Abstract
Background
For every maternal death, 20 to 30 women are estimated to have morbidities related to pregnancy or childbirth. Much of this burden of disease is in women in low- and middle-income countries. Maternal multimorbidity can include physical, psychological and social ill-health. Limited data exist about the associations between these morbidities. In order to address all health needs that women may have when attending for maternity care, it is important to be able to identify all types of morbidities and understand how each morbidity influences other aspects of women’s health and wellbeing during pregnancy and after childbirth.
Methods
We systematically reviewed published literature in English, describing measurement of two or more types of maternal morbidity and/or associations between morbidities during pregnancy or after childbirth for women in low- and middle-income countries. CINAHL plus, Global Health, Medline and Web of Science databases were searched from 2007 to 2018. Outcomes were descriptions, occurrence of all maternal morbidities and associations between these morbidities. Narrative analysis was conducted.
Results
Included were 38 papers reporting about 36 studies (71,229 women; 60,911 during pregnancy and 10,318 after childbirth in 17 countries). Most studies (26/36) were cross-sectional surveys. Self-reported physical ill-health was documented in 26 studies, but no standardised data collection tools were used. In total, physical morbidities were included in 28 studies, psychological morbidities in 32 studies and social morbidities in 27 studies with three studies assessing associations between all three types of morbidity and 30 studies assessing associations between two types of morbidity. In four studies, clinical examination and/or basic laboratory investigations were also conducted. Associations between physical and psychological morbidities were reported in four studies and between psychological and social morbidities in six. Domestic violence increased risks of physical ill-health in two studies.
Conclusions
There is a lack of standardised, comprehensive and routine measurements and tools to assess the burden of maternal multimorbidity in women during pregnancy and after childbirth. Emerging data suggest significant associations between the different types of morbidity.
Systematic review registration number
PROSPERO CRD42018079526.
Keywords: Maternal morbidity, Multimorbidity, Pregnancy and childbirth, Burden of disease, Measurement, Data collection, Low- and middle-income countries
Background
Maternal multimorbidities affect millions of women during pregnancy and after childbirth and the burden of ill-health is expected to be highest in women in low-and middle-income countries (LMIC) [1]. For every maternal death, 20 to 30 women have morbidities related to pregnancy or childbirth [2, 3]. More recent studies using new and comprehensive assessment tools suggest that the magnitude of maternal multimorbidity is much larger than previously estimated [4–6]. International targets and the Sustainable Development Goals have a new focus; in addition to preventing maternal mortality, improving health and well-being, as well as “survive and thrive” are the new goals [7]. There is international agreement that all women have the right to the highest attainable standard of health and well-being, also during pregnancy and after childbirth [7, 8]. Estimates of morbidity have until now largely focused on acute and/or severe complications such as haemorrhage, sepsis and eclampsia [9]. The current definition of health is “a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity” [10]. There are arguments that this definition needs to be re-formulated to consider health in a context of functionality, capacity, adaptability and the ability to perform activities of daily living despite having an illness or disability; but with a continued emphasis on the importance of the three domains of health: physical, psychological and social [11]. There is also debate that current definitions, measurements and timeframes for “multimorbidity”, “co-morbidity”, “morbidity burden” and related constructs are not well conceptualized [11, 12].
Regarding maternal morbidity, a suggested definition is “any health condition attributed to and/or aggravated by pregnancy and childbirth that has a negative impact on women’s well-being” [13]. In order to address all health needs that women may have when attending for maternity care, it is important to be able to identify all types of morbidities and understand how each morbidity influences other aspects of women’s health and wellbeing during pregnancy and after childbirth. To date, lack of data exist regarding measurement and burden of disease described as “maternal morbidity”, “maternal multimorbidity”, or “maternal co-morbidity”; these terms are often used interchangeably; and there is uncertainty regarding the timeframe over which maternal morbidity impacts a woman’s health and wellbeing. Additionally, there is limited understanding of best practices to measure different components of maternal ill-health and descriptions of morbidities, and if and how different types of morbidities are interlinked and associated.
Objective
A systematic review of the literature was conducted for studies from LMIC that measured two or more different types of maternal morbidity and/or associations with and between morbidities.
Methods
We included studies which assessed two or more types of maternal morbidity in women during and/or after pregnancy. For the purposes of this study we categorised maternal multimorbidity as physical (such as but not limited to medical, infectious, obstetric), psychological (such as but not limited to depression, suicidal ideation) and social co-morbidities (such as but not limited to domestic violence, substance misuse) [5]. We assessed tools that were used to collect data, including self-reported subjective measures; and/or objective measures such as clinical examination; and/or use of investigations for different types of maternal multimorbidity as reported by authors. We described how and what different types of maternal multimorbidity (physical, psychological, social) were measured and if there were any reported associations between these.
Data sources and search strategy
This protocol is registered in PROSPERO (CRD42018079526). Relevant articles published between January 2007 and December 2018 were identified using a structured search strategy in four electronic databases: CINAHL Plus, Global Health, Medline, and Web of Science. A search strategy was developed using thesaurus (including MeSH) and free-text terms for “maternal morbidity” and associated keywords, were used as main search terms. For each aspect of maternal morbidity (“physical”, “psychological” and “social”) search terms and related keywords were selected (Supplementary Table 1). Reference lists and bibliographies of key topic articles were also searched and any additional papers that met the inclusion criteria were obtained.
Inclusion and exclusion criteria
The study population was limited to women during pregnancy, childbirth or up to 12 weeks postnatal. Studies were excluded if: (i) they reported one type of maternal morbidity only, (ii) examined trend, risk factors or associations only without estimates of prevalence of types of morbidity, or (iii) reported severe or life-threatening complications of pregnancy or childbirth that would require emergency obstetric care. The review was limited to studies from LMIC as defined by the World Bank. Language was limited to English.
Selection and data extraction
One researcher screened all titles and abstracts (MMc). A sub-sample (20%) was double screened by the second researcher (SZ). Evaluation of full-text papers was done independently by these two researchers with reasons for exclusion recorded and any discrepancies were discussed with a third researcher (NvdB). Information was extracted into a pre-designed summary table and included data on location of study, study dates, study design, study population, types of maternal morbidity, methods of measurement, timing (pregnancy phase) of the assessment and whether or not associations were reported (Supplementary Table 2). Throughout the review and extraction process, articles where uncertainty existed were discussed by all researchers to reach consensus.
