Abstract
Cardiac biomarkers are an important tool for diagnosing cardiac diseases in both human and veterinary patients. Serum concentrations of N-terminal probrain natriuretic peptide (NT-proBNP) and cardiac troponin I (cTnI) have been used to indicate the presence of various cardiac diseases including hypertrophic cardiomyopathy (HCM) in various species including humans. However, these cardiac biomarkers have not been established as a diagnostic tool for detecting cardiac disease in rhesus macaques. In the rhesus macaque colony at the California National Primate Research Center, naturally occurring HCM and various other cardiac diseases have been identified. In this study, commercially available assays were used to measure serum cTnI and NT-proBNP concentrations to evaluate their utility as a diagnostic screening tool for cardiac diseases in rhesus macaques. This study revealed that the serum cTnI concentration was significantly higher in animals with echocardiographically apparent cardiac disease as compared with the animals that had no cardiac structural and functional changes (the control group). However, no significant differences were detected between animals with HCM and non-HCM cardiac disease. Because the area under the receiver operating characteristic curve was 0.81 when the serum cTnI was compared between the control and cardiac disease groups, serum cTnI was considered a moderately accurate test to predict the presence of cardiac disease. The optimal cut-off value of serum cTnI concentration for diagnosis of cardiac disease was 0.0085 ng/mL, with a sensitivity of 0.68 and specificity of 0.94. Significant but weak correlations were noted between the serum cTnI concentration and several echocardiographic parameters. Conversely, no significant differences in NT-proBNP concentrations were detected between animals with and without cardiac diseases. In conclusion, measurement of serum cTnI can be used to aid in diagnosing cardiac diseases in rhesus macaques. However, cTnI measurement does not replace echocardiographic evaluation to diagnose cardiac diseases in rhesus macaques due to the poor sensitivity of the assay and the weak correlation to with more established echocardiographic markers for cardiac disease.
Abbreviations: ACD, all cardiac disease; BNP, brain natriuretic peptide; cTnI, cardiac troponin I; E:A, ratio of the peak early to late transmitral flow velocity; Eʹ; :Aʹ; (lateral), ratio of the peak early to late lateral mitral annular motion velocity; Eʹ; :Aʹ; (medial), ratio of the peak early to late medial mitral annular motion velocity; FS, fractional shortening; HCM, hypertrophic cardiomyopathy; IVSd, interventricular septal wall thickness during diastole; LA/Ao, (lax) 2-dimensional left atrial diameter to left aortic root diameter ratio from right parasternal long-axis view; LA/Ao (sax), 2-dimensional left atrial diameter to left aortic root diameter ratio from right parasternal short-axis view; LVPWd, thickness of left ventricular posterior wall during diastole; NT-proBNP, N-terminal probrain natriuretic peptide; OCD, other cardiac disease; VAo, peak velocity of aortic flow
Diagnostic imaging, including echocardiography, is commonly used in a clinical setting to diagnose cardiac diseases, including hypertrophic cardiomyopathy (HCM). However, imaging techniques are highly operator dependent, and a sonographer must receive advanced training to be able to accurately diagnose HCM, even using advanced imaging equipment. Ideally, measurement of circulating cardiac biomarkers would enable the objective evaluation of the likely presence and severity of cardiac abnormalities such as HCM, thereby reducing the number of animals that require advanced imaging. Much of research has been performed to explore the use of cardiac biomarkers as a screening tool to correctly identify subclinical cardiac diseases in a variety of species, but this has not yet been done in rhesus macaques.14,53
Cardiac troponin I (cTnI) is a cardiomyocyte-specific troponin found on the actin filament in myocardial tissues. cTnI is released from myocardial cells that are injured or die, making it possible to use it as a circulating biomarker of myocardial injury.1,2,52 In addition, a high-sensitivity cTnI assay is commercially available, and this assay can detect very low concentrations of cTnI with high precision.5 This high sensitivity cTnI assay has been validated in various species, including healthy rhesus macaques.6 It was also measured in rhesus macaques with naturally occurring HCM in a small pilot study of animals from the same facility as the present study, but this previous study failed to show significant differences in cTnI values between HCM and control animals.25
NT-proBNP is an inactive byproduct of brain natriuretic peptide (BNP). NT-proBNP has been used as a stable surrogate marker of BNP, because of its longer plasma half-life compared with BNP.19,24 Measuring the plasma or serum concentration of NT-proBNP has been used to aid in diagnosing various cardiac diseases in both humans and other species, including dogs and cats.7,54 In humans, NT-proBNP has been used for the diagnosis of acute and chronic heart failure.10,21,26,50 In addition, serum NT-proBNP concentration has been known to be increased in patients with HCM.11,13,16,34 An elevated serum concentration of both cTnI and NT-proBNP has also been associated with various characteristics of HCM in humans and other species, including the degree of left ventricular wall thickening, changes in left atrial size, the presence and degree of left ventricular outflow tract obstruction (LVOTO).20,27,34,41
To the authors’ knowledge, no study has compared the serum concentration of NT-proBNP in rhesus macaques, either with or without cardiac disease. Although serum cTnI concentration was measured in a previous study, the numbers of HCM and control macaques were very low.25 Therefore, the objective of the present study was to measure the concentrations of cTnI and NT-proBNP in rhesus macaques, with and without cardiac disease, using commercially available assays. An association between elevated serum cTnI and NT-proBNP concentrations and various characteristics of HCM was evaluated. We hypothesized that rhesus macaques with HCM and other cardiac diseases would have higher concentrations of cTnI and NT-proBNP, and that the severity of the cardiac disease would correlate with serum cTnI and NT-proBNP concentrations.
Materials and Methods
Subject and Housing.
All study procedures were conducted at the CNPRC, which is a USDA registered and AAALAC accredited institution that maintains a Public Health Services Assurance. The protocol for the present study was approved by the Institutional Animal Care and Use Committee of the University of California-Davis. All rhesus macaques were maintained in accordance with the USDA Animal Welfare Act and recommendations in the Guide for the Care and Use of Laboratory Animals.3,4 Rhesus macaques were housed either indoors or outdoors with other rhesus macaques. Two rhesus macaques were relocated to indoor housing approximately 4 d before echocardiographic examination and blood collection. The rest of the rhesus macaques under study were relocated to indoor housing more than 8 d prior to examination and blood collection, or were housed indoors for an extended period of time. The rhesus macaques were housed outside in large field pens or small corn cribs. The rhesus macaques housed indoors, or relocated to indoor housing just prior to examination, were usually paired and housed in stainless steel cages sized according to the primary cage-space regulations. Outdoor husbandry included cleaning of all fixture surfaces, standard sanitization, and protection from the elements. All rhesus macaques housed indoors were on a 12:12 light:dark cycle as standard. Temperature, humidity, and ventilation of the rooms were maintained as a part of routine husbandry care for indoor rhesus macaques. All rhesus macaques were housed with species-appropriate environmental enrichment, fed chow twice daily (LabDiet Monkey Diet 5047, Purina Laboratory, St Louis, MO), offered water ad libitum via automatic watering devices, and had their diet supplemented with fruits and vegetables twice a week. Physical examination, weighing, tuberculosis testing, and dental prophylaxis were performed every 6 mo to 1 y, while animals were under sedation. All rhesus macaques were monitored periodically for herpes B virus, simian T-lymphotrophic virus, SIV, and simian type D retrovirus)4,30
All animals enrolled in this study had a complete physical examination, blood collection, and echocardiographic examination under sedation.6 They were divided into 3 groups based on complete echocardiographic examination: (1) control with no significant cardiac abnormalities, (2) HCM, and (3) other cardiac diseases (OCD). Rhesus macaques with HCM and OCD were also categorized into a combined group labeled all cardiac disease (ACD).
