Abstract
In recent years, dynamic, ‘click’ hydrogels have been applied in numerous biomedical applications. Owing to the mild, cytocompatible, and highly specific reaction kinetics, a multitude of orthogonal handles have been developed for fabricating dynamic hydrogels to facilitate ‘4D’ cell culture. The high degree of tunability in crosslinking reactions of orthogonal ‘click’ chemistry has enabled a bottom-up approach to install specific biomimicry in an artificial extracellular matrix. In addition to click chemistry, highly specific enzymatic reactions are also increasingly used for network crosslinking and for spatiotemporal control of hydrogel properties. On the other hand, covalent adaptable chemistry has been used to recapitulate the viscoelastic component of biological tissues and for formulating self-healing and shear-thinning hydrogels. The common feature of these three classes of chemistry (i.e., orthogonal click chemistry, enzymatic reactions, and covalent adaptable chemistry) is that they can be carried out under ambient and aqueous conditions, a prerequisite for maintaining cell viability for in situ cell encapsulation and post-gelation modification of network properties. Due to their orthogonality, different chemistries can also be applied sequentially to provide additional biochemical and mechanical control to guide cell behavior. Herein, we review recent advances in the use of orthogonal click chemistry, enzymatic reactions, and covalent adaptable chemistry for the development of dynamically tunable and biomimetic hydrogels.
Graphical Abstract
This review highlight recent advances in bio-orthogonal and dynamic hydrogels crosslinked by irreversible click chemistry, enzymatic reactions, and covalent-adaptable network.
1. Introduction
Since late-2000’s, hydrogels synthesized by click chemistry have become a unique class of biomaterials suitable for a variety of biomedical applications.1–3 In contrast to radically initiated chain-growth polymerization of (meth)acrylated macromers, which yield random crosslinks and stochastically distributed mesh size, orthogonal click chemistries are milder, more cytocompatible, and produce hydrogel networks with less structural defects. Click hydrogels also have significantly improved mechanical properties. The selective reactivity of click chemistry handles (e.g., azide/alkyne, azide/cyclooctyne, thiol/norbornene, thiol/maleimide, etc.) not only facilitate hydrogel crosslinking, but also allows for specific tailoring of gel physicochemical properties via a secondary reaction. Click chemistry affords the crosslinked network with a wide range of bond stability, such as purely covalent bonds, cleavable linkages, and covalent adaptable crosslinks.4 This level of tailorability is instrumental in modulating cell fate and tissue morphogenesis, as the stability of the crosslinks often dictates how a cell respond to its extracellular microenvironment.5 Click chemistry also provide an elegant means of tuning hydrogel physicochemical properties in a spatiotemporally controllable manner.6,7
An expansive ‘toolbox’ of click reagents are now commercially available for a multitude of bioconjugation and polymerization applications (Fig. 1). In general, click gel crosslinking starts from conjugating mutually reactive click reagents to multifunctional macromers, followed by reacting the modified macromers via simple mixing. By adjusting the concentration, molecular weight, and stoichiometric ratio/functionality of the macromers, hydrogels can be fabricated with different network connectivity or crosslinking density. This in turn affects mechanical properties of the hydrogels that may guide cell fate processes. Fabrication of click hydrogels can also be facilitated by photochemistry or enzymatic reactions, which provide an additional gelation control without sacrificing the orthogonality of robust click chemistry. Click chemistry has also been widely adapted for post-gelation modification of an otherwise inert and static hydrogel network. This is commonly achieved by a secondary photochemistry, by swelling in additional click-based macromers, or by removing molecular cage capping the otherwise reactive motifs pre-conjugated in the hydrogels. The integration of multiple click chemistries in a single hydrogel results in the formation of a smart and highly responsive hydrogel network. In this review, we highlight the recent advances in major bio-orthogonal reactions used to form cell-laden hydrogels with highly tunable physicochemical properties. In particular, we focus on the use of three types of bio-orthogonal chemistries: Irreversible click chemistry, enzymatic crosslinking, and covalent adaptable network. The common criterion for these three bio-orthogonal chemistries is that the crosslinking reactions between the macromers should have high specificity. Hence, we do not include hydrogels formed with random chain-growth polymerizations, supramolecular/host-guest interactions, and enzymatic reactions with low substrate specificity (e.g., acid-amine coupling).
Figure 1.
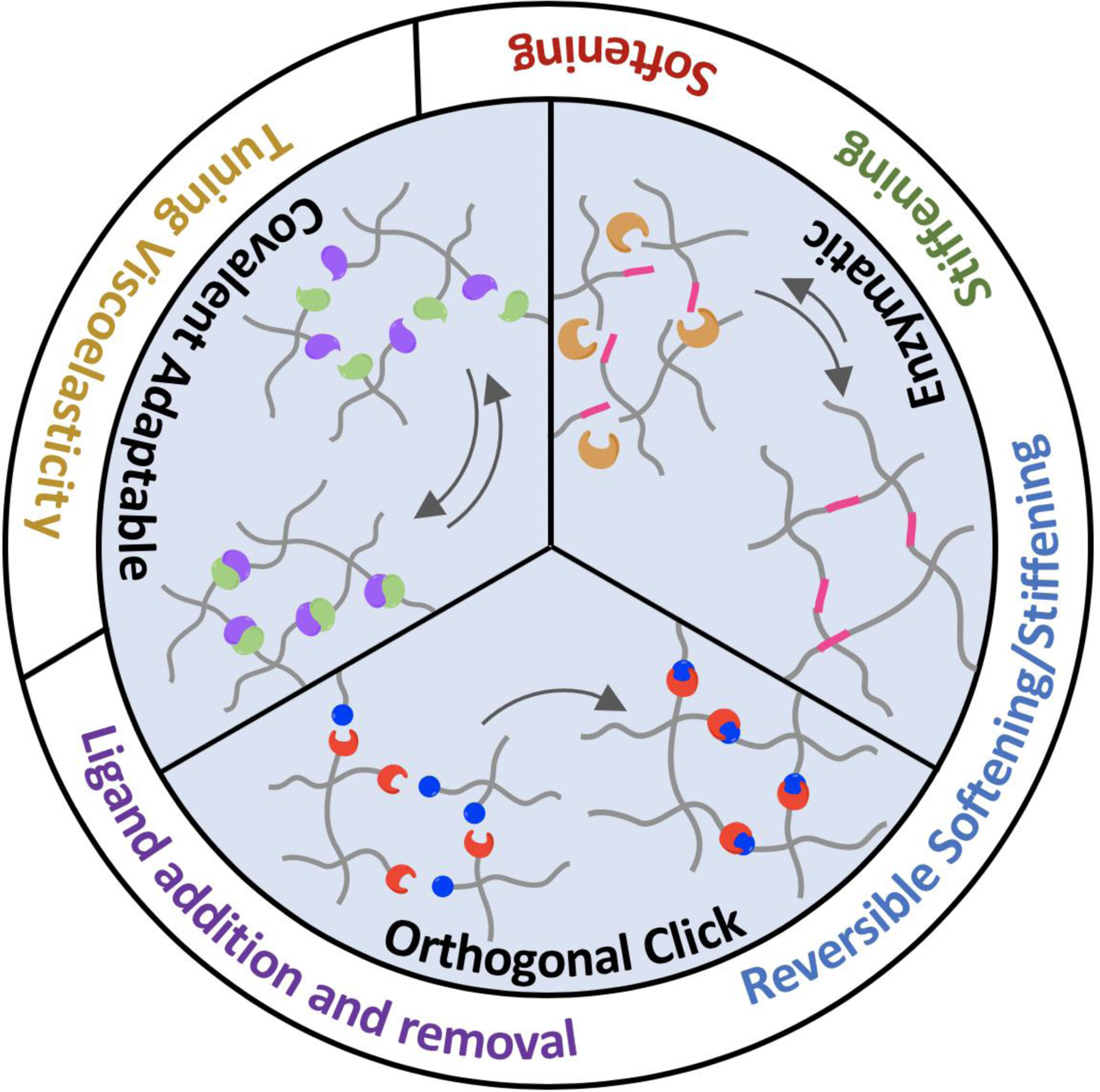
Summary of orthogonal chemistries used in dynamic hydrogel crosslinking.
2. Chemistry for crosslinking of biomimetic hydrogels
2.1. Orthogonal Irreversible Click Chemistry
2.1.1. Thiol-ene/alloc/vinyl photopolymerization of hydrogels
Bio-orthogonal thiol-norbornene click chemistry has emerged as an effective and versatile tool for crosslinking of biomimetic cell-laden hydrogels (Fig. 2A). Thiol-norbornene photoclick reactions are free-radical initiated, thereby requiring photoactivatable initiators to generate the radicals. However, thiol-norbornene crosslinking strategies do not consume oxygen, and require low concentration of initiators. Thiol-norbornene photopolymerization can be used to prepare hydrogels as microparticles8 or bulk hydrogels9 for cell culture. Uniquely, this chemistry can also be used to spatially pattern extracellular matrix (ECM) components to study cell behaviors. Typical initiation strategies include using UV light or visible light polymerization. Mechanistically, UV light irradiation cleaves a type I initiator (e.g., lithium aryl phosphinate, LAP) into free radicals, which abstract protons from sulfhydryls to create thiyl radicals. The thiyl radicals react with nearby vinyl functionalities (e.g., norbornene, NB) while regenerating additional thiyl radicals for propagation of the crosslinking reactions. Alge and colleagues utilized UV light to crosslink 4-arm PEG-NB with bis-thiol containing dithiothreitol (DTT) in the presence of LAP to generate hydrogel microgels that could be further modified with other chemistries.8 In another example highlighting the modularity of this strategy, Ki and colleagues generated schizophyllan, a water soluble β glucan, hydrogels using norbornene modified schizophyllan macromers, DTT, and LAP.10 In contrast to using UV-light, visible light-initiated crosslinking often involves the use of non-cleavage type (type II) photoinitiators (e.g., eosin-Y, rose bengal), which can be excited into a triplet state for extracting protons and initiating crosslinking. Our lab has utilized eosin-Y as a visible light photoinitiator11 and others have shown the use of additional type II initiators such as riboflavin to fabricate PEG-based hydrogels.12 Thiol-ene photopolymerization has recently been used in conjunction with other click chemistry (e.g., tetrazine-norbornene reaction) to assemble microporous annealed particle (MAP) hydrogels.13 Other materials crosslinked using thiol-ene chemistry include alginate,14 pectin,15 gelatin,9 and hyaluronic acid.16
Figure 2.
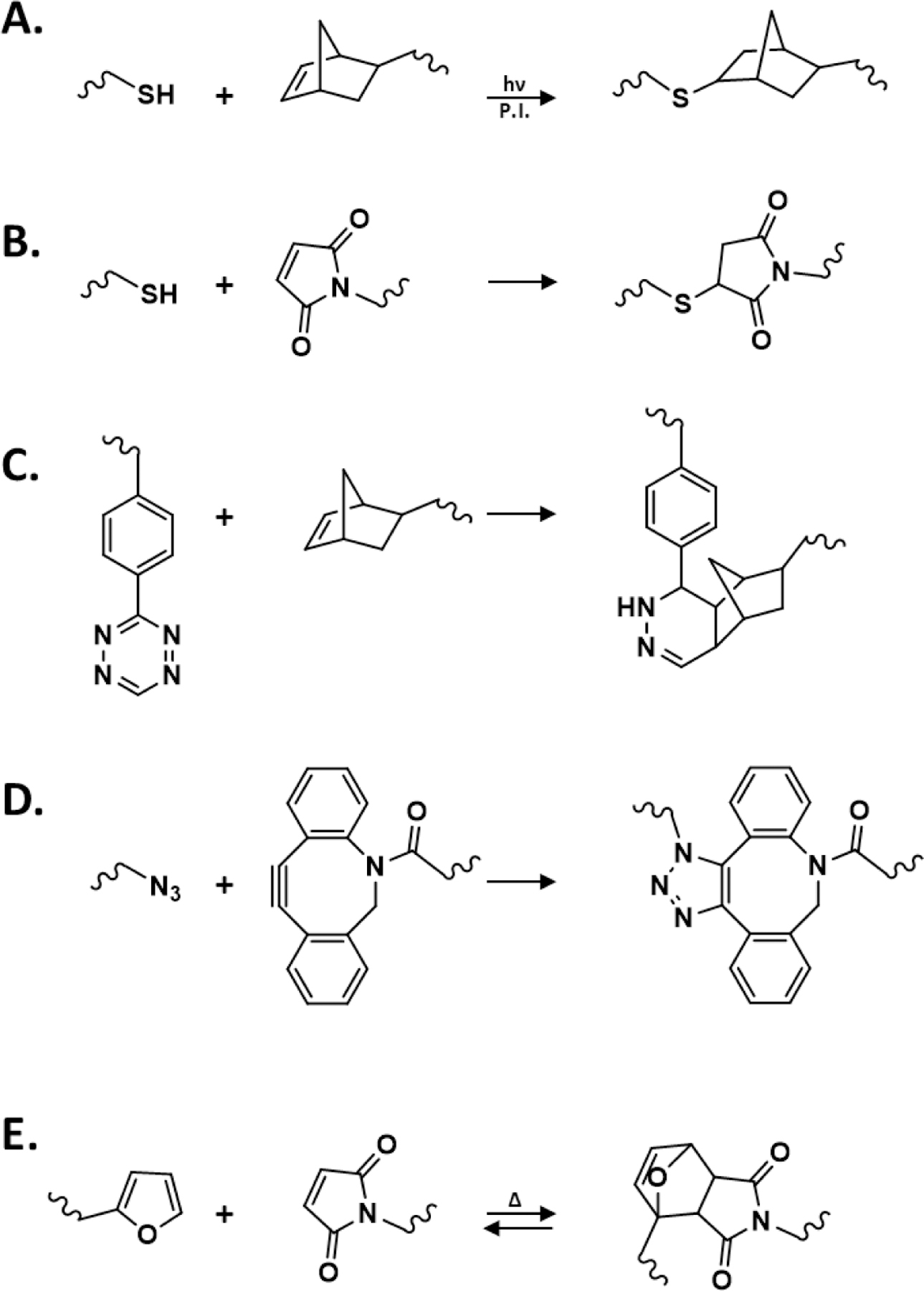
Reaction schemes of (A) thiol-norbornene photopolymerization (P.I.: photoinitiator), (B) Thiol-MAL Michael-type addition reaction, (C) iEDDA-mediated crosslinking (by Tz-NB click reaction), (D) SPAAC reaction (by azide-DBCO click reaction), and (E) Diels-Alder reactions (by furan-MAL click reaction).
In addition to thiol-norbornene photopolymerization, thiol-alloxycarbonyl (alloc) is often utilized to generate hydrogels and to tether additional molecules to a network. For example, Sawicki and Kloxin developed a protocol for encapsulation of cells using alloc-functionalized peptides and multi-arm PEGSH.17 Similar to thiol-norbornene reaction, this approach was shown to also enable photopatterning of hydrogels and achieve biocompatible crosslinking for 3D cell culture. Kloxin and colleagues also used thiol-alloc reaction to tether collagen mimetic peptides to PEG-based hydrogels for regulating mesenchymal stem cell fate processes.18 Besides fabricating hydrogels, the alloc moiety has been shown to ‘click’ to acrylate groups for modifying the crosslink network structure of PEG-acrylate hydrogels.19 Increasing the concentration of a mono-alloc-functionalized peptide significantly reduced the modulus of the hydrogels, resulted in variation of growth factor release kinetics.
