Abstract
Introduction
The prognostic 15-gene expression profile (15-GEP) test for uveal melanoma (UM) predicts metastatic risk based on primary tumor biology. Here we report outcomes from a prospective registry of 15-GEP-tested patients, and a meta-analysis with published cohorts.
Objectives
Management and 5-year clinical outcomes following 15-GEP testing were evaluated.
Methods
Eighty-nine patients with 15-GEP results were prospectively enrolled at four centers. Physician-recommended management plans were collected, and clinical outcomes tracked every 6 months.
Results
Eighty percent of Class 1 (low-risk) patients underwent low-intensity management; all Class 2 (high-risk) patients underwent high-intensity management (p < 0.0001). Median follow-up for event-free patients was 4.9 years. Five Class 1 (10%) and 23 Class 2 (58%) tumors metastasized (p < 0.0001). Five-year Class 1 and 2 metastasis-free survival rates were 90% (81–100%) and 41% (27–62%; p < 0.0001), and melanoma-specific survival rates were 94% (87–100%) and 63% (49–82%; p = 0.0007). Class 2 was the only independent predictor of metastasis and was associated with increased risk for metastasis and mortality by meta-analysis.
Conclusions
UM patient management is guided by 15-GEP testing. Class 2 patients were managed more intensely, in accordance with an observed metastatic rate of >50%; Class 1 patients were safely spared intensive surveillance, resulting in appropriate utilization of healthcare resources.
Keywords: Gene expression profiling, Uveal melanoma, Prognosis, Meta-analysis
Introduction
Uveal melanoma (UM) is a rare intraocular cancer that carries a 30–50% risk of metastasis within 5 years of diagnosis [1, 2]. Metastases are observed most commonly in the liver and less frequently in the lungs and other organs. Thus, surveillance following definitive treatment of the eye tumor in the absence of risk stratification often includes ultrasound or cross-sectional imaging of the abdomen and chest, as well as liver function testing. The goal of metastatic surveillance is to identify the development of metastatic disease as early as possible, as studies have shown that routine imaging can identify early disease amenable to resection [3, 4], and liver-directed therapies have better response rates and improved outcomes in patients with lower metastatic disease burden [5, 6, 7, 8, 9, 10, 11, 12].
However, approximately half of UM patients are expected to remain metastasis-free, making frequent imaging, laboratory testing, and medical oncology visits unnecessary, burdensome, and costly. Therefore, patients are normally stratified based on their risk of metastasis, with the goal of customizing surveillance plans to provide lower intensity for the patients with a low risk of metastasis while maintaining higher intensity surveillance for those likely to develop metastatic disease. Clinicopathologic staging has limited predictive value in UM and may be confounded by interobserver variabilities in tumor measurements [13] that are fundamental to American Joint Committee on Cancer (AJCC) staging.
A prognostic 15-gene expression profile (15-GEP) test that predicts 5-year metastatic risk with Class 1A, 1B, and 2 results indicating low-, intermediate-, and high-risk groups, respectively, has been in clinical use for several years [14, 15]. The test's accuracy has been documented in several retrospective and prospective studies involving more than 1,600 patients [14, 15, 16, 17, 18, 19, 20]; and its high technical reliability has also been reported [14, 15, 17, 21]. It has been demonstrated that physicians use the test results in a risk-specific manner to guide metastatic surveillance recommendations including imaging, specialist referrals, laboratory testing, and clinical trial participation for adjuvant therapy [19, 22, 23]. Combining these concepts, it is critical to demonstrate that patients who are considered low risk by the 15-GEP test (Class 1) and subsequently were recommended to have reduced intensity metastatic surveillance for metastatic disease do, in fact, have low rates of metastasis and superior long-term outcomes compared to Class 2 patients who received higher intensity care. We initiated a multicenter, prospective, observational registry study to evaluate this question and reported the initial results after a median follow-up time of 2.4 years in 70 patients [19]. In this study, we report the 5-year health outcomes of this study with 89 enrolled patients. This study represents the longest follow-up for 15-GEP-tested UM patients reported to date.