Quality assessment
Appraisal of the quality of studies was conducted based on descriptions of maternal morbidities, sampling methods and completeness of data. Quality of evidence for each study was assessed using the Grading of Recommendations, Assessment Development and Evaluation (GRADE) tool adapted from the Critical Analysis Skills Programme (CASP) tool [14].
Data synthesis
A narrative synthesis approach was used to describe outcomes including: types of maternal morbidity categorised as physical (such as medical, infectious, obstetric), psychological (such as depression, suicidal ideation) and social (such as domestic violence, substance misuse); approaches used to collect data (self-reported or determined by healthcare providers); data collection tools used (standardised validated tool, or study specific); measurements of maternal morbidities; and reported associations (if any) between different types of maternal morbidities.
Results
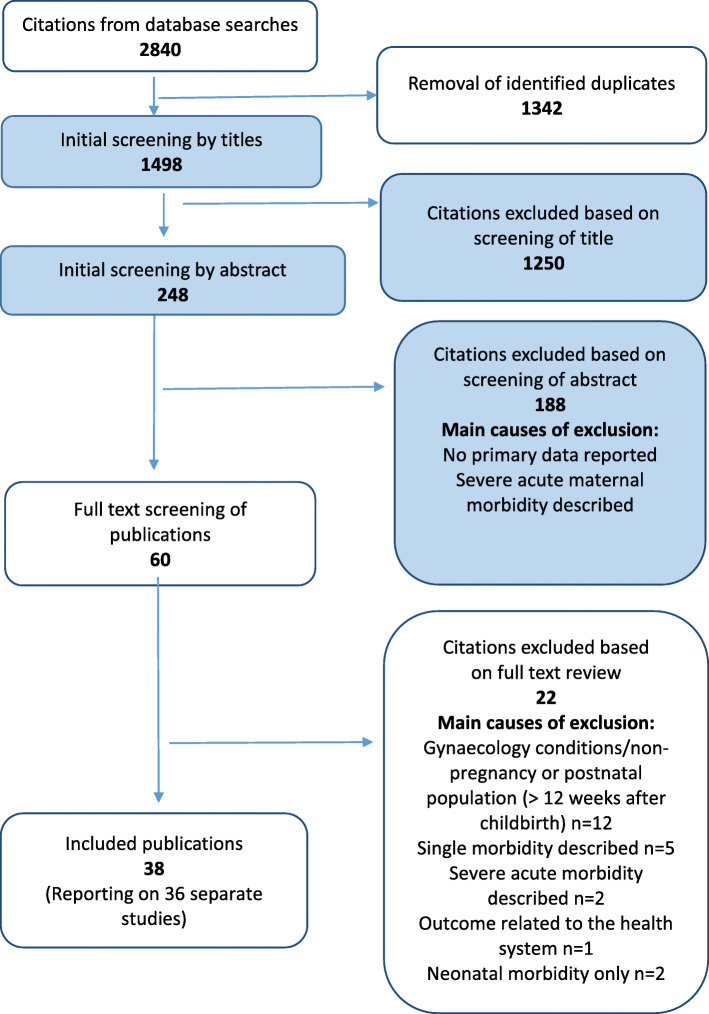
By combining the search terms, 2840 studies were identified from the four databases and after screening for relevance, 58 were retrieved for full text review (Fig. 1). Upon applying the eligibility criteria, 38 articles met the inclusion criteria. Two studies were conducted by the same group of authors [15–18]. In these publications, the same methodology was reported in two papers, but there was a different emphasis on the results and outcomes reported per publication. For the purposes of this review, the first publication is referenced in the methodology section [15, 17]. Both publications were included in the summary tables and measurements and/or associations for each publication are described in the results section. Most studies (92%; 33/36) were of medium quality, and the rest low quality (8%, 3/36).
Fig. 1.
PRISMA diagram for article selection process
Characteristics of studies
The 36 studies were from 17 different countries, with 15 from sub-Saharan Africa. Eleven were conducted in low-income countries and six in middle-income countries (four lower-middle and two upper-middle income countries).
Study design, source of data, data collection method and sample size
Twenty-six studies used cross-sectional survey study designs. Four were observational prospective cohort studies [15, 19–21]. One study was a case control [22]. Twenty five studies used face-to-face interviews or consultations to collect self-reported primary data from women using questionnaires. Most studies that collected primary data relied on women’s self-reported symptoms (n = 28). In four studies, clinical examination and/or laboratory tests were also conducted [6, 20, 23, 24]. Three studies extracted data using secondary data analysis of large databases of hospital admissions, discharges or birth registers [25–27]. In these secondary data analyses authors used their own data collection tools with little details of the variables extracted. One study extracted data from medical case notes [28] (Supplementary Table 2). A total number of 71,229 women were assessed in the 36 studies: 60,911 during pregnancy and 10,318 after childbirth. In nine studies less than 500 women were assessed [24–33]; thirteen assessed 500–999 women each [15, 18, 20, 21, 34–43]. In nine studies 1000–1999 women were assessed [19, 22, 27, 28, 44–48]; and five had sample sizes of ≥2000 women (Supplementary Table 2) [6, 25, 26, 49, 50].
Stages of pregnancy assessed
A total of 23 studies collected data from women during pregnancy: in the second trimester [16, 19, 32, 40, 47]; in the third trimester [20, 24, 37]; or at any time during pregnancy [19, 29, 32, 33]. In 11 of those, gestational age was not given [32, 36, 38–45, 48, 49]. Seven studies assessed women within 12 weeks of childbirth [18, 22, 29, 30, 32, 46, 50]. In one study, data was collected during three stages after childbirth: at 4–12 weeks; at 12–24 weeks; and at 24–56 weeks [23]. Zafar et al. collected data at three different assessment stages, during early and late antenatal period and after childbirth (Supplementary Table 2) [6].
