Abstract
Objective
Vascular calcification is prevalent in the aging population, as we know that arterial calcification is associated with aging. Recent studies have demonstrated that carnosine, a naturally occurring dipeptide, performs the treatment of aging‐related diseases, such as atherosclerosis and type 2 diabetes. Here, we investigated the role of carnosine in a calcification model of vascular smooth muscle cells (VSMCs).
Methods
In this research, we used an in vitro model of VSMC calcification to investigate the role of carnosine in the progression of rat VSMC calcification.
Results
Carnosine treatment attenuated calcium deposition in a dose‐dependent manner, detected by Alizarin Red S staining and calcium content assay. Carnosine also reduced the protein level of Runx2, bone morphogenetic protein 2 (BMP‐2), and cellular reactive oxygen species (ROS) production. Further, carnosine inhibited the activation of the mammalian target of rapamycin (mTOR) pathway.
Conclusion
Carnosine attenuated the VSMC calcification via inhibition of osteoblastic transdifferentiation and the mTOR signaling pathway.
Keywords: calcification, carnosine, vascular smooth muscle cells
1. INTRODUCTION
Vascular calcification is increasingly afflicting the aging population, driven by the dysmetabolic milieus of diabetes, chronic kidney disease (CKD), and arterial stiffness. 1 , 2 , 3 , 4 Although it has always been considered as an independent predictive factor of cardiovascular events and associated morbidity, the precise relationship between aging and vascular calcification is not well understood.
Aging has been demonstrated as a complex multifactorial process, converse to the previous models built on single factors. 5 Therefore, we focused on carnosine (β‐alanyl‐l‐histidine), a naturally occurring dipeptide that is abundant in the brain tissue and muscles. 6 It performs multiple biological functions, including anti‐inflammation, anti‐oxidation, chelating metal ions, and anti‐glycosylation. 6 , 7 , 8
As previously mentioned, the complexity of the aging process has superseded previous constructs based on single factors. Recent reports show that carnosine may improve the treatment outcome of aging‐related diseases, such as atherosclerosis, type 2 diabetes, and Alzheimer’s disease. 9 , 10 , 11 Studies show that carnosine suppresses cell senescence in cultured human fibroblasts 12 and delays aging in senescence‐accelerated mice and drosophila. 13 , 14 , 15 In addition, some in vitro/in vivo studies using human and animal models show that synthesis of carnosine decreases with age. 15
Recent research reports that carnosine can alleviate aging‐related vascular diseases, such as atherosclerosis and type 2 diabetes. Vascular calcification is a complication that also occurs during aging, in particular in association with atherosclerosis and type 2 diabetes. 1 , 2 , 4 Accordingly, this research sought to discern this hypothesis with regard to vascular calcification.
2. METHODS
2.1. Primary VSMC culture and in vitro calcification
Rat vascular smooth muscle cells (VSMCs) were obtained by a tissue explant culturing method. 16
Thoracic aortas were harvested from 8‐month‐old male Sprague‐Dawley rats. After removing the adventitial layers and intima membrane, the tunica media was cut into small pieces (1‐2 mm3) and placed in a culture dish. The explanted tissue was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone) supplemented with 15% fetal bovine serum (FBS), 100 U/mL penicillin, and 0.1 mg/mL streptomycin. The culture was incubated at 37°C under a humidified atmosphere of 5% CO2. VSMCs were obtained from the explants after 7‐10 days of culturing. The expression of the alpha smooth muscle actin was detected using immunohistochemistry methods. For this experiment, cells are not used beyond passage 8.
When the VSMC reached 95% confluence, cells were incubated for 10 days in calcification medium (DMEM containing 15% FBS and 2.6 mmol/L inorganic P [Pi]). The medium was changed every other day.
2.2. Detection and quantification of mineralization
Von Kossa staining and a calcium assay were used to evaluate the degree of calcium deposition. Briefly, cells were fixed for 15 minutes with 4% paraformaldehyde, washed thrice with ultrapure water, incubated for 1.5 hours in 5% silver nitrate under 100‐W bulb, and then rinsed thrice with ultrapure water. Cell fixation was performed at room temperature.
