Abstract
Background
18F-fluorodeoxyglucose (18F-FDG) uptake in hepatocellular carcinoma (HCC) is significantly associated with early recurrence and survival after curative surgical resection. However, there are no reports regarding the relationship between 18F-FDG uptake and outcomes after radiofrequency ablation (RFA). A prospective cohort study was conducted to evaluate the prognostic value of 18F-FDG positron emission tomography (PET) in HCC patients after RFA.
Methods
A total of 121 consecutive patients with primary HCC (≤3 tumors, of diameter ≤ 3 cm) without vascular invasion on imaging were examined by 18F-FDG-PET computed tomography prior to RFA. An HCC with a component of 18F-FDG uptake visibly stronger than that of surrounding liver was defined as 18F-FDG-PET positive.
Results
The median follow-up period was 1267 days. There were 110 18F-FDG-PET negative and 11 positive tumors. The cumulative 1-year recurrence rates in the 18F-FDG negative and positive groups were 30 and 64% (P = 0.017), respectively, and cumulative 1-year metastatic recurrence rates were 6 and 36% (P < 0.001), respectively. The cumulative 5-year survival rates were 88 and 22% (P < 0.001), respectively. Multivariate analysis revealed 18F-FDG-PET positivity and tumor size as independent factors related to metastatic recurrence and survival after RFA.
Conclusions
18F-FDG-PET positivity was significantly associated with outcomes after RFA. RFA should not be readily selected as the first-line treatment for small HCC that includes a component of visually strong 18F-FDG uptake.
Keywords: 18F-fluorodeoxyglucose positron emission tomography, Hepatocellular carcinoma, Radiofrequency ablation
Background
Radiofrequency ablation (RFA) is established as the standard of care for patients with small hepatocellular carcinoma (HCC) unsuitable for surgical resection. Clinical practice guidelines for HCC state that RFA is indicated for ≤3 tumors, of diameter ≤ 3 cm [1–3]. However, some reports have indicated that along with the pathologic differentiation that occurs in advanced HCC, there is an increased incidence of microscopic vascular invasion and intrahepatic metastasis even in small HCC [4–7], and the prognosis after RFA becomes poor [8]. In addition, numerous studies have reported an association between RFA and severe problems such as intrahepatic dissemination [9–11], aggressive recurrence with vascular invasion [12–15], and seeding [16]. Because the risks of these types of critical recurrence after RFA are related to tumor characteristics at the time of ablation (e.g., poor differentiation and vascular invasion), the histological differentiation grade should be assessed in determining the optimal treatment plan, even in patients with small HCC. However, tumor biopsy has limitations related to tumor location, sampling error, and the risk of complications such as bleeding and tumor seeding [17].
18F-FDG positron emission tomography (PET) is already in common use in screening for various cancers, including for lung and breast cancer. However, as the sensitivity of 18F-FDG-PET for detecting HCC is lower than that of contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI), 18F-FDG-PET is therefore no longer recommended as a standard imaging modality for the early diagnosis of HCC [3]. However, it has been reported that 18F-FDG-PET uptake is associated with poor prognosis after surgical resection [18, 19], and that it can predict vascular invasion and recurrence in HCC patients before liver transplantation [20, 21]. If there is a relationship also between 18F-FDG-PET uptake and outcomes after RFA, 18F-FDG-PET would be useful when considering the optimal and safe treatment strategy for small HCC. The aim of this prospective cohort study was to clarify whether 18F-FDG-PET uptake is associated with outcomes after RFA for small HCC.
Methods
Patients
Included in the study were adult patients with primarily diagnosed HCCs who underwent RFA. The exclusion criteria were any of the following: 1) four tumors or more, 2) tumor diameter > 3 cm, 3) severe decompensated cirrhosis (Child–Pugh class C). A total of 121 consecutive patients with initially diagnosed HCCs had undergone 18F-FDG-PET CT within 4 weeks before RFA between May 2008 and February 2013. Any two of contrast CT, dynamic MRI, or contrast ultrasonography were performed for the differential diagnosis of liver tumor. HCC was diagnosed based on “typical imaging finding,” which is typically defined as a nodule that is visualized as a high signal intensity area in the arterial phase and relatively low signal intensity area in the venous phase [22]. Prior to RFA, the following were recorded in all patients: tumor diameter; etiology of hepatitis; Child–Pugh classification; platelet count; serum alanine aminotransferase (ALT) level; levels of the tumor markers alpha-fetoprotein (AFP), Lens culinaris agglutinin-reactive alpha-fetoprotein (AFP-L3), des-gamma-carboxy prothrombin (DCP); and serum fibrosis markers (type IV collagen 7S and hyaluronic acid). This prospective observational study was approved by our ethics committee and conformed to the provisions of the Helsinki Declaration. Written informed consent was obtained from all patients.
18F-FDG-PET imaging protocol
All patients were imaged prior to RFA by a whole-body PET scanner (Eminence-B, Shimadzu, Kyoto, Japan) with axial resolution of 3.9 mm (full-width at half maximum) and a 20-cm field of view (z axis). Prior to scanning, patients fasted for at least 5 h, and their blood glucose level at the time of FDG injection was < 150 mg/dl. Each patient received intravenous injection of approximately 2.6 MBq 18F-FDG per kg of body weight, and was scanned from the head to the upper thigh (slice thickness, 8 mm) 50 min after injection. FDG images were corrected for attenuation with a cesium external source.
Image analysis
The 18F-FDG-PET images were independently analyzed with reference to the contrast CT or dynamic MRI images by one experienced radiologist and one experienced hepatologist, each with > 20 years of experience in liver imaging. Any disagreements in interpretation were resolved by consensus. The degree of 18F-FDG uptake in a nodule seen on 18F-FDG-PET was visually compared with that in the surrounding liver. Tumors with stronger 18F-FDG uptake, as a whole or partially, in comparison with the surrounding liver were termed PET positive (Fig. 1), and those with 18F-FDG uptake equal to the surrounding liver were termed PET negative (Fig. 2). In the case of multiple HCCs, 18F-FDG uptake in the largest tumor was analyzed.