Site of data collection
In studies that collected primary data (n = 30), data collection took place during visits for routine antenatal or postnatal care in outpatient departments of healthcare facilities: tertiary/provincial hospitals [23, 31, 35, 38, 41, 44, 46]; secondary level or district hospitals [32, 43, 51], and primary healthcare facility level [15, 18, 36, 39, 47]. For four studies the site was unclear [29, 33, 34, 42]. In 12 studies, this took place in the community or in women’s homes (Supplementary Table 2) [6, 19, 20, 22, 24, 30, 42, 45, 49–51].
Maternal multimorbidity
All three types of maternal morbidity including physical, psychological and social ill-health were assessed in 12 studies [6, 15, 16, 20, 39, 41–43, 46, 48]; psychological and social ill-health were assessed in nine studies [18, 25, 29, 31, 32, 34, 35, 39, 42]; physical and psychological ill-health in 11 [6, 18, 19, 21, 22, 24, 27, 29, 32, 33, 49]; and physical and social ill-health in six [17, 26, 44, 45, 47, 50] (Supplementary Table 2).
Physical morbidity
Twenty-nine studies reported on different types of physical morbidity; three of which assessed pre-selected populations including women with HIV [35, 42] or women with gestational diabetes [38]. A variety of data collection tools were used, but generally not well described. No study used validated questionnaires or international disease classifications. The most commonly reported physical morbidities were anaemia in six studies (prevalence range 5.0–57.7%) [6, 20, 22, 23, 32, 49], and HIV in nine (prevalence range 3.0–16.0%) [6, 16, 29, 33, 35, 36, 43, 48, 50]. There was a variety of other types of physical morbidities, with wide ranges for some conditions such as antepartum haemorrhage; nausea and vomiting; preterm birth; malaria; reproductive or sexually transmitted infection; urinary tract infection (Supplementary Table 3). Some authors used summative aggregated measures, for example “gynaecological and obstetric problems” as occurring in 10–22% of women; “multiple morbidities” in 60% of women or “at least one reported symptom” (44% occurrence) [22, 46, 49]. One study used antenatal hospitalisation as a “proxy” for physical morbidities (55.4% of women) [45] (Supplementary Table 3).
Psychological morbidity
Of the 32 studies that report psychological morbidities, the most common condition was depression with a prevalence range of 13.5–39.5% across 21 studies [18, 20–24, 27–35, 38, 41, 49, 51]. Twelve studies described more than one psychological condition [15, 16, 19, 25, 34, 37, 42, 43, 46–49]. Some authors described aggregates or a summative psychological condition; for example, “common mental disorders” and “symptoms of any mental distress” [15, 16]. There was a range of other types of psychological morbidity described, such as anxiety; suicidal ideation; and distress (Supplementary Table 4). Fourteen different data collection tools were used either alone or in combination (Table 1). The commonest tool was the Edinburgh Postnatal Depression Score (EPDS) questionnaire, used in fourteen studies [6, 18, 20, 28–31, 34–36, 38, 41, 49]. However, different studies used various cut-off scores (from ≥4 to ≥13) for the EPDS questionnaire and the Kessler scale (from > 15 to > 30) [6, 18, 20, 28–31, 34–36, 38, 41, 49, 54, 56].
Table 1.
Description of data collection tools used to assess psychological and social morbidity
| No. | Data collection tools to assess psychological morbidity | International abbreviation | Original country, author, and date |
| 1. | Aga Khan University Anxiety and Depression Scale | AKUADS | Pakistan, Ali 1988 [52] |
| 2. | Clinical Interview Schedule-Revised | CIS-R | USA, Lewis 1992 [53] |
| 3. | Edinburgh Postnatal Depression Scale | EPDS | UK, Cox 1987 [54] |
| 4. | Harvard Trauma Questionnaire | HTQ | USA, Mollica 1992 [55] |
| 5. | Kessler-10 item psychological distress scale | K-10 | USA, Kessler 2002 [56] |
| 6. | List of Threatening Experiences questionnaire | LTE-Q | UK, Brugha 1985 [57] |
| 7. | Montgomery–Åsberg Depression Rating scale | MADRA | UK, Montgomery 1979 [58] |
| 8. | Patient Health Questionnaire | PHQ-9 | USA, Spitzer 1992 [59] |
| 9. | Self-Reporting Questionnaire-(20 Items) | SRQ-20 | WHO, Switzerland, Beusenberg 1994 [60] |
| 10. | State-Trait Anxiety Inventory | STAI | USA, Spielberger 1983 [61] |
| 11. | Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition | SCI- DSM IV | USA, American Psychiatric Association 1994 [62] |
| 12. | WHO version of the Centre for Epidemiological Studies Depression scale | CES-DR | USA, Radloff 1977 [63] |
| 13. | Mini-intentional neuropsychological | MINI | USA, Sheehan, 1998 [64] |
| No. | Data collection tools to assess social morbidity | International abbreviation | Original country, author, and date |
| 1. | Alcohol Use Disorders Identification Test | AUDIT | WHO, Babor 2001 [65] |
| 2. | CAGE (Cut-annoyed-guilty-eye) Questionnaire | CAGE | USA, Ewing 1970 [66] |
| 3. | Maternity Social Support Index | MSSI | USA, Pascoe 1988 [67] |
| 4. | Social Provisions Scale | SPS | USA, Cutrona 1987 [68] |
| 5. | HIV-AIDS Stigma Instrumental PHWHA | HASI-P | WHO, Babor 2001 [69] |
Social morbidity
In total, 27 studies assessed social morbidity; the most commonly reported type of social morbidity was domestic violence in 14 studies [17, 18, 23, 25, 28, 31, 35, 38, 39, 43, 45, 47, 48, 50]. Substance abuse was assessed in nine studies (Supplementary Table 4) [16, 34, 39, 40, 42, 44–46, 50]. Three studies assessed both domestic violence and substance abuse [26, 37, 46]. Eight studies assessed other aspects of social health including husband’s alcohol intake, poor social support, food insecurity and unplanned pregnancy [20, 21, 30, 36, 41, 42, 46, 51].