Sedation.
Echocardiographic examination was performed under sedation using ketamine hydrochloride (10 mg/kg IM; Ketaject, Phoenix Pharmaceutical, St Joseph, MO). An additional dose of ketamine (5 to 10 mg/kg IM) was given if additional time under anesthesia was necessary for echocardiographic examination and blood sampling.
Echocardiography.
Rhesus macaques with HCM or other cardiac diseases were selected from the echocardiographic database developed by the authors (YU, JS). Echocardiographic examination was performed by an ACVIM board-certified cardiologist (JS) or a veterinarian (YU) trained by an ACVIM board-certified cardiologist. To perform complete echocardiography, rhesus macaques were placed in right and left lateral recumbency under sedation. All images were obtained using a 4- to 12-mHz sector-array transducer (S12-4) with color and spectral Doppler capabilities (Phillips Affiniti 50, Best, Netherland). Acquired images were analyzed by a veterinarian (YU) using a standard offline analysis software (Syngo Dynamics, Siemens, Erlangen, Germany) and reviewed by a board-certified cardiologist (JS). All echocardiographic parameters were measured in accordance with the guidelines of the American Society of Echocardiography.37
HCM was diagnosed based on the presence of a thickened ventricular wall and diastolic dysfunction on echocardiographic evaluation.41 Criteria for the diagnosis of HCM was defined as thickness of the left ventricular posterior wall during diastole (LVPWd) and/or interventricular septal wall thickness during diastole (IVSd) more than 6.5 millimeters, in rhesus macaques younger than 9 y old. Rhesus macaques older than 9 y were diagnosed with HCM if IVSd was more than 8.8 mm and/or LVPWd is more than 7.4 mm.25,58 Diastolic dysfunction was diagnosed by the ratio of the peak early to late transmitral flow velocity (E:A) being less than 0.9, or an E:A greater than 0.9 with a lateral (E’/A’ [lateral]) and/or medial (E’/A’ [medial]) peak mitral annular motion velocity during early and late filling of less than 0.9.
Rhesus macaques in the OCD group were diagnosed with systolic dysfunction, moderate to severe degenerative valvular disease, or ventricular septal defect. Systolic dysfunction was diagnosed if a fractional shortening (FS) of the left ventricle was less than 25%, and/or left ventricular ejection fraction (EF) was less than 50%. FS and EF were calculated as previously described.58 Moderate to severe aortic regurgitation was diagnosed if the ratio of jet height to left ventricular outflow tract width was greater than 25%. Moderate to severe mitral and tricuspid regurgitation was diagnosed when the regurgitant jet occupied greater than 30% of the left or right atrial area respectively.12 Rhesus macaques in the control group were free from these abnormalities; animals displaying trace to mild valve regurgitations without concurrent chamber enlargement were not excluded from the control group, as this has been shown to represent normal variation in sedated patients of various species.
Serum sample preparation.
While macaques were sedated for ECG, 3 mL of venous blood were collected into a red-top tube by venipuncture prior to echocardiographic examination. The collected blood samples were centrifuged within 20 min of collection. The supernatant serum samples were aliquoted to 2 2 mL cryogenic tubes, then frozen and stored at – 80 °C within 1 h of sample collection. The serum samples were thawed at room temperature (18 to 20 °C) shortly before performing the cardiac biomarker assays, which were performed within 6 mo of collection.
Measurement of serum cTnI concentration.
Serum cTnI was measured according to manual instructions of a commercially available assay (ADVIA Centaur CP TnI-Ultra, Siemens Healthcare Diagnostics, Tarrytown, NY). The lower detection limit of cTnI is 0.006 ng/mL, and the upper detection limit is 50 ng/mL. The 10% coefficient of variation (CV) is 0.03 ng/mL with a 99th percentile of 0.04 ng/mL.
Measurement of serum NT-proBNP concentration.
Serum NT-proBNP concentration was measured by using a commercially available monkey-specific assay (Catalog number MBS009046, MyBioSource San Diego, CA), performed according to manufacturer's instructions. This assay is a quantitative sandwich enzyme–linked immunosorbent assay (ELISA), with the range of 62.5 to 2000 pg/mL for quantitation and detection sensitivity of 10 pg/mL. Both intraassay CV and interassay CV are reported by the manufacturer as less than 15% in rhesus monkeys. To perform the assay, 50 µL of undiluted serum from the rhesus macaques was used. The standard solution was diluted to yield 62.5, 125, 250, 500, 1000, 2000 pg/mL solutions with a blank well containing only the diluent. The optical density values were measured spectrophotometrically at 450 nm within 15 min after adding the stop solution. A standard curve was constructed used the commercial curve fitting software (Excel), and the actual concentrations of NT-proBNP were calculated based on this standard curve. The samples were analyzed in duplicate measurements. For validating the ELISA kit, we assessed the CV, recovery rate and linearity of the assay. For linearity analysis, 25 µL standard solution was spiked with 25 µL of the serum sample diluted at 1:4, 1:8, and 1:16 ratios. To determine the recovery rate and lower detection limit, 25 µL of the standard solution with known NT-proBNP concentration (31.25, 62.5, 125, 250 pg/mL) was added to each of the wells with 25 µL of serum sample obtained from a healthy rhesus macaque. The CV was determined by using 8 serum samples that were from healthy rhesus macaques being used in another study. These samples were assessed in triplicate.
Statistical analysis.
Normality testing for continuous variables was performed using a D'Agostino-Pearson test in each group. The parametric variables are reported as mean ± SD, and nonparametric variables are reported as median and interquartile range (IQR) for each echocardiographic variable. The patient characteristics and echocardiographic parameters among different groups were compared using an ANOVA with Holm–Sidak posthoc comparisons for parametric variables, and Kruskal–Wallis test with Dunn posthoc comparisons for nonparametric variables. Differences in cTnI and NT-proBNP concentration between 2 groups were also examined using an unpaired Student t test for parametric and Kolmogorov–Smirnov test for nonparametric variables. Categorical variables were compared using a Chi-Squared or Fisher exact test.
Pearson correlation method was used to assess the correlations between the serum biomarker concentrations and patient characteristics (age, BW, HR) and echocardiographic parameters. Simple and multiple linear regression analyses were then performed between cTnI and NT-proBNP concentrations, patient characteristics, and echocardiographic variables when these variables were significantly correlated with the biomarker concentrations.
Receiver operating characteristic (ROC) analysis was performed to evaluate the diagnostic accuracy of these biomarkers and to determine optimal cut-off values of these biomarker concentrations in serum for distinguishing rhesus macaques with and without cardiac diseases. The accuracy was determined to be low with 0.5 ≤ AUC < 0.7, moderate with 0.7 ≤ AUC < 0.9, or high with AUC ≥ 0.9. The cut-off values were reported with statistical parameters including sensitivity and specificity, and the biomarker concentration with the maximal Youden Index (J = specificity + sensitivity – 1) was chosen as a cut-off value to distinguish rhesus macaques with and without cardiac diseases. For all statistical tests, P < 0.05 was considered significant.
Results
Patient characteristics.
A total of 129 rhesus macaques were enrolled in this study, including 101 healthy rhesus macaques without significant cardiac abnormalities. A total of 52 males and 77 females were included in the study. The median age was 7 y (range: 1.6 to 26.8 y) and the median weight was 9.1 kg (range: 3.3 to 18.6 kg). Among these macaques, 28 were diagnosed with cardiac disease and categorized into the all cardiac disease (ACD) group. Among these ACD animals, 21 were diagnosed with HCM, and 7 were diagnosed with various other types of cardiac disease, including systolic dysfunction (2), moderate valvular regurgitation (4), and ventricular septal defect (1). The 7 macaques with non-HCM cardiac diseases were categorized as the other cardiac disease (OCD) group.