Thiol-vinyl polymerization is also a commonly used reaction for generating a wide range of hydrogels requiring the use of natural materials such as chitosan or synthetic molecules like PEG.20,21 As with other thiol-based photopolymerizations, these hydrogels exhibit a high degree of tunability and modularity. For example, Hughes and coworkers developed an injectable, photocurable gelatin-based hydrogel by modifying gelatin with thiol and acrylate moieties.22 The injectability and cytocompatibility of the gelatin-based hydrogel was demonstrated using rabbit corneal wound model. Our lab has also used thiol-vinyl crosslinking to fabricate PEG-acrylate-based hydrogels for investigating the HIPPO pathway activation in hepatocellular carcinoma cells.23
2.1.2. Michael-type addition reactions and hydrogel crosslinking
Widely used for forming cell-laden hydrogels, Michael-type additions are typically base-catalyzed reaction between a Michael donor (e.g., thiols, amines) and electrophilic Michael acceptors (e.g., maleimides, vinyl sulfones, acrylates) (Fig. 2B). The gelation kinetics of Michael-type hydrogels are highly dependent on the concentration or molecular structure of the functional groups. García and coworkers have designed PEG-based thiol-maleimide hydrogels to encapsulate pancreatic islets and intestinal organoids.24–26 Segura and colleagues demonstrated the importance of controlling Michael-type reaction kinetics to achieve a crosslinked hydrogel with low degree of network defects.27 They showed that faster crosslinking kinetics (i.e., 2–5 seconds) led to heterogeneous crosslinking and peptide labeling of PEG-based hydrogels, whereas a slower gelation rate (a minute or longer) yielded hydrogels with appropriate mixing time and a more homogeneously crosslinked gel.27 On the other hand, Del Campo and coworkers demonstrated that multi-arm PEG-methylsulfone (PEG-MS), which reacted with PEG-SH with a moderate reactivity, was particularly advantageous for hydrogel crosslinking owing to a better mixing of pre-polymer solutions.28
2.1.3. Inverse electron-demand Diels-Alder (iEDDA) crosslinking of hydrogels
iEDDA reactions between diene tetrazine (Tz) and dienophiles (e.g., NB, trans-cyclooctene, TCO), are fast and irreversible click reactions that have increasingly been used for formulating hydrogels in 3D cell culture applications (Fig. 2C). Conjugating the diene and dienophile moieties to macromers for iEDDA-based gel crosslinking is synthetically simple as numerous amine-, carboxyl-, sulfhydryl-reactive derivatives are commercially available. For example, Mooney and coworkers used carbodiimide chemistry to generate Tz- and NB-alginate macromers for facile in situ cell encapsulation.29,30 Tz-NB crosslinking has been combined with other click chemistry to generate double network hydrogels with high mechanical strengths.31 Controlling Tz-NB reactivity is critical for providing appropriate mixing and pipetting time, which is essential for homogeneous cell encapsulation in bulk hydrogels. In an effort to control the initiation of Tz-NB crosslinking, Forsythe and colleagues devised a Tz moiety, dihydrogen-Tz (dHTz) that requires oxidization prior to iEDDA reaction to precisely control Tz-NB gelation, which was initiated by low concentration of Horseradish peroxidase (HRP) or red-light induced oxidation in the presence of methylene blue.32,33 Recent reports also show that tetrazine can be replaced with a more stable derivative methylphenyltetrazine to slow the gelation rate and to prevent reagent degradation.34,35 Nonetheless, for this class of reaction, macromer formulations must be carefully optimized for achieving homogeneous mixing without significantly delaying gelation speed and matrix stiffness. Another potential consideration for this reaction is the generation of nitrogen gas by-product. While nitrogen gas is inert and do not pose significant toxicity, a slowly crosslinked network typically generate larger nitrogen gas bubbles, which may cause local heterogeneity in mechanical properties of the hydrogel network.
Aside from Tz-NB crosslinking, Tz-TCO can be used for rapid generation and post-fabrication of hydrogels. Tz-TCO reaction is significantly faster than Tz-NB reaction and therefore has application in injection, interfacial polymerization, and bioprinting. Jia and colleagues utilized Tz-TCO for fibrous scaffold fabrication. This was achieved by interfacial polymerization with a trifunctional TCO monomer and a PEG-based bifunctional Tz monomer, and the scaffold was demonstrated to promote fibroblast viability and migration.36 Pei and coworkers also used Tz-TCO reaction for in situ hydrogel crosslinking and encapsulation and release of bone morphogenic protein-2 (BMP2).37
2.1.4. Strain-promoted alkyne-azide cycloaddition crosslinking (SPAAC)
Copper-catalyzed azide-alkyne cycloaddition (CuAAC) was one of the earliest utilized click chemistries in biomaterials-based applications.38 However, its use in formulating cell-laden hydrogel was limited due to the toxicity of copper. Copper-free SPAAC (Fig. 2D), initially established by Bertozzi and colleagues,39 has proven to maintain high specificity and reaction rates while exhibiting limited cytotoxicity, thus making it ideal for cell encapsulation.40–42 As with other click chemistries, numerous functional precursors have been developed to tune the gelation kinetics and reactivities of SPAAC. For example, Heilshorn et al. developed elastin-like proteins (ELPs) decorated with either azide or bicyclo[6.1.0]nonyne (BCN) moieties for SPAAC-induced gelation.43 In another example, an injectable hyaluronic acid (HA) based hydrogel was fabricated using azide-conjugated PEG and cyclooctyne-conjugated HA.44 Adronov and coworkers utilized aza-dibenzocyclooctyne (DIBAC or DBCO), which has higher reactivity than other cyclooctynes, to generate PEG-based hydrogels with tunable mechanics.45,46 Other uses of DBCO include the development of microgel scaffolds for hMSC secretome analysis as described by Anseth and colleagues.47 Kloxin and coworkers also utilized DBCO to generate PEG-based SPAAC hydrogels decorated with thrombin-cleavable fluorescent proteins.48
2.1.5. Diels-Alder reactions
Diels-Alder (DA) reactions permit spontaneous crosslinking of hydrogels at physiological pH and temperature (Fig. 2E). However, DA gelation is typically slower than other click chemistries, particularly for the commonly used diene maleimide and dienophile furan pair.49,50 Increasing the reaction rate requires an acidic environment (pH = 5.5), but this is an unfavorable condition for in situ cell encapsulation. In this regard, Schoichet and colleageues used electron-rich methylfuran to accelerate gelation of HA-methylfuran and PEG-MAL at pH 7.4.51 In another example, Heilshorn and Madl achieved a 30 second gelation rate using 8-arm PEG-fulvene and 8-arm PEG-MAL.52 DA reaction has been used in conjunction with other chemistry such as acylhydrazone bond formation or metal coordination bonding to generate double network hydrogels with self-healing properties. 53–55 Other dually crosslinked networks involving DA reaction include using a rapid thermo-responsive gelation approach, followed by slow furan-MAL crosslinking to provide the gel with enhanced mechanical stability.56
2.2. Enzymatic crosslinking
2.2.1. Horseradish Peroxidase (HRP)
Enzymatic reactions are popular strategies for in situ crosslinking of hydrogels due to their highly predictable reaction kinetics and enhanced substrate specificity. Of particular interest is the use of HRP or in situ crosslinking of phenolic polymers (e.g. hydroxyphenylacetic acid (HPA), tyramine, or tyrosine-modified polymers. Upon activation by H2O2 (Fig. 3A), HRP abstracts a hydrogen from the phenolic groups to generate radicals, which terminally join to form dityrosine crosslinks.57 In a recent example, HRP was used to polymerize tyramine-substituted silk (SF-TA) or gelatin (G-TA) to improve crosslinking and mechanical properties of silk-based hydrogel.58 Despite the use of H2O2 at 0.01% (ca. 5.6 mM), the hydrogels were reported to sustain both 2D and 3D spreading of human mesenchymal stem cells (hMSCs).58 In an effort to mitigate the cytotoxicity and enzyme inactivation effects of exogenously added H2O2, many have exploited glucose oxidase (GOX) and glucose to supply H2O2 indirectly. In a recent example, Kim and coworkers employed the GOX/HRP dual enzyme system to generate a gelatin-based hydrogel for 3D encapsulation of human dermal fibroblast.59 Gantumur et al. reported that H2O2 could be generated from self-oxidation of cysteine residues within HRP.60 To achieve self-oxidation, high concentration of HRP (300 U/ml) was used along with glucose as a reducing agent. Despise the use of high HRP concentration, the hydrogel system showed no cytotoxicity to the encapsulated 10T1/2 fibroblasts.60
Figure 3.
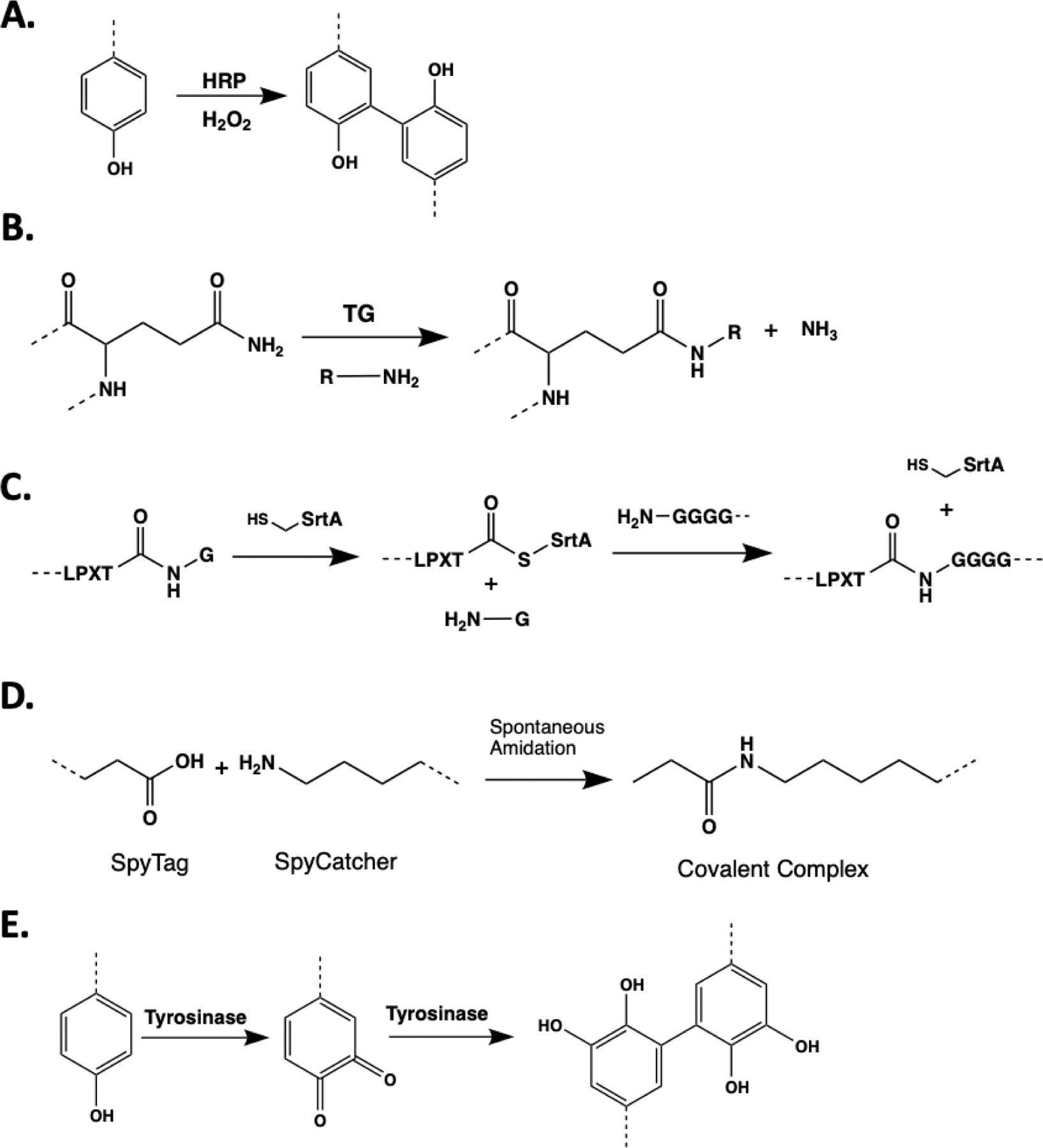
Mechanism of enzymatic hydrogel crosslinking mediated by (A) HRP and H2O2 (B) TG, (C) SrtA, (D) SpyTag-Spy Catcher protein pair, and (E) Tyrosinase.
Beside phenolic functional groups, HRP has also been shown to generate radicals from thiols to initiate disulfide bond crosslinking.61 Gelatin-based hydrogel formation via this mechanism exhibited enhanced adhesiveness for long-term culture of human dermal fibroblasts (hDF).61 Via targeting thiol residues, HRP could also initiate thiol-norbornene gelation.62 In our own recent work, we showed that HRP could be used to crosslinked 8-arm PEG-norbornene with bis-cysteine peptides for in situ cell encapsulation. Furthermore, the incorporation of tyrosine residues within a peptide crosslinker significantly increased the kinetics of HRP-mediated thiol-norbornene gelation.62 It was hypothesized that tyrosyl radicals were generated on the tyrosine residues that rapidly transferred to nearby cysteines, yielding thiyl radicals for initiating gelation. Furthermore, these tyrosine residues remained available for mushroom tyrosinase (MT) mediated dynamic gel stiffening.62
2.2.2. Transglutaminase
Transglutaminse (TG) is another useful enzyme that is popular for in situ hydrogel crosslinking. TG catalyzes the formation of isopeptide bonds between primary amine and corboxyamide group of glutamines (Fig. 3B).63 Blood coagulation factor XIII was employed to crosslink high molecular weight HA conjugated with TG substrates to form hydrogels that enabled in situ encapsulation of rat neurons.64 The encapsulated neurons displayed impressive neurite outgrowth, as well as axonal and dendritic speciation.64 TG crosslinking can be used in combination with other reactions to generate double network hydrogels. For example, Chen et al. used TG to crosslink gelatin in combination with alginate/Ca2+ to generate a cytocompatible interpenetrating network (IPN) hydrogel for 3D cell culture and bioprinting.65 In another example, microbial TG was utilized to crosslink gelatin microgels to produce a biofunctional, injectable, and macroporous hydrogel.66 The porous hydrogel supported higher proliferation and migration of hDFs than the nonporous hydrogel over 2 weeks.66 A notable recent advance of TG-based crosslinking was the use of ultrasound to liberate liposome-encapsulated Ca2+, which activated TG to achieve tunable fibrin gelation.67
2.2.3. Other enzymes
Many enzymes routinely used in cellular and molecular labeling are increasingly explored for hydrogel applications. For example, sortase A (SrtA) catalyzes the transpeptidation between LPXTG (where X is any amino acid except proline) and Gn (n > 1) sequences to yield LPXTGn and a leaving G (Fig. 3C).63,68 The high specific SrtA transpeptidation has been used for post-gelation modification,69 controlled degradation,70 and crosslinking of PEG-based hydrogels.71 These examples have demonstrated the cytocompatibility of SrtA-mediated reaction for in situ cell encapsulation. Uniquely, dynamic controls of hydrogel properties (e.g., on-demand stiffening or softening) could be achieved via designing peptide crosslinkers with orthogonal specificity to different enzymes (e.g., tyrosine residues for tyrosinase, protease-labile sequence).71
Another orthogonal enzyme ligation strategy for hydrogel crosslinking is the SpyTag-Spy Catcher system, which were engineered from the CnaB2 domain of the fibronectin adhesion protein FbaB. SpyTag is a peptide sequence (AHIVMVDAYKPTK) containing reactive D amino acid and SpyCatcher is the protein domain containing the reactive K residue.72 Mixing of SpyTag and SpyCatcher results in the formation of isopeptide bond between the reactive D and K amino acids (Fig. 3D). This reaction was applied to assemble hydrogels that can be further functionalized with adhesion proteins to modulate human mammary epithelial cells behaviors in 3D.73 In another example, a protein-based hydrogel network was created using ELPs conjugated with SpyTag and SpyCatcher.74 In addition to the SpyTag-Spy Catcher covalent crosslinks, the gels were designed to entrap engineered p53 domains, which can dimerize to produce physically entangled interchain interactions. Hence, the resulting hydrogels could be created with various dissipation modes and would be useful for investigating cell-matrix interactions.74
Thrombin, which catalyzes the cleavage of fibrinogen into fibrin, is also frequently used in hydrogels crosslinking. By cleaving fibrinogen at the specific Arg–Gly residues, fibrin polymerization domains are exposed and fibrin monomers can self-associate into insoluble fibrin.75 Taira et al. exploited this highly specific reaction for the development of an electrochemically-induced fibrin gelation for encapsulation of human lung fibroblasts.76 In another recent example, Zhao et al. used thrombin to prepare aptamer-functionalized fibrinogen injectable hydrogel that allowed for in vivo co-delivery of multiple growth factors to promote angiogenesis.77
Another promising enzyme is tyrosinase, which specifically oxidizes tyrosine residues into catechols, dihydroxyphenylalanine (DOPA)-quinones and eventually to DOPA-dimer linkages (Fig 3E). Recently, a novel tyrosinase, derived from Streptomyces avermitilis, was employed to crosslink a tissue adhesive hydrogel from tyramine-conjugated HA and gelatin.78 Comparing to tyrosinase extracted from Agaricus bisporus or Bacillus megaterium, this new tyrosinase exhibited a superior reactivity that significantly reduced gelation rate to less than 50 s.78
2.3. Covalent adaptable networks
The effects of viscoelasticity on cell fate have received increasing attention in recent literature.79–84 Several covalent adaptable networks (CANs) have been developed to mimic tissue viscoelasticity. CANs are networks containing reversible bonds that undergo changes between bound and free states over time or in response to environment stimuli.85 CANs are a great toolkit for adjusting hydrogel viscoelasticity since they offer dynamically adaptable network connectivity. Hydrogels containing CANs provide a robust yet dynamic framework, from which to study how matrix viscoelasticity affects cell fate processes.