In the 14 studies assessing domestic violence, a variety of data collection tools were used and most authors used their own definitions and questionnaires to screen for domestic violence. Five publications used all or part of internationally recognised questionnaires (Table 1) [17, 18, 39, 45, 47]. Different types of domestic violence included: disrespect, forced sex, intimate partner violence, physical assault, severe emotional and verbal abuse. Other authors used descriptions of domestic violence as aggregates or summative measures, for example, terms such as “multiple acts of physical violence” and “physical and/or sexual abuse” [38]. Nine studies assessed one or more forms of substance abuse [16, 34, 39, 40, 42, 44–46, 50], and two of these used validated questionnaires [34, 40]. In general, substance abuse related to alcohol (9 studies; prevalence range 0–49.5%) [16, 34, 39–41, 44–46, 50].
Associations between different types of morbidity
For physical morbidity, there was an association between increased psychological morbidity in women with obstetric complications (haemorrhage, infections, incontinence, prolonged labour, caesarean birth, low birthweight, stillbirth, neonatal death) (Table 2) [6, 26, 41, 45, 50]. Women with gestational diabetes were not more likely to have psychological morbidity (depression) [38], but women with HIV were more likely to have social morbidity (domestic violence) [18]. Psychological morbidity was more common in younger women [40] and among women with social morbidities such as domestic violence [25, 35], unwanted pregnancy [19, 41, 50] and poor social support (Table 2) [41]. For social morbidity, there was an association between women with substance abuse (alcohol) and domestic violence [48]; and domestic violence was also associated with neonatal death [48] and maternal complications (Table 2) [46]. Due to heterogeneity, meta-analysis of associations was not possible.
Table 2.
Associations between types of maternal morbidity
| Type of morbidity | Author, date | Associations between different types of maternal morbidity |
|---|---|---|
| Physical morbidity |
Shamu 2014 [18] |
Positive HIV status was associated with intimate partner violence for pregnant women: partially adjusted OR 1.43: (95%CI: 1.00–2.05). |
|
Surkan 2017 [26] |
In models adjusted for sociodemographic factors and co-morbidities, all postpartum illnesses were associated with an increased relative risk of depressive symptoms in women by 6 months postpartum. These morbidities included uterine prolapse (RR 1.20, 95% CI 1.04–1.39), urinary tract infection (RR 1.24, 95% CI 1.11–1.38), stress related incontinence (RR 1.49, 95% 1.33–1.67), simultaneous stress related incontinence and continuously dripping urine (RR 1.60–2.96), headache [RR 1.20 (95% CI 1.12–1.28)], convulsions (RR 1.67, 95%CI 1.36–2.06), night blindness (RR 1.33, 95% CI 1.19–1.49), anaemia (RR 1.38, 95% CI 1.31–1.46), pneumonia (RR 1.24, 95% CI 1.12–1.37), gastroenteritis (RR 1.24, 95% CI 1.17–1.31) and hepatobiliary disease (RR 2.10, 96% CI 1.69–2.60). | |
|
Zafar 2015 [6] |
Multivariate logistic regression showed that for pregnant women in Malawi, after controlling for parity and pregnancy stage, antepartum bleeding increased the odds of psychological morbidity 5-fold (OR: 5.01; 95% CI 1.60, 15.70; p = 0.006). Infective morbidity (i.e. for each additional infective morbidity) showed more than 2.5-fold increase in the odds of having psychological morbidity (OR: 2.58; 95% CI 1.92, 3.47; p = 0.000). For Pakistan, there was a 56% increase in odds of psychological morbidity due to increasing burden of infective morbidity (OR: 1.56; 95% CI 1.36, 1.79; p = 0.000), and 78% increased odds due to increasing burden of non-infective morbidity (OR: 1.78; 95% CI 1.51, 2.11; p = 0.000), when controlling for the effect of complications during previous pregnancy. Complications during previous pregnancy, infective morbidity (p < 0.001), intra or postpartum haemorrhage (p < 0.02) were associated with psychological morbidity in both settings. | |
| Psychological morbidity | Faisal-Cury 2009 [16] | Obstetric complications were independently associated with common mental disorders in pregnant women. |
| Faisal-Cury 2010 [15] | Common mental disorders during pregnancy were not associated with risk of preterm birth (adjusted OR: 1.03, 95% CI: 0.57–1.88) or low birth weight (adjusted OR: 1.09, 95% CI: 0.62–1.91). | |
| Karmaliana 2009 [19] | Psychological distress in pregnant women was associated with husband unemployment (p = 0.032), lower household wealth (p = 0.027), having 10 or more years of formal education (p = 0.002), first (p = 0.002) and unwanted pregnancies (p < 0.001). | |
| Hanlon 2009 [45] | Significant associations exist between pregnant women who report intimate partner violence and preterm labour, need for caesarean section, antenatal hospitalization and vaginal bleeding. | |
| Nasreen 2011 [37] | Increasing levels of common mental disorder symptoms in pregnant women were associated with prolonged labour (> 24 h) (SRQ 1–5: RR 1.4; 95% CI 1.0–1.9, SRQ > or = 6: RR 1.6; 95% CI 1.0–2.6). | |
| Natasha 2015 [38] | There was no association between women with depression and gestational diabetes mellitus or other obstetric factors. However, pregnant women’s level of literacy, poor household economy, poor relationship with husbands, and partner violence showed strong associations with depression and anxiety. | |
|
Prost 2012 [50] |
Unwanted pregnancy, small perceived infant size and stillbirth or neonatal deaths were all independently associated with increased risk of psychological distress in postnatal women. Loss of infants or unwanted pregnancies increased the risk of distress considerably (aORs: 7.06 95% CI: 5.51–9.04 and 1.49, 95% CI: 1.12–1.97). | |
|
Rees 2016 [47] |
For pregnant women with any mental distress, adjusted odds ratios for four or more traumatic events and severe psychological abuse was 3.60 (95% CI 2.08–6.23); for four or more traumatic events and physical abuse 7.03 (95% CI 3.23–15.29); and for four or more traumatic events and severe psychological and physical abuse the adjusted OR was 10.45 (95% CI 6.06–18.01). For pregnant women who reported four or more traumatic events, and either physical abuse alone or in combination with severe psychological abuse, there was a 10-fold increase in depressive and other mental health symptoms. | |
| Ukacukw 2009 [28] | After multivariable adjustment, intimate partner violence intensity had a strong and statistically significant association with depression symptom severity for pregnant women. | |
|
Waqas 2015 [41] |
Results of unadjusted log-binomial regression showed that unwanted pregnancy, prenatal depression and social support were associated with low birth weight. | |
|
Wong 2017 [42] |
Inferential analysis revealed that higher HADS scores were significantly associated with lower social support scores, rural background, history of harassment, abortion, caesarean birth and unplanned pregnancies (P < .05). | |
| Social morbidity | Hassan 2014 [44] | A significant association was found between pregnant women reporting intimate partner violence and preterm labour [adjusted odds ratio (adjOR) 1.54, 95% confidence interval (CI) 1.16–2.03], caesarean section (adjOR 11.84, 95% CI 6.37–22.02), antenatal hospitalization (adjOR 6.34, 95% CI 3.82–10.52) and vaginal bleeding (adjOR 1.51, 95% CI 0.9–2.3). |
|
Romero-Gutiérrez 2011 [46] |
Maternal complications were higher in pregnant women who experienced violence (30.2% vs 23.6%, p = 0.004). Pregnant women who experienced sexual violence had more maternal complications (43.2%), and pregnant women who experienced psychological violence had more neonatal complications (54.2%). | |
| Stöckl 2010 [48] | Women’s odds of drinking alcohol during pregnancy were significantly increased if they had experienced violence during pregnancy. Violence during pregnancy was also associated with having had a child or infant that died. |
Discussion
Main findings
There is emerging evidence of a high burden of multimorbidity in women living in LMIC during pregnancy and after childbirth, as well as emerging evidence of associations between physical, psychological and social morbidities, suggesting that maternal morbidities are inter-linked. There is, however, still limited data about the strengths and direction of the associations between the different types of morbidities.