The sex, age, body weight (BW) and heart rate (HR) in each group are shown in Table 1. These patient characteristics were compared among rhesus macaques in the different cardiac disease groups. Significant differences in age (P = 0.0007), BW (P = 0.0001), and HR (P = 0.0076) were noted among the 3 groups. The BW (P = 0.0081) and HR (P = 0.013) were significantly higher in the HCM group than the control group, whereas animals in the OCD group were significantly older (P = 0.037) and their BW significantly higher than those of the control group (P = 0.014). No significant differences in the age, BW, and HR were detected between the HCM and OCD groups. No significant differences in sex distributions (P = 0.22) were detected when data from all groups were combined.
Table 1.
Patient characteristics of enrolled rhesus macaques in the control, HCM, OCD groups.
| Overall | Control | HCM | OCD | P value (overall) | Control compared with HCM | Control compared with OCD | HCM compared with OCD | |
| Sex (M/F) | 52M/77F | 39M/62F | 8M/13F | 5M/2F | 0.22 | |||
| Age (year) | 7 (4.6–12.8) | 6.7 (6.7–12.5) | 11.7 (5.8–13.4) | 12.9 (8.7–19) | 0.0048a | 0.055 | 0.037a | > 0.99 |
| BW (kg) | 9.1 (7.2–11.7) | 8.6 (6.7–10.8) | 10.4 (9.4–13.8) | 13.8 (11.2–14.9) | 0.005a | 0.0081a | 0.014a | > 0.99 |
| HR (bpm) | 138 ± 26 | 135 ± 26 | 152 ± 24 | 128 ± 24 | 0.001a | 0.013a | > 0.99 | 0.086 |
These parameters were compared among the control, HCM, and OCD groups and the overall P values are listed.
significant difference (P < 0.05). BCS = body condition score; bpm = beats or breaths per minute; BW = body weight; HCM = hypertrophic cardiomyopathy; HR = heart rate; OCD = other cardiac disease; RR = respiratory rate
Echocardiographic parameters.
Various echocardiographic parameters were measured for all animals enrolled in this study and were compared among the groups (control, HCM, and OCD groups) (Table 2). IVSd, LVPWd, E’/A’ (medial), E’/A’ (lateral), E/A, FS, EF, peak velocity for aortic flow (VAo) were significantly different between the control and HCM groups. Two-dimensional left atrial diameter as compared with the left aortic root diameter ratio from the right parasternal short-axis view (LA/Ao [sax]) and from the right parasternal long-axis view (LA/Ao [lax]), LVPWd were significantly different between the control and OCD groups. Only LA/Ao (lax), FS and EF were significantly different between the HCM and OCD groups.
Table 2.
Median and interquartile ranges or mean ± SD of various echocardiographic parameters in rhesus macaques in the control, HCM, and OCD groups.
| Overall | Control | HCM | OCD | P value (overall) | Control compared with HCM | Control compared with OCD | HCM compared with OCD | |
| LA/Ao (sax) | 1.5 ± 0.21 | 1.4 ± 0.19 | 1.4 ± 0.22 | 1.64 (1.53–1.83) | 0.024a | >0.99 | 0.02a | 0.052 |
| LA/Ao (lax) | 2.1 ± 0.28 | 2.1 ± 0.25 | 2.07 ± 0.29 | 2.36 (2.33–2.72) | <0.0001a | >0.99 | <0.0001a | 0.0006a |
| IVSd (cm) | 0.51 (0.43–0.59) | 0.48 ± 0.097 | 0.68 ± 0.13 | 0.58 (0.53–0.64) | <0.0001a | <0.0001a | 0.056 | 0.79 |
| LVPWd (cm) | 0.58 (0.49–0.67) | 0.56 ± 0.1 | 0.79 ± 0.14 | 0.72 (0.58–0.77) | <0.0001a | <0.0001a | 0.014a | >0.99 |
| FS (%) | 38 ± 9.0 | 37.9 ± 8.3 | 44.4 (35.9–47.9) | 34.4 (22.3–36.5) | 0.025a | 0.03a | 0.13 | 0.0037a |
| EF (%) | 70.5 (63.3–78.0) | 70.2 (62.0–76.8) | 78.8 (68.1–81.9) | 65.2 (45.5–68.3) | 0.0043a | 0.046* | 0.18 | 0.004* |
| E’/A’ (medial) | 1.3 ± 0.43 | 0.94 ± 0.02 | 0.92 ± 0.24 | 0.98 (0.68–1.72) | 0.0019a | 0.0016a | 0.81 | 0.84 |
| E’/A’ (lateral) | 1.5 (1.0–1.8) | 0.65 ± 0.021 | 8.37 (7.6–12.3) | 1.23 (0.59–1.52) | 0.0018a | 0.0044a | 0.17 | >0.99 |
| VAo (m/s) | 1.2 (1.1–1.4) | 1.23 ± 0.17 | 1.36 (1.21–1.69) | 1.1 (1.01–1.47) | 0.0061a | 0.0058a | >0.99 | 0.11 |
| E/A | 1.3 (0.9–1.5) | 1.84 (1.44-2.1) | 0.86 (0.76–0.99) | 1.09 (0.86–1.56) | <0.0001a | <0.0001a | >0.99 | 0.23 |
significant difference (P < 0.05). E/A = ratio of the peak early to late transmitral flow velocity, E’/A’(lateral) = ratio of the peak early to late lateral mitral annular filling velocity, E’/A’(medial) = ratio of the peak early to late medial mitral annular filling velocity, HCM = hypertrophic cardiomyopathy; IVSd = interventricular septal wall thickness during diastole; LA/Ao (lax) = left atrial diameter to left aortic root diameter ratio from right parasternal long-axis view, LA/Ao (sax) = left atrial diameter to left aortic root diameter ratio from right parasternal short-axis view; LVPWd = thickness of left ventricular posterior wall during diastole, OCD = other cardiac disease, VAo = peak velocity for aortic flow
Serum cTnI concentrations.