Cellular calcium quantification was performed using a calcium assay following the manufacturer’s instructions. In brief, the VSMCs were treated for 1 hour at room temperature with 10% hydrochloric acid. The calcium content of the HCl supernatants was determined using the o‐Cresolphthalein complex one method (Beyotime Biotechnology) and normalized relative to the protein concentration of the same culture well. Experiments were performed in triplicate on three different cultures (n = 3).
2.3. Western Blot
Protein lysate preparation and immunoblotting were performed using a RIPA lysis buffer (Thermo Scientific) as previously described. 17 Here, proteins were first separated by SDS‐PAGE, transferred to Polyvinylidene Fluoride Membrane (PVDF) membranes, and then incubated with 5% skim milk. They were then incubated overnight at 4°C with primary antibodies, followed by a second incubation with secondary antibodies at room temperature. Finally, the PVDF membranes were incubated with enhanced chemiluminescence reagent (Thermo Scientific) to detect the protein level.
2.4. Intracellular level of oxidative stress
The concentration of cellular reactive oxygen species (ROS) was measured using dichlorodihydrofluorescein diacetate (DCFH‐DA). Here, VSMCs were treated with or without carnosine in calcification medium for 72 hours, washed twice with phosphate‐buffered saline, and cultured for 30 minutes with 10 uM DCFH‐DA fluorescence probes. The concentration of ROS level was quantified based on measured fluorescence intensity of eight fields per dish. The quantification was performed using an in‐built microscope system. Controls were not subjected to carnosine treatment.
2.5. Ex vivo aortic ring culture
The rat thoracic aortas were first isolated under a sterile operation. 18 After removing the adventitia and endothelium, the vessels were cut into 1‐2 mm rings and placed in either calcification culture medium (2.6 mmol/L Pi) or normal culture medium, and incubated for 10 days at 37°C under 5% CO2. The culture medium was changed every other day.
2.6. Statistical analysis
Continuous quantitative data from the three independent experimental setups were presented as SEM and compared using one‐way analysis of variance. The analyses were performed using GraphPad Prism 5 (GraphPad Software). Statistical significance between groups was set at P < 0.05 or P < 0.01.
3. RESULT
3.1. Carnosine attenuates Pi‐induced calcification in VSMCs
First, this research aimed at establishing the inhibitory effect of carnosine on mineralization of VSMCs by using a well‐established vascular calcification model. After incubating a confluent of VSMCs in a calcification culture medium, we treated VSMCs with 2.5, 5, or 10 mmol/L carnosine. Alizarin Red S staining revealed that after 10 days, carnosine treatment had attenuated calcium deposition and that the process was dose‐dependent (Figure 1A). The level of calcium and alkaline phosphatase (ALP) activity in VSMCs significantly decreased after the addition of carnosine (Figure 1B,C). In addition, carnosine treatment reduced the expression of osteogenic markers Runx2 and bone morphogenetic protein 2 (BMP‐2), but had no effect on SM22 markers on smooth muscle cells (Figure 2A‐D). These findings demonstrate that carnosine may impair Pi‐induced mineral deposition, and that the inhibition is dose‐dependent.
Figure 1.