2.3.1. Boronate Ester
Boronic acid (BA) complexes with 1,2- or 1,3-diols to produce reversible boronate ester-diol bonding (Fig. 4A). The equilibrium of the complex is sensitive to pKa of the boronic acid groups. Thus, to form stable hydrogels, the pH of the buffer solution usually has to be higher than the pKa of BA to prevent dissociation.86–88 Boronate ester bond is widely employed in cell encapsulation due to its selective and non-toxic nature. The Anseth group has recently reported a fast stress-relaxing hydrogel containing both stable azide-alkyne bonds and dynamic covalent boronate ester bonds.89 Despite the absence of chemically and hydrolytically degradable bond, this hydrogel system could promote hMSCs spreading through dynamic linkages that were sensitive to cell-induced forces. By varying the phenylboronic acid derivatives with different pKa values, the stress relaxation rate can be systematically controlled over a broad range of timescales to capture the dynamic mechanical properties of ECM in various tissues.89 In another work, PEG was functionalized with benzaldehydes and phenylboronic acid at each end of the chain to obtain multifunctional PEG (MF-PEG).90 This MF-PEG simultaneously cross-linked with poly(vinyl alcohol) (PVA) through the boronate ester bonds and glycol-chitosan via imine formation to generate an adhesive and self-healing hydrogel with a unique double dynamic network (DDN) structure under mild conditions (pH ≈ 7, 25 °C). 90
Figure 4.
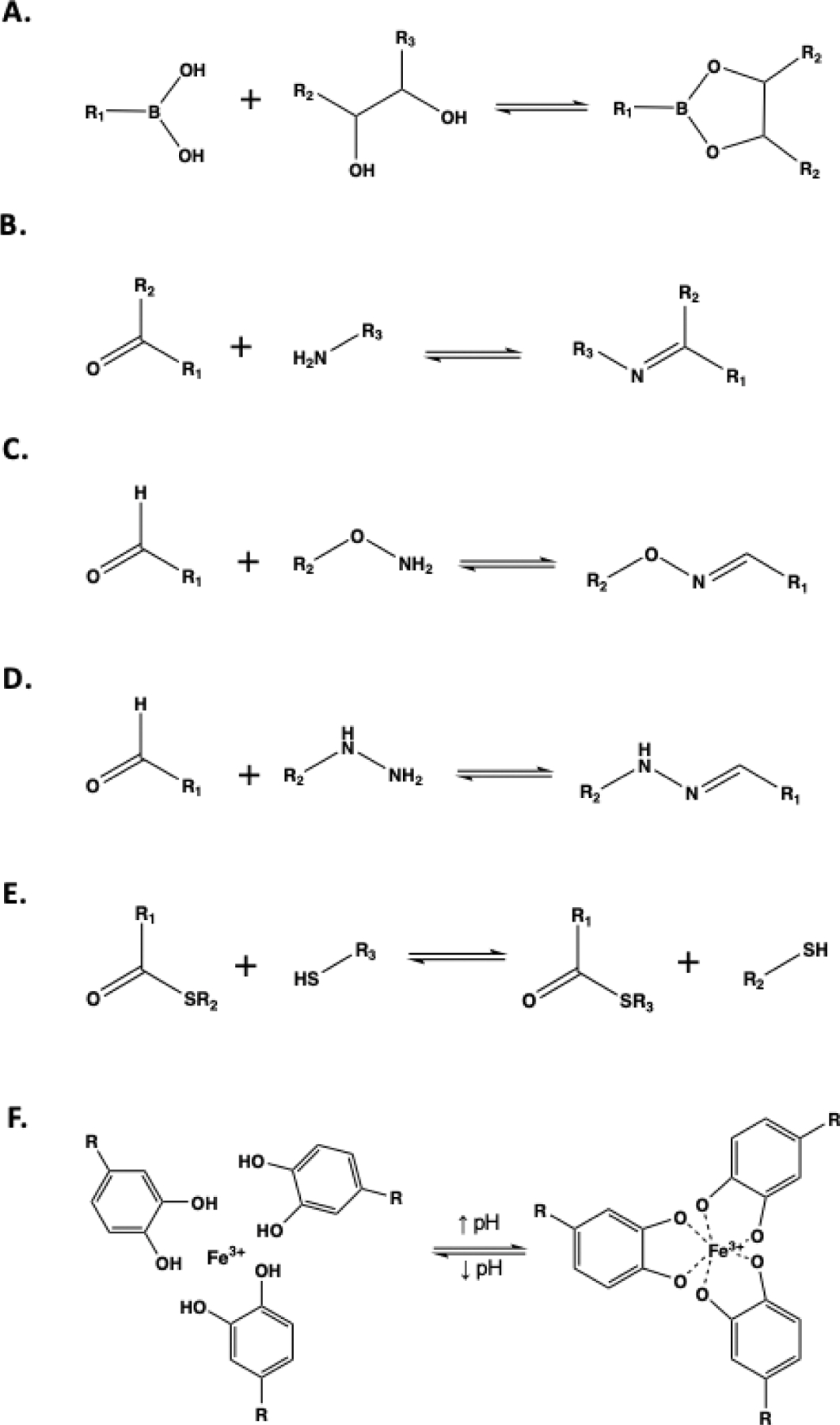
Reaction schemes of (A) Boronate-ester bond, (B) Imine formation, (C) Oxime ligation, (D) Hydrazone bond, (E) Thioester exchange, (F) Fe-catechol coordination complex.
When incorporated within hydrogel constructs, the dynamic nature of boronate ester bonds not only gives rise to viscoelasticity, but also provides hydrogels with self-healing ability, which is uniquely advantageous for generating co-culture systems. For example, co-polymer 2-acrylamidophenyl-boronic acid was crosslinked with PVA to form self-healing hydrogels.91 Two hydrogels separately encapsulated fibroblasts or breast cancer cells were fused together upon close contact due to the formation of boronic acid-diol complex at the gel interfaces. The result hydrogel was used to probe directional cell migration and invasion of the co-cultured cells.91
2.3.2. Imine/Enamine
Imines/enamines (Schiff bases) are generated from the condensation reaction between amine and aldehyde (or ketone) (Fig. 4B). In general, Schiff base bonds are more stable in neutral pH and have also been used to generate hydrogels under physiological conditions.92 For example, Zhang et al. utilized the reaction between the amino groups along the dendric oligoethylene glycol-functionalized chitosan and dialdehyde crosslinkers to generate a hydrogel at pH 7.4.93 The hydrogels were cytocompatible for 3D stem cell culture despite the use of aldehydes.93
Although promising for designing adaptable hydrogels, the rapid coupling and uncoupling of Schiff base linkages usually causes poor structural stability.94 To overcome this challenge, Wei et al. developed a dual network hydrogel formed via two chemical reactions: Schiff base and Michael-type addition.95 The reaction between the amino groups of N-carboxyethyl chitosan and the aldehyde groups on oxidized and acrylated HA (OAHA) constituted the dynamic hydrogel network. On the other hand, the Michael addition reaction of acrylates moieties on OAHA chain with the dithiol of matrix metalloproteinase-sensitive crosslinkers yielded a network with both biofunctionality and enhanced stability. Owing to the self-healing effect of the dynamic chemistry, multiple hydrogel pieces can be fabricated with different RGD concentrations then assembled together to create a hydrogel string with RGD gradient to investigate cellular response.95
2.3.3. Oxime
A special type of imine, oxime, is produced by reacting aldehyde or ketone with hydroxyl amine functional groups (Fig. 4C).96 Oxime bonds are susceptible to hydrolysis, which occurs faster under acidic conditions (pH ≤ 5).97 However, oxime bonds still possessed higher stability than regular imines.98 Therefore, comparing to other Schiff base gels, oxime-mediated hydrogels exhibited better mechanical properties, but still maintain the dynamic nature. It was reported that hydrogels created from alkoxyamine- and aldehyde-modified alginate permitted tunable viscoelasticity,99 which was adjusted by simply varying the molar ratios between oxyamine and aldehyde. This material was used to probe the growth and migration of encapsulated B-cells.99 In another case, to offer more control over the spontaneous oxime reaction, the DeForest group created photo-triggered oxime ligation by protecting alkoxyamine with 2-(2-nitrophenyl) propyloxycarbonyl (NPPOC) photocage.100 Upon UV light expose, the photocage was removed, revealing the alkoxyamine on the PEG chain for immediate crosslinking with benzadehyde-modified PEG and generating hydrogel within minutes.100
2.3.4. Hydrazone
Another dynamic covalent bond that is widely used to crosslinking biomimetic hydrogel crosslinking is hydrazone, which is also a special type of imine. Hydrazone results from the condensation reaction between hydrazine and aldehyde (Fig. 4D). Similar to oxime bond, the exchange between free and complexed state of the hydrazone bonds occurs through hydrolysis and recondensation, which constitutes the dynamic property of this chemistry. In an example, the polymerization of HA-hydrazine, HA-aldehyde/HA-benzaldehyde and collagen I rendered an IPN hydrogel system that recapitulated the viscoelasticity and fibrillary of ECM in tissues.101 The stress relaxation in the IPN hydrogels was tuned via the two modes of stress relaxation present within the network: one from collagen and the other from the hydrazone dynamic bond. It was revealed that faster relaxation promotes MSCs spreading, fiber remodeling, and focal adhesion (FA) formation.101 Light can also be employed to initiate hydrazone gelation. In this case, 4-arm PEG-hydrazine and PEG-nitrobenzyl was used to fabricate hydrogels.102 Under UV-light exposure, pendant photoreactive 2-nitrobenzyl alcohol was converted into 2-nitrobenzyladehyde and reacted with PEG-hydrazine to form a hydrogel.102
To further extend the mechanical properties as well as the applicability of this chemistry, a double-network hydrogel crosslinked via both vinyl double bonds and hydrazone was proposed by Gao’s research group.103 Since hydrazone is subjected to decomposition under acidic conditions, the hydrogel stiffness can be adjusted by incubating the gels in pH 7.4 or pH 5 buffers. This dynamic double network hydrogel was used to demonstrate the differential migratory behaviors of endothelial cells (ECs) and mesenchymal stem cells (MSCs) against gels with different stiffness. Specifically, ECs migrated much deeper into the softer hydrogel that were pre-treated with pH 5 buffer, whereas MSCs migrated more easily into the stiffer hydrogel.103
2.3.5. Other CANs
Additional dynamic covalent chemistries for generating stress relaxing hydrogels include disulfide bond, catechol-Fe complexes, and Diels-Alder cycloaddition. To generate stress-relaxing hydrogels via thioester exchange, gels were initially crosslinked by thiol-ene photo-crosslinking of 8-arm PEG thiol macromers with the thioester di(vinyl ether) (TEDVE) crosslinker with an off-stoichiometry ratio.104 The excess thiols reacted with the backbone thioester in a thioester exchange reaction to afford tunable viscoelasticity (Fig 4E).104 Using an alternative chemistry, four-arm PEG modified with dopamine was reacted with Fe3+ to form reversible bonds (Fig 4F).105 In addition to the dynamic bond formation, the spontaneous oxidation of catechol resulted in formation of irreversible covalent bonds causing the hydrogel to have both self-heal and shape memory properties.105 Lastly, the orthogonal DA bonds mentioned in section 2.1 also possesses dynamic nature and could potentially be used to generate self-healing, adaptable hydrogels. As mentioned above, a recent study demonstrated that HA was modified with methylfuran, a more electron-rich diene than furan, can couple with maleimide (dienophile) under physiological pH to form more stable hydrogels.51 The improvement in stability suggests slower forward and reverse DA reactions, which might affect the time-dependent mechanical properties and the self-healing effect of the hydrogels.
Even though dynamic covalent network provides a promising means for cell encapsulation, solely relying on this chemistry in the network structure does not provide a stable network, especially for dynamic bonds with fast dissociation constants. Furthermore, gel degradability and bio-adhesivity should also be incorporated in the network to support cell fate processes. In one example, DeForest and colleagues utilized gels with oxime bonds to encapsulate 3T3 fibroblasts.100 They noticed that the lack of cell adhesion ligands and biodegradable network resulted in cells with rounded morphologies. Similarly, by encapsulating cells using Schiff base chemistry, the Gerecht group found that cells within regions of high RGD and MMP-sensitive crosslinker concentration were significantly more elongated than regions without any bioactive peptides.95 In addition, the reversibility of dynamic covalent bonds will also affect how fast cells can spread. In particular, the Anseth group reported hMSCs encapsulated within hydrazone hydrogels demonstrated no extensive spreading due to low hydrolysis rate of aromatic hydrazone crosslinking, which restricted cell mobility.106 It was proposed that the use of more dissociative bonds would be beneficial in decreasing physical confinement and promoting cell expansion and migration. Finally, a study by Lee and coworkers reported that gels with high viscosity would not always promote cell spreading.107 Chondrocytes encapsulated within high viscosity gel secreted more ECM and spread less than those in gels with a lower viscosity. The effect of viscosity on cells behavior is still relatively unexplored and not yet fully understood, further development of hydrogels with tunable viscoelasticity would certainly further decipher the physiological significance of matrix viscoelasticity.
3. Methods for dynamic tuning of hydrogel crosslinking
Tuning the crosslinking density of cell-laden hydrogels post-gelation is of paramount importance for studying dynamic biological processes, including tissue fibrosis, tumor progression, or stem cell differentiation. Dynamic stiffening of ECM is a result of excess deposition and crosslinking of matrix proteins (e.g., collagen fibers).108 Strategies to control dynamic hydrogel crosslinking post-gelation include photopolymerizations, orthogonal click chemistries, and enzymatic reactions.
