Abstract
Background:
Prioritizing HIV prevention for adolescent girls and young women (AGYW) at high risk of HIV acquisition in sub-Saharan Africa (typically considered ≥3 per 100 person years [PYs]) is urgently needed, but identifying these AGYW is challenging. We sought to assess and, if needed, enhance a risk assessment tool from the VOICE trial for identifying AGYW at high risk for HIV in Lilongwe, Malawi.
Methods:
A multisite prospective cohort study was conducted among sexually active AGYW 15–24 years old at four health centers in 2016–2017. The VOICE tool was first applied and then updated by excluding variables that were not predictive and adding variables that were. Incidence rates (IR), incidence rate ratios (IRR), ninety-five percent confidence intervals (CIs), area under the receiver-operator characteristic curve (AUC), sensitivity, and specificity were calculated.
Results:
Seven-hundred ninety-five participants experienced 14 seroconversions over 672 person-years (IR: 2.08 per 100 PYs, CI: 1.23–3.52). The VOICE tool had moderate predictive ability (AUC: 0.64, CI: 0.52, 0.75). Maintaining two variables (genital ulcers and vaginal discharge), removing five socio-demographic variables, and adding two variables (ever pregnant and >5 year male-female age gap) enhanced performance (AUC=0.79, 95% CI: 0.69–0.89). Thirty-five percent had a score of 0, 41% had a score of 1–2, and 24% had a score ≥3. A score ≥1 resulted in 100% sensitivity, 35.9% specificity, and an IR of 3.25 per 100 PYs. A score ≥3 resulted in 64.3% sensitivity, 76.8% specificity, and an IR of 5.89 per 100 PYs.
Conclusions:
A simple risk assessment tool identified a subset of AGYW in Malawi at high risk of HIV acquisition who may benefit from biomedical HIV prevention.
Keywords: HIV, adolescent, prevention, incidence, risk
Short Summary
A simple, feasible clinical risk assessment tool was developed that could accurately predict Malawian adolescent girls and young women at highest risk of HIV acquisition.
Introduction
Adolescent girls and young women (AGYW) 15–24 years old in sub-Saharan Africa (SSA) experience high HIV incidence rates. These rates are nearly three times higher than their male counterparts, and many times higher than adolescents in other regions of the world.1 Even though AGYW only account for 10% of the population in southern and eastern Africa, they account for 26% of new HIV infections.2 Although general population HIV incidence has declined in SSA, gains have been slower for AGYW.3
The World Health Organization (WHO) recommends offering oral pre-exposure prophylaxis (PrEP) to reduce the risk of HIV acquisition in populations with high HIV incidence, defined as a rate of ≥3 per 100 person-years.4 Although HIV incidence among AGYW exceeds this threshold in select SSA populations, at a regional level, AGYW HIV incidence is estimated at 0.3 per 100 person years.5 In both a national survey of Malawian women of reproductive age and a large survey of pregnant Malawian AGYW, HIV incidence was 0.6 per 100 person years.6,7 In such moderate incidence settings, it is critical to identify sub-populations of AGYW at highest risk of HIV who may benefit most from PrEP, as it is not economical or feasible to offer PrEP to the 100 million AGYW in SSA.
Risk assessment tools are predictive models that can be used to identify those at elevated risk of a particular outcome.8 In SSA, they have been developed to identify patients with sexually transmitted infections (STI) at high risk for acute HIV infection,9 pregnant women at risk for HIV acquisition11, and HIV-discordant couples at risk of HIV-seroconversion.10 A risk assessment tool was developed from the VOICE trial (a large multisite oral PrEP trial conducted from 2009–2011 in South Africa, Zimbabwe, and Uganda)12 to identify women at high risk for HIV acquisition, and then validated in two other large network studies.13 However, this tool did not perform well when it was applied to a cohort of adolescent girls 13–20 years old from HIV Prevention Trials Network (HPTN) 068, a randomized conditional cash transfer study conducted in South Africa.14 Validating this tool outside of a clinical trial setting and potentially enhancing its performance is the next step.
In a cohort of AGYW in Malawi, we first identify factors that predict HIV incidence. Then, we apply the VOICE risk assessment tool to this cohort and assess its performance characteristics. Finally, we use standard updating methods to enhance the performance of this risk assessment tool.
Materials and Methods
Study Overview and Setting
Girl Power was a multi-site quasi-experimental prospective cohort study conducted at four clinics in Lilongwe, Malawi and four clinics in Western Cape, South Africa.15 Due to differences in HIV testing protocols in the two countries that resulted in infrequent testing in South Africa, this analysis focused on the Malawi setting. Girl Power-Malawi was conducted from February 2016 to August 2017 in four comparable public-sector health centers. All centers were located on a main road, had antenatal clinic volumes ≥200 women per month, and had antenatal HIV prevalence levels of approximately 10%, levels well above those in the surrounding areas.