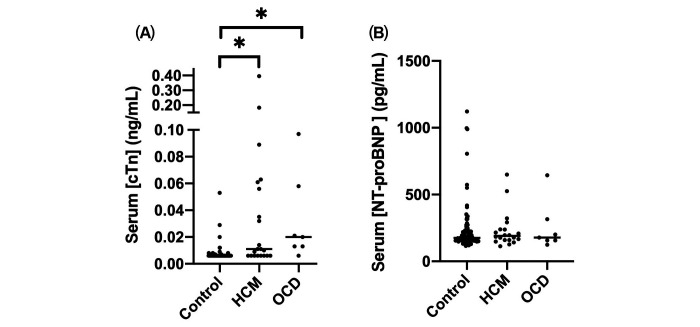
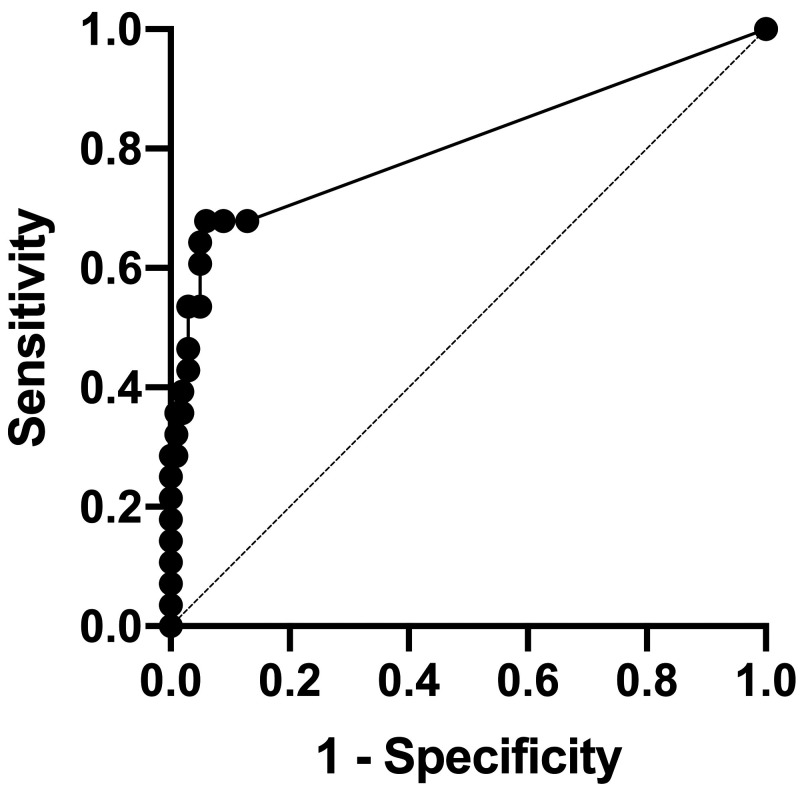
The median serum cTnI concentration for all rhesus macaques was 0.006 (range: < 0.006 to 0.395 ng/mL). No significant differences were noted between males and females (P = 0.078). The serum cTnI concentration in rhesus macaques relocated to indoor housing less than 30 d and in those that were housed indoors for more than 30 d prior to examination and blood collection were compared in 101 control macaques (n = 48 and 53 rhesus macaques, respectively). No significant differences in serum cTnI concentration was noted between these 2 groups (P = 0.23). The serum cTnI concentration was significantly higher in the ACD group as compared with the control group (P < 0.0001) (Figure 1). In the ACD group, macaques in the both HCM (P < 0.0001) and OCD groups (P < 0.0001) had significantly higher serum cTnI concentration than the control group. However, a significant difference in the cTnI concentration was not detected between HCM and OCD groups (P > 0.99) (Table 3). The ROC analysis was performed to determine the accuracy of serum cTnI measurement as a predictor of the presence of cardiac diseases in rhesus macaques. Because the area under the ROC curve (AUC) was 0.81 (95% CI, 0.69. – 0.92) when comparing the serum cTnI between the control and ACD groups, serum cTnI was considered moderately accurate for predicting the presence of cardiac disease (P < 0.0001). The optimal cut-off value of serum cTnI concentration for identifying cardiac disease was 0.085 ng/mL based on the maximal Youden Index (J = 1.62), with sensitivity of 0.68 (95% CI, 0.49 to 0.82) and specificity of 0.94 (95% CI, 0.88 to 0.97) (Figure 2, Table 4).
Figure 1.
Serum (A) cTnI and (B) NT-proBNP concentrations in rhesus macaques with hypertrophic cardiomyopathy (HCM), other cardiac diseases (OCD), or without cardiac disease. Each black circle indicates the biomarker value. The bar indicates medians for each group. Rhesus macaques were categorized into the control, HCM, and OCD groups. cTnI = cardiac troponin I; HCM = hypertrophic cardiomyopathy; OCD = other cardiac disease; NT-proBNP = N-terminal probrain natriuretic peptide. *P < 0.0001.
Table 3.
Median and interquartile ranges of cTnI and NT-proBNP concentrations in rhesus macaques in the control, ACD, HCM, OCD groups.
| Control | ACD | HCM | OCD | |
| cTnI (ng/mL) | 0.006 (0.006–0.006) | 0.013* (0.006–0.058) | 0.011* (0.006–0.059) | 0.02* (0.013–0.058) |
| NT-proBNP (pg/mL) | 175.7 (155.8–224.9) | 184.9 (156.4–239.5) | 190.1 (159.1–239) | 178.1 (156.4–315.4) |
significant difference (P < 0.05) compared with the control groups. ACD = all cardiac disease; cTnI = cardiac troponin I; HCM = hypertrophic cardiomyopathy; NT-proBNP = n-terminal probrain natriuretic peptide; OCD = other cardiac diseases.
Figure 2.
ROC analysis of cTnI in the control and ACD groups. The sensitivity and specificity of cTnI at the cut-off value of 0.0085 was 68% and 94%, respectively. ROC = receiver operative characteristic curve; cTnI = cardiac troponin I; ACD = all cardiac disease
Table 4.
cTnI cut-off values with the sensitivity, specificity, and likelihood ratio to differentiate rhesus macaques with and without any cardiac diseases.
| cTnI (ng/mL) | Sensitivity | 95% CI | Specificity | 95% CI | Likelihood ratio |
| > 0.0065 | 0.68 | 0.49–0.82 | 0.87 | 0.79–0.92 | 5.3 |
| > 0.0075 | 0.68 | 0.49–0.82 | 0.91 | 0.84–0.95 | 7.6 |
| > 0.0085 | 0.68 | 0.49–0.82 | 0.94 | 0.88–0.97 | 11 |
| > 0.0095 | 0.64 | 0.46–0.79 | 0.95 | 0.89–0.98 | 13 |
| > 0.011 | 0.61 | 0.42–0.76 | 0.95 | 0.89–0.98 | 12 |
| > 0.012 | 0.54 | 0.36–0.70 | 0.95 | 0.89–0.98 | 11 |
| > 0.013 | 0.54 | 0.36–0.70 | 0.97 | 0.92–0.99 | 18 |
| > 0.014 | 0.46 | 0.30–0.64 | 0.97 | 0.92–0.99 | 16 |
| > 0.017 | 0.43 | 0.27–0.61 | 0.97 | 0.92–0.99 | 14 |
| > 0.021 | 0.39 | 0.24–0.58 | 0.98 | 0.93–1.0 | 20 |
| > 0.025 | 0.36 | 0.21–0.54 | 0.98 | 0.93–1.0 | 18 |
| > 0.031 | 0.36 | 0.21–0.54 | 0.99 | 0.93–1.0 | 36 |
| > 0.034 | 0.32 | 0.18–0.51 | 0.99 | 0.93–1.0 | 32 |
| > 0.044 | 0.29 | 0.15–0.47 | 0.99 | 0.93–1.0 | 29 |
| > 0.055 | 0.29 | 0.15–0.47 | 1 | 0.93–1.0 | |
| > 0.057 | 0.25 | 0.13–0.43 | 1 | 0.93–1.0 | |
| > 0.060 | 0.21 | 0.10–0.40 | 1 | 0.93–1.0 | |
| > 0.062 | 0.18 | 0.079–0.36 | 1 | 0.93–1.0 | |
| > 0.076 | 0.14 | 0.057–0.31 | 1 | 0.93–1.0 | |
| > 0.093 | 0.11 | 0.037–0.27 | 1 | 0.93–1.0 | |
| > 0.14 | 0.071 | 0.013–0.23 | 1 | 0.93–1.0 | |
| > 0.29 | 0.036 | 0.0018–0.18 | 1 | 0.93–1.0 |
The optimal cut-off value of cTnI was determined to be 0.0085 with the sensitivity of 68% and the specificity of 94% based on the Youden Index J. cTnI = cardiac troponin I
A significant but weak correlation was detected between BW and serum cTnI concentration measured in all rhesus macaques (r = 0.27, P = 0.0017). No significant correlations were detected between the cTnI concentration and age (P = 0.55) and HR (P = 0.84) of all rhesus macaques. With regard to echocardiographic parameters, significant but weak correlations were detected between the serum cTnI concentration and LA/Ao (sax), IVSd, LVPWd, and FS in all rhesus macaques (Table 5). In the ACD group, La/Ao (sax) (r = 0.39, P = 0.041) was significantly correlated to the cTnI concentration; in the HCM group, La/Ao (sax) (r = 0.52, P = 0.016) and FS (r = -0.5, P = 0.021) were also significantly correlated to the cTnI concentration. In the OCD group, the serum cTnI concentration was not significantly correlated to any echocardiographic parameters.