3.1. Dynamic stiffening of hydrogels
Irreversible stiffening of hydrogels is useful to mimic tissue fibrosis and tumor stiffening. This is often achieved by performing secondary crosslinking in a cell-laden hydrogel in the presence of additional macromers and initiators. Anseth and colleagues developed dynamic PEG-based thiol-norbornene hydrogels by diffusing additional 8-arm PEG-NB, 8-arm PEG-SH, and LAP, followed by UV-light induced secondary crosslinking.109 Also utilizing light-based secondary crosslinking in the presence of additional photoinitiator LAP, Burdick and coworkers achieved dynamic stiffening in thiol-methacrylate Michael-type hydrogels crosslinked with excessed methacrylates.110 Alternatively, Engler and colleagues implemented a two stage photopolymerization to stiffen HA-methacrylate hydrogels by simply varying the UV-light irradiation time.111 In a recent study, the Anseth group exploited the ability of DBCO group to undergo radical-induced photocrosslinking for preparing stiffening hydrogels to probe myoblast mechanotransduction.104 This approach eliminated the need to diffuse additional macromers as long as the initial network contained excess cyclooctyne (relative to azide). Rosales and coworkers used o-nitrobenzyl crosslinkers and free methacrylate groups within a hydrogel fabricated by Michael-type addition to achieve UV-mediated photocleavage and visible-light and LAP-mediated stiffening.112 In another example, Bryant and colleagues demonstrated that through sequential hydrogel deposition and thiol-norbornene photopolymerization in a cylindrical mold, hydrogels with multiple, discrete regions of stiffness can be created.113 Alge and coworkers used microgel packing in conjunction with thiol-norbornene photopolymerization to generate microporous annealed particle (MAP) hydrogels with an E gradient between 9.8 to 29.2 kPa.114 Using the MAP gels, the researchers revealed the influence of gradient stiffness on hMSC proliferation and cell volume. Anthracene photodimerization can also be used to stiffen PEG-based hydrogels.115 Specifically, PEG-anthracene hydrogels were formed by UV light-mediated [4+4] photodimerization of anthracene functional groups, and stiffening was achieved by additional dimerization through further UV light treatment.
In addition to UV-light based photo-stiffening, our lab has developed a visible light induced tyrosine dimerization approach for achieving secondary crosslinking of PEG-peptide hydrogels.116 Dynamic stiffening was initiated by diffusing Flavin mononucleotide (FMN) into the PEG-peptide hydrogels containing tyrosine residues, followed by visible light exposure. Additional methods to achieve spatial stiffening of hydrogels include the use of protecting groups.117 For example, the otherwise reactive thiol groups were protected by photocleavable molecular cages. Upon localized illumination, the protecting group was cleaved, exposing the free thiols that could react with pendant VS groups in the network. Enzymes can also be employed to stiffen hydrogels. In contrast to photopolymerization which requires a light source and radical species for initiating secondary crosslinking, enzymatic reactions rely only on diffusion-reaction kinetics. Enzymatic stiffening of hydrogels is a cytocompatible strategy that can be modeled using Michaelis-Menten kinetics. Our lab has reported several tyrosinase-induced dynamic hydrogel stiffening.62,71,118–120 Tyrosinase, which converts mono-phenolic motifs (e.g., tyrosine, hydroxyphenylacetic acid or HPA) into DOPA dimers, was used to dynamically stiffen PEG-peptide hydrogels and HPA-decorated gelatin/HA hybrid hydrogels. The later study revealed a synergistic effect of hydrogel stiffening and presence of HA on epithelial-to-mesenchymal transition (EMT) of pancreatic cancer cells (Fig. 5A). In another example, Jia and colleagues explored Tz-TCO reactions to stiffen hydrogels121 TCO-modified HA was diffused into a hydrogel network crosslinked by thiol-acrylate reaction. Notably, the gels were prepared with excess Tz moieties, which permitted secondary crosslinking in the presence of additional TCO-modified HA. Interestingly, while the same strategy was effective in dynamic labeling of cell adhesive ligands (e.g., TCO-RGDS), stiffening with TCO-HA was not as significant.
Figure 5.
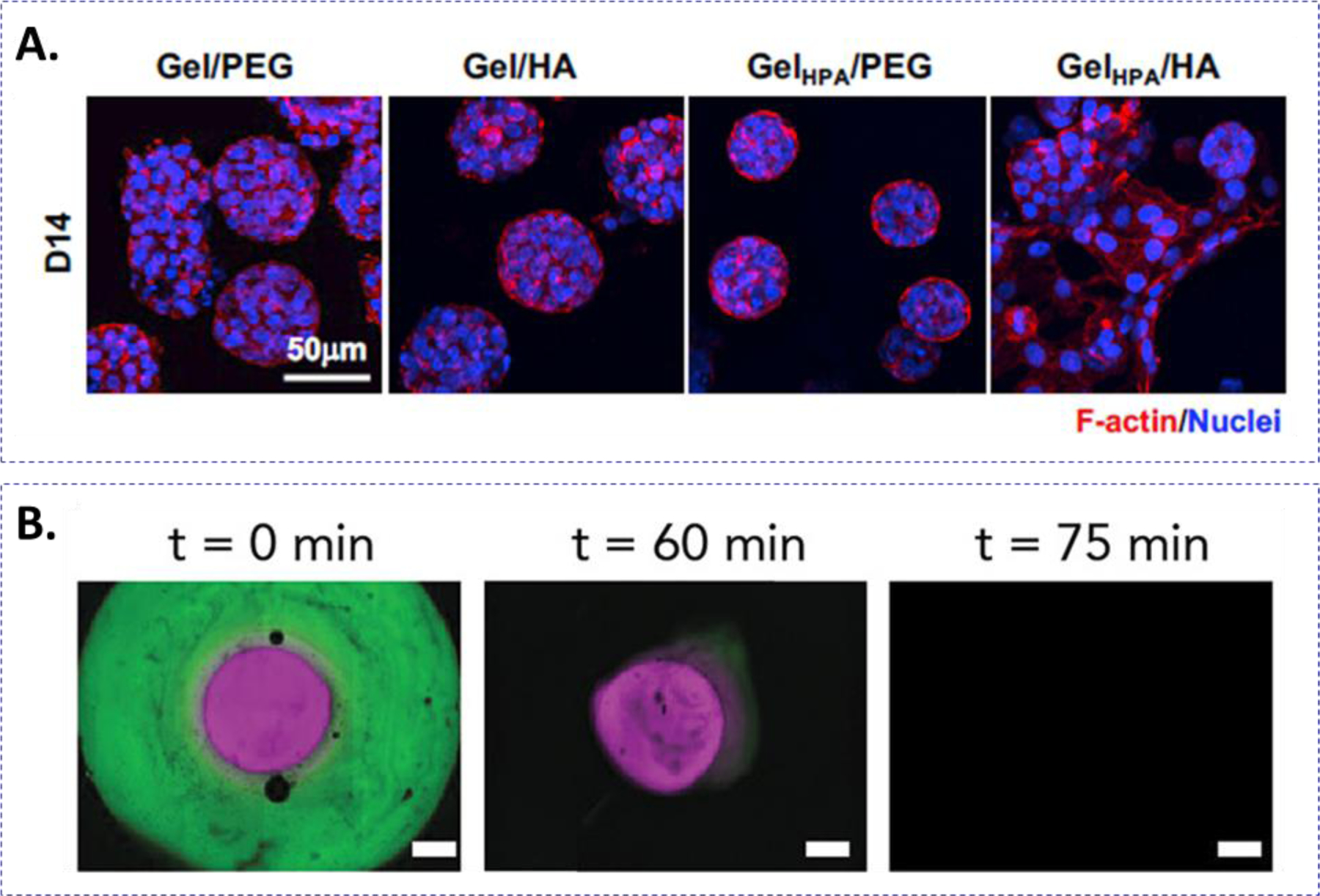
(A) Representative confocal images of pancreatic cancer spheroids in gelatin-based hydrogels with or without HA and with or without stiffening. Stiffening and HA induced EMT of the pancreatic cancer cells. Reprinted with permission.116 Copyright 2018, Elsevier. (B) Modification of the o-nitrobenzyl linker yields hydrogels with different rates of photo- and hydrolytic degradation. BSA-AF488 labeled nitrobenzyl-ester and BSA-AF647 labeled nitrobenzyl-carbamate concentric hydrogels were formed and incubated in basic (pH = 10) buffer to accelerate ester hydrolysis. UV-light treatment at 60 min released the protein from the nitrobenzyl-carbamate layer. Reprinted with permission.124 Copyright 2020, American Chemical Society.
3.2. Dynamic softening of click crosslinked hydrogels
Irreversible softening of hydrogels relies on hydrolysis, enzymatic cleavage, or photo-lysis of the crosslinkers. Hydrolysis and enzymatic linker cleavages have been extensively studied. On the other hand, incorporation of o-nitrobenzyl functional groups into a hydrogel network has been increasingly used for controlled hydrogel softening.122 For example, Forsythe and coworkers synthesized o-nitrobenzyl ester-containing PEG-methacrylate for crosslinking with gelatin-methacrylate into photodegradable hydrogels.123 The o-nitrobenzyl groups were cleaved upon exposure to 365 nm light, causing a reduction of crosslinking density and softening of the hydrogels. In an effort to tune the photodegradation rate of hydrogels, nitrobenzyl-ester, -amide, -carbonate, and -carbamate were synthesized and incorporated into hydrogels.124 It was determined that carbamate linkers had the fastest photodegradation rate, which was useful in temporal control of protein release (Fig. 5B). Anseth and colleagues used addition-fragmentation chain transfer to degrade PEG-based hydrogels crosslinked by allyl sulfide-containing linker.125 Photodegradation was controlled by LAP and mono-functional PEG-SH induced thiol- allyl sulfide exchange.
Similar to stiffening strategies, softening of hydrogels can be attained using visible light irradiation. In particular, ruthenium polypyridyl complexes were used as a photolabile linker in CuAAC-crosslinked PEG hydrogels.126 The linker was cleaved upon exposure with visible light (400–500 nm), resulting in hydrogel degradation. Perylene, which is cleaved at 520 nm light, is another example of a visible light photosensitizer used for hydrogel degradation. Forsythe et al. used perylene in conjunction with o-nitrobenzyl groups to induced wavelength-dependent photodegradation of SPAAC-crosslinked hydrogels.127
3.3. Reversible chemistry for tuning hydrogel crosslinking
Many tissue morphogenesis events involve both matrix stiffening and softening at different timescales and magnitudes. A few reversible conjugation chemistries are exploited for recapitulating these dynamic events, including light-mediated azobenzene cis-trans isomerization and enzymatic reactions.
Photoswitchable chemistry has been used for reversible stiffening of cell-laden hydrogels. For example, Anseth and colleagues formulated Michael-type addition hydrogels with PEG-VS and thiol-containing peptide-azobenzene crosslinkers.128 By exposing the hydrogel to 365 nm light, the azobenzene-containing linkers underwent isomerization into the cis configuration, resulting in hydrogel softening. On the other hand, when exposed to heat or visible light, azobenzene was converted to the trans-configuration, thus stiffening the hydrogel. Other uses of azobenzene isomerization to reversibly stiffen and soften hydrogels showed the effect of stiffness on hMSC spreading on poly(acrylamide) hydrogels (Fig. 6A).129
Figure 6.
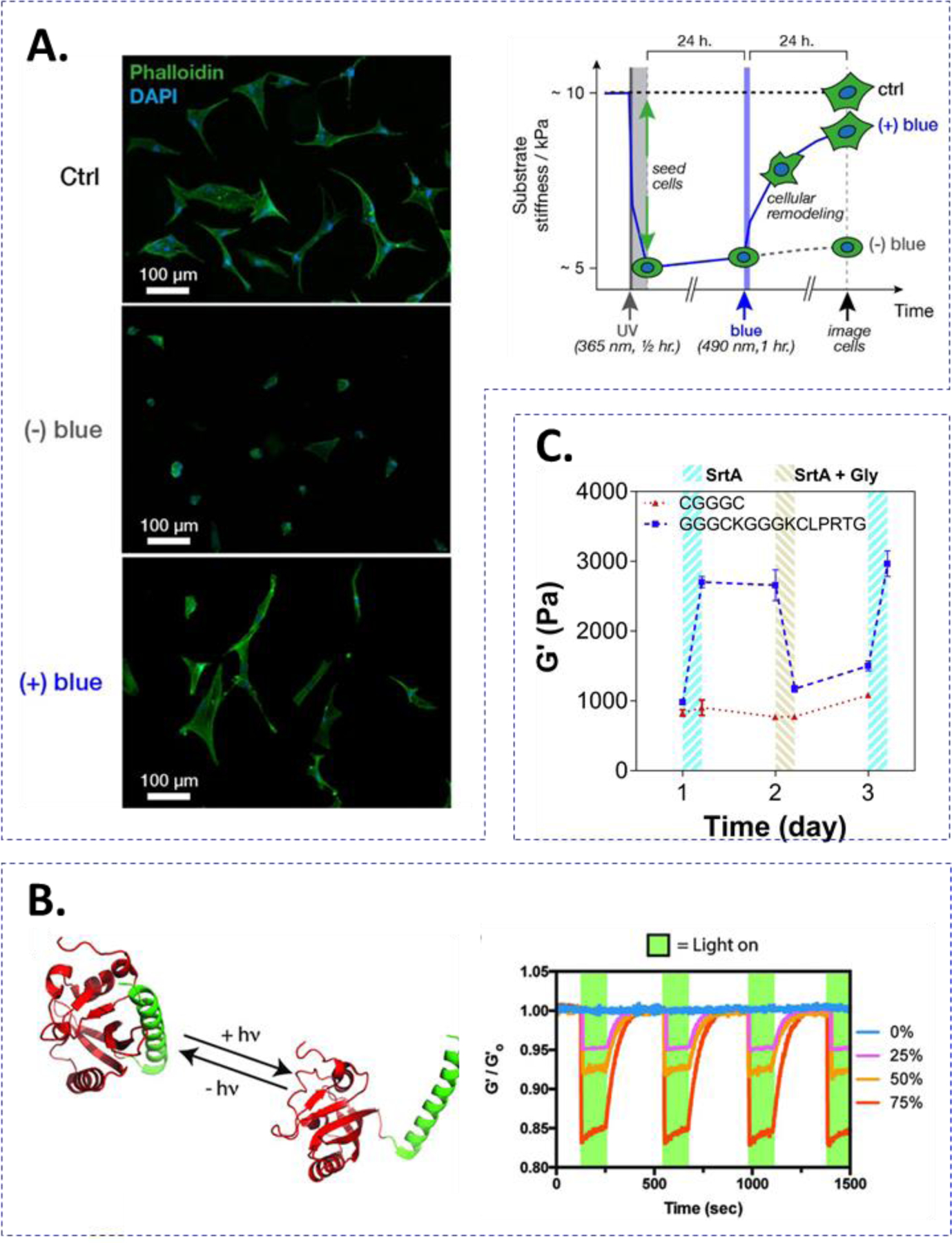
Strategies for reversibly stiffening and softening hydrogels. (A) azobenzene cis-trans isomerization-mediated stiffening and softening. Upon UV light exposure (365 nm), azobenzene is shifted to cis configuration softening the network. Treating with blue light (490 nm) leads to trans configuration of the azobenzene moiety thus stiffening the network. hMSCs cultured on top of the softened and stiffened gel exhibited more spreading than the cells on the softened gel alone. Reprinted with permission.129 Copyright 2018, American Chemical Society. (B) LOV2-Jα-mediated stiffening and softening of hydrogels. LOV2-Jα undergoes a conformational change after blue light (470 nm) treatment. LOV2-Jα interactions are recovered in the dark. Reversible photo-mediated conformational changes were cycled with minimal material fatigue. Reprinted with permission.131 Copyright 2018, Wiley. (C) SrtA-mediated reversible stiffening of hydrogels. Treating with SrtA leads to crosslinking of flanking SrtA peptide substrates. Follow-up treatment with additional SrtA and soluble glycine cleaves the peptide crosslinker reducing the crosslinking density. Reprinted with permission.134 Copyright 2019, Elsevier.