Methods
15-GEP Analysis of Tumor Specimens
UM tumor specimens were collected by either fine needle aspiration biopsy (FNAB) or by preparing six 5-μm section slides from the formalin-fixed paraffin-embedded (FFPE) tumor block after enucleation. Frozen FNABs or FFPE slides were sent to a CAP-accredited, CLIA-certified laboratory (Castle Biosciences, Inc., Phoenix, AZ, USA) as part of routine clinical care for 15-GEP testing. Tumor tissue processing and 15-GEP testing (DecisionDx-UM) were performed using previously described methods [14, 19, 21].
Study Design and Patient Enrollment
The CLEAR Registry Study (clinical trials identifier NCT02376920 at clinicaltrials.gov) was a prospective, multicenter registry study that included patients who had prognostic 15-GEP testing performed as part of their clinical care [19]. The study was approved by the Institutional Review Board at each participating center. All enrolled patients provided written informed consent. Physician treatment plans for surveillance, referral, and adjuvant therapy (clinical trials) were entered into a secure case report form upon receipt of 15-GEP test results. Baseline clinical status was also entered at this time, and patients' clinical outcomes were updated every 6 months prospectively. The date of data censor for this analysis was April 17, 2019.
Surveillance Categorization
Intensity of metastatic screening (liver function testing and surveillance imaging) was categorized as previously described [19, 22]. A low-intensity schedule was defined as annual surveillance by liver function testing and/or imaging, and a high-intensity schedule was defined as quarterly or biannual liver function testing and/or imaging.
Statistical Analyses
Class 1 and 2 patients and tumor characteristics were compared using Pearson χ2 or Kruskal-Wallis F tests as indicated. Metastasis-free survival (MFS) and melanoma-specific survival (MSS) rates were estimated using the Kaplan-Meier method and compared by log-rank test. Cox proportional hazards were used to compare the 15-GEP test and clinical covariates. For all comparisons, a p value of <0.05 was considered significant.
Meta-Analysis of Study and Published Cohorts
Given the limitations of the small cohort in this study, we aimed to determine a better estimate of relative risk associated with a Class 2 result compared to a Class 1 result based on cohorts from multiple studies. Univariate hazard ratios (HRs) associated with the 15-GEP test result (Class 2 compared to Class 1 patients) were extracted as reported or, if not directly published, were estimated from Kaplan-Meier curves [24]. The resulting HRs are shown in online supplementary Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000508382. Because some of the study cohorts have substantial patient overlap, such as a later analysis from the same ocular oncology practice [18] or specifically indicated in the text [20], the most recent of these studies were used for the meta-analysis. In the final meta-analysis, 3 studies were included in the assessment of metastasis and 4 studies were included to analyze melanoma-specific or all-cause mortality. The “meta” package in R.3.6 was used to perform the analysis with fixed and random effects model weighting.
Results
Patient and Tumor Characteristics and 15-GEP Test Results
Clinical and tumor characteristics for the 89 patients enrolled in the CLEAR study are shown in Table 1. There were 49 (55%) Class 1 tumors and 40 (45%) Class 2 tumors. Of the Class 1 tumors, 38 (78%) were Class 1A and 11 (22%) were Class 1B. The median patient age of the cohort was 61 years (range 29–87 years) and age was not significantly different between Class 1 and 2 patients. The only tumor feature significantly different between Class 1 and 2 was the median largest basal diameter (LBD; 12.8 and 15.8, respectively, p = 0.01).
Table 1.
Patient and tumor characteristics
| Feature | All patients (n = 89) | Class 1 (n = 49) | Class 2 (n = 40) |
p value |
|---|---|---|---|---|
| Age at diagnosis, years | 61 (29–87) | 60 (29–84) | 62.5 (31–87) | nsa |
| Ciliary body involvement | 34 (38) | 20 (41) | 14 (35) | nsb |
| Largest basal diameter, mm | 13.7 (3.6–27) | 12.8 (3.6–25.5) | 15.8 (4.9–27) | 0.01a |
| Thickness, mm | 5.5 (l.4–16.4) | 5.0 (1.4–16.4) | 6.85 (2–16.1) | nsa |
| Treatment type | ||||
| Plaque | 48 (54) | 33 (67) | 15 (38) | 0.006c |
| Proton beam | 4 (4) | 3 (6) | 1 (3) | nsc |
| Enucleation | 31 (35) | 11 (22) | 20 (50) | 0.008c |
| TTT | 1 (1) | 0 (0) | 1 (2) | nsc |
| None specified | 5 (6) | 2 (4) | 3 (8) | |
Data are presented as median (range) or n (%), as appropriate.