Table 5.
Correlations between cTnI or NT-proBNP value and various echocardiographic parameters obtained from all rhesus macaques enrolled.
| cTnI | NT-proBNP | |||
| r | P-value | r | P-value | |
| LA/Ao (sax) | 0.25 | 0.0041a | 0.11 | |
| LA/Ao (lax) | 0.95 | 0.48 | ||
| IVSd (cm) | 0.23 | 0.0078a | 0.38 | |
| LVPWd (cm) | 0.27 | 0.0022a | 0.39 | |
| FS (%) | −0.18 | 0.04a | 0.27 | |
| E’/A’ medial | 0.68 | 0.99 | ||
| E’/A’ lateral | 0.34 | 0.22 | ||
| VAo (m/s) | 0.66 | 0.38 | ||
| E/A | 0.19 | 0.19 | 0.049a | |
significant correlation (P < 0.05). E/A = ratio of the peak early to late transmitral flow velocity; E’/A’(lateral) = ratio of the peak early to late lateral mitral annular motion velocity; E’/A’(medial) = ratio of the peak early to late medial mitral annular motion velocity; HCM = hypertrophic cardiomyopathy; IVSd = interventricular septal wall thickness during diastole, LA/Ao (lax) = left atrial diameter to left aortic root diameter ratio from right parasternal long-axis view; LA/Ao (sax) = left atrial diameter to left aortic root diameter ratio from right parasternal short-axis view; LVPWd = thickness of left ventricular posterior wall during diastole; OCD = other cardiac disease, VAo = peak velocity for aortic flow
Serum NT-proBNP concentrations.
The NT-proBNP immunoassay was validated with an average spiking and recovery rate of 106.5%. The linearity was characterized as a linear equation with a slope of 0.0005, an intercept of 0.0307, and an R2 value of 0.997. The CV was 10.8%, and the minimum detection limit was 31.25 pg/mL. The median serum NT-proBNP concentration for all rhesus macaques was 178.1 (range 113.4 to 1121 pg/mL). No significant differences were noted between males and females (P = 0.86). No significant differences were noted between the control rhesus macaques relocated to indoor housing for less than 30 d and the control animals housed indoors for over 30 d before examination (P = 0.45). Serum NT-proBNP concentrations were not significantly different between the control and ACD groups (P = 0.81) (Figure 1). No significant differences of NT-proBNP concentration were detected among control, HCM and OCD groups (Table 3).
No significant correlation was detected between age (P = 0.85), BW (P = 0.99) or HR (P = 0.18) and NT-proBNP concentration. In all macaques, significant but weak correlations were detected between the serum NT-proBNP concentration and E/A (Table 5). No significant correlations were detected between the NT-proBNP concentrations and any echocardiographic parameters in the ACD and HCM groups. In the OCD group, the serum NT-proBNP concentration was significantly correlated to VAo (r = 0.76, P = 0.046).
Discussion
The aim of this study was to determine if the cardiac biomarkers, cTnI and NT-proBNP, are elevated in rhesus macaques with various cardiac diseases, including HCM, as compared with animals without cardiac disease, and whether patient characteristics and echocardiographic parameters are correlated to the serum concentrations of these cardiac biomarkers. The results of the present study showed that the serum cTnI concentration was elevated significantly in the rhesus macaques with various cardiac diseases, including HCM, as compared with control animals. However, no significant difference of the serum cTnI concentration was detected between rhesus macaques with HCM and other cardiac diseases. Conversely, no significant difference was found in the serum NT-proBNP concentrations between rhesus macaques with and without cardiac diseases. Therefore, the results of the present study suggest measuring serum cTnI concentration, but not NT-proBNP concentration, could be useful for identifying cardiac disease in rhesus macaques, but does not distinguish between HCM and other cardiac diseases. In addition, the serum cTnI concentration was significantly, but weakly, correlated with BW and several echocardiographic parameters. No significant correlation was detected between the serum NT-proBNP concentrations and patient characteristics or echocardiographic parameters, except for E/A, which demonstrated a weak and clinically irrelevant correlation.
Serum cTnI is an excellent biomarker for detecting myocardial ischemic injury caused by various cardiac and noncardiac diseases, and has been used to assist diagnosing acute myocardial infarction,18,44,57 acute coronary syndrome,15,51 and other myocardial injuries.8,40 It is also elevated in human and feline patients with HCM.17,27,29,35 In addition, the serum cTnI concentration has been reported to correlate with the severity of myocardial injury, and has been used to diagnose occult cardiac diseases in humans and to conduct preclinical safety assessment studies with drug-induced cardiac injury in various animal models.45,46,59,61 Genetic and amino acid sequences of cTnI are also known to be highly conserved among species that include humans, nonhuman primates, dogs, and cats.43,49,55 These data suggest that cTnI may be useful for the assessment of cardiac diseases in various species, including rhesus macaques.
The current study identified significant but weak correlations between serum cTnI concentration and several echocardiographic parameters including LA/Ao (sax), IVSd, LVPWd, and FS. These findings indicate that the degree of serum cTnI elevation could be correlated with the severity of cardiac disease with regard to the severity of left atrial enlargement and systolic function and the thickness of left ventricular wall. However, the correlations were weak, and serum cTnI was not a good predictor of the severity of cardiac disease. This could be due to limited severity of cardiac diseases in the animals enrolled in this study. All rhesus macaques in the HCM and OCD groups were clinically healthy, without any signs of cardiac symptoms such as congestive heart failure. Enrolling animals with a broader spectrum of disease severity might help determine the true correlation between the serum cTnI concentration and the severity of cardiac disease. In human patients with HCM, elevated cTnI concentration is also a predictor of sudden cardiac death due to HCM.28,48 This relationship would be valuable if present in rhesus macaques because the most commonly reported clinical consequences from HCM in rhesus macaques is sudden cardiac death. Elevation of serum cTnI concentration in rhesus macaques could be associated with increased risk of sudden cardiac death. Further longitudinal study of these HCM-affected rhesus macaques is warranted.
Serum cTnI concentrations were measured in rhesus macaques with HCM in a previous small study.25 This study did not find significant differences in serum cTnI concentration between the control and HCM rhesus macaques but this failure may be due to Type II error, as few control and HCM cases were enrolled. In addition, the previous study noted that outdoor rhesus macaques had significantly higher cTnI concentration than indoor rhesus macaques.25 This could be due to the exercise performed by rhesus macaques housed in large outdoor enclosures. In human patients, cTnI concentration could be elevated for 24 h to 8 d after exercise.9,60 In the present study, all but 2 control rhesus macaques enrolled in the study were housed indoors for at least 8 d prior to echocardiographic examination. Thus, the housing condition and physical activity levels were unlikely to affect cTnI concentrations in our study. Furthermore, the development and expression of cardiac diseases are known to be influenced by environment and activity level. In the present study, determining the role of the environment is difficult because many of the macaques enrolled in this study spend time in both the indoor and outdoor housing environment. Future research should explore the influences of these environmental factors on the development of cardiac diseases, including HCM.
