Abstract
Bladder cancer (BC) is the 10th most common cancer worldwide. Approximately one quarter of patients with BC have muscle-invasive disease (MIBC). Muscle-invasive disease carries a poor prognosis and choosing the optimal treatment option is critical to improve patients’ outcomes. Ongoing research supports the role of 2-deoxy-2-(18F)fluoro-D-glucose positron emission tomography (18F-FDG PET) in guiding patient-specific management decisions throughout the course of MIBC. As an imaging modality, 18F-FDG PET is acquired simultaneously with either computed tomography (CT) or MRI to offer a hybrid approach combining anatomical and metabolic information that complement each other. At initial staging, 18F-FDG PET/CT enhances the detection of extravesical disease, particularly in patients classified as oligometastatic by conventional imaging. 18F-FDG PET/CT has value in monitoring response to neoadjuvant and systemic chemotherapy, as well as in localizing relapse after treatment. In the new era of immunotherapy, 18F-FDG PET/CT may also be useful to monitor treatment efficacy as well as to detect immune-related adverse events. With the advent of artificial intelligence techniques such as radiomics and deep learning, these hybrid medical images can be mined for quantitative data, providing incremental value over current standard-of-care clinical and biological data. This approach has the potential to produce a major paradigm shift toward data-driven precision medicine with the ultimate goal of personalized medicine. In this review, we highlight current literature reporting the role of 18F-FDG PET in supporting personalized management decisions for patients with MIBC. Specific topics reviewed include the incremental value of 18F-FDG PET in prognostication, pre-operative planning, response assessment, prediction of recurrence, and diagnosing drug toxicity.
Keywords: PET – Positron Emission Tomography, bladder cancer, muscle invasive bladder cancer, immunotherapy, staging
Introduction
Bladder cancer (BC) is the 10th most common cancer worldwide, with approximately half a million new cases diagnosed globally and 200,000 related deaths per year (1). At diagnosis, 75% of the patients have non-muscle invasive BC whereas the remaining 25% have muscle invasive disease (MIBC). While non-muscle invasive BC is characterized by frequent recurrence (50–70%) but a relatively low propensity to progress (10–15%), MIBC has a poor prognosis with high rates of metastasis and 5-year survival <50% despite radical surgery (2).
The current standard treatment for MIBC is based on radical cystectomy (RC) with prior cisplatin-based neoadjuvant chemotherapy (NAC) in eligible patients (cT2-T4aN0M0) (3). For patients with more advanced stage disease or for those who recur after radical surgery, cisplatin-based combination chemotherapy remains the standard of care for first-line systemic treatment (3).
Recent advances in the field of immunotherapy are reshaping the therapeutic landscape for patients with BC. Specifically, immune checkpoints inhibitors (ICIs) have demonstrated promising results in both localized and metastatic settings (4–6). To date, five anti-PD(L)1 monoclonal antibodies have been approved by the US Food and Drug Administration in the second-line setting. This current shift in treatment strategy has created an unmet need to re-evaluate the clinical use of existing imaging techniques.
Although traditionally imaging of patients with BC has predominantly focused on CT (including CT urography) and MRI, 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) may have the potential to offer additional diagnostic information due to its unique ability to image metabolism. Indeed, urothelial carcinoma, much like many other solid tumors, is characterized by alterations of glucose metabolism and overexpression of glucose transporters (GLUT-1 and GLUT-3) (7).
This review presents the most recent advances in 18F-FDG PET-guided personalized medicine in the context of MIBC. Through a review of the current literature, we present the potential clinical value of 18F-FDG PET/CT and PET/MRI for risk stratification at diagnosis, monitoring of treatment response, and detection of recurrence during follow-up.
Risk Stratification at Diagnosis
Data supporting the potential role of 18F-FDG PET/CT for initial staging of MIBC are continuously increasing. Current data suggest that 18F-FDG PET/CT improves the detection of extravesical disease and can therefore substantially improve management decisions.