Fig. 1.
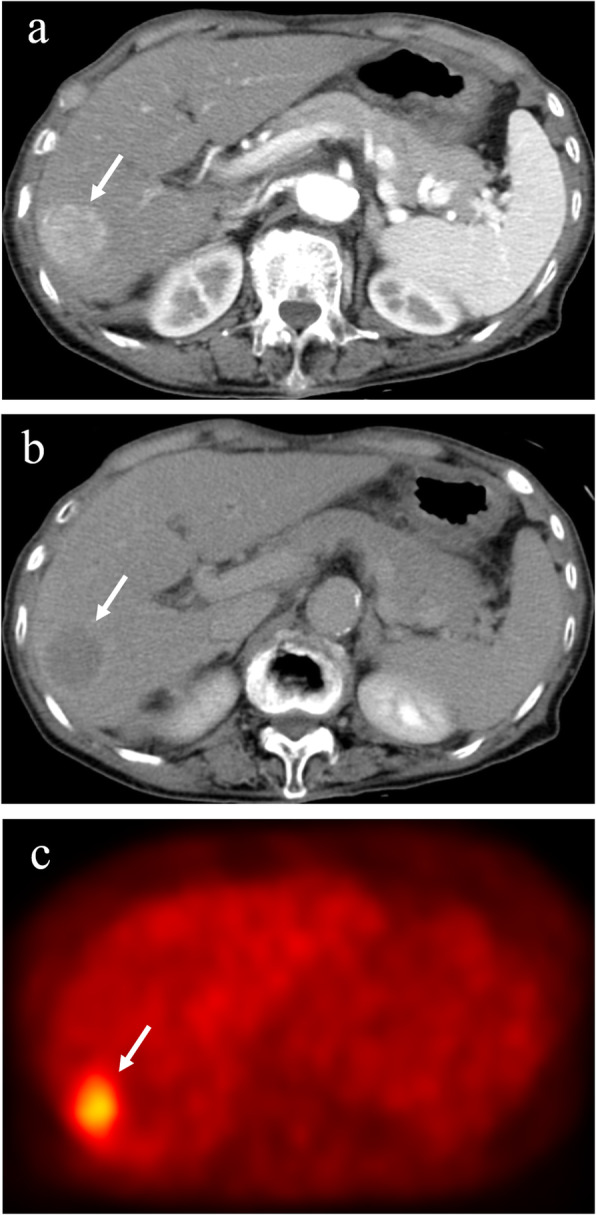
Hepatocellular carcinoma with positive 18F-fluorodeoxyglucose uptake on positron emission tomography. The hepatocellular carcinoma (diameter, 3.0 cm) in segment 5 exhibits staining during the arterial phase of contrast computed tomography (a) and washout during the equilibrium phase (b). The tumor has higher 18F-fluorodeoxyglucose uptake than that of surrounding liver on positron emission tomography (c). Arrows indicate the tumor. 18F-FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography
Fig. 2.
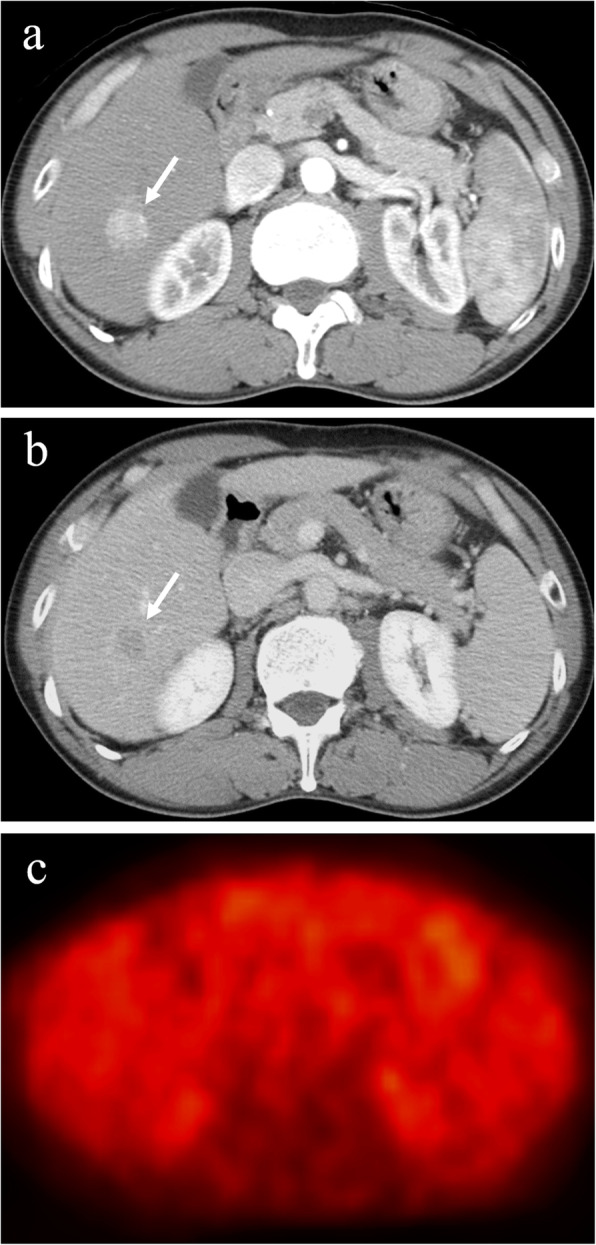
Hepatocellular carcinoma with negative 18F-fluorodeoxyglucose uptake on positron emission tomography. The hepatocellular carcinoma (diameter, 2.0 cm) in segment 6 exhibits staining during the arterial phase of contrast computed tomography (a) and washout during the equilibrium phase (b). The tumor has equal 18F-fluorodeoxyglucose uptake to that of surrounding liver on positron emission tomography (c). Arrows indicate the tumor
RFA technique
Percutaneous RFA was performed in all patients, using the Cool-tip RF system (COVIDEN, Boulder, CO, USA) under ultrasound guidance. An artificial pleural effusion or artificial ascites was produced, using saline when necessary. Impedance control mode was used with a 17-G cooled-tip electrode with a 2- or 3-cm exposed tip. Ablation was started at 40 W for the 2-cm exposed tip and at 60 W for the 3-cm exposed tip, and power was increased at a rate of 10 W/min. When a rapid increase in impedance occurred, the output was automatically stopped and ablation was restarted after a short time at an output 10 W lower. The duration of a single ablation was 6 min for the 2-cm electrode and 12 min for the 3-cm electrode. After RF exposure, the temperature of the needle tip was measured. When the temperature was < 65℃, additional ablation was performed.