Kruskal-Wallis test.
Pearson χ2 test.
Fisher's exact test. TTT, transpupillary thermotherapy.
15-GEP Test Result Impact on Patient Management
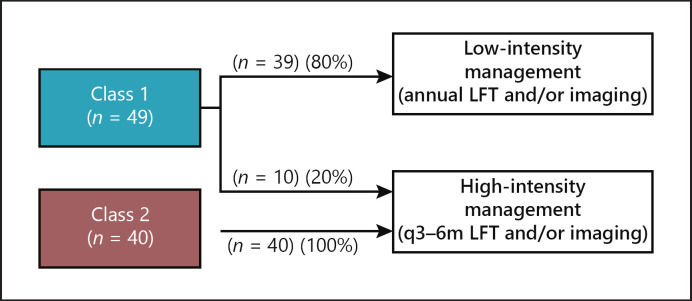
Physician recommendations for metastatic surveillance after receipt of 15-GEP test results were categorized into low- and high-intensity management based on the frequency of screening as previously published [19, 22]. All (100%) patients with Class 2 tumors were managed with high-intensity surveillance, while 80% of Class 1 patients had low-intensity surveillance (p < 0.0001) (Fig. 1). Of the 10 Class 1 patients who were managed by surveillance every 3–6 months (high-intensity), seven (70%) of them were Class 1B.
Fig. 1.
Management intensity for patients according to 15-GEP test results. Of the 89 patients in the study, 39 (80%) patients with a Class 1 result had low-intensity management (defined as imaging and/or liver function tests [LFTs] on an annual basis) and 20% had high-intensity management (defined as imaging and/or liver function tests every 3–6 months [q3–6 months]). All (100%) Class 2 patients had high-intensity surveillance, and they accounted for 80% of those undergoing high-intensity surveillance.
Clinical Outcomes in Class 1 and 2 Patients
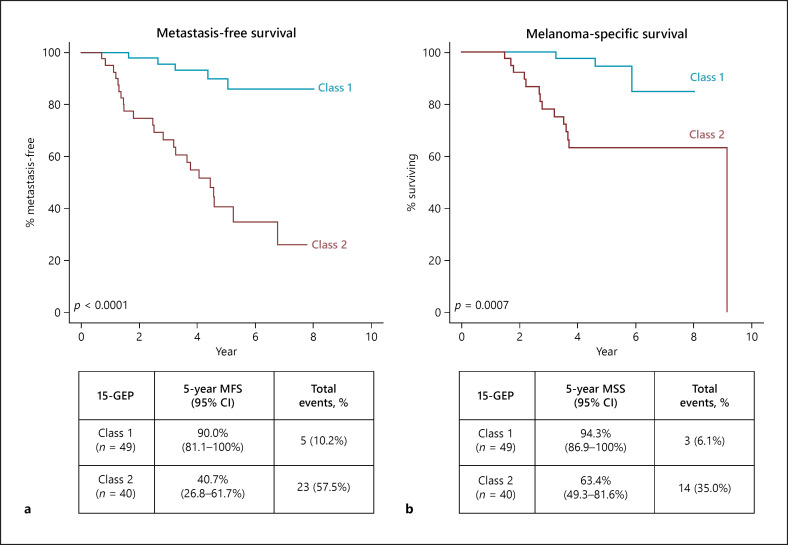
As of the censor date of April 2019, the median follow-up time for patients who remained metastasis-free was 4.9 years (1.5–8.0 years). Twenty-three patients (58%) with Class 2 tumors and 5 patients (10%) with Class 1 tumors experienced metastasis (p < 0.0001; Fig. 2a). The 5 Class 1 tumors with metastasis were Class 1A subtype. The median time to metastasis was 2.5 years for Class 2 and 3.2 years for Class 1 tumors (p = 0.34 by Mann-Whitney U test). Five-year MFS rates for Class 1 and Class 2 tumors was 90% (81–100%) and 41% (27–62%; p < 0.0001), respectively, as shown in Figure 2a. Five-year MSS rates were 94% (87–100%) and 63% (49–82%) for Class 1 and 2 patients, respectively (p = 0.0007; Fig. 2b).
Fig. 2.