During the period of study implementation, each of the four clinics offered a different model of service delivery. Clinic 1 offered the standard of care: HIV testing, family planning, and STI syndromic management provided in separate spaces without any youth-friendly modifications. Clinics 2–4 offered an integrated model of youth-friendly health services with many youth-focused clinical modifications. Clinics 3 and 4 also offered a monthly small-group behavioral intervention and clinic 4 also offered a monthly $5.50 cash transfer conditional on attending the monthly small group intervention session. Study design is described in more detail elsewhere.15 The primary research questions in the parent study were how these four models of care impacted service uptake and HIV risk behaviors.15–17
In this analysis, we sought to assess which combination of self-reported baseline factors were predictive of HIV incidence over the one-year study period, irrespective of study clinic. We focused on self-reported factors because 1) we did not routinely collect STI or pregnancy biomarker data and 2) this is what is available in most SSA settings, and thus generalizable to settings without laboratory capacity.
Study Population and Procedures
Two hundred fifty AGYW were recruited to each health center and followed for one year (N=1000 total). Persons were eligible if they were female, 15–24 years old, from the clinic’s catchment area, and willing to participate for one year. We actively recruited AGYW who had experienced sexual debut. Recruitment occurred through community outreach, participant referral, and self-referral. Phone and physical tracing were conducted for participants who missed six- and twelve-month research visits.
Data collection
Once enrolled, participants responded to a detailed behavioral survey. Young female research officers administered surveys on Android tablets using Open Data Kit software. The survey included questions about demographics, socio-economic status, behaviors, the presence of recent genital ulcers and abnormal vaginal discharge, past pregnancy, and sexual partnerships. It also included a question about whether the participant had ever been tested for HIV, the approximate test date, and the test result.
Health services were recorded on study-specific clinic cards collected by nurses, HIV testing staff, and peer educators. The card contained one record per clinic visit and had space to record each HIV test, its date, and test result: confirmed HIV-positive, new HIV-positive, HIV-negative, or indeterminate. HIV testing was encouraged every three months, but was not a formal study procedure.
Outcome of Interest
The primary outcome of interest was HIV acquisition. Participants who were HIV-positive at baseline were excluded. The clinic card was used to determine whether HIV acquisition had occurred. An HIV acquisition event was recorded if a participant 1) first tested HIV-negative and then had a “confirmed HIV-positive,” result, 2) had a “new HIV-positive” result after baseline, or 3) self-reported being HIV-positive at six or twelve months with an earlier HIV-negative result. Follow-up started at the time of enrollment. Time of HIV acquisition was the midpoint between the last HIV-negative test and the first HIV-positive test. For persons without an HIV acquisition event, person-time was calculated from enrollment to their last HIV-negative test. Persons without a recorded HIV test after baseline were excluded.
Variables of interest
Two sets of candidate predictors were explored separately. The first set included variables associated with HIV prevalence at baseline.18 These factors included five socio-demographic characteristics (age 20–24 years, non-completion of primary school, no running water at home, ≤2 household assets, and being a double orphan); two individual behaviors (multiple sexual partners in the last year and heavy alcohol use); three biologic/clinical factors (self-reported vaginal discharge or genital ulcers and past pregnancy), and six partner factors (partner travel, transactional sex, uncircumcised partner, perceived partner concurrency, an older partner, and partner known to be HIV-positive).18 We added one variable (being separated, divorced, or widowed).
The second set of variables were based on the VOICE risk score and included two socio-demographic characteristics (age <25 and marriage); two biologic factors (having a laboratory-confirmed bacterial STI or HSV-2); one individual behavioral variable (alcohol consumption in the last three months); and two partner factors (partner not providing financial or material support and believing a partner may have other partners). These variables were constructed to the extent possible with our dataset. Because laboratory-based STI testing was not conducted in Girl Power-Malawi, self-reported abnormal vaginal discharge in the last six months was used as a proxy for a bacterial STI and self-reported genital sores in the last six months was used as a proxy for HSV-2. Alcohol consumption in the last year was used as a substitute for alcohol consumption in the last three months, as we did not ask about alcohol consumption in the last three months. Given that alcohol is often asked about within a much shorter time period (e.g. one week), the responses at three months and one year would likely be similar.
Statistical Analysis
We first performed bivariable analyses to test the associations between the first set of candidate predictors and HIV acquisition using Fisher’s exact tests (Table 1). Variables associated with HIV acquisition (p-value≤0.15) were then explored in bivariable and multivariable Poisson regression analysis. The relaxed alpha level was used due to the small number of events, a common practice in prediction modeling in small datasets19,20. Incidence rates (IRs), incidence rate ratios (IRRs), and ninety-five percent confidence intervals (CI) were reported.