Assessment of response and follow up
Treatment response was assessed by contrast CT or MRI at 1–3 days after the final session. Complete response was defined as no enhancement in the entire lesion on imaging, with a safety margin. Additional ablation was performed until complete ablation was confirmed in each nodule. All patients were followed up on an outpatient basis every 3–4 months, including contrast CT or MRI and measurement of tumor marker levels. In the case of extrahepatic metastasis, diagnosis was performed by 18F-FDG-PET CT or biopsy. As imaging methods cannot distinguish whether multiple intrahepatic recurrences are metastatic or multicentric non-concurrent primary lesions, intrahepatic metastatic recurrence was defined as the presence of at least three hypervascular intrahepatic recurrences, as stated as the criteria for metastatic recurrences attributed to advanced stage cancer as an indication for RFA (≤3 tumors, of diameter ≤ 3 cm).
Statistical analysis
Values are expressed as the median (range). The Mann–Whitney U test was used to analyze continuous variables, and Fisher’s exact test or the χ2 test was used to analyze categorical variables. Cumulative recurrence-free survival rates, cumulative metastatic recurrence-free survival rates, and cumulative survival rates according to the 18F-FDG-PET positivity classification were calculated using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate analyses for factors related to recurrence, including metastatic recurrence, and survival related to HCC were performed using a Cox proportional hazards regression model. The results are expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Values of p < 0.05 were considered significant. All analyses were performed using the SPSS 21.0 software package (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
The median follow-up period was 1267 days. Table 1 lists the characteristics of the enrolled patients.
Table 1.
Patients’ baseline characteristics (n = 121)
| Age (years) | 69 (45–87) |
| Sex (male/female) | 73/48 |
| Etiology (HCV/non-HCV) | 91/30 |
| Fibrosis stage (F0/1/2/3/4) | 2/0/12/30/77 |
| Tumor size (mm) | 18 (8–30) |
| AFP (ng/mL) | 18.0 (1.8–1594.5) |
| AFP-L3 (%) | 7.4 (0.0–80.0) |
| DCP (mAU/mL) | 64 (5–9489) |
HCV hepatitis C virus, AFP alpha-fetoprotein, AFP-L3 Lens culinaris agglutinin-reactive alpha-fetoprotein, DCP Des-gamma-carboxyprothrombin. Data are expressed as medians (range)
Comparison of baseline characteristics between 18F-FDG-PET negative and 18F-FDG-PET positive groups
Table 2 shows a comparison of the baseline characteristics of patients in the 18F-FDG-PET positive and negative groups. Tumor size, AFP, AFP-L3, and DCP values were significantly higher in the 18F-FDG-PET positive group than the negative group. No significant differences were seen in terms of age, sex, etiology, Child–Pugh classification, platelet count, ALT level, fibrosis markers, or number of tumors between the 18F-FDG-PET positive and negative groups.
Table 2.
Comparison of baseline characteristics between the PET negative and PET positive groups
| PET negative (n = 110) | PET positive (n = 11) | p value | |
|---|---|---|---|
| Age (years) | 69 (45–87) | 76 (58–83) | 0.401 |
| Sex (male/female) | 68/42 | 5/6 | 0.341 |
| Etiology (HCV/non-HCV) | 82/28 | 9/2 | 0.730 |
| Fibrosis stage (F0–3/4) | 39/71 | 5/6 | 0.526 |
| Child–Pugh class (A/B) | 87/23 | 10/1 | 0.691 |
| Platelets (× 104/μL) | 9.0 (2.4–75.9) | 10.4 (2.9–21.2) | 0.351 |
| ALT (IU/L) | 37 (10–171) | 45 (16–133) | 0.339 |
| Hyaluronic acid (ng/mL) | 216.3 (23.2–1851.9) | 265.5 (85.0–884.9) | 0.438 |
| Type IV collagen 7S (ng/mL) | 7.7 (3.0–18.0) | 7.3 (4.1–12.9) | 0.487 |
| Tumor size (mm) | 18 (8–30) | 25 (15–30) | 0.001 |
| Number of tumors (1/2/3) | 91/14/5 | 8/2/1 | 0.413 |
| AFP (ng/mL) | 16.1 (1.8–1594.5) | 112.1 (3.1–1545.8) | 0.011 |
| AFP-L3 (%) | 7.3 (0–72.5) | 28.9 (0–80.0) | 0.011 |
| DCP (mAU/mL) | 58 (5–9489) | 177 (22–2615) | 0.008 |
HCV hepatitis C virus, ALT alanine aminotransferase, HCC hepatocellular carcinoma, AFP alpha-fetoprotein, AFP-L3 Lens culinaris agglutinin-reactive alpha-fetoprotein, DCP Des-gamma-carboxyprothrombin, PET positron emission tomography. Data are expressed as medians (range)
Comparison of recurrence and metastatic recurrence after RFA between 18F-FDG-PET negative and 18F-FDG-PET positive groups
Recurrence-free survival curves according to 18F-FDG-PET positivity are shown in Fig. 3. Recurrence-free survival was significantly shorter in the 18F-FDG-PET positive group than the negative group (p = 0.017). The cumulative 1-year recurrence rates of the 18F-FDG-PET negative and positive groups were 30 and 64%, respectively.