Five-year clinical outcomes in patients with Class 1 and 2 tumors. a Five-year metastasis-free survival (MFS) rates for Class 1 and 2 patients estimated by the Kaplan-Meier method and significance evaluated by log-rank test. b Five-year melanoma-specific survival (MSS) rates for Class 1 and 2 patients estimated by the Kaplan-Meier method and significance evaluated by log-rank test.
Relative Risks Associated with Clinicopathologic Features and 15-GEP Test Results
Tumor thickness, LBD, ciliary body involvement, age, and 15-GEP test result were included as covariables in multivariate analysis, which showed that a Class 2 result was the only independent predictor of metastasis (Table 2; HR 7.53, 95% CI 2.79–20.3, p < 0.0001). For melanoma-specific mortality, a Class 2 result had an HR of 6.02 (95% CI 1.62–22.3, p = 0.007), while the only other independent predictor was ciliary body involvement which, contrary to its reported role as a poor prognostic feature, was associated with a reduced risk of death (HR 0.24, 95% CI 0.06–1.00, p = 0.049) in this cohort. Multivariate analysis was also performed with LBD and tumor thickness as categorical variables. LBD was categorized as ≥12 mm and <12 mm, and tumor thickness by the median. In this analysis, Class 2 was still the only independent predictor of metastasis (Class 2 HR 7.19, 95% CI 2.67–19.2, p < 0.0001) and also the only independent predictor of melanoma-specific mortality (Class 2 HR 5.30, 95% CI 1.46–19.3, p = 0.01).
Table 2.
Multivariate analysis of molecular and clinicopathologic covariates
| Covariate | Hazard ratio (95% CI) | p value |
|---|---|---|
| Metastasis | ||
| Age | 1.00 (0.97–1.03) | 0.94 |
| Ciliary body involvement | 0.56 (0.21–1.49) | 0.25 |
| Largest basal diameter | 1.03 (0.91–1.16) | 0.64 |
| Thickness | 1.08 (0.92–1.27) | 0.34 |
| 15-GEP Class 2 | 7.53 (2.79–20.31) | <0.0001 |
| Melanoma-specific mortality | ||
| Age | 1.00 (0.96–1.05) | 0.77 |
| Ciliary body involvement | 0.24 (0.06–1.00) | 0.049 |
| Largest basal diameter | 1.03 (0.88–1.21) | 0.72 |
| Thickness | 1.17 (0.04–1.46) | 0.18 |
| 15-GEP Class 2 | 6.02 (1.62–22.3) | 0.007 |
Largest basal diameter, age, and thickness are considered as continuous variables. 15-GEP, 15-gene expression profile.
Comparison and Meta-Analysis of 15-GEP Test Results across Studies
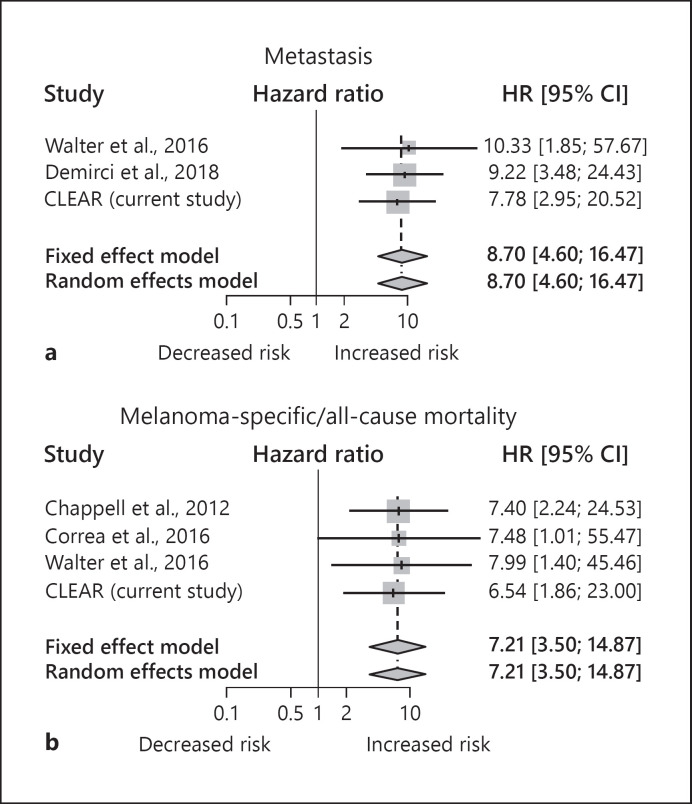
Several prior studies have evaluated clinical outcomes (metastasis, melanoma-specific mortality, and all-cause mortality) of patients tested with the 15-GEP test [14, 16, 17, 18, 20, 25]. A summary of these studies and the current study are shown in online supplementary Table 1. A meta-analysis of the studies was performed for relative risk of metastasis and mortality (either all-cause or melanoma-specific), and the resulting forest plots are shown in Figure 3. For patients with Class 2 melanoma the HR was 8.70 (95% CI 4.60–16.47, p < 0.0001) for metastasis and 7.21 (95% CI 3.50–14.87, p < 0.0001) for mortality. Heterogeneity was not detected in either analysis as indicated by I2 tests, and thus results from the random and fixed effects weighting were the same.