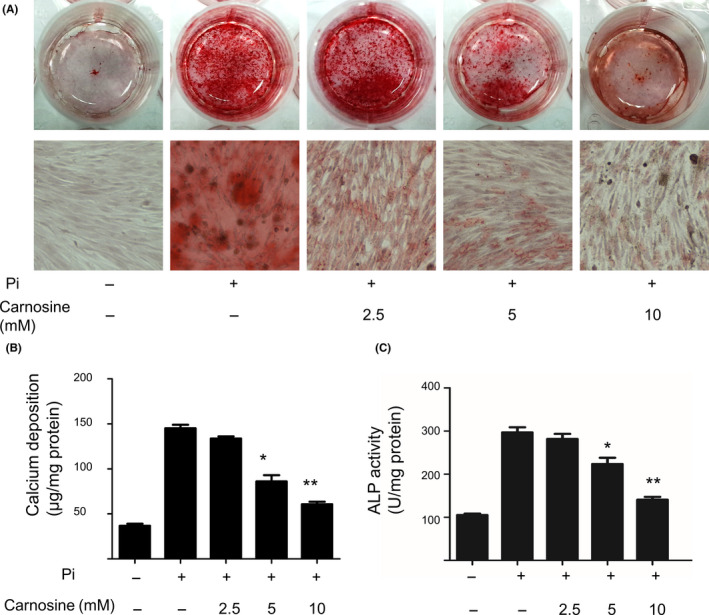
Carnosine attenuation of vascular smooth muscle cell (VSMC) calcification. (A) Alizarin Red S staining of VSMCs treated with inorganic P (Pi) with or without carnosine (2.5, 5, and 10 mmol/L) for 10 d. (B) Calcium content and (C) alkaline phosphatase (ALP) activity of VSMCs at Day 10. *P < 0.05,**P < 0.001, compared with the Pi group.
Figure 2.
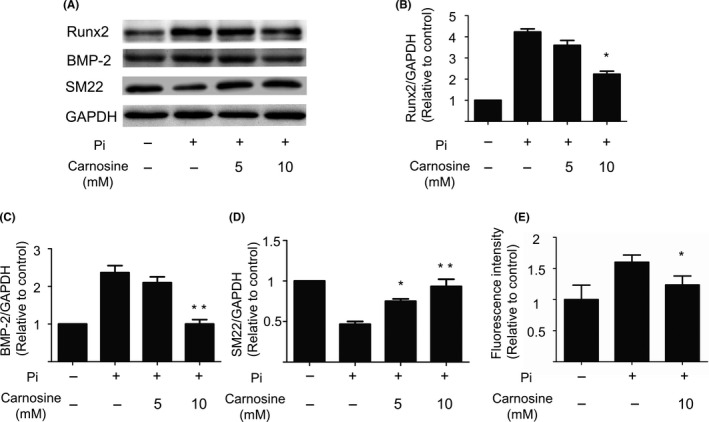
The inhibition effect of carnosine on the osteogenic differentiation of vascular smooth muscle cells (VSMCs). (A) Western blotting analysis of Runx2, bone morphogenetic protein 2 (BMP‐2), and SM22 markers on VSMCs after a 7‐d exposure to inorganic P (Pi; 2.5 mmol/L). Semi‐quantitative analysis of (B) Runx2, (C) BMP‐2, and (D) SM22. Relative levels of target proteins were quantified using the program Image J. *P < 0.05,**P < 0.001, compared with the Pi group. (E) The oxidative status of VSMCs by dichlorodihydrofluorescein diacetate fluorescence after a 3‐d calcification of VSMCs in 10 mmol/L of carnosine (mean ± SEM, n = 6). GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.
3.2. Carnosine inhibits Pi‐induced oxidative stress
To explore the mechanism with which carnosine attenuates calcification, we tested its effects of cellular oxidation. After culturing VSMCs in calcification medium for 6 days, we found that, compared with controls, 10 mmol/L of carnosine significantly decreased the production of ROS (Figure 2B).
3.3. Carnosine inhibits the activation of mTOR‐signaling pathway
Previous studies show that carnosine regulates mammalian target of rapamycin (mTOR) signaling. This pathway on its part regulates vascular osteoblastic differentiation. Consequently, we investigated whether carnosine regulates mTOR signaling.
Carnosine inhibited mTOR phosphorylation in a time‐dependent manner (Figure 3A) and in a concentration‐dependent manner (Figure 3B). To test whether carnosine‐mediated calcification inhibition is dependent on the mTOR‐signaling pathway, VSMCs were treated with leucine, an amino acid that activates mTOR. Western blot analysis showed that leucine augmented phosphorylation of mTOR and deposition of calcium. This finding demonstrated that leucine reverses the suppression of mTOR by carnosine (Figure 3C). Taken together, these findings revealed that carnosine prevents osteogenic differentiation of VSMCs by suppressing mTOR signaling.