Fig. 3.
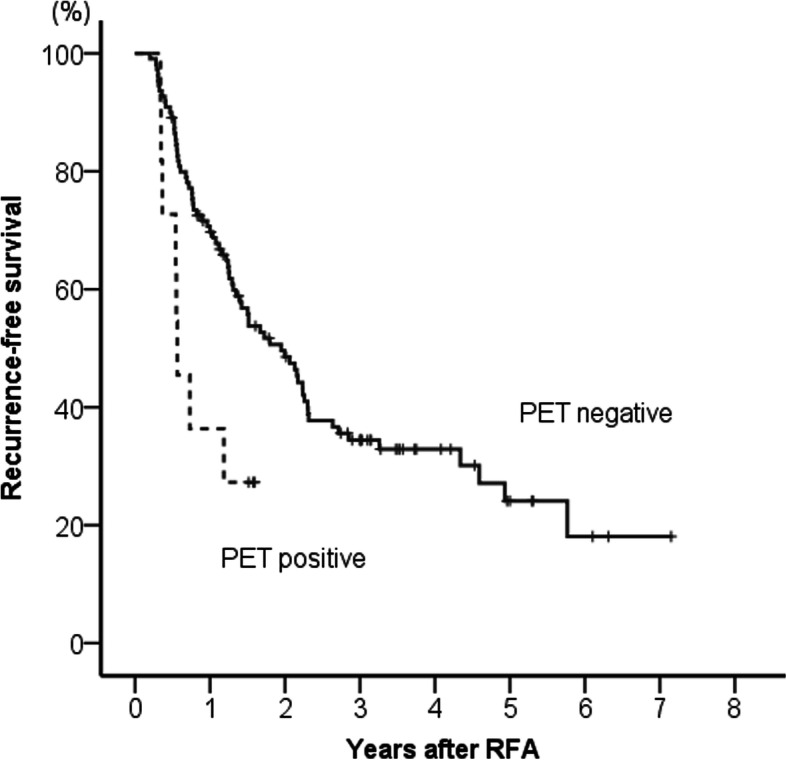
Comparison of recurrence-free survival between 18F-fluorodeoxyglucose positron emission tomography positive and negative groups. Recurrence-free survival was significantly shorter in the 18F-fluorodeoxyglucose positron emission tomography positive group than in the negative group (p = 0.017)
Metastatic recurrence-free survival curves according to 18F-FDG-PET positivity are shown in Fig. 4. Metastatic recurrence-free survival was significantly shorter in the 18F-FDG-PET positive group than in the negative group (p < 0.001). The cumulative 1-year metastatic recurrence rates of the 18F-FDG-PET negative and positive groups were 6 and 36%, respectively. Metastatic recurrences occurred in 30 patients during the follow-up period, as follows: intrahepatic metastasis (n = 18); extrahepatic and intrahepatic metastases (n = 2); extrahepatic metastasis (n = 2); intrahepatic metastasis and portal invasion (n = 4); extrahepatic metastasis and portal invasion (n = 2); extrahepatic metastasis and hepatic vein invasion (n = 1); and intrahepatic metastasis, extrahepatic metastasis, and portal invasion (each n = 1). During the follow-up period, 48% (15/31) of patients with metastatic recurrences died from HCC.
Fig. 4.
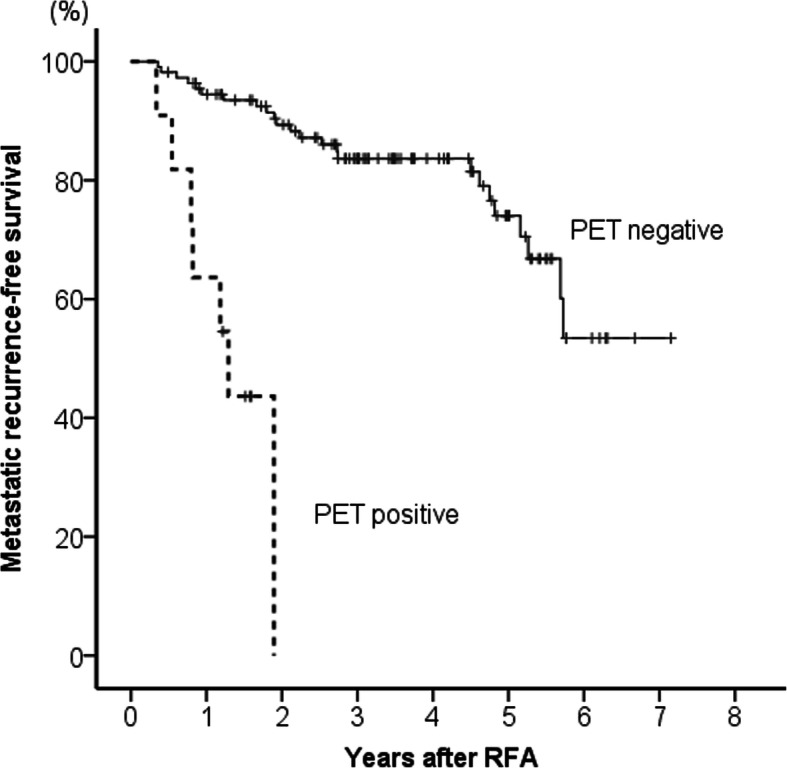
Comparison of metastatic recurrence-free survival rates between 18F-fluorodeoxyglucose positron emission tomography positive and negative groups. Metastatic recurrence-free survival was significantly shorter in the 18F-fluorodeoxyglucose positron emission tomography positive uptake group than in the negative group (p < 0.001)
Comparison of survival between 18F-FDG-PET negative and 18F-FDG-PET positive groups
Survival curves according to 18F-FDG-PET positivity are shown in Fig. 5. Survival was significantly shorter in the 18F-FDG-PET positive group than in the negative group (p < 0.001). The cumulative 5-year survival rate was 88% in the 18F-FDG-PET negative group and 22% in the 18F-FDG-PET positive group.