Tumor Detection
2-Deoxy-2-(18F)fluoro-D-glucose positron emission tomography/computed tomography performance for tumor detection in the bladder is hampered by urinary excretion of 18F-FDG (8). Several studies have proposed using adapted protocols (hyperhydration, forced diuresis and refilling, or filling the bladder in a retrograde manner). Utilizing these techniques, authors report sensitivities between 50 and 96% (9–17). However, to date, 18F-FDG PET/CT has not be shown to improve primary tumor detection and staging, when compared to cystoscopy and morphological imaging alone performed with CT and especially MRI (18).
Lymph Node Staging
Currently, the benefit of 18F-FDG PET/CT over contrast-enhanced CT (CECT) alone for lymph node (LN) staging remains controversial, since both modalities have excellent specificity but relatively poor sensitivity.
A recent meta-analysis including 14 studies and 785 patients reported that the pooled sensitivity and specificity of 18F-FDG PET/CT for initial pelvic LN staging, in a per patient analysis, were 57% [95% CI 49–64%] and 92% [95% CI 87–95%], respectively (19). One major limitation of the current literature is the heterogeneous methodologies across published studies, notably regarding study designs, inclusion criteria, administration of NAC, injection–acquisition time in PET, the use of forced diuresis, the administration of contrast media for PET/CT, and interpretation criteria for both CECT and PET/CT (20). Studies comparing performance of CT to hybrid 18F-FDG PET/CT for pelvic LN staging with pathological analysis of extended pelvic LN dissection samples as a reference are presented in Table 1. Pooled sensitivities for CT and PET/CT are 38% [95% CI 29–47%] and 52% [95% CI 45–60%], respectively, while the pooled specificities are 91% [95% CI 88–94%] and 92% [95% CI 89–95%], respectively. Few studies have suggested a potential role for metabolic analysis by ruling out nodal disease in PET negative enlarged pelvic LNs (21, 22) (Figure 1A). Moreover, higher standardized uptake values (SUVmax) in LNs has been correlated with higher recurrence risk, independent of pathological findings (23).
TABLE 1.
CT alone and 18F-FDG PET/CT performances for LN staging, with pathology as a gold standard, without preoperative chemotherapy.
| First author | Year | CT criteria | PET/CT criteria | Sensitivity |
Specificity |
Accuracy |
|||
| CT | PET/CT | CT | PET/CT | CT | PET/CT | ||||
| Girard (22) | 2019 | SA > 8 mm | SUVmax > 4, and/or SA > 10 mm, and/or SUVmax > 2 and SA > 8 mm | 7/17 | 8/17 | 38/44 | 42/44 | 45/61 | 50/61 |
| Pichler (91) | 2017 | SA > 8 mm | SA > 10mm, and/or PET subjective analysis | 5/11 | 7/11 | 54/59 | 52/59 | 59/70 | 59/70 |
| Uttam (92) | 2016 | SA > 10 mm | SUVmax > 2.5 | 3/3 | 3/3 | 6/12 | 7/12 | 9/15 | 10/15 |
| Jeong (93) | 2015 | SA > 10 mm or necrosis | SUVmax > 2.5 | 5/17 | 8/17 | 43/44 | 41/44 | 48/61 | 49/61 |
| Aljabery (94) | 2015 | LA ≥ 10 mm | SUVmax > 2.5 | 7/17 | 7/17 | 33/37 | 32/37 | 40/54 | 39/54 |
| Rouanne (95) | 2014 | SA > 10 mm | PET subjective analysis | NA | 13/26 | NA | 74/76 | NA | 87/102 |
| Goodfellow (26) | 2014 | SA > 8 mm | SA > 8 mm and/or PET subjective analysis | 13/28 | 19/28 | 64/65 | 62/65 | 77/93 | 81/93 |
| Hitier-Berthault (96) | 2013 | LA > 10 mm | PET subjective analysis | 2/22 | 8/22 | 27/30 | 26/30 | 29/52 | 34/52 |
| Swinnen (97) | 2010 | NA | PET subjective analysis | 6/13 | 6/13 | 35/38 | 37/38 | 41/51 | 43/51 |
| Kibel (33) | 2009 | NA | PET subjective analysis | NA | 7/10 | NA | 30/32 | NA | 37/42 |
| Pooled [95% CI] | 37.5% (48/128) [29.1–46.5%] | 52.4% (86/164) [44.5–60.3%] | 91.2% (300/329) [87.6–94.0%] | 92.2% (403/437) [89.3–94.6%] | 76.1% (348/457) [72.0–80.0%] | 81.4% (489/601) [78.0–84.4%] | |||
SA, short axis; LA, long axis; LN, lymph node; SUVmax, maximum standardized uptake value; NA, not available.