There was an apparent lack of standardisation of definitions and data collection tools used to measure maternal multimorbidities. The EPDS was the most common validated data collection tool to assess psychological morbidity in the studies, but with different cut-off scores to determine the risk of “depression” (ranging from 4 to 13) making comparisons difficult. Similarly, a variety of different validated data collection tools were used to assess domestic violence and/or substance abuse as components of social morbidity. Physical, psychological and social morbidities were often described as aggregates or summative measures, limiting comparability of findings.
Strengths and limitations
To the best of our knowledge, this is the first systematic review to assess maternal multimorbidities and types and levels of association between the different types. Many studies relied on recall of experience of morbidity and many primary data were symptom-based rather than “diagnosed”. Only four studies triangulated self-reported symptoms with findings from clinical examination and/or basic laboratory investigations. Assessments of measurements of ill-health based on self-reporting may be valid regarding ill-health as experienced by women, but do not provide accurate burden of disease estimates. No study described or used internationally recognised disease classifications to assess physical morbidity. Internationally recognised data collection tools were used to assess psychological and social morbidity, but these often used different cut-off scores making comparisons difficult. A limitation of this review is that studies that explored maternal multimorbidity using qualitative methodology were excluded.
Interpretation
Valid comparable measurements of maternal multimorbidity are limited to date, and this study confirms the need for a new approach and focus [70–72]. It will be important for future healthcare practice and research to agree and apply: (a) common identification criteria for maternal multimorbidity taking into account the different types of physical, psychological and social morbidity; (b) standardised and validated data collection tools that can be used in different languages and at all levels of healthcare; with, (c) validation of self-reported measurements of maternal morbidity compared to clinical assessment, investigations and diagnosis determined by healthcare providers [6, 70–72]. More recognition must be given that maternal morbidity is a complex concept with important associations between different morbidities. This has implications for screening and management of all different types of ill-health during pregnancy and after childbirth. There is a need to incorporate women’s understanding, perceptions and lived experience of maternal multimorbidity into public health approaches to improve maternal health and wellbeing during pregnancy and after childbirth in LMIC [73, 74].
Conclusion
To date a range of methods and tools have been used to assess maternal multimorbidity. Maternal multimorbidity estimates using these methodologies and tools, while useful as a guide, cannot be considered truly representative of the burden and range of maternal multimorbidity that have negative impact on women’s wellbeing during pregnancy and after childbirth. The suggested WHO definition of maternal morbidity provides such a framework in principle, but challenges remain to map out comprehensive, feasible and acceptable assessment tools, approaches and timeframes [11]. Comprehensive and routine measurements of maternal multimorbidity are necessary to inform policy and program decisions and for resource allocation for antenatal and postnatal care [5]. Improved standardised measurements of maternal multimorbidity will also allow for comparison of the burden of disease across settings within and between countries. There is a need for a sustainable way to provide good baseline maternity care for all and targeted individualised care for women who need extra care to prevent development and progression of maternal multimorbidity.
Supplementary information
Additional file 1: Supplementary Table 1. MeSH terms and keywords used in the search.
Additional file 2: Supplementary Table 2. Summary table for studies reporting maternal multimorbidity during pregnancy and after childbirth and /or associations between the multimorbidities.
Additional file 3: Supplementary Table 3. Summary table of types of physical morbidity reported in included studies.
Additional file 4: Supplementary Table 4. Summary table of measurements of psychological and social morbidities reported.
Acknowledgements
Not applicable.
Abbreviations
- EPDS
Edinburgh Postnatal Depression Score
- CASP
Critical Analysis Skills Programme
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- LMIC
Low- and middle-income countries
- MeSH
Medical Subject Headings
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- WHO
World Health Organization
Authors’ contributions
MMc and NvdB were responsible for the study inception and design. MMc and SZ performed the data extraction. MMc and NvdB interpreted the data and wrote the manuscript. All authors have read, critiqued and approved the final manuscript.
Funding
This study was funded by a grant from the Department of International Development, London UK, under the Making it Happen programme (202945–101) and Global Fund (20168770) and the EGA Hospital Charity Travelling Fellowship in Memory of Anne Boutwood from the Royal College of Obstetricians and Gynaecologists. The funders played no role in the writing of the manuscript or the decision to submit it for publication.
Availability of data and materials
All the sources of data are publicly available and referenced in the document.