Fig. 5.
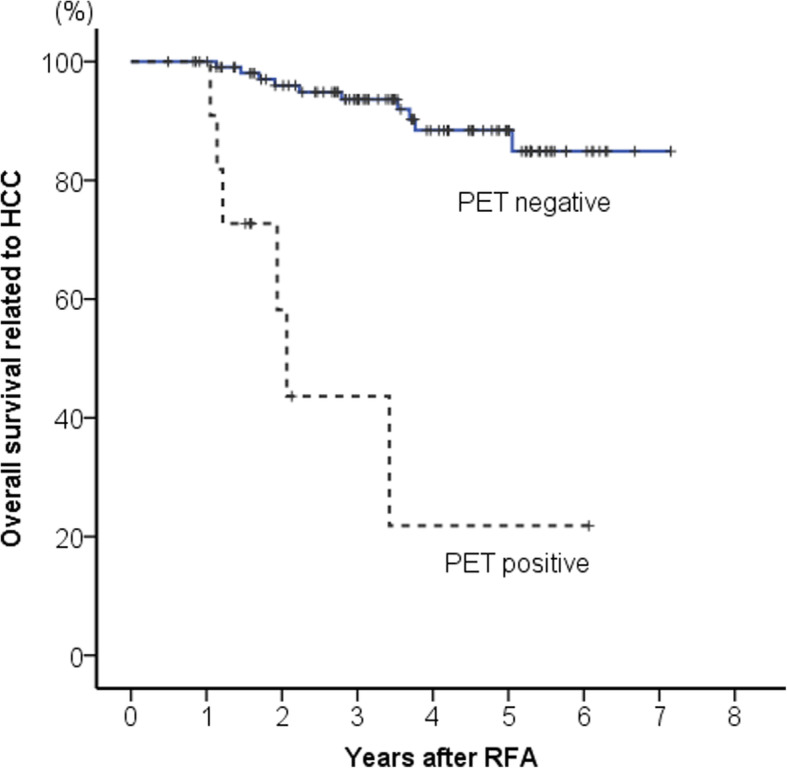
Comparison of survival between 18F-fluorodeoxyglucose positron emission tomography positive and negative groups. Survival was significantly shorter in the 18F-fluorodeoxyglucose positron emission tomography positive group than in the negative group (p < 0.001)
Univariate and multivariate analyses of factors related to recurrence
Table 3 lists the results of univariate and multivariate analyses of background variables associated with overall recurrence. Univariate analysis identified the factors of etiology (hepatitis C virus), Child–Pugh classification, ALT level, hyaluronic acid level, type IV collagen 7S level, tumor size, number of tumors, and 18F-FDG-PET positivity as being significantly associated with recurrence after RFA. In multivariate analysis, etiology (hepatitis C virus), Child–Pugh classification, and number of tumors were identified as independent factors.
Table 3.
Univariate and multivariate analyses of factors related to recurrence
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| p value | HR | 95%CI | p value | HR | 95%CI | |
| Age (years) | 0.520 | 1.008 | 0.983–1.034 | |||
| Sex (female) | 0.878 | 1.036 | 0.660–1.627 | |||
| Etiology (HCV) | 0.004 | 2.426 | 1.334–4.411 | 0.006 | 2.438 | 1.293–4.599 |
| Child–Pugh class (A) | 0.007 | 0.492 | 0.294–0.823 | 0.042 | 0.524 | 0.281–0.976 |
| Platelets (×104/μL) | 0.742 | 0.994 | 0.957–1.031 | |||
| ALT (IU/L) | 0.048 | 1.006 | 1.000–1.012 | 0.873 | 1.001 | 0.993–1.008 |
| Hyaluronic acid (ng/mL) | < 0.001 | 1.001 | 1.001–1.002 | 0.575 | 1.000 | 0.999–1.001 |
| Type IV collagen 7S (ng/mL) | 0.000 | 1.146 | 1.073–1.224 | 0.130 | 1.080 | 0.978–1.192 |
| Tumor size (mm) | 0.037 | 1.040 | 1.002–1.079 | 0.573 | 1.012 | 0.971–1.054 |
| Number of tumors | < 0.001 | 2.172 | 1.459–3.235 | 0.005 | 1.930 | 1.219–3.057 |
| AFP (ng/mL) | 0.120 | 1.001 | 1.000–1.001 | |||
| AFP-L3 (> 15%) | 0.084 | 1.725 | 0.929–3.205 | |||
| DCP (mAU/mL) | 0.282 | 1.000 | 1.000–1.000 | |||
| PET positive | 0.021 | 2.425 | 1.144–5.138 | 0.112 | 1.969 | 0.854–4.540 |
HR hazard ratio, CI confidence interval, HCV hepatitis C virus, ALT alanine aminotransferase, HCC hepatocellular carcinoma, AFP alpha-fetoprotein, AFP-L3 Lens culinaris agglutinin-reactive alpha-fetoprotein, DCP, Des-gamma-carboxyprothrombin, PET positron emission tomography
Table 4 lists the results of univariate and multivariate analyses of the background variables associated with metastatic recurrence. Univariate analysis identified the factors of Child–Pugh classification, type IV collagen 7S level, tumor size, number of tumors, AFP level, AFP-L3 (> 15%) level, and 18F-FDG-PET positivity as being significantly associated with metastatic recurrence after RFA. In multivariate analysis, 18F-FDG-PET positivity and tumor size were identified as independent factors.