FIGURE 1.
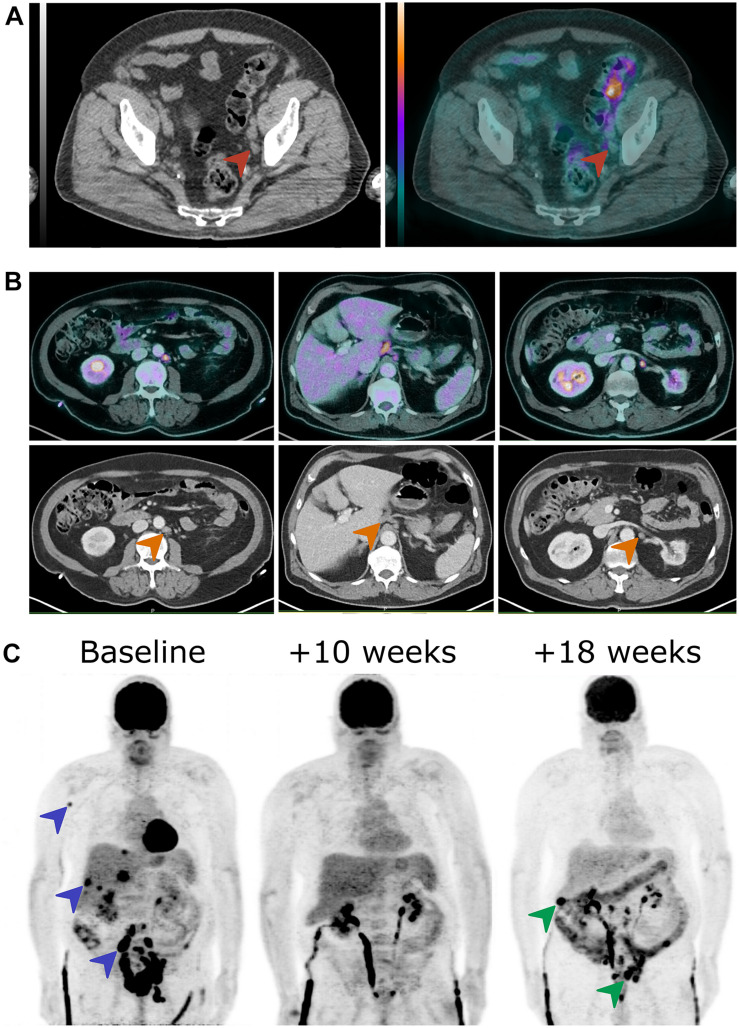
18F-FDG PET/CT images of three patients at different time points in the course of MIBC. (A) A 76-year-old man presented with an enlarged external iliac lymph node at initial staging without any 18F-FDG uptake (red arrowheads). The N0 status was confirmed by pathological examination of pelvic lymph node dissection sample. (B) A 67-year-old man was oligometastatic with one enlarged latero-aortic lymph node on CT at initial staging and 18F-FDG PET/CT revealed several other retroperitoneal lymph node metastases (orange arrowheads). (C) In a 62-year-old man who presented MIBC with osseous, hepatic, and nodal metastases at diagnosis (blue arrowheads), 18F-FDG PET/CT demonstrated complete metabolic response after three cycles of chemotherapy and then a locoregional and hepatic relapse after the fifth chemotherapy cycle (green arrowheads). 18F-FDG PET/CT, 2-deoxy-2-(18F)fluoro-D-glucose positron emission tomography/computed tomography; MIBC, muscle invasive bladder cancer.
To our knowledge, there is only a single study of 18 patients comparing 18F-FDG PET/CT and conventional MRI for pelvic LN staging in MIBC. With pathology as the gold standard, authors reported sensitivity and specificity of 80% and 80% for MRI, and 93 and 88% for 18F-FDG PET/CT, respectively. There was no statistically significant difference. However, this study was limited by small sample size and the lack of multiparametric MRI sequences such as diffusion-weighted imaging (DWI) (24).