Table 1:
Population characteristics at baseline (N=795 AGYW)
| HIV-negative (n, %) (n=781) | New HIV infections (n, %) (n=14) | Fisher’s exact p-value | ||
|---|---|---|---|---|
| Socioeconomic variables | ||||
| Age | ||||
| 15–19 years | 462 (59) | 5 (36) | 0.069* | |
| 20–24 years | 319 (41) | 9 (64) | ||
| Primary school completion | ||||
| No | 213 (28) | 3 (21) | 0.435 | |
| Yes | 559 (72) | 11 (79) | ||
| Running water in the home | ||||
| No | 431 (55) | 9 (64) | 0.346 | |
| Yes | 350 (45) | 5 (36) | ||
| >2 household assets | ||||
| No | 298 (38) | 6 (43) | 0.459 | |
| Yes | 483 (62) | 8 (57) | ||
| Double orphan | ||||
| No | 717 (92) | 14 (100) | 0.306 | |
| Yes | 64 (8) | 0 (0) | ||
| Divorced, separated or widowed | ||||
| No | 732 (94) | 11 (79) | 0.055* | |
| Yes | 48 (6) | 3 (21) | ||
| Individual behavioral variable | ||||
| Heavy alcohol use | ||||
| No | 683 (88) | 13 (93) | 0.468 | |
| Yes | 97 (12) | 1 (7) | ||
| ≥2 sexual partners in the last year | ||||
| No | 625 (80) | 9 (64) | 0.133* | |
| Yes | 156 (20) | 5 (36) | ||
| Individual biological variables | ||||
| Report of vaginal discharge | ||||
| No | 641 (82) | 8 (57) | 0.028* | |
| Yes | 139 (18) | 6 (43) | ||
| Report of genital sores | ||||
| No | 691 (88) | 10 (71) | 0.072* | |
| Yes | 90 (12) | 4 (29) | ||
| Ever pregnant | ||||
| No | 460 (59) | 3 (21) | 0.005* | |
| Yes | 318 (41) | 11 (79) | ||
| Partnership variables | ||||
| Transactional sex | ||||
| No | 625 (80) | 8 (57) | 0.046* | |
| Yes | 156 (20) | 6 (43) | ||
| At least one known HIV-positive partner | ||||
| No | 778 (>99%) | 14 (100) | 0.948 | |
| Yes | 3 (<1%) | 0 | ||
| Partner ≥ 5 years older | ||||
| No | 546 (70) | 5 (36) | 0.009* | |
| Yes | 235 (30) | 9 (64) | ||
| All partners circumcised | ||||
| No or unknown | 720 (92) | 12 (86) | 0.306 | |
| Yes | 61 (8) | 2 (14) | ||
| Believes partner may have other partners | ||||
| No | 303 (39) | 3 (21) | 0.147* | |
| Yes | 478 (61) | 11 (79) | ||
| Partner slept away ≥3 nights in the last year | ||||
| No | 724 (93) | 12 (86) | 0.266 | |
| Yes | 55 (7) | 2 (14) | ||
p≤0.15
To develop the risk assessment tool, we first applied the VOICE risk assessment tool to the Girl Power-Malawi cohort using all original variables and original score values (Table 2). We calculated IRs, IRRs, and CIs for all levels of the final score and calculated performance characteristics. We planned to consider this model final if the area-under the receiver-operator characteristics curve (AUC) was ≥0.70 (comparable to the VOICE score performance) and to pursue model updating otherwise.21, 22 Model updating started with stepwise backward elimination to determine which VOICE variables to retain using a likelihood ratio test p-value ≤0.15. After conducting backward elimination, we added in additional Girl Power candidate predictors associated with HIV incidence and again applied likelihood ratio tests to assess contribution to model fit. This model-building process was repeated until we arrived at a final parsimonious model.