Switchable proteins (i.e., proteins that can be reversibly converted between monomeric and oligomeric states) are also exploited to reversibly tune hydrogel stiffness. A photoswitchable protein known as Dronpa145N, which can be converted to a tetrameric state under 400 nm light and back to a monomeric state under 500 nm light was tethered to 4-arm PEG molecules.130 By alternating the light exposure, the hydrogel crosslinking density could be significantly modulated to approximately 5-fold the initial modulus. Another photoswitchable pair used for modulating the hydrogel crosslinking density includes LOV2 and Jα helices.131 The molecules were expressed in bacteria as azide-LOV2-Jα-azide and crosslinked into a hydrogel network using SPAAC (Fig. 6B). Treating the hydrogels with UV-light created a conformational change between the LOV2-Jα pair, lengthening the molecule on the order of tens of Angstroms. The lengthening of the engineered proteins softened the bulk hydrogel mechanics. The LOV2-Jα domain was returned to its initial length upon removal of light exposure, leading to recovery of the initial hydrogel stiffness.
Supramolecular interactions, particularly guest-host interactions, lend themselves to dynamic modulation of hydrogel properties. Our lab and others have used supramolecular interactions to reversibly stiffen and soften hydrogels. For example, thiol-norbornene hydrogels immobilized with β-cyclodextrin (β-CD) could be stiffened by diffusing multi-functional PEG-adamantane.132 The additional supramolecular crosslinks were stripped by subsequent diffusion of soluble β-CD, which competed with immobilized β-CD for the adamantane crosslinkers. Azobenzene also interacts with CD functionalities at differing binding affinities based on the state of azobenzene isomerization. Cheng and colleagues exploited this phenomenon for stiffening and softening hydrogels consisting of α-CD, azobenzene, and N-isopropylacrylamide (NIPAAm). Inducing trans-azobenzene to cis-azobenzene by UV-light treatment softened the gel whereas infrared radiation (IR) thermally induced stiffening of the hydrogel by converting NIPAAm to a hydrophobic state.133
Certain enzymes exhibit reversible conjugation/cleavage of the peptide substrates. For example, the transpeptidase SrtA reversibly ligates LPXTG and Gn sequences. Our lab has reported PEG-based hydrogels with unique peptide designs that rendered the hydrogels susceptible to reversible SrtA-mediated stiffening and softening (Fig. 6C).134 Specifically, the hydrogels were crosslinked by multi-arm PEG-NB and bis-cysteine-containing peptide linkers franked with the two SrtA substrates (i.e., GGG-C-GGG-C-LPRTG). Following thiol-norbornene gelation, the two SrtA substrates became pendant peptides that were accessible for SrtA-induced ligation, which increased the crosslinking density of the hydrogel. Subsequent incubation with SrtA and soluble glycine peptides or glycinamide resulted in cleavage of the additional LPRTGGG crosslinks, thus softening the hydrogels.
3.4. Methods and examples to tune stress-relaxation
It has been widely accepted that cells actively respond to viscoelasticity; however, the mechanisms and biological pathways responsible for these behaviors are still not well understood. Engineered hydrogels with tunable relaxation timescales would greatly facilitate the study of mechano-responsive molecular pathways. Typically, hydrogel stress relaxation can be controlled by using a network containing dynamic reversible bonds In one example, PEG hydrogel networks were formed from mixtures of two phenylboronic acid derivatives with different pKa, 4-carboxyphenylboronic acid, and o-aminomethylphenylboronic acid.135 Since the stability of the networks depended heavily on the pKa of the BA groups, the relaxation time and the mechanical response to dynamic shear stress were exquisitely controlled by the concentrations of the two phenylboronic acid derivatives.135 Similarly, a hydrazone hydrogel was formed with two hydrazone-derivatives of different binding constants: alkyl-hydrazone (aHz) and benzyl-hydrazone (bHz).136 The average stress relaxation times, ranging from hours (4.01×103 s) to months (2.78×106 s), were modulated by varying the percentage of aHz and bHz within the same hydrogel network. Chrondrocytes encapsulated in hydrogels exhibiting faster stress relaxation deposited more collagen and glycosaminoglycans as compared with gels with slower relaxation time.136
Photochemistry can also be exploited to control hydrogel viscoelasticity. Tamate et al. described a cytocompatible and dynamic hydrogel comprised of ABA triblock copolymers, where A blocks contained NIPAAm and coumarin acrylate and B block contained PEG (Fig. 7A).137 The hydrogel was initially formed via physical crosslinking through thermo-responsive NIPAAm. Under UV light irradiation, the coumarin moieties dimerized, causing a significant increase in elasticity to the stress-relaxing hydrogels.137 Likewise, Carberry et al. established a technique to turn a stress-relaxing hydrogel into an elastic gel.138 In this contribution, viscoelastic hydrogels were prepared with off-stoichiometric ratio of 8-arm PEG thiol and 8-arm PEG thioester norbornene via photoinitiated thiol-ene polymerization. The excess thiols served as a trigger to induce radical-mediated dynamic thioester exchange This was achieved by swelling the viscoelastic thioester gels in LAP and 5-norbornene-2-carboxylic acid, followed by UV-light exposure to consume the remaining thiols in the gels. Consequently, the degree of thiol-thioester exchange was reduced, so was the hydrogel viscoelasticity, in the presence of cells. The utility of this unique dynamic hydrogel was demonstrated by examining viscoelasticity-induced YAP/TAZ nuclear translocation in fibroblasts.138 The usage of photochemistry has, therefore, further expanded the toolkits for tunable viscoelastic hydrogels where user-defined spatial and temporal control can be incorporated. An alternative approach that could potentially be used for dynamically tuning viscoelasticity is reported by Accardo et al., where hydrogel mechanics were tuned by light-induced dynamic covalent diol-boronic acid crosslinking (Fig. 7B).139 Under light exposure, the BA conjugated with azobenzene can switch between E and Z isomers, resulting in changes in pK of boronic acid groups. Using this method, the dynamic covalent bonds can break and reform irreversibly on demand, which would be beneficial in isolating the cell response specifically to changes in viscoelasticity.139
Figure 7.
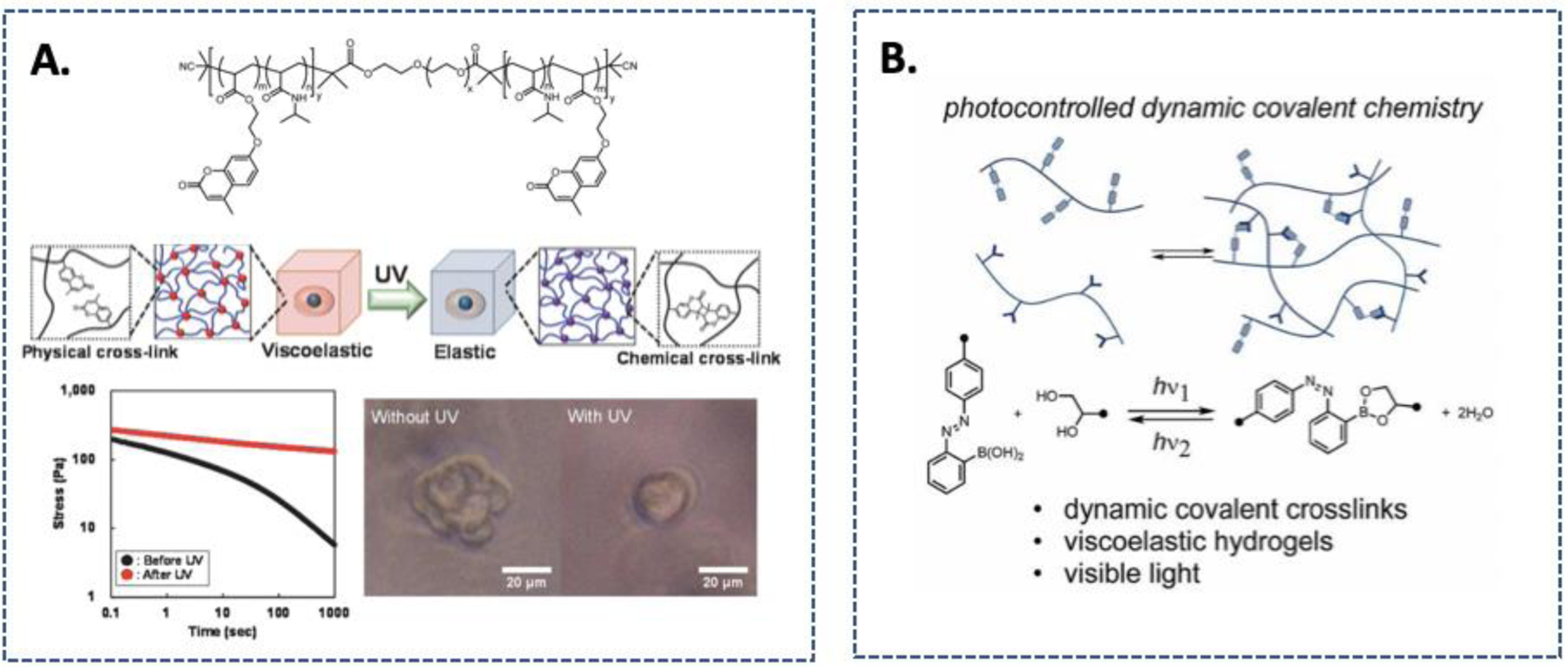
Strategies for tuning hydrogel viscoelasticity. (A) Chemical structure of the ABA triblock polymer and the modulation of viscoelasticity through photo-induced dimerization of coumarin. Reprinted with permission from 137. Copyright 2016, American Chemical Society. (B). Photo-switch control of a dynamic covalent crosslink stability using equilibrium constants for esterification of an o-azobenzene boronic acid. Reprinted with permission from 139. Copyright 2018, Royal Society of Chemistry.
Viscoelasticity can also be dynamically introduced in a hydrogel matrix. The Caliari group devised a method where viscoelasticity and stiffening of a hydrogel was introduced simultaneously via the formation of cyclodextrin-adamantane host-guest bonding and additional thiol-ene crosslinking (Fig. 8A).140 To achieve this, an elastic hydrogel was first generated with dual-functional HA-βCD-norbornene via photo-mediated thiol-ene chemistry. To trigger stiffening and increase viscoelasticity, hydrogels were incubated with LAP and adamantane conjugated-thiol peptide (Fig. 8B). Following UV light exposure, adamantane-thiol peptide reacted with left-over norbornene within the hydrogels. Since stiffening was achieved with the introduction of additional βCD-adamantane binding, both the storage and loss moduli of the hydrogel were increased simultaneously.
Figure 8.
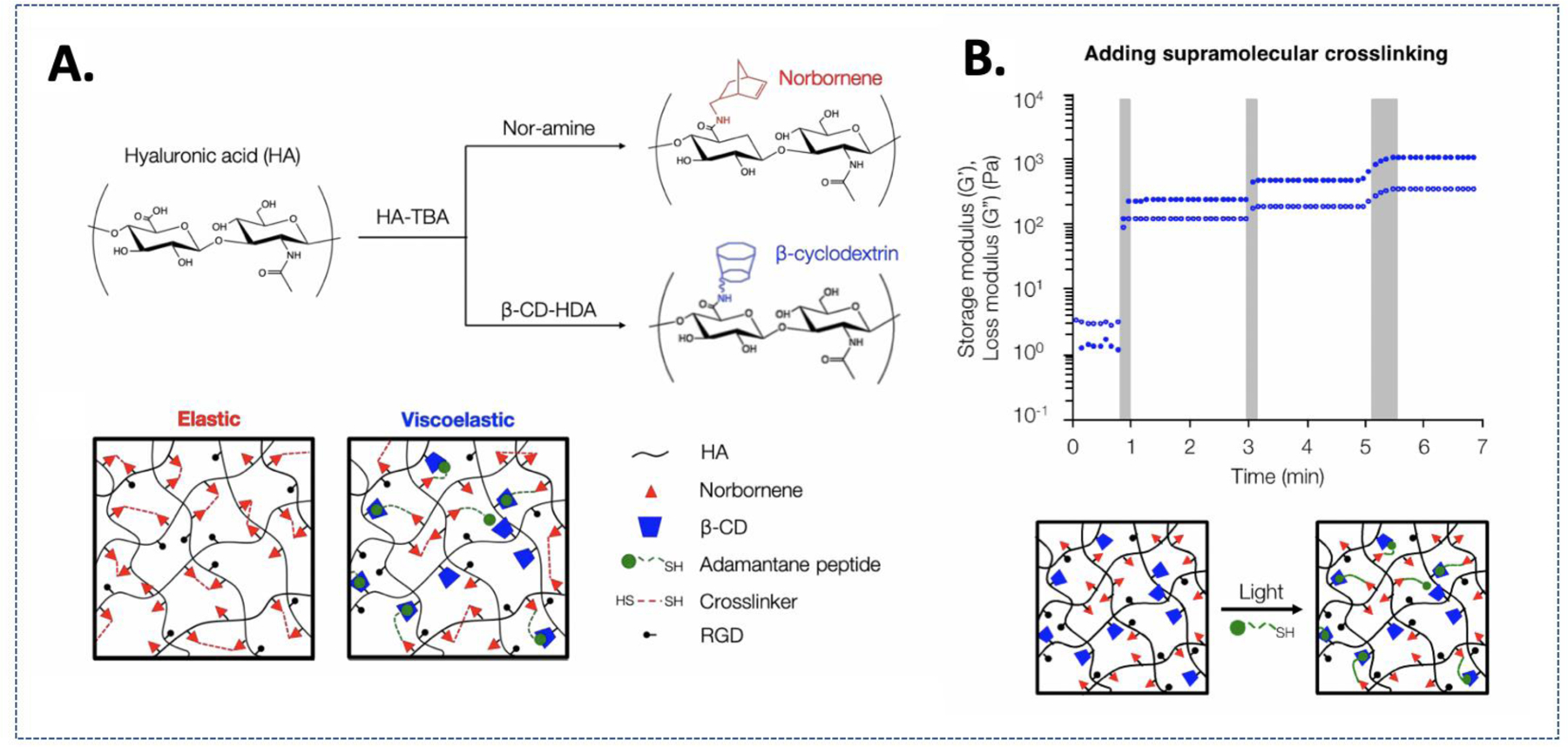
(A) Elastic hydrogel system formed by covalent thiol-norbornene crosslinks vs. Viscoelastic hydrogel system formed by thiol-ene photochemistry and supramolecular interactions between CD-HA and Ad-thiolated peptide. (B) Secondary introduction of covalent and supramolecular crosslinks to modulate stiffness and viscoelastic properties. Reprinted with permission from 140. Copyright 2019, American Chemical Society
4. Methods to control presentation of bioactive ligands
As with modulation of crosslinking density, designing materials to recapitulate dynamic biomolecule interactions would prove invaluable for modeling disease progression and for promoting tissue morphogenesis. In principle, tuning ligand presentation is similar to controlling the crosslinking density of hydrogels. Instead of introducing additional crosslinking, a chemically reactive moiety can be attached to the biomolecule of interest for its tethering into the hydrogel network. On the other hand, a chemically labile linker can be used to permit cleavage of immobilized biomolecules. Reversible chemistry can also be employed to achieve cyclic addition and removal of biomolecules.