Fig. 3.
Cumulative risk of metastasis and mortality associated with a Class 2 result across studies. a Forest plot of studies reporting 15-GEP test result and metastasis. b Forest plots of studies reporting 15-GEP test result and all-cause or melanoma-specific mortality. For both panels, univariate hazard ratios (HRs) with 95% CIs are shown to the right of each study. Gray boxes reflect the weight of the study in the aggregated estimate (diamonds) based on study error and effect size (where error is inversely related to weighting), vertical lines represent hazard ratio, and horizontal lines represent 95% CI. Dotted vertical line and center of the diamond represent the aggregated hazard ratios of fixed and random effect models, and diamond width indicates the overall confidence interval in both fixed and random effects models.
Discussion/Conclusion
In oncology, the goal of a surveillance regimen after definitive treatment of the primary tumor is to identify asymptomatic metastatic disease early while the tumor burden is low and the therapeutic response can be maximized [26, 27]. In UM, lower tumor burden at the initiation of liver-directed therapies, as well as liver resection, has been associated with improved response rates and survival outcomes [7, 8, 9, 10, 12, 28]. Given these data − the relatively high risk of metastasis in UM and estimated metastatic tumor doubling times − high-intensity follow-up for metastatic screening may include screenings every 4–6 months [29, 30]. In patients considered to be at high risk for metastasis, metastatic surveillance with biannual MRI has been shown to be effective at identifying asymptomatic liver metastases [3, 4]. However, as many as 50% of patients with UM will not experience metastasis, and thus this high-intensity surveillance will not be beneficial and is not necessary.
The 15-GEP test categorizes patients as likely versus unlikely to experience disease progression based on intrinsic differences in tumor biology [14]. With documented accuracy, the test has been integrated into routine practice during the last decade [19, 22]. Unlike prior studies which have found LBD to add independent prognostic value in addition to the 15-GEP test result [18, 20, 25], in the current study GEP was the only statistically significant clinicopathologic factor to predict both MFS and MSS in multivariate analysis. Similarly, several published studies have reported that the 15-GEP test was the strongest independent prognostic factor in multivariate analysis with clinicopathologic features [13, 14, 18, 19, 20]. As demonstrated in this study, patients with tumors identified to be high-risk Class 2 are directed to undergo intensive surveillance, as described above, and the majority of those with low-risk Class 1 tumors have follow-up at a reduced or de-escalated intensity, with annual metastatic surveillance. When data in this study are combined with those of a previous retrospective chart review [22] for a total of 177 patients tested with 15-GEP, 90% of Class 1 patients received annual metastatic surveillance, while all Class 2 patients had quarterly to biannual surveillance. Recently, Davanzo et al. [23] reported similar results on management recommendations and showed that patient adherence to these recommendations were better in Class 2 patients.
This study reports the longest follow-up for 15-GEP-tested patients to date. Given the estimated HRs for metastasis and melanoma-specific mortality associated with Class 2 melanoma in this and a previously published prospective cohort [14], a statistical sample size calculation shows that only 29 total patients are necessary to demonstrate a statistically significant difference in metastasis between Class 1 and 2 and only 47 patients are necessary to show significance in melanoma-specific mortality. Therefore, the cohort of 89 patients reported here has sufficient statistical power to evaluate differences in clinical outcomes between Class 1 and 2 patients.