Table 2:
VOICE Risk Score Applied to the Girl Power Dataset
| Characteristics | New HIV infections (n=14) | PYs | IR (CI) | IRR (CI) | Score | |
|---|---|---|---|---|---|---|
| Age | ||||||
| Age ≤25 | 14 | 672 | 2.08 (1.23, 3.52) | NA | 2 | |
| Marital status | ||||||
| Married/cohabiting | 2 | 140 | 1.43 (0.36, 5.72) | 1 | 0 | |
| Not married | 12 | 530 | 2.26 (1.29, 3.99) | 1.58 (0.35, 7.07) | 2 | |
| Alcohol use | ||||||
| No alcohol use | 10 | 426 | 2.35 (1.26, 4.37) | 1.44 (0.45, 4.58) | 0 | |
| Any alcohol use | 4 | 245 | 1.63 (0.61, 4.35) | 1 | 1 | |
| Curable STI (vaginal discharge, self-report) | ||||||
| No | 8 | 548 | 1.46 (0.73, 2.92) | 1 | 0 | |
| Yes | 6 | 122 | 4.90 (2.20, 10.92) | 3.36 (1.17, 9.68) | 1 | |
| HSV-2 (genital ulcers, self-report) | ||||||
| No | 10 | 590 | 1.69 (0.91, 3.15) | 1 | 0 | |
| Yes | 4 | 82 | 4.90 (1.84, 13.06) | 2.89 (0.91, 9.22) | 2 | |
| Partner providing economic support | ||||||
| No | 0 | 37 | 0 | 1 | ||
| Yes | 14 | 634 | 2.21 (1.31, 3.73) | NA | 0 | |
| Partner has other partners | ||||||
| No | 3 | 263 | 1.14 (0.37, 3.54) | 1 | 0 | |
| Possibly or yes | 11 | 409 | 2.69 (1.49, 4.86) | 2.35 (0.66, 8.44) | 2 | |
PYs: Person Years, IR: Incidence Rate, IRR: Incidence Rate Ratio, CI: Confidence Interval.
Variables in the final multivariable model were assigned a score proportional to their β-coefficients: each β-coefficient was divided by the lowest β-coefficient and rounded to the nearest integer.23 These integers were added together to create a final value for each observation. IRs, CIs, sensitivity, and specificity for all levels of the VOICE score and final score were calculated. The AUC was calculated for the VOICE tool and the updated tool. To internally validate the tool, we used statistical bootstrapping,8, 22 randomly sampling with replacement 1000 times, recalculating the AUC in each sample, and then pooling the results.
To explore whether participants with and without HIV acquisition information differed, we calculated the risk score values for persons with missing HIV outcomes. The primary source of missing data was the absence of an HIV test result at follow-up. The distributions of scores were compared between those with and without missing data.
Ethical approval
The study was approved by the University of North Carolina Institutional Review Board and the Malawi National Health Sciences Research Committee. AGYW ≥18 years old provided informed consent. AGYW 15–17 years provided assent and had consent provided by a parent, guardian, or authorized representative.
Results
Descriptive characteristics
One thousand AGYW were enrolled in the study. Thirty-three participants tested or reported being HIV positive at baseline and were excluded from the HIV incidence analyses and 172 had insufficient outcome information and were excluded. Of the remaining 795 participants, 14 participants experienced HIV incidence (1.8%) and 781 did not (98.2%). These 795 participants contributed 672 person-years of follow-up (mean=10.2 months/participant). The overall IR was 2.08 per 100 person years (95% CI: 1.23, 3.52). HIV IRs were similar in the standard of care clinic (2.57, 95% CI: 0.96, 6.84) than in intervention clinics (1.94, 95% CI: 1.04, 3.60).
Factors associated with HIV acquisition
Among the Girl Power candidate variables, eight were associated with HIV incidence in bivariable analysis (see Table 1). This included one socio-demographic variable (age 20–24); one individual behavioral variable (≥2 sexual partners); three biologic/clinical variables (report of genital ulcers, report of vaginal discharge, and a past pregnancy); and three sexual partnership variables (transactional sex, believing a partner may have more than one partner, and having at least one partner >5 years older). In addition, being divorced, separated, or widowed was associated with HIV acquisition. Persons with at least three of these variables were 15.2 (2.0, 115.9) times as likely to acquire HIV as those with <3 of these variables (see Table 3).