4.1. Photopatterning and cleavage
Photopatterning and/or cleavage of proteins and small molecules have been increasing used for dynamic cell culture applications. Compared with other approaches, photopolymerization affords spatial control of ligand immobilization by using diffusion of initiators and a photomask. While patterning cysteine-bearing peptides is well established,141,142 whole-protein conjugation is increasingly investigated. For example, Dai and colleagues demonstrated spatial-temporal patterning of whole protein by conjugating thiolated vascular endothelial growth factor (VEGF) in thiol-norbornene hydrogels with excess norbornene groups.143 Reversible photochemistries can also be employed to provide further control of ligand presentation. For example, Tirell and DeForest used a photodeprotection oxime-ligation regime along with o-nitrobenzyl ester functional groups to tether and remove bovine serum albumin (BSA) into a SPAAC-crosslinked hydrogel network.144 Photoaddition of the protein occurred by incorporating caged-alkoxyamines that were removed by light exposure, which permitted reaction with the aldehyde-functionalized proteins. As the proteins are also functionalized with o-nitrobenzyl moieties, they were readily cleaved with additional light exposure. In another example, Anseth and colleagues showed that thiol-ene chemistry can be used to conjugate thiyl-radical-conjugated protein (i.e., transferrin) to allyl sulfide containing hydrogels.145 In particular, thiolated proteins were tethered via UV-light initiated thiol-ene reaction with the allyl sulfide moiety. Release of protein was achieved by a second thiol-ene reaction with monofunctionalized PEG-SH. Incorporating bromohydroxycoumarin, a photocage molecule, into a hydrogel network also allows controlled protein release.146 Upon treatment with UV-light and near-IR, the photocage is cleaved releasing the payload. Deforest and colleagues used photochemistry to tether numerous proteins, enzymes, and growth factors site-specifically to hydrogels (Fig. 9A). As opposed to random modification, the proteins were engineered with site-specific reactive handle. Under visible light exposure, the photocleavable protein (PhoCl) underwent irreversible β-elimination and peptide backbone cleavage.147
Figure 9.
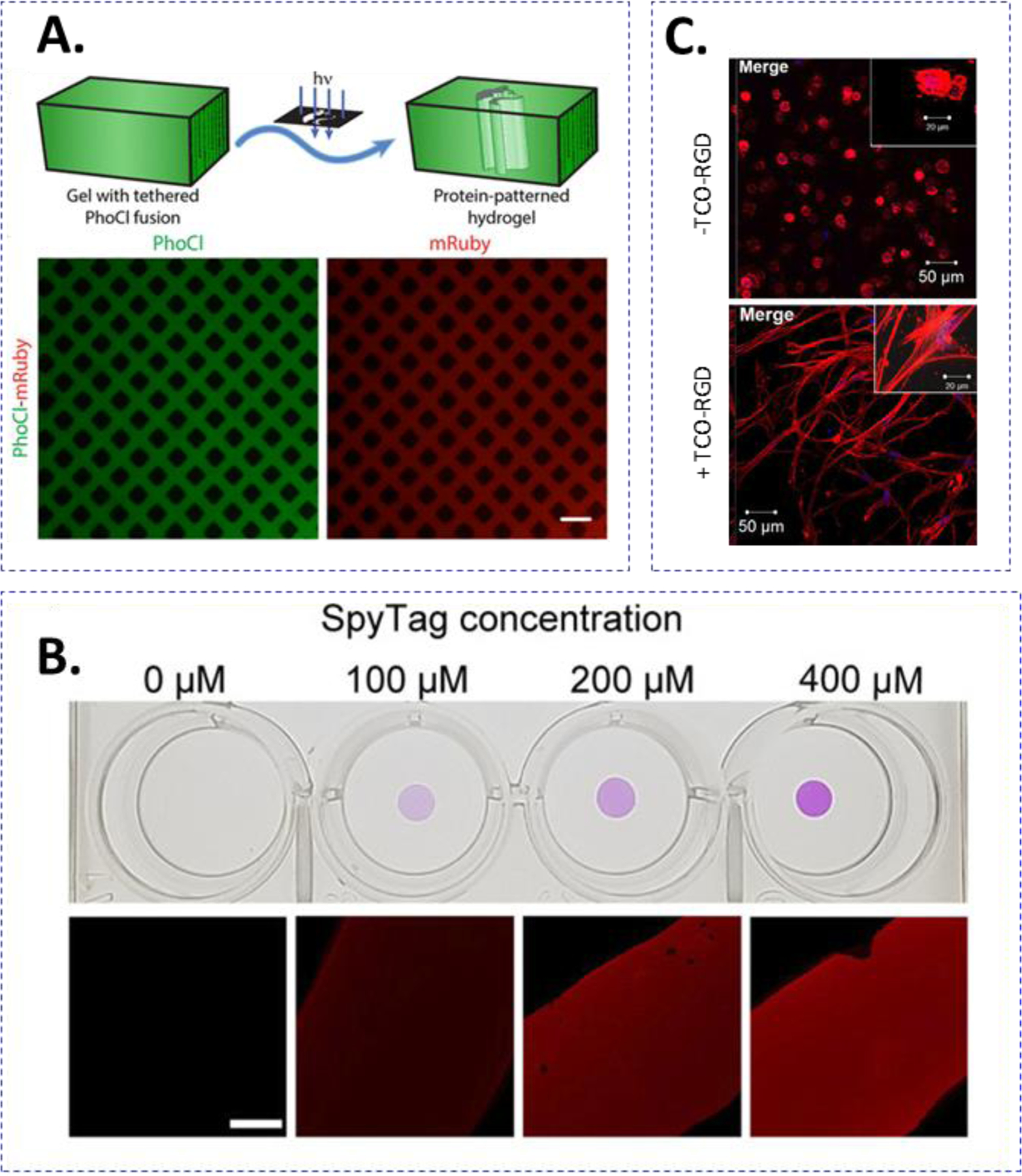
(A) Strategies for adding or removing bioactive ligands from crosslinked hydrogels. (A) PhoCl-mediated, patterned photoscission of ligands from hydrogel. Treatment with 400 nm light cleaves the PhoCl molecule releasing the molecule of interest. Reprinted with permission.147 Copyright 2019, American Chemical Society. (B) SpyTag/SpyCatcher-mediated tethering of biomolecules. Reprinted with permission.150 Copyright 2019, American Chemical Society. (C) Introduction of hyaluronic acid (HA) or RGD into a hydrogel network using Tz-TCO reactions. Reprinted with permission.121 Copyright 2018, American Chemical Society.
4.2. Enzymatic addition and removal of ligands
Enzymatic reactions can be used to immobilize and remove whole proteins that have been tagged with relatively simple substrates. Particular examples include SrtA-mediated transpeptidation, SpyTag/Spycatcher, and SnoopTag/SnoopTCatcher interactions. As alluded to earlier, enzymatic reactions require biological substrates; therefore, sequences can be translationally added to proteins of interest for site-specific labeling. Deforest and coworkers utilized sortase-mediated transpeptidation to install a variety of orthogonal click chemistry reactants.148 For example, green fluorescent protein (GFP) was tethered to a hydrogel and later removed using the above mentioned combined photodeprotection-oxime and o-nitrobenzyl regimes. The sortase-sensitive sequence conferred site-specific labeling to reduce any potential detrimental effects of non-specific protein labeling that may occur. Sortase A has also been used for patterning nerve growth factor (NGF) into hydrogels containing photocaged oligoglycine substrate.149 Conducting two-photon uncaging at precise locations within the hydrogel enabled the reaction between LPXTG moieties conjugated to Avidin/Biotin-NGF and available GGG moieties in the hydrogel. SpyTag-SpyCatcher reaction also enables the tethering of bioactive molecules in hydrogels. For example, West and colleagues generated hydrogels that could be tagged with RGDS molecules by incorporating SpyTag molecules in the network. Dynamic ligand tethering occurred when additional SpyCatcher-RGDS was diffused into the hydrogel (Fig. 9B).150 In another examole, SnoopTag/SnoopCatcher was used in conjunction with SpyTag/SpyCatcher for crosslinking and decorating HA-based hydrogels with EpCAM and E-cadherin.73 Through genetic engineering, Griffith and colleagues exploited the reversible SrtA transpeptidation for addition and removal of network-immobilized epidermal growth factor (EGF), which was tagged with SrtA substrate GGG at the protein N-terminus (i.e., NH2-GGG-EGF).69 By incubating the gel with additional glycine and sortase, GGG-EGF was cleaved and removed from the hydrogels.
Invented by the Hahn lab, LOVTRAP is a pair of light sensitive proteins that consist of LOV2 domain that binds to a small protein Zdark (Zdk) only in the dark.151 Upon irradiation with blue light wavelength (400–500 nm), Zdk readily dissociates from LOV2 and would release any protein that is conjugated to this domain. Utilizing this light-sensing protein system, the West lab developed a PEG-based hydrogel system immobilized with the LOV domain, which provides light-sensitive presentation of proteins within the PEG hydrogel.152 In particular, the PEG-LOV hydrogel network were able to reversibly bind and release mCherry-Zdk in response to blue light irradiation. When the light was removed, LOV2 underwent thermal relaxation and allowed additional Zdk-mCherry to rebind to the network. Since the LOVTRAP protein pair is highly orthogonal and can be controlled within the visible light range, it can be used in the presence of cells to modulate biochemical changes within the hydrogels.
4.3. iEDDA-mediated addition of ligands
Tetrazine-norbornene reaction is a synthetically accessible strategy for immobilization of bioactive moieties within a hydrogel. This can be achieved through simple diffusion of a tetrazine-modified protein into a hydrogel with excess norbornene functional groups. Jia and colleagues utilized Tz-TCO reaction to tether RGD dynamically into a hydrogel crosslinked with excessed Tz motifs. The infiltration of TCO-RGD molecules led to bioactive modification of the otherwise inert hydrogel (Fig. 9C).121 Additionally, Alge and colleagues showed that Tz-NB crosslinking could be used in conjunction with thiol-ene photochemistry to generate and label microgels fabricated with excess NB functionalities.8
5. Conclusion and future outlook
The needs for creating biomimetic microenvironments for cell-laden hydrogels are rapidly evolving. Up until the early 2000s, research efforts were focused on synthesizing hydrogels with pre-determined properties (e.g., crosslinking density and hydrolytic degradability).153–155 The next decade (2000 to 2010) had witness the rapid development of cell-responsive hydrogels where the materials properties could be modulated by cellular activity (e.g., protease-labile network and cell traction force induced matrix deformation).156–160 In the past 10 years, a diverse array of chemistries has been exploited for creating cell-laden hydrogels with on-demand tunable properties (e.g., spatiotemporal patterning of matrix mechanics and presentation of bioactive signals).161–164 This new class of dynamic and biomimetic hydrogels are able to recapitulate multiple aspects of cellular fate processes and these materials have been widely used for disease treatment and modeling, tissue regeneration, and even diagnostic applications.
The ability to tune the physicochemical properties of cell-laden hydrogels at will stems from the elegant integration of multiple orthogonal chemistries. A recent report from the Anseth group exemplifies this strategy, where a photoinitiated thiol-yne reaction, an azide-alkyne cycloaddition, and a methyltetrazine-TCO iEDDA reaction were integrated in a hydrogel network to independently control the presentation of several bioactive proteins to valvular interstitial cells (VICs).165 Going forward, careful considerations must be taken when and removal of residual, potentially immunogenic initiators and unreacted monomers prior to using the hydrogel as an injectable or implant. Dynamic tuning viscoelasticity strategies can be applied to build tissue models that experience loss of elasticity, or elastin-related disease (for example, valvular aortic stenosis that caused by elastin mutations leading to reduced levels of functional elastin). Double network (DN) crosslinking and interpenetrating hydrogels using both reversible and irreversible chemistry to help increased stability, mechanical properties, and allow for integrating different biopolymers to better mimic the hierarchical structures and heterogeneity of ECM. For example, one potential is the use the first network of the DN hydrogels to afford tunable elastic moduli while using the second network to afford controllability in matrix stress-relaxation. Another integrated strategy is to engineer therapeutically relevant growth factors or cytokines with clickable handles for their spatiotemporal presentation in the cell-laden hydrogels.166 This will increase the biomimicry of the hydrogels as whole proteins contain information that may be lost in short biomimetic peptides. Other dynamic properties such as self-healing, shear-thinning, or shear-thickening can also be integrated within the orthogonally crosslinked hydrogels to support multi-tissue co-culture, bioprinting, and tissue regeneration. Reversible host-guest interaction or metal ion chelation may be integrated with any of the bio-orthogonal click chemistries reviewed above to create dynamic matrices with sustained release of therapeutically relevant or immuno-regulatory molecules.167–169 Host-guest interactions may also be designed within a click hydrogel to generate hydrogels with tunable mechanics and bioactive ligands (e.g., RGD peptide).170,171
To broaden the applications of dynamic and biomimetic cell-laden hydrogels in biomedicine, there is a need to simplify the chemical synthesis process using non-toxic reagents. In order to fully capitalize on the increasing synthetic ability to modular hydrogel properties, there is also a need to utilize non-destructive imaging modality that permits longitudinal monitoring of cell fate processes. Methods to increase image resolution like machine learning, deep neural network can be utilized to analyze and extract more information from images. While traditional molecular biology techniques were adequate for hypothesis-driven studies, the benefit of dynamic hydrogel platform can only be realized with high content analysis of cell fate. This can be accomplished via integrating the dynamic hydrogels with high throughput screening, 3D printing, and other automation tools. Finally, in the age of a global pandemic, there exist an opportunity for using click hydrogels for targeted DNA or RNA delivery, as biosensors for pathogen detection and treatment (e.g., CRISPR controlled release and diagnostic devices).
Acknowledgement
This work was supported in part by the National Institutes of Health (R01CA227737) and the National Science Foundation (CAREER award #1452390 to CL, Graduate Research Fellowship to MRA).