Ethics approval and consent to participate
This systematic review did not involve contact with any human participants, and therefore no ethical approval was needed. This study was conducted in compliance with the established ethical guidelines of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12884-020-03303-1.
References
- 1.Graham W, Woodd S, Byass P, Fillipi V, Gon G, Virgo S, et al. Diversity and divergence: the dynamic burden of poor maternal health. Lancet. 2016;388(10056):2164–2175. doi: 10.1016/S0140-6736(16)31533-1. [DOI] [PubMed] [Google Scholar]
- 2.Ashford L. Hidden suffering: disabilities from pregnancy and childbirth in less developed countries. Washington, DC: Population Reference Bureau, MEASURE Communication; 2002. [Google Scholar]
- 3.Datta KK, Sharma RS, Razack PMA, Ghosh TK, Arora RR. Morbidity pattern among rural women in Alwar-Rajasthan - a cohort study. Health Popul Perspect Issues. 1980;3(4):282–292. [Google Scholar]
- 4.Barreix M, Barbour K, McCaw-Binns A, Chou D, Petzold M, Gichuhi G, et al. Standardizing the measurement of maternal morbidity: pilot study results. Int J Gynecol Obstet. 2018;141(Supp 1):10–19. doi: 10.1002/ijgo.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCauley M, Madaj B, White SA, Dickinson F, Bar-Zeev S, Aminu M, et al. Burden of physical, psychological and social ill-health during and after pregnancy among women in India, Pakistan, Kenya and Malawi. BMJ Glob Health. 2018;3(3):e000625. doi: 10.1136/bmjgh-2017-000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafar S, Jean-Baptiste R, Rahman A, Neilson JP, van den Broek NR. Non-life threatening maternal morbidity: cross sectional surveys from Malawi and Pakistan. PLoS One. 2015;10(9):e0138026. doi: 10.1371/journal.pone.0138026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United Nations . Transforming our world: the 2030 Agenda for Sustainable Development. New York: World Health Organization; 2015. [Google Scholar]
- 8.United Nations . Every Woman, Every Child: Global Strategy. 2015. [Google Scholar]
- 9.Vos T, Barber RM, Bell B, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Constitution of the World Health Organization. Geneva: World Health Organization; 1948. [Google Scholar]
- 11.Huber M, Knottnerus JA, Green L, et al. How should we define health? BMJ. 2011;343:d416. doi: 10.1136/bmj.d4163. [DOI] [PubMed] [Google Scholar]
- 12.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7(4):357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firoz T, Chou D, von Dadelszen P, Agrawal P, Vanderkruik R, Tunçalp Ö, et al. Measuring maternal health: focus on maternal morbidity. Bull World Health Organ. 2013;91(10):794–796. doi: 10.2471/BLT.13.117564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches. The GRADE Working Group. BMC Health Serv Res. 2004;4(1):38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faisal-Cury A, Araya R, Marcelo Z, Menezes P. Common mental disorders during pregnancy and adverse obstetric outcomes. J Psychosom Obstet Gynecol. 2010;31(4):229–235. doi: 10.3109/0167482X.2010.512404. [DOI] [PubMed] [Google Scholar]
- 16.Faisal-Cury A, Menezes P, Araya R, Zugaib M. Common mental disorders during pregnancy: prevalence and associated factors among low-income women in São Paulo, Brazil. Arch Womens Ment Health. 2009;12(5):335. doi: 10.1007/s00737-009-0081-6. [DOI] [PubMed] [Google Scholar]
- 17.Shamu S, Zarowsky C, Roelens K, Temmerman M, Abrahams K. High-frequency intimate partner violence during pregnancy, postnatal depression and suicidal tendencies in Harare, Zimbabwe. Gen Hosp Psychiatry. 2016;38(Jan-Feb):109–114. doi: 10.1016/j.genhosppsych.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Shamu S, Zarowsky C, Shefer T, Temmerman M, Abrahams N. Intimate partner violence after disclosure of HIV test results among pregnant women in Harare, Zimbabwe. PLoS One. 2014;9(10):e109447. doi: 10.1371/journal.pone.0109447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karmaliani R, Asad N, Bann CM, Moss N, McClure EM, Pasha O, et al. Prevalence of anxiety, depression and associated factors among pregnant women of Hyderabad. Pakistan Int J Soc Psychiatry. 2009;55(5):10. doi: 10.1177/0020764008094645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran TD, Biggs B-A, Tran T, Casey GJ, Hanieh S, Simpson JA, et al. Psychological and social factors associated with late pregnancy iron deficiency anaemia in rural Viet Nam: A population-based prospective study. PLoS One. 2013;8(10):e78162. doi: 10.1371/journal.pone.0078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wado YD, Afework MF, Hindin MJ. Effects of maternal pregnancy intention, depressive symptoms and social support on risk of low birth weight: a prospective study from southwestern Ethiopia. PLoS One. 2014;9(5):e96304.20. doi: 10.1371/journal.pone.0096304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assarag B, Dubourg D, Maaroufi A, Dujardin B, De Brouwere V. Maternal postpartum morbidity in Marrakech: what women feel what doctors diagnose? BMC Pregnancy Childbirth. 2013;13:225. doi: 10.1186/1471-2393-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chersich MF, Kley N, Luchters SMF, Njeru C, Yard E, Othigo MJ, et al. Maternal morbidity in the first year after childbirth in Mombasa Kenya; a needs assessment. BMC Pregnancy Childbirth. 2009;9:51. doi: 10.1186/1471-2393-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman A, Bunn J, Lovel H, Creed F. Association between antenatal depression and low birthweight in a developing country. Acta Psychiatr Scand. 2007;115(6):481–486. doi: 10.1111/j.1600-0447.2006.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaksen AB, Østbye T, Mmbaga BT, Daltveit AK. Alcohol consumption among pregnant women in northern Tanzania 2000–2010: a registry-based study. BMC Pregnancy Childbirth. 2015;15:205. doi: 10.1186/s12884-015-0630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surkan PJ, Sakyi KS, Christian P, Mehra S, Labrique A, Ali H, et al. Risk of depressive symptoms associated with morbidity in postpartum women in rural Bangladesh. Matern Child Health J. 2017;21(10):1890–1900. doi: 10.1007/s10995-017-2299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai AC, Tomlinson M, Comulada WS, Rotheram-Borus MJ. Intimate partner violence and depression symptom severity among south African women during pregnancy and postpartum: population-based prospective cohort study. PLoS Med. 2016;13(1):e1001943. doi: 10.1371/journal.pmed.1001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ukachukwu V, Unger H, Onoka C, Nduka C, Maina S, Ngugi N. Maternal morbidity and mortality in peri-urban Kenya-assessing progress in improving maternal healthcare. East Afr J Public Health. 2009;6(2):112–118. [PubMed] [Google Scholar]