Table 3:
Factors Associated with HIV incidence in the Girl Power Cohort
| Baseline Characteristics | New HIV infections (n=14) | Person years (n=672) | IR (CI) | Bivariable IRR (CI) | multivariable IRR (CI) | Score | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| 15–19 years | 5 | 407 | 1.23 (0.51, 2.95) | 1 | |||
| 20–24 years | 9 | 265 | 3.40 (1.77, 6.53) | 2.76 (0.93, 8.24) | |||
| Divorced or widowed | |||||||
| No | 11 | 631 | 1.74 (0.97, 3.15) | 1 | |||
| Yes | 3 | 40 | 7.56 (2.44, 23.44) | 4.34 (1.21, 15.55) | |||
| Partnerships in the last year | |||||||
| <2 | 9 | 543 | 1.66 (0.86, 3.19) | 1 | |||
| ≥2 | 5 | 129 | 3.88 (1.62, 9.32) | 2.34 (0.78, 6.99) | |||
| Any vaginal discharge in last 6 months | |||||||
| No | 8 | 548 | 1.46 (0.73, 2.92) | 1 | 1 | 0 | |
| Yes | 6 | 122 | 4.90 (2.20, 10.92) | 3.36 (1.17, 9.68) | 2.61 (0.84, 8.09) | 1 | |
| Any genital sores in last 6 months | |||||||
| No | 10 | 590 | 1.69 (0.91, 3.15) | 1 | 1 | 0 | |
| Yes | 4 | 82 | 4.90 (1.84, 13.06) | 2.89 (0.91, 9.22) | 1.94 (0.57, 6.62) | 1 | |
| Ever pregnant | |||||||
| No | 3 | 403 | 0.75 (0.24, 2.31) | 1 | 1 | 0 | |
| Yes | 11 | 267 | 4.12 (2.28, 7.45) | 5.53 (1.54, 19.84) | 4.55 (1.23, 16.85) | 2 | |
| Transactional sex | |||||||
| No | 8 | 539 | 1.48 (0.74, 2.97) | 1 | |||
| Yes | 6 | 132 | 4.54 (2.03, 10.10) | 3.06 (1.06, 8.81) | |||
| Any partner over ≥5 years | |||||||
| No | 5 | 466 | 1.07 (0.45, 2.58) | 1 | 1 | 0 | |
| Yes | 9 | 205 | 4.38 (2.28, 8.42) | 4.09(1.37, 12.20) | 2.42 (0.77, 7.56) | 1 | |
| Believes partner may have other partners | |||||||
| No | 3 | 263 | 1.14 (0.37, 3.54) | 1 | |||
| Yes | 11 | 409 | 2.69 (1.49, 4.86) | 2.35 (0.66, 8.44) | |||
| Overall | |||||||
| Number of above variables (0–9) | |||||||
| < 3 variables | 1 | 362 | 0.28 (0.04, 1.96) | 1 | |||
| ≥3 variables | 13 | 310 | 4.20 (2.43, 7.22) | 15.16 (1.98, 115.91) | |||
Risk score
Three of the variables associated with HIV in the VOICE cohort were also associated with HIV incidence in our cohort (genital sores, vaginal discharge, and believing a sexual partner may have other partners) (see Table 3). Two variables were not associated with HIV incidence in our cohort (alcohol use and being unmarried). Two variables had limited variability (being ≤25 years and receiving material support from a partner). VOICE risk score values ranged from 2 to 11. Median score was 6 (interquartile range 4, 7). Based on the VOICE cutoff ≥5, the sensitivity was 92.9%, specificity was 32.1%, and AUC was 0.64 (95% CI: 0.52, 0.75), lower than the 0.70 a priori threshold.
The model updating process resulted in a final risk assessment tool with two variables from the VOICE risk score (genital ulcers and vaginal discharge), as well as two additional variables (>5 years partner age difference and pregnancy history) (see Table 3). All variables were assigned a value of 1 except for pregnancy history, which was assigned a value of 2. Scores ranged from 0 to 5 with a median of 1 (IQR: 0, 2). Thirty-five percent had a score of 0, 41% had a score of 1–2, and 24% had a score ≥3. A monotonic dose-response relationship between score and HIV incidence was observed (Fig. 1). Each one-unit increase in score was associated with 2.25 times the risk of HIV acquisition (95% CI: 1.51, 3.33). The AUC was 0.79 (95% CI: 0.69, 0.89). Those with a score ≥1 experienced an HIV incidence rate of 3.25/100 PYs. At this threshold, 100% of AGYW who acquired HIV were captured, but specificity was only 35.9%. Those with a score ≥3 experienced an HIV incidence rate of 5.89/100 PYs. This cutoff had 64.3% sensitivity and 76.8% specificity. The AUC from the bootstrapping process was 0.79 (0.69, 0.89), essentially identical to the main analysis.
Figure 1a and 1b.