Table 4.
Univariate and multivariate analyses of factors related to metastatic recurrence
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| p value | HR | 95%CI | p value | HR | 95%CI | |
| Age (years) | 0.619 | 0.989 | 0.949–1.032 | |||
| Sex (female) | 0.592 | 0.818 | 0.391–1.708 | |||
| Etiology (HCV) | 0.631 | 1.246 | 0.508–3.052 | |||
| Child–Pugh class (A) | 0.027 | 0.426 | 0.200–0.906 | 0.087 | 0.449 | 0.180–1.123 |
| Platelets (×104/μL) | 0.588 | 1.014 | 0.965–1.064 | |||
| ALT (IU/L) | 0.367 | 1.005 | 0.994–1.015 | |||
| Hyaluronic acid (ng/mL) | 0.145 | 1.001 | 1.000–1.001 | |||
| Type IV collagen 7S (ng/mL) | 0.018 | 1.141 | 1.023–1.274 | 0.454 | 1.057 | 0.914–1.222 |
| Tumor size (mm) | 0.000 | 1.132 | 1.062–1.207 | 0.017 | 1.089 | 1.015–1.169 |
| Number of tumors | 0.014 | 1.940 | 1.145–3.285 | 0.197 | 1.492 | 0.812–2.740 |
| AFP (ng/mL) | 0.012 | 1.001 | 1.000–1.002 | 0.534 | 1.000 | 0.998–1.001 |
| AFP-L3 (> 15%) | 0.030 | 2.559 | 1.096–5.972 | 0.329 | 1.703 | 0.585–4.961 |
| DCP (mAU/mL) | 0.775 | 1.000 | 1.000–1.000 | |||
| PET positive | < 0.001 | 12.941 | 4.646–36.047 | < 0.001 | 10.297 | 3.128–33.898 |
HR hazard ratio, CI confidence interval, HCV hepatitis C virus, ALT alanine aminotransferase, HCC hepatocellular carcinoma, AFP alpha-fetoprotein, AFP-L3, Lens culinaris agglutinin-reactive alpha-fetoprotein, DCP Des-gamma-carboxyprothrombin, PET positron emission tomography
Univariate and multivariate analyses of factors related to survival
Table 5 lists the results of univariate and multivariate analyses of background variables associated with survival. Univariate analysis identified the factors of tumor size, number of tumors, AFP level, AFP-L3 (> 15%) level, and 18F-FDG-PET positivity as being significantly associated with survival after RFA. In multivariate analysis, 18F-FDG-PET positivity and tumor size were identified as independent factors.
Table 5.
Univariate and multivariate analyses of factors related to survival
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| p value | HR | 95%CI | p value | HR | 95%CI | |
| Age (years) | 0.648 | 1.014 | 0.955–1.076 | |||
| Sex (female) | 0.253 | 0.517 | 0.167–1.603 | |||
| Etiology (HCV) | 0.963 | 0.974 | 0.313–3.025 | |||
| Child–Pugh class (A) | 0.147 | 0.456 | 0.158–1.316 | |||
| Platelets (×104/μL) | 0.139 | 1.033 | 0.990–1.078 | |||
| ALT (IU/L) | 0.365 | 1.006 | 0.993–1.020 | |||
| Hyaluronic acid (ng/mL) | 0.681 | 1.000 | 0.999–1.002 | |||
| Type IV collagen 7S (ng/mL) | 0.070 | 1.150 | 0.989–1.338 | |||
| Tumor size (mm) | 0.001 | 1.174 | 1.071–1.287 | 0.044 | 1.112 | 1.003–1.232 |
| Number of tumors | 0.037 | 2.035 | 1.044–3.965 | 0.535 | 1.315 | 0.554–3.122 |
| AFP (ng/mL) | 0.003 | 1.002 | 1.001–1.003 | 0.436 | 0.999 | 0.998–1.001 |
| AFP-L3 (> 15%) | 0.007 | 4.274 | 1.474–12.390 | 0.074 | 3.774 | 0.879–16.210 |
| DCP (mAU/mL) | 0.698 | 1.000 | 1.000–1.000 | |||
| PET positive | < 0.001 | 12.783 | 4.456–36.671 | 0.004 | 7.300 | 1.920–27.751 |
HR hazard ratio, CI confidence interval, HCV hepatitis C virus, ALT alanine aminotransferase, HCC hepatocellular carcinoma, AFP alpha-fetoprotein, AFP-L3 Lens culinaris agglutinin-reactive alpha-fetoprotein, DCP Des-gamma-carboxyprothrombin, PET positron emission tomography
Discussion
To the best of our knowledge, the present study is the first to evaluate the prognostic value of 18F-FDG-PET in patients with small HCC treated by RFA. Although some studies have reported the prognostic value of 18F-FDG uptake in HCC patients who underwent liver resection or liver transplantation, most of these studies were retrospective [23]. Many previous studies have used visual analysis, standardized uptake value (SUV), and tumor-to-nontumor liver uptake ratio (TLR) as parameters for evaluating 18F-FDG uptake, and showed that 18F-FDG-PET can predict the risk of early recurrence or poor survival after surgical resection or liver transplantation. For example, Hatano et al. reported that overall survival after resection was significantly longer in the lower SUV ratio group (SUV ratio < 2) than in the higher SUV ratio group (SUV ratio > 2) [18]. Seo et al. also reported that overall and disease-free survival rates were significantly lower in the high SUV or TLR group than in the low SUV or TLR group [19]. Hyun et al. reported that higher TLR (≥2) was significantly associated with death in patients underwent curative treatments including resection, liver transplantation, and radiofrequency ablation [24]. Lim et al. showed that visual positivity of 18F-FDG-PET was an independent predictor for early recurrence after liver resection in their prospective observation study [25]. In the present study, visual qualitative analysis was used to evaluate 18F-FDG uptake because in clinical practice, visual analysis is easier than quantitative analysis. Furthermore, as SUV is a relative value that is influenced by the imaging conditions, there is no accepted optimal cut-off value and most of the currently published data on SUVs in tumors are of little or no value to investigators outside the laboratory where the investigation was conducted [26]. In this study, there was no significant correlation between recurrence and visual 18F-FDG-PET positivity. It is known that HCCs have several recurrence patterns such as local or multicentric or intrahepatic or extrahepatic metastasis. Especially multicentric recurrence is not affected by tumor factors such as visual 18F-FDG-PET positivity. Several kinds of recurrence were also included in this study, so visual 18F-FDG-PET positivity may not have been related to recurrence including all recurrence patterns. The present study found a significant association of visual 18F-FDG-PET positivity with early metastatic recurrence and survival after RFA. Metastatic recurrence is difficult to treat curatively, and leads to cancer death. The poor survival of 18F-FDG-PET positive patients with small HCC treated by RFA is probably attributable to the high-grade malignant potential of the HCC.