- 29.Chibanda D, Mangezi W, Tshimanga M, Woelk G, Rusakaniko S, Stranix-Chibanda L, et al. Postnatal depression by HIV status among women in Zimbabwe. J Women's Health. 2010;19(11):2071–2077. doi: 10.1089/jwh.2010.2012. [DOI] [PubMed] [Google Scholar]
- 30.Dewing S, Tomlinson M, Le Roux IM, Chopra M, Tsai AC. Food insecurity and its association with co-occurring postnatal depression, hazardous drinking, and suicidality among women in peri-urban South Africa. J Affect Disord. 2013;150(2):460–465. doi: 10.1016/j.jad.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalifa DS, Glavin K, Bjertness E, Lien L. Determinants of postnatal depression in Sudanese women at 3 months postpartum: a cross-sectional study. BMJ Open. 2016;6:e009443. doi: 10.1136/bmjopen-2015-009443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukose A, Ramthal A, Thomas T, Bosch R, Kurpad AV, Duggan C, et al. Nutritional factors associated with antenatal depressive symptoms in the early stage of pregnancy among urban south Indian women. Matern Child Health J. 2014;18(1):161–170. doi: 10.1007/s10995-013-1249-2. [DOI] [PubMed] [Google Scholar]
- 33.Natamba BK, Achan J, Arbach A, Oyok TO. Ghosh S, Mehta S, et al. Reliability and validity of the Center for Epidemiologic Studies Depression Scale in screening for depression among HIV-infected and uninfected pregnant women attending antenatal Services in Northern Uganda: a cross-sectional study. BMC Psychiatr. 2014;14:2197. doi: 10.1186/s12888-014-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vythilingum B, Roos A, Faure SC, Geerts L, Stein DJ. Risk factors for substance use in pregnant women in South Africa. S Afr Med J. 2012;102(11 Pt 1):851–854. doi: 10.7196/SAMJ.5019. [DOI] [PubMed] [Google Scholar]
- 35.Yator O, Mathai M, Vander Stoep A, Rao D, Kumar M. Risk factors for postpartum depression for women living with HIV attending the prevention of mother to child transmission clinic at Kenyatta National Hospital at Nairobi, Kenya. AIDS Care. 2016;28(7):884–889. doi: 10.1080/09540121.2016.1160026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brittain K, Mellins CA, Phillips T, Zerbe A, Abrams EJ, Myer L, et al. Social support, stigma and antenatal depression among HIV-infected pregnant women in South Africa. AIDS Behavior. 2017;21(1):274–282. doi: 10.1007/s10461-016-1389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasreen HE, Kabir ZN, Forsell Y, Edhborg M. Prevalence and associated factors of depressive and anxiety symptoms during pregnancy: a population based study in rural Bangladesh. BMC Womens Health. 2011;11:22. doi: 10.1186/1472-6874-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natasha K, Hussain A, Khan AKA. Prevalence of depression among subjects with and without gestational diabetes mellitus in Bangladesh: a hospital based study. J Diabetes Metab Disord. 2015;14:64. doi: 10.1186/s40200-015-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ntaganira J, Muula AS, Masaisa F, Dusabeyezu F, Siziya S, Rudatskikira E. Intimate partner violence among pregnant women in Rwanda. BMC Womens Health. 2008;8:17. doi: 10.1186/1472-6874-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran TD, Tran T, Wynter K, Fisher J. Interactions among alcohol dependence, perinatal common mental disorders and violence in couples in rural Vietnam: a cross-sectional study using structural equation modeling. BMC Psychiatry. 2012;12:148. doi: 10.1186/1471-244X-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waqas A, Raza N, Lodhi HW, Muhammad Z, Jamal M, Rehman A. Psychosocial factors of antenatal anxiety and depression in Pakistan: is social support a mediator? PLoS One. 2015;10(1):e0116510. doi: 10.1371/journal.pone.0116510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong M, Myer L, Zerbe A, Phillips T, Petro G, Mellins CA, et al. Depression, alcohol use, and stigma in younger versus older HIV-infected pregnant women initiating antiretroviral therapy in Cape Town, South Africa. Arch Womens Ment Health. 2017;20(1):149–159. doi: 10.1007/s00737-016-0688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart RC, Umar E, Tomenson B, Creed F. A cross-sectional study of antenatal depression and associated factors in Malawi. Arch Womens Ment Health. 2014;17(2):145–154. doi: 10.1007/s00737-013-0387-2. [DOI] [PubMed] [Google Scholar]
- 44.Hassan M, Kashanian M, Hassan M, Roohi M, Yousefi H. Maternal outcomes of intimate partner violence during pregnancy: study in Iran. Public Health. 2014;128(5):410–415. doi: 10.1016/j.puhe.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Hanlon C, Medhin G, Alem A, Tesfaye F, Lakew Z, Worku B, et al. Impact of antenatal common mental disorders upon perinatal outcomes in Ethiopia: the P-MaMiE population-based cohort study. Tropical Med Int Health. 2009;14:156–166. doi: 10.1111/j.1365-3156.2008.02198.x. [DOI] [PubMed] [Google Scholar]
- 46.Romero-Gutiérrez G, Cruz-Arvizu VH, Regalado-Cedillo CA. Ponce-Ponce de Leon AL. Prevalence of violence against pregnant women and associated maternal and neonatal complications in Leon, Mexico. Midwifery. 2011;27(5):750–753. doi: 10.1016/j.midw.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Rees SJ, Tol W, Mohammad M, Tay AK, Tam N, dos Reis N, et al. A high-risk group of pregnant women with elevated levels of conflict-related trauma, intimate partner violence, symptoms of depression and other forms of mental distress in post-conflict Timor-Leste. Transl Psychiatry. 2016;6:e725. doi: 10.1038/tp.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stöckl H, Watts C, Kilonzo Mbwambo JK. Physical violence by a partner during pregnancy in Tanzania: prevalence and risk factors. Reprod Health Matters. 2010;18(36):171–180. doi: 10.1016/S0968-8080(10)36525-6. [DOI] [PubMed] [Google Scholar]