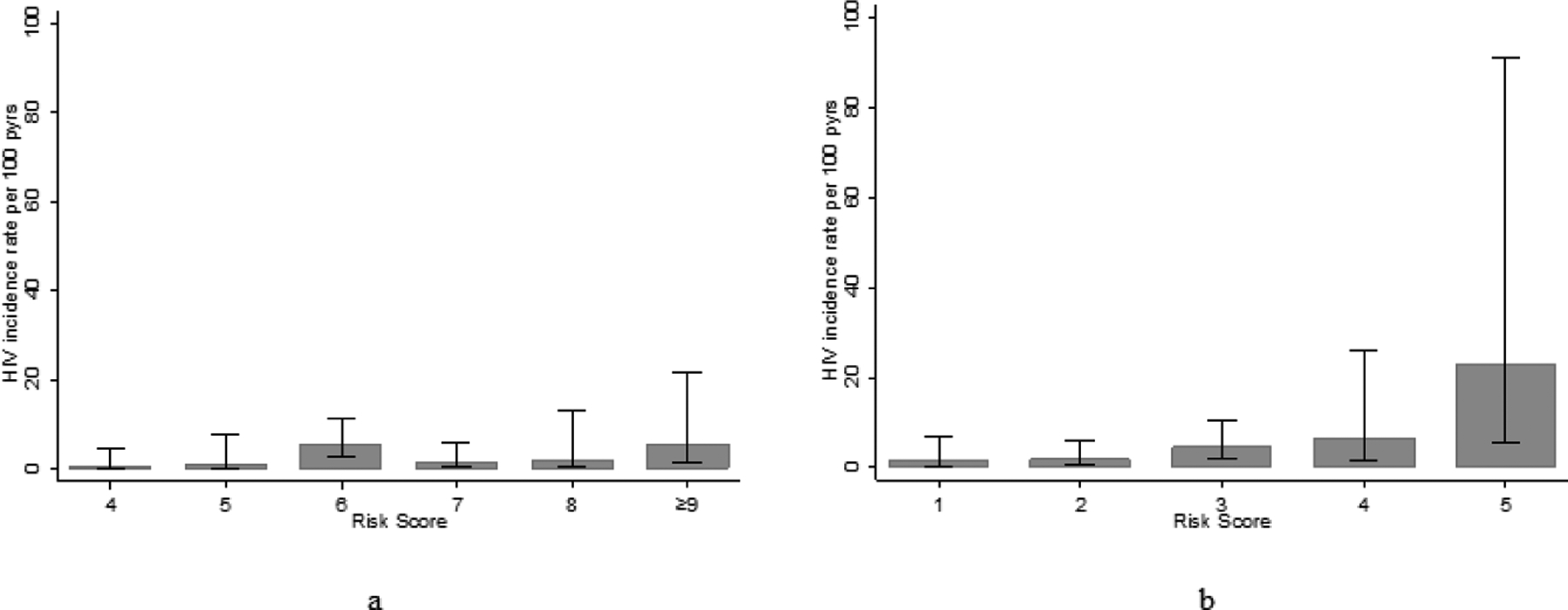
Relationship between the VOICE (a) and Girl Power (b) Risk Scores and HIV Incidence
The 172 participants with missing outcome data had a median score of 1 (IQR=0, 2), which was the same as those who had complete outcome data (p=0.34). The missing data was driven primarily by lower testing rates in our standard of care clinic, a population with lower access to HIV testing services.
Discussion
In a cohort of sexually active AGYW in Lilongwe, Malawi the overall HIV incidence rate was 2.1/100 PYs, but risk was not uniform in all participants. By updating a regional risk assessment tool, we identified subsets of AGYW with HIV incidence rates above 3 per 100 PYs. In this cohort, the updated tool had fewer variables and performed better than the VOICE-derived tool with a higher AUC, and exhibited very good performance characteristics. Less than one quarter of the population had a score ≥3, but this segment accounted for two thirds of the infections. HIV incidence in this population was nearly 6/100 PYs.
The final risk assessment tool had several desirable characteristics. First, we built on past work to the extent possible, updating a previous risk assessment tool, rather than developing a new one. This decision moves the field towards a common regional tool with high performance in multiple settings. Second, the updated tool is parsimonious with only four variables and five points. This simplicity is important for overburdened healthcare setting, as it could feasibly be administered quickly without laboratory delays or lengthy risk assessments. Third, it is appropriate for clinical settings as it primarily assesses clinical information—history of pregnancy and STI symptoms, minimizing potentially stigmatizing questions about sexual behavior. Finally, there is a monotonic relationship between score and HIV incidence, allowing for multiple meaningful thresholds.
These results have potential policy implications for the Malawian HIV program, as the Ministry of Health initiates PrEP implementation in AGYW. Given millions of AGYW and moderate HIV incidence in this population, offering PrEP to all AGYW is not feasible. Our risk assessment tool provides an epidemiologically-driven way of identifying the subset who would benefit most. The Girl Power population had higher behavioral risk than the general AGYW population in Lilongwe.24 The result was an HIV incidence rate 3–4 times higher than the estimated incidence rate among pregnant AGYW in Lilongwe (2.08 versus 0.57/100 PYs)8. This suggests that although PrEP may be indicated for a large share of the Girl Power population, it would be appropriate for a smaller share of the overall AGYW population.
Policy considerations must be addressed prior to implementing this risk assessment tool. First, an optimal “high risk” threshold is needed. A cut-point ≥1 would identify all sero-converters, but with a large share of the population offered PrEP, most of whom would not acquire HIV. Alternatively, a score ≥3 would identify two thirds of sero-converters, and allow for more targeted PrEP use. Such decisions must be made by weighing resources and program goals, as well as the tradeoffs between sensitivity and specificity. A second consideration is that the WHO recommends that such tools be used to guide higher-risk persons towards receiving PrEP, rather than to deny lower-risk persons from receiving it. Setting is a third consideration. The tool could be implemented in a range of clinical settings that attend to AGYW including STI, HIV testing, antenatal, and family planning clinics or in community-based programs. Determining which of these settings is most appropriate is an area for implementation research. Finally, our study population was 15–24 years old, and it is important to determine whether this assessment tool is appropriate in older or younger age groups. This is especially important given the absence of women over 25 in our population and the limited performance of the VOICE tool in the younger HPTN 068 population.