Our study measured the cTnI concentration in a large number of animals including control and HCM cases. In addition, several rhesus macaques with non-HCM cardiac diseases were also enrolled to determine if the elevation of cTnI was associated with any specific type of cardiac disease. Our study revealed significantly higher serum cTnI in the ACD group than in the control group. The differences of the cTnI levels remained significant when values were compared among control, HCM and OCD groups. However, no significant differences in the cTnI concentrations were found between the HCM and OCD groups. This finding is consistent with those reported in human and feline patients with HCM.1,38,46 The serum cTnI concentration is increased secondary to myocardial injury, and an elevation of this cardiac biomarker was not specific to HCM, as it is also elevated in patients with any type of significant underlying disease leading to primary and secondary myocardial injury. Therefore, measuring serum cTnI concentration is unlikely to be a specific diagnostic tool for HCM, and further diagnostics, including echocardiography, are warranted in animals with elevated serum cTnI to determine the etiology of their myocardial injury.
NT-proBNP is released from myocytes after prohormone BNP is cleaved by the enzymes furin and corin into NT-proBNP and BNP. The synthesis of prohormone and the release of NT-proBNP and BNP are caused by cardiac wall stress, including stretching, ischemic and hypoxic stimuli of myocytes. Serum NT-proBNP concentration has been measured in humans and some animal species to aid in diagnosing and prognosticating various cardiac diseases, including acute and chronic congestive heart failure,32,36,50 myocardial infarction, dilated cardiomyopathy39,42 and hypertrophic cardiomyopathy.7,20,33,34 It has also been used to differentiate cardiac origin of respiratory distress from noncardiac origins.22,23,31,62 However, no studies have been reported in rhesus macaques with cardiac diseases. In the present study, a commercially available monkey-specific NT-proBNP quantitative sandwich ELISA kit was used to measure the serum concentration of NT-proBNP. This kit was validated based on the spiking and recovery test, as well as linearity test performed by the authors. However, no significant differences in the serum NT-proBNP concentrations were noted between rhesus macaques with and without cardiac diseases in this study. Increased serum NT-proBNP concentrations have been identified in human HCM patients, and NT-proBNP concentrations correlate positively with the severity of ventricular hypertrophy, left ventricular diastolic dysfunction, left ventricular outflow tract obstruction, and left atrial diastolic volume.7,13,33,47,56 In the present study, no correlations between the serum concentration of NT-proBNP and various echocardiographic parameters were identified. Based on these findings, the NT-proBNP concentrations measured by this ELISA kit cannot be used to assess the presence or severity of cardiac disease. Failure to show significant differences and correlations between the NT-proBNP concentrations and the severity of the disease may be due to nonspecific binding of an antibody used in this assay, resulting in false measurement of NT-proBNP in rhesus macaques. Another possible reason for the failure to detect the significant differences of the NT-proBNP concentration in this study is its low sensitivity when discriminating between the control and cardiac disease in animals that are not showing clinical symptoms. Therefore, further studies should be done with rhesus macaques who are displaying apparent clinical signs due to cardiac diseases. However, because normal NT-proBNP concentrations were detected in some rhesus macaques in the OCD group that had apparently enlarged left atria, while severely elevated NT-proBNP concentrations were detected in some animals with normal cardiac conditions, the NT-proBNP ELISA kit used in this study might not provide an accurate reflection of true NT-proBNP concentration. Alternatively, the rhesus macaque may differ in response to cardiac disease or cardiac injury, rendering NT-proBNP a poor cardiac biomarker.
Several limitations to the present study must be considered. First, the number of rhesus macaques in the OCD group was small, and the failure to show significant differences in the biomarker assays between the HCM and OCD group could therefore be due to type II error. Second, cardiac diseases in the rhesus macaques in this study were all subclinical. This could underly the weak correlations between the cTnI and echocardiographic parameters that were reported. Measuring serum cTnI concentrations in rhesus macaques with various degrees of cardiac diseases in a longitudinal study could reveal greater correlations between the cTnI level and echocardiographic parameters. These study findings could also enable us to use the serum cTnI concentration to determine and monitor the severity and progression of cardiac diseases, including HCM. Finally, only one type of NT-proBNP assay was used in this study, and failure to find differences of NT-proBNP between the control and cardiac disease groups could be due to inadequate sensitivity and specificity of this particular assay. Further study, using different manufacturers of NT-proBNP assays, and measuring this biomarker in rhesus macaques with a broader spectrum of disease severity should be investigated to determine the true utility of NT-proBNP measurement when assessing the presence and severity of cardiac disease.
In conclusion, the present study demonstrated that serum cTnI concentration is elevated in rhesus macaques with HCM and other types of cardiac disease as compared with control rhesus macaques. However, no significant differences of cTnI levels were found between rhesus macaques with HCM and OCD. In addition, significant but weak correlations were identified between some key echocardiographic variables and the serum cTnI level. Therefore, measuring serum cTnI concentration using the commercially available high sensitivity cTnI assay can be useful to assist in diagnosing HCM and OCD and may be useful for monitoring the severity and progression of cardiac disease. However, cTnI measurement does not replace echocardiographic evaluation when attempting to diagnose cardiac diseases in rhesus macaques, due to the poor sensitivity of the assay and its weak correlations with echocardiographic parameters.
Acknowledgments
We acknowledge the expertise of Deborah Kent, Kami Elliott, Nancy Gee, and Ross Allen who aided in the completion of this study. This study was supported by the California National Primate Research Center Base Grant Award Number CNPRC-P51 OD011107.
References
- 1.Adams JE, 3rd, Bodor GS, Dávila-Román VG, Delmez JA, Apple FS, Ladenson JH, Jaffe AS. 1993. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation 88:101–106. 10.1161/01.CIR.88.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Adams JE, 3rd, Schechtman KB, Landt Y, Ladenson JH, Jaffe AS. 1994. Comparable detection of acute myocardial infarction by creatine kinase MB isoenzyme and cardiac troponin I. Clin Chem 40:1291–1295. 10.1093/clinchem/40.7.1291. [DOI] [PubMed] [Google Scholar]
- 3.Animal Welfare Act as Amended. 2013. 7 USC S2131–2159.
- 4.Animal Welfare Regulations. 2013. 9 CFR:S3.129.