The risk of recurrence after ablation is related to the tumor characteristics at the time of therapy, which include size, degree of differentiation, and the presence or absence of lymphovascular invasion [17]. In the present study, tumor size and 18F-FDG-PET positivity were independent factors related to metastatic recurrence after RFA. Some reports have already indicated that 18F-FDG-PET has high predictive value as a surrogate for the presence of microvascular invasion [27]. Ochi et al. identified SUV max as the only independent predictive factor for microsatellite distance > 1 cm from the primary tumor lesion [28]. It would be difficult to achieve a complete cure by RFA in 18F-FDG-PET positive HCCs because these tumors are already locally advanced, with microvascular invasion and intrahepatic metastasis. Therefore, even though 18F-FDG-PET positive HCCs are within the indications for RFA, these tumors should not be treated by RFA as the first-line treatment. Park et al. found that compared with a surgical margin of < 1 cm, a surgical margin of > 1 cm significantly improved overall survival in 18F-FDG-PET positive patients but not in 18F-FDG-PET negative patients [29]. Accordingly, segmental hepatectomy with a sufficient surgical margin, or positron beam therapy that can obtain a wider safety margin than RFA, could be considered as first-line treatment. However, the outcome of hepatectomy for 18F-FDG-PET positive HCC is also poor. Further investigation is required to clarify whether a margin of > 1 cm is sufficient to improve the prognosis of patients with 18F-FDG-PET positive HCC. Combination therapy with RFA and transcatheter arterial chemoembolization or adjuvant therapy could also be considered; however, the routine use of adjuvant therapy for patients with HCC following successful resection or ablation is not recommended [17]. Further study is needed to determine the optimal treatment strategy for 18F-FDG-PET positive small HCC.
Several limitations must be considered when interpreting the results of the present study. First, the sample size is small because of the low proportion of 18F-FDG-PET positive HCCs among small HCCs. Especially, the proportion of 18F-FDG-PET positive HCCs was lower compared to 18F-FDG-PET negative HCCs which would have affected the statistical power. Therefore, a larger scale study is needed to validate our results. Second, histological evaluation of HCC was not performed. Intrahepatic cholangiocarcinoma (ICC) is also positive on 18F-FDG-PET, and we were unable to completely exclude ICC or combined ICC and HCC. Third, the optimal treatment strategy for 18F-FDG-PET positive small HCC cannot be derived from our results.
Conclusions
In conclusion, 18F-FDG-PET positivity was significantly associated with outcomes after RFA. As RFA for 18F-FDG-PET positive small HCC has a high risk of metastatic recurrence and poor prognosis, RFA should not be readily selected as the first-line treatment, even though it is within the indications for RFA. 18F-FDG-PET should be performed in considering the optimal treatment strategy for small hypervascular HCCs diagnosed by contrast CT or MRI.
Acknowledgements
Not applicable.
Abbreviations
- 18F-FDG
18F-fluorodeoxyglucose
- HCC
Hepatocellular carcinoma
- RFA
Radiofrequency ablation
- PET
Positron emission tomography
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- ALT
Alanine aminotransferase
- AFP
Alpha-fetoprotein
- AFP-L3
Lens culinaris agglutinin-reactive alpha-fetoprotein
- DCP
Des-gamma-carboxy prothrombin
- HRs
Hazard ratios
- CIs
Confidence intervals
- HCV
Hepatitis C virus
- SUV
Standardized uptake value
- TLR
Tumor-to-nontumor liver uptake ratio
- ICC
Intrahepatic cholangiocarcinoma
Authors’ contributions
Yoshiyuki Ida and Hideyuki Tamai designed the study, analyzed the data, and wrote the article; Naoki Shingaki performed the statistical analysis; Ryo Shimizu and Shuya Maeshima acquired the data; Takao Maekita and Mikitaka Iguchi reviewed the results; Masaki Terada analyzed the 18F-FDG-PET images and reviewed the methods; and Masayuki Kitano reviewed and edited the article. The author(s) read and approved the final manuscript.
Funding
This research did not receive any specific funding.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. However, there is no additional data available.