Figure 3.
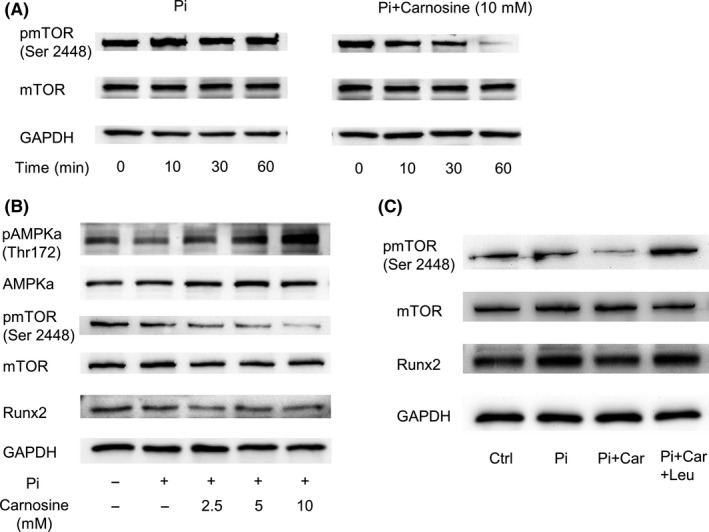
Effects of carnosine (Car) on the expression of AMPK/mammalian target of rapamycin (mTOR) pathway of vascular smooth muscle cells (VSMCs). (A) Western blots showing the expression of protein in VSMCs treated for 0, 10, 30, and 60 min, with or without 10 mmol/L carnosine. (B) Representative Western blots of protein expression level in VSMCs treated for 60 min with different concentrations of carnosine and inorganic P (Pi). (C) Effects of leucin (Leu, 4 mmol/L) on Pi‐induced mTOR level in VSMCs. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.
3.4. Carnosine attenuates calcification of organ culture
We then investigated whether carnosine could inhibit mineralization in vessels exposed to a high phosphorus environment. It was found that phosphorous significantly increased mineralization in rat aortic rings. However, carnosine also prevented deposition of minerals in the aortic rings (Figure 4).
Figure 4.
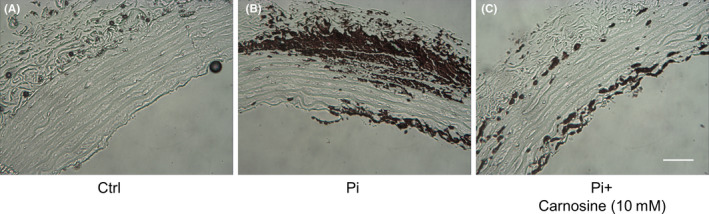
Carnosine inhibits inorganic P (Pi)‐induced mineralization of aortic rings. Aortic rings from Sprague‐Dawley rats were incubated in (A) control medium (Dulbecco’s modified Eagle’s medium [DMEM] + 15% fetal bovine serum [FBS]), (B) phosphate‐enriched culture medium (DMEM + 15% FBS + 2.8 mmol/L Pi), or (C) phosphate medium plus carnosine (10 mmol/L) for 10 d. Von Kossa staining shows the degree of calcium deposit (bar = 100 µm).
4. DISCUSSION
This study revealed that carnosine inhibition of VSMC calcification occurs in a concentration‐dependent manner. Mechanistically, carnosine inhibited osteogenic differentiation and oxidative stress by impairing the expression of mTOR. To our knowledge, this is the first study to show how carnosine prevents vascular calcification.
Carnosine is a naturally occurring dipeptide with multiple beneficial properties, such as modulation of inflammation, oxidation, and glycolysis. Recent in vivo and in vitro studies suggested that carnosine may prevent development of cardiovascular disease linked to vascular calcification, including atherosclerosis, diabetes, and chronic renal failure. However, the effects of carnosine on vascular calcification in VSMC have not been established.