References:
- 1.Malkoch M et al. Synthesis of well-defined hydrogel networks using click chemistry. Chem Commun (Camb), 2774–2776, doi: 10.1039/b603438a (2006). [DOI] [PubMed]
- 2.Crescenzi V, Cornelio L, Di Meo C, Nardecchia S & Lamanna R Novel hydrogels via click chemistry: synthesis and potential biomedical applications. Biomacromolecules 8, 1844–1850, doi: 10.1021/bm0700800 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Polizzotti BD, Fairbanks BD & Anseth KS Three-dimensional biochemical patterning of click-based composite hydrogels via thiolene photopolymerization. Biomacromolecules 9, 1084–1087, doi: 10.1021/bm7012636 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Kloxin CJ & Bowman CN Covalent adaptable networks: smart, reconfigurable and responsive network systems. Chem Soc Rev 42, 7161–7173, doi: 10.1039/c3cs60046g (2013). [DOI] [PubMed] [Google Scholar]
- 5.Ooi H, Hafeez S, Van Blitterswijk C, Moroni L & Baker M Hydrogels that listen to cells: a review of cell-responsive strategies in biomaterial design for tissue regeneration. Materials Horizons 4, 1020–1040 (2017). [Google Scholar]
- 6.Brown TE & Anseth KS Spatiotemporal hydrogel biomaterials for regenerative medicine. Chemical Society Reviews 46, 6532–6552 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruskowitz ER & DeForest CA Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nature Reviews Materials 3, 1–17 (2018). [Google Scholar]
- 8.Jivan F et al. Sequential thiol–ene and tetrazine click reactions for the polymerization and functionalization of hydrogel microparticles. Biomacromolecules 17, 3516–3523 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Shih H, Greene T, Korc M & Lin C-C Modular and adaptable tumor niche prepared from visible light initiated thiol-norbornene photopolymerization. Biomacromolecules 17, 3872–3882 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Tae H & Ki CS Fabrication of schizophyllan hydrogel via orthogonal thiol-ene photopolymerization. Carbohydrate polymers 167, 270–279 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Shih H, Liu H-Y & Lin C-C Improving gelation efficiency and cytocompatibility of visible light polymerized thiol-norbornene hydrogels via addition of soluble tyrosine. Biomaterials science 5, 589–599 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Batchelor R, Kwandou G, Spicer P & Stenzel M (−)-Riboflavin (vitamin B2) and flavin mononucleotide as visible light photo initiators in the thiol–ene polymerisation of PEG-based hydrogels. Polymer Chemistry 8, 980–984 (2017). [Google Scholar]
- 13.Darling NJ et al. Click by Click Microporous Annealed Particle (MAP) Scaffolds. Adv Healthc Mater, e1901391, doi: 10.1002/adhm.201901391 (2020). [DOI] [PMC free article] [PubMed]
- 14.Ooi HW et al. Thiol–ene alginate hydrogels as versatile bioinks for bioprinting. Biomacromolecules 19, 3390–3400 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira RF, Barrias CC, Bártolo PJ & Granja PL Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta biomaterialia 66, 282–293 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y et al. Cytocompatible and non-fouling zwitterionic hyaluronic acid-based hydrogels using thiol-ene “click” chemistry for cell encapsulation. Carbohydrate Polymers 236, 116021 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Sawicki LA & Kloxin AM Light-mediated formation and patterning of hydrogels for cell culture applications. JoVE (Journal of Visualized Experiments), e54462 (2016). [DOI] [PMC free article] [PubMed]
- 18.Hilderbrand AM, Ford EM, Guo C, Sloppy JD & Kloxin AM Hierarchically structured hydrogels utilizing multifunctional assembling peptides for 3D cell culture. Biomaterials Science 8, 1256–1269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweller RM, Wu ZJ, Klitzman B & West JL Stiffness of protease sensitive and cell adhesive PEG hydrogels promotes neovascularization in vivo. Annals of biomedical engineering 45, 1387–1398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmakah AM et al. A fast-degrading thiol–acrylate based hydrogel for cranial regeneration. (2017). [DOI] [PubMed]
- 21.Zhou Y et al. Photopolymerized maleilated chitosan/thiol-terminated poly (vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydrate polymers 184, 383–389 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Li L et al. Gelatin-based photocurable hydrogels for corneal wound repair. ACS applied materials & interfaces 10, 13283–13292 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Lin TY, Bragg JC & Lin CC Designing visible light-cured thiol-acrylate hydrogels for studying the HIPPO pathway activation in hepatocellular carcinoma cells. Macromolecular bioscience 16, 496–507 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz-Acuna R et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol 19, 1326–1335, doi: 10.1038/ncb3632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz-Acuña R et al. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nature protocols 13, 2102–2119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver JD et al. Design of a vascularized synthetic poly (ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 172, 54–65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darling NJ, Hung Y-S, Sharma S & Segura T Controlling the kinetics of thiol-maleimide Michael-type addition gelation kinetics for the generation of homogenous poly (ethylene glycol) hydrogels. Biomaterials 101, 199–206 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Paez JI, Farrukh A, Valbuena-Mendoza R, Włodarczyk-Biegun MK & del Campo A. n. Thiol-Methylsulfone-Based Hydrogels for 3D Cell Encapsulation. ACS Applied Materials & Interfaces 12, 8062–8072 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Desai RM, Koshy ST, Hilderbrand SA, Mooney DJ & Joshi NS Versatile click alginate hydrogels crosslinked via tetrazine–norbornene chemistry. Biomaterials 50, 30–37 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Lueckgen A et al. Hydrolytically-degradable click-crosslinked alginate hydrogels. Biomaterials 181, 189–198 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Rodell CB, Dusaj NN, Highley CB & Burdick JA Injectable and Cytocompatible Tough Double-Network Hydrogels through Tandem Supramolecular and Covalent Crosslinking. Adv Mater 28, 8419–8424, doi: 10.1002/adma.201602268 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carthew J, Frith J, Forsythe J & Truong V Polyethylene glycol–gelatin hydrogels with tuneable stiffness prepared by horseradish peroxidase-activated tetrazine–norbornene ligation. Journal of Materials Chemistry B 6, 1394–1401 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Truong VX, Tsang KM, Ercole F & Forsythe JS Red Light Activation of Tetrazine–Norbornene Conjugation for Bioorthogonal Polymer Cross-Linking across Tissue. Chemistry of Materials 29, 3678–3685 (2017). [Google Scholar]
- 34.Delplace V et al. Nonswelling, Ultralow Content Inverse Electron-Demand Diels–Alder Hyaluronan Hydrogels with Tunable Gelation Time: Synthesis and In Vitro Evaluation. Advanced Functional Materials 30, 1903978 (2020). [Google Scholar]
- 35.Delplace V et al. Inverse Electron-Demand Diels–Alder Methylcellulose Hydrogels Enable the Co-delivery of Chondroitinase ABC and Neural Progenitor Cells. Biomacromolecules (2020). [DOI] [PubMed]
- 36.Liu S et al. Cellular interactions with hydrogel microfibers synthesized via interfacial tetrazine ligation. Biomaterials 180, 24–35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y et al. Fast-forming BMSC-encapsulating hydrogels through bioorthogonal reaction for osteogenic differentiation. Biomaterials science 6, 2578–2581 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Lutz J-F & Zarafshani Z Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide–alkyne “click” chemistry. Advanced drug delivery reviews 60, 958–970 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Agard NJ, Prescher JA & Bertozzi CR A strain-promoted [3+ 2] azide− alkyne cycloaddition for covalent modification of biomolecules in living systems. Journal of the American Chemical Society 126, 15046–15047 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Filion TM, Prifti F & Song J Cytocompatible Poly (ethylene glycol)-co-polycarbonate Hydrogels Cross-Linked by Copper-Free, Strain-Promoted Click Chemistry. Chemistry–An Asian Journal 6, 2730–2737 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Zheng J, Wang H, Becker ML & Leipzig ND Neural stem cell encapsulation and differentiation in strain promoted crosslinked polyethylene glycol-based hydrogels. Journal of biomaterials applications 32, 1222–1230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truong VX et al. Photodegradable gelatin-based hydrogels prepared by bioorthogonal click chemistry for cell encapsulation and release. Biomacromolecules 16, 2246–2253 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Madl CM, Katz LM & Heilshorn SC Bio-Orthogonally Crosslinked, Engineered Protein Hydrogels with Tunable Mechanics and Biochemistry for Cell Encapsulation. Advanced functional materials 26, 3612–3620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu S, Dong H, Deng X, Zhuo R & Zhong Z Injectable hyaluronic acid/poly (ethylene glycol) hydrogels crosslinked via strain-promoted azide-alkyne cycloaddition click reaction. Carbohydrate polymers 169, 332–340 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Hodgson SM, Bakaic E, Stewart SA, Hoare T & Adronov A Properties of Poly (ethylene glycol) Hydrogels Cross-Linked via Strain-Promoted Alkyne–Azide Cycloaddition (SPAAC). Biomacromolecules 17, 1093–1100 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Hodgson SM et al. Reproducible dendronized PEG hydrogels via SPAAC cross-linking. Biomacromolecules 18, 4054–4059 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Caldwell AS, Rao VV, Golden AC & Anseth KS Porous bio-click microgel scaffolds control hMSC interactions and promote their secretory properties. Biomaterials 232, 119725 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao F et al. An injectable particle-hydrogel hybrid system for glucose-regulatory insulin delivery. Acta Biomaterialia 64, 334–345, doi: 10.1016/j.actbio.2017.09.044 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Nimmo CM, Owen SC & Shoichet MS Diels− Alder click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules 12, 824–830 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Yu F et al. Diels–Alder crosslinked HA/PEG hydrogels with high elasticity and fatigue resistance for cell encapsulation and articular cartilage tissue repair. Polymer Chemistry 5, 5116–5123 (2014). [Google Scholar]
- 51.Smith LJ et al. Diels–Alder click-cross-linked hydrogels with increased reactivity enable 3D cell encapsulation. Biomacromolecules 19, 926–935 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Madl CM & Heilshorn SC Rapid Diels–Alder Cross-linking of Cell Encapsulating Hydrogels. Chemistry of Materials 31, 8035–8043 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y & Hsu S.-h. Synthesis and biomedical applications of self-healing hydrogels. Frontiers in chemistry 6, 449 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S, Wang L, Yu X, Wang C & Wang Z Synthesis and characterization of a novel double cross-linked hydrogel based on Diels-Alder click reaction and coordination bonding. Materials Science and Engineering: C 82, 299–309 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Yu F, Cao X, Du J, Wang G & Chen X Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining Diels–Alder click reaction and acylhydrazone bond. ACS applied materials & interfaces 7, 24023–24031 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Bi B, Ma M, Lv S, Zhuo R & Jiang X In-situ forming thermosensitive hydroxypropyl chitin-based hydrogel crosslinked by Diels-Alder reaction for three dimensional cell culture. Carbohydrate polymers 212, 368–377 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi S, Uyama H & Kimura S Enzymatic Polymerization. Chemical Reviews 101, 3793–3818, doi: 10.1021/cr990121l (2001). [DOI] [PubMed] [Google Scholar]
- 58.Hasturk O, Jordan KE, Choi J & Kaplan DL Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 232, 119720, doi: 10.1016/j.biomaterials.2019.119720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim B, Lee Y, Son J, Park K & Park K Dual Enzyme-Triggered In Situ Crosslinkable Gelatin Hydrogels for Artificial Cellular Microenvironments. Macromolecular Bioscience 16, 1570–1576, doi: 10.1002/mabi.201600312 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Gantumur E, Sakai S, Nakahata M & Taya M Cytocompatible Enzymatic Hydrogelation Mediated by Glucose and Cysteine Residues. ACS Macro Letters, 485–488, doi: 10.1021/acsmacrolett.7b00122 (2017). [DOI] [PubMed]
- 61.Thi T, Lee Y, Ryu S, Nguyen D & Park K Enhanced tissue adhesiveness of injectable gelatin hydrogels through dual catalytic activity of horseradish peroxidase. Biopolymers 109, doi: 10.1002/bip.23077 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Nguyen HD, Liu H-Y, Hudson BN & Lin C-C Enzymatic Cross-Linking of Dynamic Thiol-Norbornene Click Hydrogels. ACS Biomaterials Science & Engineering 5, 1247–1256, doi: 10.1021/acsbiomaterials.8b01607 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milczek EM Commercial Applications for Enzyme-Mediated Protein Conjugation: New Developments in Enzymatic Processes to Deliver Functionalized Proteins on the Commercial Scale. Chemical Reviews 118, 119–141, doi: 10.1021/acs.chemrev.6b00832 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Broguiere N, Isenmann L & Zenobi-Wong M Novel enzymatically cross-linked hyaluronan hydrogels support the formation of 3D neuronal networks. Biomaterials 99, 47–55, doi: 10.1016/j.biomaterials.2016.04.036 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Chen Q et al. An Interpenetrating Alginate/Gelatin Network for Three-Dimensional (3D) Cell Cultures and Organ Bioprinting. Molecules 25, 756, doi: 10.3390/molecules25030756 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou S et al. Injectable Macroporous Hydrogel Formed by Enzymatic Cross-Linking of Gelatin Microgels. ACS Applied Bio Materials 1, 1430–1439, doi: 10.1021/acsabm.8b00380 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nele V et al. Ultrasound-Triggered Enzymatic Gelation. Advanced Materials 32, 1905914, doi: 10.1002/adma.201905914 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spicer CD, Pashuck ET & Stevens MM Achieving Controlled Biomolecule-Biomaterial Conjugation. Chem Rev 118, 7702–7743, doi: 10.1021/acs.chemrev.8b00253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cambria E et al. Covalent Modification of Synthetic Hydrogels with Bioactive Proteins via Sortase-Mediated Ligation. Biomacromolecules 16, 2316–2326, doi: 10.1021/acs.biomac.5b00549 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valdez J et al. On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks. Biomaterials 130, 90–103, doi: 10.1016/j.biomaterials.2017.03.030 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arkenberg MR & Lin C-C Orthogonal enzymatic reactions for rapid crosslinking and dynamic tuning of PEG-peptide hydrogels. Biomaterials science 5, 2231–2240, doi: 10.1039/c7bm00691h (2017). [DOI] [PubMed] [Google Scholar]
- 72.Zakeri B et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proceedings of the National Academy of Sciences of the United States of America 109, 7, doi: 10.1073/pnas.1115485109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wieduwild R & Howarth M Assembling and decorating hyaluronan hydrogels with twin protein superglues to mimic cell-cell interactions. Biomaterials 180, 253–264 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Yang Z et al. Genetically Programming Stress-Relaxation Behavior in Entirely Protein-Based Molecular Networks. ACS Macro Letters 7, 1468–1474, doi: 10.1021/acsmacrolett.8b00845 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Echalier C, Valot L, Martinez J, Mehdi A & Subra G Chemical cross-linking methods for cell encapsulation in hydrogels. Materials Today Communications 20, 100536, doi: 10.1016/j.mtcomm.2019.05.012 (2019). [DOI] [Google Scholar]
- 76.Taira N, Ino K, Kumagai T, Nashimoto Y & Shiku H Electrochemical fabrication of fibrin gels via cascade reaction for cell culture. Chemical Communications 55, 5335–5338, doi: 10.1039/C9CC01576K (2019). [DOI] [PubMed] [Google Scholar]
- 77.Zhao N et al. Dual Aptamer-Functionalized in Situ Injectable Fibrin Hydrogel for Promotion of Angiogenesis via Codelivery of Vascular Endothelial Growth Factor and Platelet-Derived Growth Factor-BB. ACS Applied Materials & Interfaces 11, 18123–18132, doi: 10.1021/acsami.9b02462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim S-H et al. Tissue adhesive, rapid forming, and sprayable ECM hydrogel via recombinant tyrosinase crosslinking. Biomaterials 178, 401–412, doi: 10.1016/j.biomaterials.2018.04.057 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Bauer A et al. Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts. Acta Biomaterialia 62, doi: 10.1016/j.actbio.2017.08.041 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cameron AR, Frith JE & Cooper-White JJ The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979–5993, doi: 10.1016/j.biomaterials.2011.04.003 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Charrier EE, Pogoda K, Wells RG & Janmey PA Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nature communications 9, 449, doi: 10.1038/s41467-018-02906-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaudhuri O Viscoelastic hydrogels for 3D cell culture. Biomaterials Science 5, 1480–1490, doi: 10.1039/C7BM00261K (2017). [DOI] [PubMed] [Google Scholar]
- 83.Chaudhuri O et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature Materials 15, 326–334, doi: 10.1038/nmat4489 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ooi HW, Hafeez S, van Blitterswijk CA, Moroni L & Baker MB Hydrogels that listen to cells: a review of cell-responsive strategies in biomaterial design for tissue regeneration. Materials Horizons 4, 1020–1040, doi: 10.1039/C7MH00373K (2017). [DOI] [Google Scholar]
- 85.Fredrik Schaufelberger BJJT, Olof Ramström (Eds.:Wei Zhang, Yinghua Jin). Principles of Dynamic Covalent Chemistry. 1–30 (2017).