Ethics approval and consent to participate
This study was approved by the ethics committee of Wakayama Medical University, Japan. Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Makuuchi M, Kokudo N. Clinical practice guidelines for hepatocellular carcinoma - the Japan Society of Hepatology 2009 update. Hepatol Res. 2010;40(Suppl 1):2–144. doi: 10.1111/j.1872-034X.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931–1937. doi: 10.1002/cncr.10892. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda S, Itamoto T, Nakahara H, Kohashi T, Ohdan H, Hino H, et al. Clinicopathologic features and prognostic factors of resected solitary small-sized hepatocellular carcinoma. Hepatogastroenterology. 2005;52:1163–1167. [PubMed] [Google Scholar]
- 6.Imai K, Beppu T, Nakayama Y, Ishiko T, Horino K, Komori H, et al. Preoperative prediction of poorly differentiated components in small-sized hepatocellular carcinoma for safe local ablation therapy. J Surg Oncol. 2009;100:121–126. doi: 10.1002/jso.21302. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita Y, Tsuijita E, Takeishi K, Fujiwara M, Kira S, Mori M, et al. Predictors for microinvasion of small hepatocellular carcinoma </= 2 cm. Ann Surg Oncol. 2012;19:2027–2034. doi: 10.1245/s10434-011-2195-0. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Lim HK, Choi D, Lee WJ, Kim MJ, Kim CK, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: effect of histologic grade on therapeutic results. AJR Am J Roentgenol. 2006;186(Suppl):327–333. doi: 10.2214/AJR.05.0350. [DOI] [PubMed] [Google Scholar]
- 9.Nicoli N, Casaril A, Hilal MA, Mangiante G, Marchiori L, Ciola M, et al. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165–167. doi: 10.1016/j.amjsurg.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 10.Masuda T, Beppu T, Ishiko T, Horino K, Baba Y, Mizumoto T, et al. Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepato-Biliary-Pancreat Surg. 2008;15:589–595. doi: 10.1007/s00534-007-1288-4. [DOI] [PubMed] [Google Scholar]
- 11.Mori Y, Tamai H, Shingaki N, Moribata K, Shiraki T, Deguchi H, et al. Diffuse intrahepatic recurrence after percutaneous radiofrequency ablation for solitary and small hepatocellular carcinoma. Hepatol Int. 2009;3:509–515. doi: 10.1007/s12072-009-9131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seki T, Tamai T, Ikeda K, Imamura M, Nishimura A, Yamashiki N, et al. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastroenterol Hepatol. 2001;13:291–294. doi: 10.1097/00042737-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol. 2003;8:332–335. doi: 10.1007/s10147-003-0328-6. [DOI] [PubMed] [Google Scholar]
- 14.Portolani N, Tiberio GA, Ronconi M, Coniglio A, Ghidoni S, Gaverini G, et al. Aggressive recurrence after radiofrequency ablation of liver neoplasms. Hepatogastroenterology. 2003;50:2179–2184. [PubMed] [Google Scholar]
- 15.Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, et al. Rapid progression of hepatocellular carcinoma after radiofrequency ablation. World J Gastroenterol. 2004;10:1137–1140. doi: 10.3748/wjg.v10.i8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llovet JM, Vilana R, Bru C, Bianchi L, Salmeron JM, Boix L, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 17.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 18.Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, et al. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surg. 2006;30:1736–1741. doi: 10.1007/s00268-005-0791-5. [DOI] [PubMed] [Google Scholar]
- 19.Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427–433. doi: 10.1158/1078-0432.CCR-06-1357. [DOI] [PubMed] [Google Scholar]
- 20.Hong G, Suh KS, Suh SW, Yoo T, Kim H, Park MS, et al. Alpha-fetoprotein and (18) F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol. 2016;64:852–859. doi: 10.1016/j.jhep.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Lin CY, Liao CW, Chu LY, Yen KY, Jeng LB, Hsu CN, et al. Predictive value of 18F-FDG PET/CT for vascular invasion in patients with hepatocellular carcinoma before liver transplantation. Clin Nucl Med. 2017;42:e183–e1e7. doi: 10.1097/RLU.0000000000001545. [DOI] [PubMed] [Google Scholar]
- 22.Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee SM, Kim HS, Lee S, Lee JW. Emerging role of (18) F-fluorodeoxyglucose positron emission tomography for guiding management of hepatocellular carcinoma. World J Gastroenterol. 2019;25:1289–1306. doi: 10.3748/wjg.v25.i11.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyun SH, Eo JS, Lee JW, Choi JY, Lee KH, Na SJ, et al. Prognostic value of (18) F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with Barcelona clinic liver Cancer stages 0 and a hepatocellular carcinomas: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging. 2016;43:1638–1645. doi: 10.1007/s00259-016-3348-y. [DOI] [PubMed] [Google Scholar]
- 25.Lim C, Salloum C, Chalaye J, Lahat E, Costentin CE, Osseis M, et al. 18F-FDG PET/CT predicts microvascular invasion and early recurrence after liver resection for hepatocellular carcinoma: a prospective observational study. HPB (Oxford) 2019;21:739–747. doi: 10.1016/j.hpb.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Keyes JW. SUV: standard uptake or silly useless value? J Nucl Med. 1995;36:1836–1839. [PubMed] [Google Scholar]
- 27.Kornberg A, Friess H. (18) F-fludeoxyglucose positron emission tomography for diagnosis of HCC: implications for therapeutic strategy in curative and non-curative approaches. Ther Adv Gastroenterol. 2019. 10.1177/1756284819836205. [DOI] [PMC free article] [PubMed]
- 28.Ochi H, Hirooka M, Hiraoka A, Koizumi Y, Abe M, Sogabe I, et al. (18) F-FDG-PET/CT predicts the distribution of microsatellite lesions in hepatocellular carcinoma. Mol Clin Oncol. 2014;2:798–804. doi: 10.3892/mco.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JH, Kim DH, Kim SH, Kim MY, Baik SK, Hong IS. The clinical implications of liver resection margin size in patients with hepatocellular carcinoma in terms of positron emission tomography positivity. World J Surg. 2018;42:1514–1522. doi: 10.1007/s00268-017-4275-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. However, there is no additional data available.