Self-report of pregnancy, genital ulcers, and vaginal discharge were all associated with HIV incidence. These factors all amplify the probability of HIV acquisition25,26 and are indicators of unprotected sexual activity. The co-occurrence of STIs, pregnancy, and HIV underscores the importance of holistically addressing AGYW sexual and reproductive health, something we achieved in the larger Girl Power study16.
The absence of laboratory-ascertained STI biomarkers is an important feature of our study that merits discussion. Unlike VOICE, a well-funded, clinical trial,27 Girl Power-Malawi did not have the resources to directly collect STI biomarkers. Instead, we included self-reported genital tract symptoms, which were predictive. Using self-reported STI symptoms is problematic for definitive diagnosis of STIs: STIs are often asymptomatic28 and other vaginal infections, such as bacterial vaginosis, can also cause abnormal vaginal discharge.29,30 Nonetheless, using self-report in a risk assessment tool is warranted, if it is predictive, which it was in our cohort. Validating this finding in other sub-Saharan African cohorts of AGYW is an important next step.
None of the HIV acquisition events occurred among women with partners known to be HIV-infected, the most salient HIV risk factor. This finding stems, in part, from the small number of women (n=3) who reported a known HIV-infected partner. It is not known how the score would perform in AGYW known to be in HIV-discordant relationships, an area for future research. Furthermore, this finding highlights the need to support mutual disclosure of HIV status in this age group, such as recruitment for couple HIV testing and counseling or secondary distribution of HIV self-test kits.31,32
Because the primary aim of Girl Power was not to measure HIV incidence, the cohort had few HIV acquisition events, and ultimately imprecise estimates, an important set of limitations. It is possible that the model was over-fit to these data and that the tool would not perform as well in other populations. External validation in a larger population and in similar settings is an important next step to assess generalizability.
A related limitation is that a substantial number of HIV outcomes were missing. These missing data contribute to imprecise estimates, but also introduce the possibility of bias. We believe this source of bias is minimal because the distribution of the risk score values was similar in those with and without missing data. Furthermore, missing outcomes were most common in the standard of care arm in which testing was more challenging. Because study arm was randomly assigned, there is a high likelihood that missing data were missing at random.
In summary, although all AGYW in SSA are often referred to as being at high-risk for HIV, we show that risk is not uniform, and those with elevated risk are identifiable. This is an important finding as Malawi and other countries in the region determine how best to roll out PrEP to AGYW. Identifying and supporting those at highest risk for HIV is possible and essential.
Acknowledgments:
NER, MCH, AP, JP, and LGB conceptualized the overall study and this secondary analysis. EK and MC were responsible for data analysis. NER drafted the manuscript. All authors made revisions to the manuscript and approved the final draft.
We would like to thank the District Health Office, the Lighthouse, and Girl Power-Malawi participants and staff for their integral contributions to this study.
Footnotes
Conflicts of Interest and Source of Funding
The study was funded by Evidence for HIV Prevention in Southern Africa (EHPSA), a DFID program managed by Mott MacDonald. NER is funded by the National Institute of Mental Health (R00 MH104154) and the National Institute of Allergy and Infectious Diseases (P30 AI50410). EK is funded by NIH-National Cancer Institute (U54CA190152). MC (D43TW010060, P30 AI50410) and JTP (K01TW010857) are funded by the Fogarty International Center.
References
- 1.Joint United Nations Programme on HIV/AIDS. The Gap Report. In; 2014. [Google Scholar]
- 2.Abdool Karim SS, Passmore JS, Baxter C. The microbiome and HIV prevention strategies in women. Curr Opin HIV AIDS 2018,13:81–87. [DOI] [PubMed] [Google Scholar]
- 3.Idele P, Gillespie A, Porth T, Suzuki C, Mahy M, Kasedde S, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr 2014,66 Suppl 2:S144–153. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. In; 2015. [Google Scholar]
- 5.UNAIDS. Number of New HIV Infections, Young Women (15–24). 2020. http://aidsinfo.unaids.org/ (accessed January 16 2020).