- 86.Gennari A et al. Revisiting Boronate/Diol Complexation as a Double Stimulus-Responsive Bioconjugation. Bioconjug Chem 28, 1391–1402, doi: 10.1021/acs.bioconjchem.7b00080 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Furikado Y et al. Universal reaction mechanism of boronic acids with diols in aqueous solution: kinetics and the basic concept of a conditional formation constant. Chemistry (Weinheim an der Bergstrasse, Germany) 20, 13194–13202, doi: 10.1002/chem.201403719 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Guan Y & Zhang Y Boronic acid-containing hydrogels: synthesis and their applications. Chemical Society Reviews 42, 8106–8121, doi: 10.1039/c3cs60152h (2013). [DOI] [PubMed] [Google Scholar]
- 89.Tang S et al. Adaptable Fast Relaxing Boronate-Based Hydrogels for Probing Cell–Matrix Interactions. Advanced Science 5, 1800638, doi: 10.1002/advs.201800638 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y et al. Self-Healing Hydrogel with a Double Dynamic Network Comprising Imine and Borate Ester Linkages. Chemistry of Materials, doi: 10.1021/acs.chemmater.9b01301 (2019). [DOI]
- 91.Smithmyer ME et al. Self-Healing Boronic Acid-Based Hydrogels for 3D Co-cultures. ACS Macro Letters, 1105–1110, doi: 10.1021/acsmacrolett.8b00462 (2018). [DOI] [PMC free article] [PubMed]
- 92.Tseng TC et al. An injectable, self-healing hydrogel to repair the central nervous system. Advanced materials 27, 3518–3524 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Zhang X et al. Thermoresponsive dendronized chitosan-based hydrogels as injectable stem cell carriers. Polymer Chemistry, doi: 10.1039/C9PY00256A (2019). [DOI]
- 94.Wu X, He C, Wu Y & Biomaterials C-X Synergistic therapeutic effects of Schiff’s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials (2016). [DOI] [PubMed]
- 95.Wei Z, Lewis DM, Xu Y & Gerecht S Dual cross-linked biofunctional and self-healing networks to generate user-defined modular gradient hydrogel constructs. Advanced Healthcare Materials 6, 1700523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karimi F, Collins J, Heath DE & Connal LA Dynamic Covalent Hydrogels for Triggered Cell Capture and Release. Bioconjugate Chemistry 28, 2235–2240, doi: 10.1021/acs.bioconjchem.7b00360 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Kölmel DK & Kool ET Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chemical Reviews 117, 10358–10376, doi: 10.1021/acs.chemrev.7b00090 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalia J & Edition R-RT Hydrolytic stability of hydrazones and oximes. Angewandte Chemie International Edition (2008). [DOI] [PMC free article] [PubMed]
- 99.Baker AEG, Tam RY & Shoichet MS Independently Tuning the Biochemical and Mechanical Properties of 3D Hyaluronan-Based Hydrogels with Oxime and Diels–Alder Chemistry to Culture Breast Cancer Spheroids. Biomacromolecules 18, 4373–4384, doi: 10.1021/acs.biomac.7b01422 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Farahani PE, Adelmund SM, Shadish JA & DeForest CA Photomediated oxime ligation as a bioorthogonal tool for spatiotemporally-controlled hydrogel formation and modification. Journal of Materials Chemistry B 5, 4435–4442 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lou J, Stowers R, Nam S, Xia Y & Chaudhuri O Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 154, 213–222, doi: 10.1016/j.biomaterials.2017.11.004 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Azagarsamy MA, Marozas IA, Spaans S & Anseth KS Photoregulated Hydrazone-Based Hydrogel Formation for Biochemically Patterning 3D Cellular Microenvironments. ACS Macro Letters 5, 19–23, doi: 10.1021/acsmacrolett.5b00682 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Duan Y et al. Migration of endothelial cells and mesenchymal stem cells into hyaluronic acid hydrogels with different moduli under induction of pro-inflammatory macrophages. Journal of Materials Chemistry B 7, 5478–5489, doi: 10.1039/C9TB01126A (2019). [DOI] [PubMed] [Google Scholar]
- 104.Brown TE et al. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 178, 496–503, doi: 10.1016/j.biomaterials.2018.03.060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu L, Tian T, Wu S, Xiang T & Zhou S A pH-induced self-healable shape memory hydrogel with metal-coordination cross-links. Polymer Chemistry 10, 1920–1929, doi: 10.1039/C9PY00015A (2019). [DOI] [Google Scholar]
- 106.Azagarsamy MA, Marozas IA, Spaans S & Anseth KS Photoregulated hydrazone-based hydrogel formation for biochemically patterning 3D cellular microenvironments. ACS Macro Letters 5, 19–23 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Lee K et al. Solution viscosity regulates chondrocyte proliferation and phenotype during 3D culture. Journal of Materials Chemistry B 7, 7713–7722 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Herrera J, Henke CA & Bitterman PB Extracellular matrix as a driver of progressive fibrosis. The Journal of clinical investigation 128, 45–53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mabry KM, Lawrence RL & Anseth KS Dynamic stiffening of poly (ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials 49, 47–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caliari SR et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Scientific reports 6, 21387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ondeck MG et al. Dynamically stiffened matrix promotes malignant transformation of mammary epithelial cells via collective mechanical signaling. Proceedings of the National Academy of Sciences 116, 3502–3507 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rosales AM, Vega SL, DelRio FW, Burdick JA & Anseth KS Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angewandte Chemie International Edition 56, 12132–12136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aziz AH et al. Mechanical characterization of sequentially layered photo-clickable thiol-ene hydrogels. Journal of the mechanical behavior of biomedical materials 65, 454–465 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Xin S, Dai J, Gregory CA, Han A & Alge DL Creating Physicochemical Gradients in Modular Microporous Annealed Particle Hydrogels via a Microfluidic Method. Advanced Functional Materials 30, 1907102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Günay KA et al. PEG–Anthracene Hydrogels as an On-Demand Stiffening Matrix To Study Mechanobiology. Angewandte Chemie 131, 10017–10021 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu H-Y, Korc M & Lin C-C Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials 160, 24–36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mosiewicz KA, Kolb L, Van Der Vlies AJ & Lutolf MP Microscale patterning of hydrogel stiffness through light-triggered uncaging of thiols. Biomaterials Science 2, 1640–1651 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Liu HY & Lin CC A Diffusion-Reaction Model for Predicting Enzyme-Mediated Dynamic Hydrogel Stiffening. Gels 5, doi: 10.3390/gels5010017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu HY, Nguyen HD & Lin CC Dynamic PEG-Peptide Hydrogels via Visible Light and FMN-Induced Tyrosine Dimerization. Adv Healthc Mater 7, e1800954, doi: 10.1002/adhm.201800954 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu H-Y et al. Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells. Acta biomaterialia 48, 258–269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hao Y et al. Rapid Bioorthogonal Chemistry Enables in Situ Modulation of the Stem Cell Behavior in 3D without External Triggers. ACS applied materials & interfaces 10, 26016–26027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kloxin AM, Kasko AM, Salinas CN & Anseth KS Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63, doi: 10.1126/science.1169494 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsang KM et al. Facile One-Step Micropatterning Using Photodegradable Gelatin Hydrogels for Improved Cardiomyocyte Organization and Alignment. Advanced functional materials 25, 977–986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.LeValley PJ et al. Photolabile linkers: exploiting labile bond chemistry to control mode and rate of hydrogel degradation and protein release. Journal of the American Chemical Society 142, 4671–4679 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brown TE, Marozas IA & Anseth KS Amplified Photodegradation of Cell-Laden Hydrogels via an Addition–Fragmentation Chain Transfer Reaction. Advanced Materials 29, 1605001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rapp TL, Wang Y, Delessio MA, Gau MR & Dmochowski IJ Designing photolabile ruthenium polypyridyl crosslinkers for hydrogel formation and multiplexed, visible-light degradation. RSC advances 9, 4942–4947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Truong VX, Li F & Forsythe JS Photolabile hydrogels responsive to broad spectrum visible light for selective cell release. ACS applied materials & interfaces 9, 32441–32445 (2017). [DOI] [PubMed] [Google Scholar]
- 128.Rosales AM, Mabry KM, Nehls EM & Anseth KS Photoresponsive elastic properties of azobenzene-containing poly (ethylene-glycol)-based hydrogels. Biomacromolecules 16, 798–806 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee I-N et al. Photoresponsive hydrogels with photoswitchable mechanical properties allow time-resolved analysis of cellular responses to matrix stiffening. ACS applied materials & interfaces 10, 7765–7776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu X et al. Reversible hydrogels with tunable mechanical properties for optically controlling cell migration. Nano Research 11, 5556–5565 (2018). [Google Scholar]
- 131.Liu L et al. Cyclic stiffness modulation of cell-laden protein–polymer hydrogels in response to user-specified stimuli including light. Advanced Biosystems 2, 1800240 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shih H & Lin C-C Tuning stiffness of cell-laden hydrogel via host–guest interactions. Journal of Materials Chemistry B 4, 4969–4974 (2016). [DOI] [PubMed] [Google Scholar]
- 133.Zheng Z et al. Dynamic softening or stiffening a supramolecular hydrogel by ultraviolet or near-infrared light. ACS applied materials & interfaces 9, 24511–24517 (2017). [DOI] [PubMed] [Google Scholar]
- 134.Arkenberg MR, Moore DM & Lin C-C Dynamic control of hydrogel crosslinking via sortase-mediated reversible transpeptidation. Acta biomaterialia 83, 83–95 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yesilyurt V et al. Mixed Reversible Covalent Crosslink Kinetics Enable Precise, Hierarchical Mechanical Tuning of Hydrogel Networks. Advanced Materials 29, 1605947, doi: 10.1002/adma.201605947 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Richardson BM, Wilcox DG, Randolph MA & Anseth KS Hydrazone covalent adaptable networks modulate extracellular matrix deposition for cartilage tissue engineering. Acta Biomaterialia 83, 71–82, doi: 10.1016/j.actbio.2018.11.014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tamate R et al. Photo-Dimerization Induced Dynamic Viscoelastic Changes in ABA Triblock Copolymer-Based Hydrogels for 3D Cell Culture. Chemistry of Materials 28, 6401–6408, doi: 10.1021/acs.chemmater.6b02839 (2016). [DOI] [Google Scholar]
- 138.Carberry BJ, Rao VV & Anseth KS Phototunable Viscoelasticity in Hydrogels Through Thioester Exchange. Annals of biomedical engineering, doi: 10.1007/s10439-020-02460-w (2020). [DOI] [PMC free article] [PubMed]
- 139.Accardo JV & Kalow JA Reversibly tuning hydrogel stiffness through photocontrolled dynamic covalent crosslinks. Chemical Science 9, 5987–5993, doi: 10.1039/C8SC02093K (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hui E, Gimeno KI, Guan G & Caliari SR Spatiotemporal Control of Viscoelasticity in Phototunable Hyaluronic Acid Hydrogels. Biomacromolecules, doi: 10.1021/acs.biomac.9b00965 (2019). [DOI] [PMC free article] [PubMed]
- 141.Fairbanks BD et al. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv Mater 21, 5005–5010, doi: 10.1002/adma.200901808 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Benton JA, Fairbanks BD & Anseth KS Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials 30, 6593–6603, doi: 10.1016/j.biomaterials.2009.08.031 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dorsey TB et al. Evaluation of photochemistry reaction kinetics to pattern bioactive proteins on hydrogels for biological applications. Bioactive materials 3, 64–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.DeForest CA & Tirrell DA A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nature materials 14, 523–531 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Grim JC et al. A reversible and repeatable thiol–ene bioconjugation for dynamic patterning of signaling proteins in hydrogels. ACS central science 4, 909–916 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Gracia Lux C et al. Short soluble coumarin crosslinkers for light-controlled release of cells and proteins from hydrogels. Biomacromolecules 16, 3286–3296 (2015). [DOI] [PubMed] [Google Scholar]
- 147.Shadish JA, Strange AC & DeForest CA Genetically encoded photocleavable linkers for patterned protein release from biomaterials. Journal of the American Chemical Society 141, 15619–15625 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shadish JA, Benuska GM & DeForest CA Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat Mater 18, 1005–1014, doi: 10.1038/s41563-019-0367-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Broguiere N et al. Morphogenesis guided by 3D patterning of growth factors in biological matrices. bioRxiv, 828947 (2019). [DOI] [PubMed]
- 150.Hammer JA, Ruta A, Therien AM & West JL Cell-Compatible, Site-Specific Covalent Modification of Hydrogel Scaffolds Enables User-Defined Control over Cell–Material Interactions. Biomacromolecules 20, 2486–2493 (2019). [DOI] [PubMed] [Google Scholar]
- 151.Wang H et al. LOVTRAP: an optogenetic system for photoinduced protein dissociation. Nature methods 13, 755–758 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hammer JA, Ruta A & West JL Using Tools from Optogenetics to Create Light-Responsive Biomaterials: LOVTRAP-PEG Hydrogels for Dynamic Peptide Immobilization. Annals of Biomedical Engineering, 1–10 (2019). [DOI] [PubMed]
- 153.Cruise GM, Scharp DS & Hubbell JA Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials, 1287–1294, doi: 10.1016/S0142-9612(98)00025-8 (1998). [DOI] [PubMed]
- 154.West JL & Hubbell JA Polymeric Biomaterials with Degradation Sites for Proteases Involved in Cell Migration. Macromolecules, 241–244, doi: 10.1021/ma981296k (1999). [DOI]
- 155.Hern DL & Hubbell JA Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. Journal of biomedical materials research 39, 266–276, doi: (1998). [DOI] [PubMed] [Google Scholar]
- 156.Burdick JA, Chung C, Jia X, Randolph MA & Langer R Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 6, 386–391, doi: 10.1021/bm049508a (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang Y, Onyeri S, Siewe M, Moshfeghian A & Madihally SV In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 26, 7616–7627, doi: 10.1016/j.biomaterials.2005.05.036 (2005). [DOI] [PubMed] [Google Scholar]
- 158.Lutolf MP et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proceedings of the National Academy of Sciences, 5413–5418, doi: 10.1073/pnas.0737381100 (2003). [DOI] [PMC free article] [PubMed]
- 159.Burdick JA & Anseth KS Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 23, 4315–4323, doi: 10.1016/s0142-9612(02)00176-x (2002). [DOI] [PubMed] [Google Scholar]
- 160.Nichol JW et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544, doi: 10.1016/j.biomaterials.2010.03.064 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stowers RS, Allen SC & Suggs LJ Dynamic phototuning of 3D hydrogel stiffness. Proc Natl Acad Sci U S A 112, 1953–1958, doi: 10.1073/pnas.1421897112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gandavarapu NR, Azagarsamy MA & Anseth KS Photo-click living strategy for controlled, reversible exchange of biochemical ligands. Adv Mater 26, 2521–2526, doi: 10.1002/adma.201304847 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guvendiren M & Burdick JA Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun 3, 792, doi: 10.1038/ncomms1792 (2012). [DOI] [PubMed] [Google Scholar]
- 164.DeForest CA, Polizzotti BD & Anseth KS Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater 8, 659–664, doi: 10.1038/nmat2473 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma H, Caldwell AS, Azagarsamy MA, Rodriguez AG & Anseth KS Bioorthogonal click chemistries enable simultaneous spatial patterning of multiple proteins to probe synergistic protein effects on fibroblast function. Biomaterials, 120205 (2020). [DOI] [PMC free article] [PubMed]
- 166.Shadish JA, Benuska GM & DeForest CA Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nature materials 18, 1005–1014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Feng Q et al. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials 101, 217–228 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Feng Q et al. Dynamic and cell-infiltratable hydrogels as injectable carrier of therapeutic cells and drugs for treating challenging bone defects. ACS central science 5, 440–450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang K et al. Adaptable Hydrogels Mediate Cofactor-Assisted Activation of Biomarker-Responsive Drug Delivery via Positive Feedback for Enhanced Tissue Regeneration. Advanced Science 5, 1800875 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hong KH & Song S-C 3D hydrogel stem cell niche controlled by host-guest interaction affects stem cell fate and survival rate. Biomaterials 218, 119338 (2019). [DOI] [PubMed] [Google Scholar]
- 171.Shih H & Lin C-C Photoclick hydrogels prepared from functionalized cyclodextrin and poly (ethylene glycol) for drug delivery and in situ cell encapsulation. Biomacromolecules 16, 1915–1923 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