In the current study, patients with Class 2 results, all of whom had high-intensity surveillance, had a 58% overall rate of metastasis, a 35% overall rate of melanoma-specific death, and 5-year MFS and disease-specific survival rates of 41 and 63%, respectively. Conversely, 80% of Class 1 patients had low-intensity surveillance, and Class 1 patients had significantly better outcomes compared to Class 2 patients as reflected in 5-year MFS and disease-specific survival rates of 90 and 94%, respectively. Overall rates of metastasis and melanoma-specific mortality in Class 1 patients were 10% and 6%, respectively. Lower rates of metastasis for Class 1 tumors have been reported in previously published studies, ranging from 1 to 6% (online suppl. Table 1). However, most of these studies had shorter follow-up (mean: 28.6 months; median: 23 months). The higher metastatic rate for both Class 1 and 2 patients in the current analysis could be reflective of a smaller cohort and longer follow-up time compared to other published reports.
Of the 10 Class 1 patients who were recommended to have high-intensity surveillance, 7 had Class 1B tumors with an expected intermediate risk of metastasis. While none of these patients had metastasis at the last follow-up, it has been suggested that Class 1B tumors have latent metastasis compared to Class 2 tumors, and thus it is possible that the follow-up time was not long enough to detect potentially latent metastatic disease in Class 1B patients. Of the 5 Class 1A patients who experienced metastasis during the study period, 4 patients were recommended to have annual liver function tests with liver and/or chest imaging, and 1 patient had only annual liver function testing recommended. Of note, 11 other Class 1A patients who did not experience metastasis were screened with liver function testing alone, while no Class 1B or Class 2 patients were screened with liver function testing alone. Two cases within the cohort had a documented local recurrence, but none of the Class 1 patients who experienced a distant metastasis had evidence of a local recurrence. While the risk associated with a Class 1A tumor is low, it is not expected to be 0%, and thus annual metastatic screening, as was generally recommended by physicians participating in this study, may still be warranted as it spared the 90% of patients who did not metastasize from more intensive screening.
The results of this 5-year analysis suggest that intensive screening was appropriate for Class 2 patients and that reduced-intensity surveillance in Class 1 patients was also appropriate given that 90% of these patients were metastasis free at the last follow-up and were safely spared extra imaging, laboratory testing, and clinical visits. These results are aligned with current National Comprehensive Cancer Network (NCCN) recommendations for surveillance imaging based on the 15-GEP test result, wherein Class 1 patients have less frequent imaging for metastatic screening while Class 2 patients have more frequent imaging [30]. However, whether more frequent screening should be recommended to Class 1A patients to identify those that recur earlier, and whether this would affect survival, is a question that should be addressed by ocular oncology experts.
Since the initial prospective validation of the 15-GEP test, several single and multicenter studies have reported clinical outcomes associated with tested tumors [16, 17, 18, 20, 25]. A meta-analysis including those studies and the current one shows the consistent performance of the 15-GEP test to predict risk of metastasis and melanoma-specific mortality in UM. This is consistent with the test's incorporation into routine clinical care and its inclusion in both the AJCC [31] and NCCN guidelines for UM to help guide schedules for metastatic screening, as previously mentioned [30].
In conclusion, this study supports that the 15-GEP test is used to appropriately guide metastatic surveillance in UM patients based on risk for metastasis. Optimization of surveillance plans based on individual metastatic risk leads to improved health outcomes by reducing the burden of surveillance for Class 1 patients. A reduction in this surveillance burden, coupled with a lower frequency of visits and a potential reduction in exposure to radiation, are demonstrable healthcare outcome benefits for those who have a low rate of metastatic disease. For Class 2 patients, the test allows for increased surveillance in patients who are more likely to develop metastatic disease, thereby improving the utilization of healthcare resources by focusing care on those who need it most.
Statement of Ethics
This study was approved by the Institutional Review Board at each participating center, and all enrolled patients provided their written informed consent. This research complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Disclosure Statement
K.R.C., K.M.P., K.M.A., K.M.O., and F.A.M. are employees and shareholders of Castle Biosciences, Inc.
Funding Sources
This study was sponsored by Castle Biosciences, Inc.
