higaki H, Sugihara H, Kato M, Murakami I, Hayashi K. 2011. In vitro Epstein-Barr virus infection model of rabbit lymphocytes from peripheral blood or spleen. Intervirology 54:17–24. 10.1159/000318882. [DOI] [PubMed] [Google Scholar]
- 23.Khan G, Ahmed W, Philip PS, Ali MH, Adem A. 2015. Healthy rabbits are susceptible to Epstein-Barr virus infection and infected cells proliferate in immunosuppressed animals. Virol J 12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kvarnhammar AM, Veitonmaki N, Hagerbrand K, Dahlman A, Smith KE, Fritzell S, von Schantz L, Thagesson M, Werchau D, Smedenfors K, Johansson M, Rosen A, Aberg I, Winnerstam M, Nyblom E, Barchan K, Furebring C, Norlen P, Ellmark P. 2019. The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J Immunother Cancer 7:1–14. 10.1186/s40425-019-0570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangsbo SM, Broos S, Fletcher E, Veitonmaki N, Furebring C, Dahlen E, Norlen P, Lindstedt M, Totterman TH, Ellmark P. 2015. The human agonistic CD40 antibody ADC-1013 eradicates bladder tumors and generates T-cell-dependent tumor immunity. Clin Cancer Res 21:1115–1126. 10.1158/1078-0432.CCR-14-0913. [DOI] [PubMed] [Google Scholar]
- 26.Moghaddam A, Rosenzweig M, Lee-Parritz D, Annis B, Johnson RP, Wang F. 1997. An animal model for acute and persistent Epstein-Barr virus infection. Science 276:2030–2033. 10.1126/science.276.5321.2030. [DOI] [PubMed] [Google Scholar]
- 27.Mosier DE, Baird SM, Kirven MB, Gulizia RJ, Wilson DB, Kubayashi R, Picchio G, Garnier JL, Sullivan JL, Kipps TJ. 1990. EBV-associated B-cell lymphomas following transfer of human peripheral-blood lymphocytes to mice with severe combined immune-deficiency. Curr Top Microbiol Immunol 166:317–323. [DOI] [PubMed] [Google Scholar]
- 28.Okuno K, Takashima K, Kanai K, Ohashi M, Hyuga R, Sugihara H, Kuwamoto S, Kato M, Sano H, Sairenji T, Kanzaki S, Hayashi K. 2010. Epstein-Barr virus can infect rabbits by the intranasal or peroral route: an animal model for natural primary EBV infection in humans. J Med Virol 82:977–986. 10.1002/jmv.21597. [DOI] [PubMed] [Google Scholar]
- 29.Peng X, Griffith JW, Han RC, Lang CM, Kreider JW. 1999. Development of keratoacanthomas and squamous cell carcinomas in transgenic rabbits with targeted expression of EJras oncogene in epidermis. Am J Pathol 155:315–324. 10.1016/S0002-9440(10)65125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu L, Green M, Webber S, Reyes J, Ellis D, Rowe D. 2000. Epstein-Barr virus gene expression in the peripheral blood of transplant recipients with persistent circulating virus loads. J Infect Dis 182:1013–1021. 10.1086/315828. [DOI] [PubMed] [Google Scholar]
- 31.Raab-traub N, Dambaugh T, Kieff E. 1980. DNA of Epstein-Barr Virus-Viii - B95-8, the previous prototype, is an unusual deletion derivative. Cell 22:257–267. 10.1016/0092-8674(80)90173-7. [DOI] [PubMed] [Google Scholar]
- 32.Rajčáni J, Szenthe K, Durmanová V, Tóth A, Asványi B, Pitlik E, Stipkovits L, Szathmary S. 2014. Epstein-Barr Virus (HHV-4) inoculation to rabbits by intranasal and oral routes results in subacute and/or persistent infection dissimilar to human disease. Intervirology 57:254–269. 10.1159/000360223. [DOI] [PubMed] [Google Scholar]
- 33.Sergent SR, Johnson SM, Ashurst J, Johnston G. 2015. Epstein-Barr virus-associated atraumatic spleen laceration presenting with neck and shoulder pain. Am J Case Rep 16:774–777. 10.12659/AJCR.893919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smithberg SR, Fogle JE, Mexas AM, Reckling SK, Lankford SM, Tompkins MB, Dean GA. 2008. In vivo depletion of CD4+CD25+ regulatory T cells in cats. J Immunol Methods 329:81–91. 10.1016/j.jim.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straus SE, Cohen JI, Tosato G, Meier J. 1993. Epstein-Barr-virus infections: biology, pathogenesis, and management. Ann Intern Med 118:45–58. 10.7326/0003-4819-118-1-199301010-00009. [DOI] [PubMed] [Google Scholar]
- 36.Takashima K, Ohashi M, Kitamura Y, Ando K, Nagashima K, Sugihara H, Okuno K, Sairenji T, Hayashi K. 2008. A new animal model for primary and persistent Epstein-Barr virus infection: Human EBV-infected rabbit characteristics determined using sequential imaging and pathological analysis. J Med Virol 80:455–466. 10.1002/jmv.21102. [DOI] [PubMed] [Google Scholar]
- 37.Tang Y, He R, Zhang Y, Liu F, Cheng A, Wu Y, Gan R. 2011. Human-derived IgG level as an indicator for EBV-associated lymphoma model in Hu-PBL/SCID chimeras. Virol J 8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walstra K, Gratwohl A, Riederer I, Speck B. 1985. B/T cell ratio of rabbit peripheral-blood lymphocytes. Influence of separation technique on results. J Immunol Methods 79:143–147. 10.1016/0022-1759(85)90400-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Yi X, Du L, Wang H, Tang J, Wang ML, Qi CL, Li H, Lai YJ, Xia W, Tang AZ. 2017. A study of Epstein-Barr virus infection in the Chinese tree shrew (Tupaia belangeri chinensis). Virol J 14:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willard-Mack CL, Elmore SA, Hall WC, Harleman J, Kuper CF, Losco P, Rehg JE, Ruhl-Fehlert C, Ward JM, Weinstock D, Bradley A, Hosokawa S, Pearse G, Mahler BW, Herbert RA, Keenan CM. 2019. Nonproliferative and proliferative lesions of the rat and mouse hematolymphoid system. Toxicol Pathol 47:665–783. 10.1177/0192623319867053. [DOI] [PMC free article] [PubMed] [Google Scholar]