Distant Metastatic Staging
Along with detecting nodal involvement, 18F-FDG PET/CT’s greatest strength is probably in detecting distant metastases during initial staging. In a patient-based analysis, 18F-FDG PET/CT sensitivity ranges between 54 and 87%, while specificity ranges between 90 and 97% for the detection of distant metastases from BC (15, 25–27). One study reported 18F-FDG PET/CT to be more sensitive than CT alone for detection of distant metastases, with sensitivities of 54 versus 41%, respectively. Both modalities showed similar very high specificities of 97 and 98%, respectively (26). Thus, 18F-FDG PET/CT revealed more lesions suspected to be metastasis or second primary cancer than conventional imaging (26, 28–30). 18F-FDG PET/CT changed management over conventional imaging in a range of 18–68% of patients (25, 29, 31, 32), and resulted in less additional tests in 70% of patients (25). The presence of FDG-avid regional LNs or extra pelvic lesions was an independent predictor of overall survival (28, 33), whereas it was not statistically significant for the counterpart conventional CECT findings (28).
In conclusion, the current literature suggests that 18F-FDG PET/CT provides an incremental value for nodal and distant staging in MIBC at initial diagnosis. However, due to its significant cost for healthcare systems (34), prospective studies with high evidence level are still needed before its use can be formally adopted into consensus guidelines and recommendations (3, 18, 35, 36). In support of its role in distant staging, a recent consensus statement revealed that 18F-FDG PET/CT is the imaging modality of choice to avoid over-treatment in oligometastatic patients with an agreement of 88% of participants (37) (Figure 1B).
Metabolic Prognostic Factors
Several prognostic biomarkers can be extracted from 18F-FDG PET using routine clinical workstations. The mainstream prognostic imaging biomarkers are baseline metabolic tumor burden (TMTV), baseline total lesion glycolysis (TLG), and SUVmax. All have been associated with overall survival in a wide range of cancers and treatments (38–40). With the paradigm shift of immunotherapy, authors have evaluated the potential role of appraising host metabolism in lymphoid tissues such as the bone marrow. They have demonstrated that increased bone marrow metabolism is associated with shorter overall survival and systemic immune suppression (41, 42).
In patients with advanced BC, such prognostic factors have not been reported as of yet. Additionally, there are no studies demonstrating that these biomarkers can predict response to chemotherapy or ICIs. In a proof of concept study, Chen et al. suggested that higher 18F-FDG uptake by BC may be associated with elevated PD-1/PD-L1 expression, potentially guiding the decision to select patients for ICIs (43, 44).
Response Evaluation
Based on a limited number of studies, 18F-FDG PET/CT appears to be a valuable tool for monitoring response to chemotherapy both in the neoadjuvant and metastatic settings. While the performance of 18F-FDG PET/CT in evaluating tumor sensitivity to immunotherapy has been demonstrated in several solid malignancies, it remains to be established for MIBC.
Neoadjuvant or Induction Treatment
There have been a few studies that have focused on the performance of 18F-FDG PET/CT in monitoring the response of BC to NAC. In terms of primary bladder tumor evaluation after NAC, 18F-FDG PET/CT has demonstrated 75% sensitivity and 90% specificity in identifying patients with complete pathologic response (45). After induction chemotherapy in pelvic LN metastatic patients, responders were distinguished from non-responders with a sensitivity of 83–100% and a specificity of 67–94% with 18F-FDG PET/CT, compared to 88 and 33%, respectively, with conventional CECT. Complete responders were correctly identified with 67–75% sensitivity and 46–90% specificity with 18F-FDG PET/CT, compared to 64 and 60% with CECT, respectively (45–47). These results suggest that 18F-FDG PET/CT may be useful to assess response to neoadjuvant or induction chemotherapy, but is of limited interest to select patients for RC due to low predictive value in detecting residual LN involvement (47, 48).
To our knowledge, there is no published study investigating the role of 18F-FDG PET/CT in evaluating response after preoperative immunotherapy.