- 5.Apple FS, Collinson PO, IFCC Task Force on Clinical Applications of Cardiac Biomarkers. 2012. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 58:54–61. 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 6.Apple FS, Murakami MM, Ler R, Walker D, York M, HESI Technical Committee of Biomarkers Working Group on Cardiac Troponins. 2008. Analytical characteristics of commercial cardiac troponin I and T immunoassays in serum from rats, dogs, and monkeys with induced acute myocardial injury. Clin Chem 54:1982–1989. 10.1373/clinchem.2007.097568. [DOI] [PubMed] [Google Scholar]
- 7.Arteaga E, Araujo AQ, Buck P, Ianni BM, Rabello R, Mady C. 2005. Plasma amino-terminal pro-B-type natriuretic peptide quantification in hypertrophic cardiomyopathy. Am Heart J 150:1228–1232. 10.1016/j.ahj.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Babuin L, Jaffe AS. 2005. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ 173:1191–1202. 10.1503/cmaj/051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker P, Leckie T, Harrington D, Richardson A. 2019. Exercise-induced cardiac troponin elevation: An update on the evidence, mechanism and implications. Int J Cardiol Heart Vasc 22:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. 2004. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 110:2168–2174. 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 11.Binder J, Ommen SR, Chen HH, Ackerman MJ, Tajik AJ, Jaffe AS. 2007. Usefulness of brain natriuretic peptide levels in the clinical evaluation of patients with hypertrophic cardiomyopathy. Am J Cardiol 100:712–714. 10.1016/j.amjcard.2007.03.089. [DOI] [PubMed] [Google Scholar]
- 12.Boon JA. 1998. Acquired heart disease. In: Manual of veterinary echocardiography. Pennsylvania (PA): Williams and Wilkins. [Google Scholar]
- 13.Brito D, Matias JS, Sargento L, Cabral MJ, Madeira HC. 2004. Plasma N-terminal pro-brain natriuretic peptide: a marker of left ventricular hypertrophy in hypertrophic cardiomyopathy. Rev Port Cardiol 23:1557–1582. [PubMed] [Google Scholar]
- 14.Cambronero F, Marín F, Roldán V, Hernández-Romero D, Valdés M, Lip GY. 2009. Biomarkers of pathophysiology in hypertrophic cardiomyopathy: implications for clinical management and prognosis. Eur Heart J 30:139–151. 10.1093/eurheartj/ehn538. [DOI] [PubMed] [Google Scholar]
- 15.Chapman AR, Hesse K, Andrews J, Ken Lee K, Anand A, Shah ASV, Sandeman D, Ferry AV, Jameson J, Piya S, Stewart S, Marshall L, Strachan FE, Gray A, Newby DE, Mills NL. 2018. High-sensitivity cardiac troponin I and clinical risk scores in patients with suspected acute coronary syndrome. Circulation 138:1654–1665. 10.1161/CIRCULATIONAHA.118.036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coats CJ, Gallagher MJ, Foley M, O'Mahony C, Critoph C, Gimeno J, Dawnay A, McKenna WJ, Elliott PM. 2013. Relation between serum N-terminal pro-brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur Heart J 34:2529–2537. 10.1093/eurheartj/eht070. [DOI] [PubMed] [Google Scholar]
- 17.Connolly DJ, Cannata J, Boswood A, Archer J, Groves EA, Neiger R. 2003. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg 5:209–216. 10.1016/S1098-612X(03)00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daubert MA, Jeremias A. 2010. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vasc Health Risk Manag 6:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lemos JA, McGuire DK, Drazner MH. 2003. B-type natriuretic peptide in cardiovascular disease. Lancet 362:316–322. 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 20.Efthimiadis GK, Hitoglou-Makedou A, Giannakoulas G, Mitakidou A, Karamitsos T, Karvounis H, Mochlas S, Styliadis I, Stefanidis H, Parcharidis G, Louridas G. 2007. Clinical significance of N-terminal-probrain natriuretic peptide in hypertrophic cardiomyopathy. Heart Vessels 22:322–327. 10.1007/s00380-007-0976-y. [DOI] [PubMed] [Google Scholar]
- 21.Fonarow GC, Peacock WF, Phillips CO, Givertz MM, Lopatin M, ADHERE Scientific Advisory Committee and Investigators. 2007. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 49:1943–1950. 10.1016/j.jacc.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Fox PR, Oyama MA, Hezzell MJ, Rush JE, Nguyenba TP, DeFrancesco TC, Lehmkuhl LB, Kellihan HB, Bulmer B, Gordon SG, Cunningham SM, MacGregor J, Stepien RL, Lefbom B, Adin D, Lamb K. 2014. Relationship of plasma N-terminal pro-brain natriuretic peptide concentrations to heart failure classification and cause of respiratory distress in dogs using a 2nd generation ELISA assay. J Vet Intern Med 29:171–179. 10.1111/jvim.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox PR, Oyama MA, Reynolds C, Rush JE, DeFrancesco TC, Keene BW, Atkins CE, Macdonald KA, Schober KE, Bonagura JD, Stepien RL, Kellihan HB, Nguyenba TP, Lehmkuhl LB, Lefbom BK, Moise NS, Hogan DF. 2009. Utility of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) to distinguish between congestive heart failure and non-cardiac causes of acute dyspnea in cats. J Vet Cardiol 11 Suppl 1:S51–S61. 10.1016/j.jvc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Goetze JP. 2004. Biochemistry of pro-B-type natriuretic peptide-derived peptides: the endocrine heart revisited. Clin Chem 50:1503–1510. 10.1373/clinchem.2004.034272. [DOI] [PubMed] [Google Scholar]
- 25.Haertel AJ, Stern JA, Reader JR, Spinner A, Roberts JA, Christe KL. 2016. Antemortem screening for left ventricular hypertrophy in rhesus macaques (Macaca mulatta). Comp Med 66:333–342. [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Anker SD, Amann-Zalan I, Hoersch S, Katus HA. 2004. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation 110:1780–1786. 10.1161/01.CIR.0000143059.68996.A7. [DOI] [PubMed] [Google Scholar]
- 27.Hertzsch S, Roos A, Wess G. 2019. Evaluation of a sensitive cardiac troponin I assay as a screening test for the diagnosis of hypertrophic cardiomyopathy in cats. J Vet Intern Med 33:1242–1250. 10.1111/jvim.15498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hładij R, Rajtar-Salwa R, Dimitrow PP. 2017. Troponin as ischemic biomarker is related with all three echocardiographic risk factors for sudden death in hypertrophic cardiomyopathy (ESC Guidelines 2014). Cardiovasc Ultrasound 15:1–5. 10.1186/s12947-017-0115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hori Y, Iguchi M, Heishima Y, Yamashita Y, Nakamura K, Hirakawa A, Kitade A, Ibaragi T, Katagi M, Sawada T, Yuki M, Kanno N, Inaba H, Isayama N, Onodera H, Iwasa N, Kino M, Narukawa M, Uchida S. 2018. Diagnostic utility of cardiac troponin I in cats with hypertrophic cardiomyopathy. J Vet Intern Med 32:922–929. 10.1111/jvim.15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 31.Januzzi JL, Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, Lloyd-Jones DM, Brown DF, Foran-Melanson S, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB. 2005. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 95:948–954. 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 32.Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, Pinto YM, Richards M. 2006. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 27:330–337. 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 33.Kahveci G, Bayrak F, Mutlu B, Basaran Y. 2009. Determinants of elevated NT-proBNP levels in patients with hypertrophic cardiomyopathy: an echocardiographic study. Heart Lung Circ 18:266–270. 10.1016/j.hlc.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Kim SW, Park SW, Lim SH, Kwon SU, Choi YJ, Park MK, Lee SC, Lee SH, Park JE, Jeon ES. 2006. Amount of left ventricular hypertrophy determines the plasma N-terminal pro-brain natriuretic peptide level in patients with hypertrophic cardiomyopathy and normal left ventricular ejection fraction. Clin Cardiol 29:155–160. 10.1002/clc.4960290406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubo T, Kitaoka H, Yamanaka S, Hirota T, Baba Y, Hayashi K, Iiyama T, Kumagai N, Tanioka K, Yamasaki N, Matsumura Y, Furuno T, Sugiura T, Doi YL. 2013. Significance of high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J Am Coll Cardiol 62:1252–1259. 10.1016/j.jacc.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 36.Lainchbury JG, Troughton RW, Strangman KM, Frampton CM, Pilbrow A, Yandle TG, Hamid AK, Nicholls MG, Richards AM. 2009. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: results from the BATTLESCARRED (NT-proBNP-assisted treatment to lessen serial cardiac readmissions and death) trial. J Am Coll Cardiol 55:53–60. 10.1016/j.jacc.2009.02.095. [DOI] [PubMed] [Google Scholar]