Osteoblastic differentiation of VSMCs is critical in the development of vascular calcification. 19 Differentiation of VSMCs to osteoblast‐like cells that express osteoblast transcription factors and bone matrix‐related proteins has been confirmed in vitro and in vivo. VSMCs exposed to high phosphorus lose expression of the smooth muscle contractile proteins SM22α and SM α‐actin and express the bone markers Runx2, osteopontin, osteocalcin, and ALP.
In this study, we showed that carnosine inhibited Pi‐induced VSMC calcification by attenuating VSMC calcium levels and ALP activity. Vascular calcification is not just a passive process of calcium and phosphate absorption, but an active process of regulation. 1 It shares many similarities with skeletal mineralization, osteogenic gene expression, and loss of VSMC markers. In this study, carnosine treatment significantly suppressed expression of two osteogenic markers (Runx2 and BMP‐2).
The mTOR is a serine/threonine kinase that regulates cellular metabolism. 20 It also regulates cell growth and proliferation in response to a wide range of cues. 21 The mTOR signaling pathway is deregulated under many human diseases. Its signaling is also involved in osteoblastic differentiation of VSMCs. 22 , 23 , 24 A recent study demonstrated that carnosine inhibited the activation of mTOR. These findings suggest that carnosine inhibits phosphorylation of mTOR via osteoblastic differentiation of VSMCs. 25
Increased production of ROS is a contributing factor in the development and progression of vascular calcification. To explore the effect of carnosine in high Pi‐induced oxidative stress, we used the DCFH‐DA probe to determine the level of reactive oxygen (Figure 2E). It is interesting that previous studies have also documented mTOR‐pathway participation in ROS production. Rapamycin is an mTORC1 inhibitor and glutathione levels and lipid peroxidation are significantly reduced in rapamycin‐treated db/db mice hearts. 26 For instance, this drug protects the mitochondria against oxidative stress and improves endothelial function 27 and vascular contractility 28 by reducing oxidative stress.
Although further studies are required to verify the mechanisms by which carnosine attenuates vascular calcification of VSMCs, our experiments have shown that carnosine can indeed inhibit both in vitro and ex vivo vascular calcification. As such, it is potentially therapeutic against vascular calcification.
CONFLICTS OF INTEREST
There are no conflicts of interest to be reported by the authors of this study.
AUTHOR CONTRIBUTIONS
All authors: Writing of paper. Professor Cuntai Zhang: Design, literature review, coordination. Yi Huang: Design, trial, data collection, literature review. Jinli Wang: Data collection and statistical analysis. Dan Yan and Mandi Luo: Trial, data collection.
Huang Y, Wang J, Luo M, Yan D, Zhang C. Carnosine attenuates vascular smooth muscle cells calcification through mTOR signaling pathway. Aging Med. 2020;3:153–158. 10.1002/agm2.12125
REFERENCES
- 1. Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114(4):590‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vervloet M, Cozzolino M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 2017;91(4):808‐817. [DOI] [PubMed] [Google Scholar]
- 3. Yahagi K, Kolodgie FD, Lutter C, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37(2):191‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viegas C, Araujo N, Marreiros C, Simes D. The interplay between mineral metabolism, vascular calcification and inflammation in chronic kidney disease (CKD): challenging old concepts with new facts. Aging. 2019;11(12):4274‐4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernandez‐Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436‐453. [DOI] [PubMed] [Google Scholar]
- 6. Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013;93(4):1803‐1845. [DOI] [PubMed] [Google Scholar]