Author Contributions
T.M.A., T.T., and Y.S. contributed to the patient enrollment and data acquisition, and provided critical review of the manuscript, tables, and figures. K.R.C. contributed to the data analysis and interpretation, and provided critical review of the manuscript, tables, and figures. K.M.P. contributed to the study conception and design, data analysis and interpretation, and critical review of the manuscript, tables, and figures. K.M.A. contributed to the study design and critical review of the manuscript, tables, and figures. K.M.O. contributed to the study design and reviewed the manuscript, tables, and figures. F.A.M. contributed to the study conception and design, data analysis and interpretation, and provided critical review of the manuscript, tables, and figures. All authors agreed to the final, submitted version of the paper and have agreed to be accountable for the accuracy and integrity of the study.
Supplementary Material
Supplementary data
References
- 1.Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Collaborative Ocular Melanoma Study Group Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005 Dec;123((12)):1639–43. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 2.Collaborative Ocular Melanoma Study Group The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006 Dec;124((12)):1684–93. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- 3.Gomez D, Wetherill C, Cheong J, Jones L, Marshall E, Damato B, et al. The Liverpool uveal melanoma liver metastases pathway: outcome following liver resection. J Surg Oncol. 2014 May;109((6)):542–7. doi: 10.1002/jso.23535. [DOI] [PubMed] [Google Scholar]
- 4.Marshall E, Romaniuk C, Ghaneh P, Wong H, McKay M, Chopra M, et al. MRI in the detection of hepatic metastases from high-risk uveal melanoma: a prospective study in 188 patients. Br J Ophthalmol. 2013 Feb;97((2)):159–63. doi: 10.1136/bjophthalmol-2012-302323. [DOI] [PubMed] [Google Scholar]
- 5.Akyuz M, Yazici P, Dural C, Yigitbas H, Okoh A, Bucak E, et al. Laparoscopic management of liver metastases from uveal melanoma. Surg Endosc. 2016 Jun;30((6)):2567–71. doi: 10.1007/s00464-015-4527-9. [DOI] [PubMed] [Google Scholar]
- 6.Frenkel S, Nir I, Hendler K, Lotem M, Eid A, Jurim O, et al. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br J Ophthalmol. 2009 Aug;93((8)):1042–6. doi: 10.1136/bjo.2008.153684. [DOI] [PubMed] [Google Scholar]
- 7.Gonsalves CF, Eschelman DJ, Sullivan KL, Anne PR, Doyle L, Sato T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol. 2011 Feb;196((2)):468–73. doi: 10.2214/AJR.10.4881. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Bedikian AY, Ahrar J, Ensor J, Ahrar K, Madoff DC, et al. Hepatic artery chemoembolization in patients with ocular melanoma metastatic to the liver: response, survival, and prognostic factors. Am J Clin Oncol. 2010 Oct;33((5)):474–80. doi: 10.1097/COC.0b013e3181b4b065. [DOI] [PubMed] [Google Scholar]
- 9.Hsueh EC, Essner R, Foshag LJ, Ye X, Wang HJ, Morton DL. Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer. 2004 Jan;100((1)):122–9. doi: 10.1002/cncr.11872. [DOI] [PubMed] [Google Scholar]
- 10.Huppert PE, Fierlbeck G, Pereira P, Schanz S, Duda SH, Wietholtz H, et al. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur J Radiol. 2010 Jun;74((3)):e38–44. doi: 10.1016/j.ejrad.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 11.Leyvraz S, Spataro V, Bauer J, Pampallona S, Salmon R, Dorval T, et al. Treatment of ocular melanoma metastatic to the liver by hepatic arterial chemotherapy. J Clin Oncol. 1997 Jul;15((7)):2589–95. doi: 10.1200/JCO.1997.15.7.2589. [DOI] [PubMed] [Google Scholar]
- 12.Mariani P, Piperno-Neumann S, Servois V, Berry MG, Dorval T, Plancher C, et al. Surgical management of liver metastases from uveal melanoma: 16 years' experience at the Institut Curie. Eur J Surg Oncol. 2009 Nov;35((11)):1192–7. doi: 10.1016/j.ejso.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Char DH, Kroll S, Stone RD, Harrie R, Kerman B. Ultrasonographic measurement of uveal melanoma thickness: interobserver variability. Br J Ophthalmol. 1990 Mar;74((3)):183–5. doi: 10.1136/bjo.74.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onken MD, Worley LA, Char DH, Augsburger JJ, Correa ZM, Nudleman E, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012 Aug;119((8)):1596–603. doi: 10.1016/j.ophtha.