- 49.Hamadani JD, Tofail F, Hilaly A, Mehrin F, Shiraji S, Banu S, et al. An association of postpartum maternal morbidities with children's mental, psychomotor and language development in rural Bangladesh. J Health Popul Nutr. 2012;30(2):193–204. doi: 10.3329/jhpn.v30i2.11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prost A, Lakshminarayana R, Nair N, Tripathy P, Copas A, Mahapatra R, et al. Predictors of maternal psychological distress in rural India: a cross-sectional community-based study. J Affect Disord. 2012;138(3):277–286. doi: 10.1016/j.jad.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rwakarema M, Premji SS, Nyanza EC, Riziki P, Palacios-Derflingher L. Antenatal depression is associated with pregnancy-related anxiety, partner relations, and wealth in women in northern Tanzania: a cross-sectional study. BMC Womens Health. 2015;15:68. doi: 10.1186/s12905-015-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali BS, Reza H, Khan MM, Jehan I. Development of an indigenous screening instrument in Pakistan: the Aga Khan University anxiety and depression scale. J Pak Med Assoc. 1998;48(9):261–265. [PubMed] [Google Scholar]
- 53.Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med. 1992;22(2):465–486. doi: 10.1017/S0033291700030415. [DOI] [PubMed] [Google Scholar]
- 54.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Brit J Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 55.Mollica RF, Caspi-Yavin Y, Bollini P, Truong T, Tor S, Lavelle J. The Harvard Trauma Questionnaire. Validating a cross-cultural instrument for measuring torture, trauma, and posttraumatic stress disorder in Indochinese refugees. J Nerv Ment Dis. 1992;180(2):111–116. doi: 10.1097/00005053-199202000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalence and trends in non-specific psychological distress. Psychol Med. 2002;32(6):959–976. doi: 10.1017/S0033291702006074. [DOI] [PubMed] [Google Scholar]
- 57.Brugha T, Bebbington P, Tennant C, Hurry J. The list of threatening experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Med. 1985;15(1):189–194. doi: 10.1017/S003329170002105X. [DOI] [PubMed] [Google Scholar]
- 58.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Brit J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 59.Spitzer RL, Williams JBW, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID) Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 60.Beusenberg M, Orley JH. A User's guide to the self-reporting questionnaire Geneva: World Health Organization, Division of Mental Health. 1994. [Google Scholar]
- 61.Spielberger CD. State-trait anxiety inventory STAI. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 62.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 63.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 64.Sheehan DV, Lecrubier Y, Sheehan KH, Anorm P, Janavs J, Weiller E, et al. The Mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Supp 20):22–57. [PubMed] [Google Scholar]
- 65.Babor TF, Biddle-Higgins JC, Saunders JB, Monteiro MG. AUDIT: the alcohol use disorders identification test: guidelines for use in primary health care. Geneva: World Health Organization; 2001. [Google Scholar]
- 66.Ewing J, Rouse BA. Identifying the hidden alcoholic. Paper presented at: the 29th International Congress on Alcohol and Drug Dependence, 1970; Sydney Australia. 1970. [Google Scholar]
- 67.Pascoe JM, Ialongo NS, Horn WF, Reinhart MA, Perradatto D. The reliability and validity of the maternal social support index. Fam Med. 1988;20(4):271–276. [PubMed] [Google Scholar]
- 68.Cutrona CE, Russell DW, Jones WH, Perlman D. Advances in Personal Relationships. Greenwich: JAI Press; 1987. The provisions of social relationships and adaptation to stress; pp. 37–67. [Google Scholar]
- 69.Holzemer WL, Uys LR, Chirwa ML, Greeff M, Makoae LN, Kohi TW. Validation of the HIV/AIDS stigma instrument - PLWA (HASI-P) AIDS Care. 2007;19(8):1002–1012. doi: 10.1080/09540120701245999. [DOI] [PubMed] [Google Scholar]
- 70.Chou D, Tunçalp Ö, Firoz T, Barreix M, Filippi V, von Dadelszen P, et al. Constructing maternal morbidity – towards a standard tool to measure and monitor maternal health beyond mortality. BMC Pregnancy Childbirth. 2016;16:45. doi: 10.1186/s12884-015-0789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Say L, Chou D, Barbour K, Barreix M, Cecatti J, Costa M, et al. Maternal morbidity: time for reflection, recognition, and action. Int J Gynecol Obstet. 2018;141(S1):1–3. doi: 10.1002/ijgo.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vanderkruik RC, Tunçalp Ö, Chou D, Say L. Framing maternal morbidity: WHO scoping exercise. BMC Pregnancy Childbirth. 2013;13:213. doi: 10.1186/1471-2393-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lange IL, Gherissi A, Chou D, Say L, Filippi V. What maternal morbidities are and what they mean for women: A thematic analysis of twenty years of qualitative research in low and lower-middle income countries. PLoS One. 2019;14(4):e0214199. doi: 10.1371/journal.pone.0214199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCauley M, Avais A, Agrawal R, Saleem S, Zafar S, van den Broek N. “Good health means being mentally, socially, emotionally and physically fit”: Women’s understanding of health and ill-health during and after pregnancy. BMJ Open. 2020;10:e028760. doi: 10.1136/bmjopen-2018-028760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. MeSH terms and keywords used in the search.
Additional file 2: Supplementary Table 2. Summary table for studies reporting maternal multimorbidity during pregnancy and after childbirth and /or associations between the multimorbidities.
Additional file 3: Supplementary Table 3. Summary table of types of physical morbidity reported in included studies.
Additional file 4: Supplementary Table 4. Summary table of measurements of psychological and social morbidities reported.
Data Availability Statement
All the sources of data are publicly available and referenced in the document.