- 37.Lang RM, Badano LP, Mor_Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. 2015. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular imaging. Eur Heart J Cardiovasc Imaging 16:233–270. 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 38.Langhorn R, Willesen JL. 2015. Cardiac troponins in dogs and cats. J Vet Intern Med 30:36–50. 10.1111/jvim.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luchner A, Hengstenberg C, Lowel H, Trawinski J, Baumann M, Riegger GA, Schunkert H, Holmer S. 2002. N-terminal pro-brain natriuretic peptide after myocardial infarction: a marker of cardio-renal function. Hypertension 39:99–104. 10.1161/hy0102.100537. [DOI] [PubMed] [Google Scholar]
- 40.Mair J, Genser N, Morandell D, Maier J, Mair P, Lechleitner P, Calzolari C, Larue C, Ambach E, Dienstl F, Pau B, Puschendorf B. 1996. Cardiac troponin I in the diagnosis of myocardial injury and infarction. Clin Chim Acta 245:19–38. 10.1016/0009-8981(95)06168-1. [DOI] [PubMed] [Google Scholar]
- 41.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. 1995. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation 92:785–789. 10.1161/01.CIR.92.4.785. [DOI] [PubMed] [Google Scholar]
- 42.Mayr A, Mair J, Schocke M, Klug G, Pedarnig K, Haubner BJ, Nowosielski M, Grubinger T, Pachinger O, Metzler B. 2011. Predictive value of NT-pro BNP after acute myocardial infarction: relation with acute and chronic infarct size and myocardial function. Int J Cardiol 147:118–123. 10.1016/j.ijcard.2009.09.537. [DOI] [PubMed] [Google Scholar]
- 43.Mclvor ME, Cummings CE, Mower MM, Wenk RE, Lustgarten JA, Baltazar RF, Salomon J. 1987. Sudden cardiac death from acute fluoride intoxication: the role of potassium. Ann Emerg Med 16:777–781. 10.1016/S0196-0644(87)80573-5. [DOI] [PubMed] [Google Scholar]
- 44.Neumann JT, Sorensen NA, Schwemer T, Ojeda F, Bourry R, Sciacca V, Schaefer S, Waldeyer C, Sinning C, Renne T, Than M, Parsonage W, Wildi K, Makarova N, Schnabel RB, Landmesser U, Mueller C, Cullen L, Greenslade J, Zeller T, Blankenberg S, Karakas M, Westermann D. 2016. Diagnosis of myocardial infarction using a high-sensitivity Troponin I 1-hour algorithm. JAMA Cardiol 1:397–404. 10.1001/jamacardio.2016.0695. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien PJ. 2006. Blood cardiac troponin in toxic myocardial injury: archetype of a translational safety biomarker. Expert Rev Mol Diagn 6:685–702. 10.1586/14737159.6.5.685. [DOI] [PubMed] [Google Scholar]
- 46.O'Brien PJ, Smith DE, Knechtel TJ, Marchak MA, Pruimboom-Brees I, Brees DJ, Spratt DP, Archer FJ, Butler P, Potter AN, Provost JP, Richard J, Snyder PA, Reagan WJ. 2006. Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab Anim 40:153–171. 10.1258/002367706776319042. [DOI] [PubMed] [Google Scholar]
- 47.Park JR, Choi JO, Han HJ, Chang SA, Park SJ, Lee SC, Choe YH, Park SW, Oh JK. 2011. Degree and distribution of left ventricular hypertrophy as a determining factor for elevated natriuretic peptide levels in patients with hypertrophic cardiomyopathy: insights from cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 28:763–772. 10.1007/s10554-011-9876-4. [DOI] [PubMed] [Google Scholar]
- 48.Rajtar-Salwa R, Hladij R, Dimitrow PP. 2017. Elevated level of Troponin but not N-terminal probrain natriuretic peptide is associated with increased risk of sudden cardiac death in hypertrophic cardiomyopathy calculated according to the ESC guidelines 2014. Dis Markers 2017:1–5. 10.1155/2017/9417908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schober KE, Kirbach B, Oechtering G. 1999. Noninvasive assessment of myocardial cell injury in dogs with suspected cardiac contusion. J Vet Cardiol 1:17–25. 10.1016/S1760-2734(06)70030-3. [DOI] [PubMed] [Google Scholar]
- 50.Schou M, Gustafsson F, Corell P, Kistorp CN, Kjaer A, Hildebrandt PR. 2007. The relationship between N-terminal pro-brain natriuretic peptide and risk for hospitalization and mortality is curvilinear in patients with chronic heart failure. Am Heart J 154:123–129. 10.1016/j.ahj.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Scirica BM, Morrow DA. 2004. Troponins in acute coronary syndromes. Prog Cardiovasc Dis 47:177–188. 10.1016/j.pcad.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Sharma S, Jackson PG, Makan J. 2004. Cardiac troponins. J Clin Pathol 57:1025–1026. 10.1136/jcp.2003.015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh V, Martinezclark P, Pascual M, Shaw ES, O'Neill WW. 2010. Cardiac biomarkers—the old and the new: a review. Coron Artery Dis 21:244–256. 10.1097/MCA.0b013e328338cd1f. [DOI] [PubMed] [Google Scholar]
- 54.Sisson DD. 2004. Neuroendocrine evaluation of cardiac disease. Vet Clin North Am Small Anim Pract 34:1105–1126. 10.1016/j.cvsm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Sleeper MM, Clifford CA, Laster LL. 2001. Cardiac troponin I in the normal dog and cat. J Vet Intern Med 15:501–503. 10.1111/j.1939-1676.2001.tb01582.x. [DOI] [PubMed] [Google Scholar]
- 56.Tesic M, Seferovic J, Trifunovic D, Djordjevic-Dikic A, Giga V, Jovanovic I, Petrovic O, Marinkovic J, Stankovic S, Stepanovic J, Ristic A, Petrovic M, Mujovic N, Vujisic-Tesic B, Beleslin B, Vukcevic V, Stankovic G, Seferovic P. 2017. N-terminal pro-brain natriuretic peptide is related with coronary flow velocity reserve and diastolic dysfunction in patients with asymmetric hypertrophic cardiomyopathy. J Cardiol 70:323–328. 10.1016/j.jjcc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Tucker JF, Collins RA, Anderson AJ, Hauser J, Kalas J, Apple FS. 1997. Early diagnostic efficiency of cardiac troponin I and Troponin T for acute myocardial infarction. Acad Emerg Med 4:13–21. 10.1111/j.1553-2712.1997.tb03637.x. [DOI] [PubMed] [Google Scholar]
- 58.Ueda Y, Gunther-Harrington CT, Cruzen CL, Roberts JA, Stern JA. 2017. Echocardiographic parameters of clinically normal geriatric rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 56:361–368. [PMC free article] [PubMed] [Google Scholar]
- 59.Wallace KB, Hausner E, Herman E, Holt GD, MacGregor JT, Metz AL, Murphy E, Rosenblum IY, Sistare FD, York MJ. 2004. Serum troponins as biomarkers of drug-induced cardiac toxicity. Toxicol Pathol 32:106–121. 10.1080/01926230490261302. [DOI] [PubMed] [Google Scholar]
- 60.Wilhelm M, Zueger T, De Marchi S, Rimoldi SF, Brugger N, Steiner R, Stettler C, Nuoffer JM, Seiler C, Ith M. 2012. Inflammation and atrial remodeling after a mountain marathon. Scand J Med Sci Sports 24:519–525. 10.1111/sms.12030. [DOI] [PubMed] [Google Scholar]
- 61.Wong GC, Morrow DA, Murphy S, Kraimer N, Pai R, James D, Robertson DH, Demopoulos LA, DiBattiste P, Cannon CP, Gibson CM. 2002. Elevations in troponin T and I are associated with abnormal tissue level perfusion: a TACTICS-TIMI 18 substudy. Treat angina with aggrastat and determine cost of therapy with an invasive or conservative strategy—Thrombolysis in myocardial infarction. Circulation 106:202–207. 10.1161/01.CIR.0000021921.14653.28. [DOI] [PubMed] [Google Scholar]
- 62.Worster A, Balion CM, Hill SA, Santaguida P, Ismaila A, McKelvie R, Reichert SM, McQueen MJ, Booker L, Raina PS. 2008. Diagnostic accuracy of BNP and NT-proBNP in patients presenting to acute care settings with dyspnea: a systematic review. Clin Biochem 41:250–259. 10.1016/j.clinbiochem.2007.08.008. [DOI] [PubMed] [Google Scholar]