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010 Jul;12((4)):461–8. doi: 10.2353/jmoldx.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chappell MC, Char DH, Cole TB, Harbour JW, Mishra K, Weinberg VK, et al. Uveal melanoma: molecular pattern, clinical features, and radiation response. Am J Ophthalmol. 2012 Aug;154((2)):227–232.e2. doi: 10.1016/j.ajo.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correa ZM, Augsburger JJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications. Graefes Arch Clin Exp Ophthalmol. 2014 Jan;252((1)):131–5. doi: 10.1007/s00417-013-2515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corrêa ZM, Augsburger JJ. Independent Prognostic Significance of Gene Expression Profile Class and Largest Basal Diameter of Posterior Uveal Melanomas. Am J Ophthalmol. 2016 Feb;162:20–27.e1. doi: 10.1016/j.ajo.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Plasseraud KM, Cook RW, Tsai T, Shildkrot Y, Middlebrook B, Maetzold D, et al. Clinical Performance and Management Outcomes with the DecisionDx-UM Gene Expression Profile Test in a Prospective Multicenter Study. J Oncol. 2016;2016:5325762. doi: 10.1155/2016/5325762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter SD, Chao DL, Feuer W, Schiffman J, Char DH, Harbour JW. Prognostic Implications of Tumor Diameter in Association With Gene Expression Profile for Uveal Melanoma. JAMA Ophthalmol. 2016 Jul;134((7)):734–40. doi: 10.1001/jamaophthalmol.2016.0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plasseraud KM, Wilkinson JK, Oelschlager KM, Poteet TM, Cook RW, Stone JF, et al. Gene expression profiling in uveal melanoma: technical reliability and correlation of molecular class with pathologic characteristics. Diagn Pathol. 2017 Aug;12((1)):59. doi: 10.1186/s13000-017-0650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaberg TM, Jr, Cook RW, Oelschlager K, Maetzold D, Rao PK, Mason JO., 3rd Current clinical practice: differential management of uveal melanoma in the era of molecular tumor analyses. Clin Ophthalmol. 2014 Dec;8:2449–60. doi: 10.2147/OPTH.S70839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davanzo JM, Binkley EM, Bena JF, Singh AD. Risk-stratified systemic surveillance in uveal melanoma. Br J Ophthalmol. 2019 Jan;31:313569. doi: 10.1136/bjophthalmol-2018-313569. [DOI] [PubMed] [Google Scholar]
- 24.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007 Jun;8((1)):16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demirci H, Niziol LM, Ozkurt Z, Slimani N, Ozgonul C, Liu T, et al. Do Largest Basal Tumor Diameter and the American Joint Committee on Cancer's Cancer Staging Influence Prognostication by Gene Expression Profiling in Choroidal Melanoma. Am J Ophthalmol. 2018 Nov;195:83–92. doi: 10.1016/j.ajo.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Freeman M, Laks S. Surveillance imaging for metastasis in high-risk melanoma: importance in individualized patient care and survivorship. Melanoma Manag. 2019 Apr;6((1)):MMT12. doi: 10.2217/mmt-2019-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salama AK, de Rosa N, Scheri RP, Pruitt SK, Herndon JE, 2nd, Marcello J, et al. Hazard-rate analysis and patterns of recurrence in early stage melanoma: moving towards a rationally designed surveillance strategy. PLoS One. 2013;8((3)):e57665. doi: 10.1371/journal.pone.0057665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel K, Sullivan K, Berd D, Mastrangelo MJ, Shields CL, Shields JA, et al. Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: results of a phase II study. Melanoma Res. 2005 Aug;15((4)):297–304. doi: 10.1097/00008390-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Eskelin S, Pyrhonen S, Summanen P, Hahka-Kemppinen M, Kivela T. Tumor Doubling Times in Metastatic Malignant Melanoma of the Uvea. Ophthalmology. 2000;107((8)):7. doi: 10.1016/s0161-6420(00)00182-2. [DOI] [PubMed] [Google Scholar]
- 30.Coit D, Thompson JA, Albertini M, et al. Uveal Melanoma NCCN Guidelines National Comprehensive Cancer Network. 2019.
- 31.Kivela T, Simpson ER, Grossniklaus HE, et al. Uveal Melanoma. In: Amin MB, Edge MB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, editors. AJCC cancer staging manual. ed. 8. New York (NY): Springer; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data