- 6.President’s Emergency Plan for AIDS Relief, ICAP, CDC, Center for Social Responsibility, Naitonal Statistics Office, COM-JHP. Malawi Population-Based HIV Impact Assessment, MPHIA 2015–2016. Malawi Population-Based HIV Impact Assessment: A Drop that Counts 2016. [Google Scholar]
- 7.Payne D, Maher A, Curran K, Agyemang E, Kim E, Kilembe F, et al. Recent HIV infection surveillance among adolescent girls and young women in Malawi. Conferences on Retroviruses and Opportunistic Infectious 2019. [Google Scholar]
- 8.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 2000,284:79–84. [DOI] [PubMed] [Google Scholar]
- 9.Powers KA, Miller WC, Pilcher CD, Mapanje C, Martinson FE, Fiscus SA, et al. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. AIDS 2007,21:2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pintye J, Drake AL, Kinuthia J, Unger JA, Matemo D, Heffron RA, et al. A Risk Assessment Tool for Identifying Pregnant and Postpartum Women Who May Benefit From Preexposure Prophylaxis. Clin Infect Dis 2017,64:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahle EM, Hughes JP, Lingappa JR, John-Stewart G, Celum C, Nakku-Joloba E, et al. An empiric risk scoring tool for identifying high-risk heterosexual HIV-1 serodiscordant couples for targeted HIV-1 prevention. J Acquir Immune Defic Syndr 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. The New England Journal of Medicine 2015; 372(6): 509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkus JE, Brown E, Palanee T, Nair G, Gafoor Z, Zhang J, et al. An Empiric HIV Risk Scoring Tool to Predict HIV-1 Acquisition in African Women. J Acquir Immune Defic Syndr 2016,72:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovenco D, Pettifor A, MacPhail C, Kahn K, Wagner R, Piwowar-Manning E, et al. Assessing risk for HIV infection among adolescent girls in South Africa: an evaluation of the VOICE risk score (HPTN 068). J Int AIDS Soc 2019,22:e25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg NE, Pettifor AE, Myers L, Phanga T, Marcus R, Bhushan NL, et al. Comparing four service delivery models for adolescent girls and young women through the ‘Girl Power’ study: protocol for a multisite quasi-experimental cohort study. BMJ Open 2017,7:e018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg NE, Bhushan NL, Vansia D, Phanga T, Maseko B, Nthani T, et al. Comparing Youth-Friendly Health Services to the Standard of Care Through “Girl Power-Malawi”: A Quasi-Experimental Cohort Study. J Acquir Immune Defic Syndr 2018,79:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg NE, Gichane MW, Vansia D, Phanga T, Bhushan NL, Bekker LG, et al. Assessing the Impact of a Small-Group Behavioral Intervention on Sexual Behaviors Among Adolescent Girls and Young Women in Lilongwe Malawi: A Quasi-Experimental Cohort Study. AIDS Behav 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price JT, Rosenberg NE, Vansia D, Phanga T, Bhushan NL, Maseko B, et al. Predictors of HIV, HIV Risk Perception, and HIV Worry Among Adolescent Girls and Young Women in Lilongwe, Malawi. J Acquir Immune Defic Syndr 2018,77:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amrhein V, Greenland S, McShane B and 800 signatories “A call for an end to hyped claims and the dismissal of possibly crucial effects” Nature, 2019,567:307 [Google Scholar]
- 20.Leamer EE, Specification Searches, 1978, p 92. [Google Scholar]
- 21.Moons KG, Kengne AP, Grobbee DE, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012,98:691–698. [DOI] [PubMed] [Google Scholar]
- 22.Moons KG, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012,98:683–690. [DOI] [PubMed] [Google Scholar]
- 23.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA 1997,277:488–494. [PubMed] [Google Scholar]
- 24.National Statistics Office. Malawi Demographic and Health Survey 2015–16. In. Zomba, Malawi and Rockville, Maryland; 2017. [Google Scholar]
- 25.Thomson KA, Hughes J, Baeten JM, John-Stewart G, Celum C, Cohen CR, et al. Increased Risk of HIV Acquisition Among Women Throughout Pregnancy and During the Postpartum Period: A Prospective Per-Coital-Act Analysis Among Women With HIV-Infected Partners. J Infect Dis 2018,218:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis 1992,19:61–77. [PubMed] [Google Scholar]
- 27.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015,372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnabas SL, Dabee S, Passmore JS, Jaspan HB, Lewis DA, Jaumdally SZ, et al. Converging epidemics of sexually transmitted infections and bacterial vaginosis in southern African female adolescents at risk of HIV. Int J STD AIDS 2018,29:531–539. [DOI] [PubMed] [Google Scholar]
- 29.Torrone EA, Morrison CS, Chen PL, Kwok C, Francis SC, Hayes RJ, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med 2018,15:e1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passmore JA, Jaspan HB, Masson L. Genital inflammation, immune activation and risk of sexual HIV acquisition. Curr Opin HIV AIDS 2016,11:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg NE, Mtande TK, Saidi F, Stanley C, Jere E, Paile L, et al. Recruiting male partners for couple HIV testing and counselling in Malawi’s option B+ programme: an unblinded randomised controlled trial. Lancet HIV 2015,2:e483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV 2016,3:e266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]