- 7. Hipkiss AR. Aging, proteotoxicity, mitochondria, glycation, NAD and carnosine: possible inter‐relationships and resolution of the oxygen paradox. Front Aging Neurosci. 2010;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caruso G, Fresta CG, Fidilio A, et al. Carnosine decreases PMA‐induced oxidative stress and inflammation in murine macrophages. Antioxidants. 2019;8(8):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menini S, Iacobini C, Ricci C, et al. D‐Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br J Pharmacol. 2012;166(4):1344‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albrecht T, Schilperoort M, Zhang S, et al. Carnosine attenuates the development of both type 2 diabetes and diabetic nephropathy in BTBR ob/ob mice. Sci Rep. 2017;7:44492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caruso G, Caraci F, Jolivet RB. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog Neurobiol. 2019;175:35‐53. [DOI] [PubMed] [Google Scholar]
- 12. Shao L, Li QH, Tan Z. L‐carnosine reduces telomere damage and shortening rate in cultured normal fibroblasts. Biochem Biophys Res Commun. 2004;324(2):931‐936. [DOI] [PubMed] [Google Scholar]
- 13. Kwolek‐Mirek M, Molon M, Kaszycki P, Zadrag‐Tecza R. L‐carnosine enhanced reproductive potential of the Saccharomyces cerevisiae yeast growing on medium containing glucose as a source of carbon. Biogerontology. 2016;17(4):737‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuneva AO, Kramarenko GG, Vetreshchak TV, Gallant S, Boldyrev AA. Effect of carnosine on Drosophila melanogaster lifespan. Bull Exp Biol Med. 2002;133(6):559‐561. [DOI] [PubMed] [Google Scholar]
- 15. Dai Z, Lu XY, Zhu WL, et al. Carnosine ameliorates age‐related dementia via improving mitochondrial dysfunction in SAMP8 mice. Food Funct. 2020;11(3):2489‐2497. [DOI] [PubMed] [Google Scholar]
- 16. Patel JJ, Srivastava S, Siow RC. Isolation, culture, and characterization of vascular smooth muscle cells. Methods Mol Biol. 2016;1430:91‐105. [DOI] [PubMed] [Google Scholar]
- 17. Yan J, Wang J, Huang H, et al. Fibroblast growth factor 21 delayed endothelial replicative senescence and protected cells from H2O2‐induced premature senescence through SIRT1. Am J Transl Res. 2017;9(10):4492‐4501. [PMC free article] [PubMed] [Google Scholar]
- 18. Akiyoshi T, Ota H, Iijima K, et al. A novel organ culture model of aorta for vascular calcification. Atherosclerosis. 2016;244:51‐58. [DOI] [PubMed] [Google Scholar]
- 19. Leopold JA. Vascular calcification: mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 2015;25(4):267‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sciarretta S, Forte M, Frati G, Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122(3):489‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol. 2007;47:443‐467. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Y, Zhao MM, Cai Y, et al. Mammalian target of rapamycin signaling inhibition ameliorates vascular calcification via Klotho upregulation. Kidney Int. 2015;88(4):711‐721. [DOI] [PubMed] [Google Scholar]
- 23. Zhan JK, Wang YJ, Wang Y, et al. The mammalian target of rapamycin signalling pathway is involved in osteoblastic differentiation of vascular smooth muscle cells. Can J Cardiol. 2014;30(5):568‐575. [DOI] [PubMed] [Google Scholar]
- 24. Zhan JK, Wang YJ, Wang Y, et al. Adiponectin attenuates the osteoblastic differentiation of vascular smooth muscle cells through the AMPK/mTOR pathway. Exp Cell Res. 2014;323(2):352‐358. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Z, Miao L, Wu X, et al. Carnosine inhibits the proliferation of human gastric carcinoma cells by retarding Akt/mTOR/p70S6K signaling. J Cancer. 2014;5(5):382‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Das A, Durrant D, Koka S, Salloum FN, Xi L, Kukreja RC. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem. 2014;289(7):4145‐4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajapakse AG, Yepuri G, Carvas JM, et al. Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: inhibition by resveratrol. PLoS One. 2011;6(4):e19237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao G, Li JJ, Li Y, et al. Rapamycin inhibits hydrogen peroxide‐induced loss of vascular contractility. Am J Physiol Heart Circ Physiol. 2011;300(5):H1583‐H1594. [DOI] [PubMed] [Google Scholar]