Metastatic Bc: Chemotherapy
2-Deoxy-2-(18F)fluoro-D-glucose positron emission tomography/computed tomography is useful for evaluating response to systemic chemotherapy (Figure 1C). In one study, 18F-FDG PET/CT using the European Organization for Research and Treatment of Cancer (EORTC) criteria outperformed CT interpretation alone based on Response Criteria in Solid Tumors (RECIST) criteria for the prediction of response to first-line systemic chemotherapy (cisplatin and gemcitabine) (49). Furthermore, early response assessment using 18F-FDG PET/CT predicted progression-free survival and overall survival after two cycles of combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) in first-line metastatic chemotherapy (50).
Metastatic Bc: Immunotherapy
The long-term benefit of first-line immunotherapy compared to carboplatin-based chemotherapy was recently reported in patients with metastatic or locally advanced urothelial carcinoma (5). In this new era of immuno-oncology, the treatment paradigm is shifting toward restoring tumor elimination by the immune system. This new treatment paradigm has introduced novel patterns of response and progression, such as pseudo-progression and hyperprogression, which have been observed in a wide range of cancers, particularly using 18F-FDG PET/CT (51).
Pseudo-progression is a well-described novel immune-related pattern of response. Pseudo-progression is defined as a transient increase followed by a decrease in apparent total tumor burden. Its incidence is highly variable between studies, ranging between 2 and 10%, depending on tumor type, treatment, and patients (52, 53). Pseudo-progression incidence rates have been reported as ranging between 1.5 and 17% of patients with advanced urothelial carcinoma on immunotherapy (54). Thus, the majority of apparent early progressive disease visualized on 18F-FDG PET/CT represents true progression rather than pseudo-progression (55).
Hyperprogression is defined as a rapid increase in tumor growth rate (≥2-fold) compared to the expected growth rate in cancer patients treated with Anti-PD-1/PD-L1 agent. Hyperprogression occurs with an incidence of 9% in solid tumors and is associated with a poor outcome (56). The definition of hyperprogression on 18F-FDG PET/CT is currently controversial since metrics differ between institutions (57).
Immune-related adverse events (IrAEs) are well-known side effects of ICIs that can involve almost all organs. IrAEs in patients with MIBC treated with ICIs occur in up to 23% of patients, similar to patients with other solid malignancies (6). 2-Deoxy-2-(18F)fluoro-D-glucose positron emission tomography/computed tomography performed during the treatment for restaging and/or response assessment can also reveal a wide range of IrAEs, (e.g., sarcoidosis-like syndrome, thyroiditis, hypophysitis, enterocolitis, interstitial pneumonitis, pancreatitis, and arthritis) with an accuracy of 83% (58, 59). Authors recently reported that shared genetic factors impact risk for IrAEs and survival on immunotherapy in BC (60).
To date, there have been no studies investigating the specific role of 18F-FDG PET/CT in evaluating the response of advanced BC to immunotherapy.
Recurrent Disease
Few published studies have evaluated the diagnostic performance of 18F-FDG PET/CT in detecting relapse of BC after systemic chemotherapy and/or RC (Figure 1C). Sensitivity is reported to be between 87 and 92% and specificity between 83 and 94%, with a significant change in the management of up to 40% of patients compared to conventional imaging alone (61–63). Alongi et al. reported SUVmax > 6 and TLG > 8.5 of recurrent bladder tumors as the most significant predictors of 2-year progression-free survival (61).
Technical Considerations, New Tracers, and Devices
Although several studies have demonstrated the clinical utility of 18F-FDG PET/CT throughout the management of patients with BC, technical caveats should be considered.
Pet Technology
Over the past decade the gradual spread of 3D PET equipped with time-of-flight technology and using iterative reconstruction algorithms including point-spread-function correction has significatively improved image quality. More specifically, these recently developed technologies have enhanced detectability of subcentimeter foci of disease (64) and therefore may significantly influence performances of PET/CT in regard to nodal disease (65).
Rationale for Diuretic Protocols
Although detection and characterization of primary bladder tumors is not the aim of 18F-FDG PET/CT, adapted protocols are of interest in terms of improving LN staging in pelvic malignancies by lowering artifacts caused by concentrated urinary radioactivity (66). Due to its diagnostic performance with respect to nodal disease, 18F-FDG PET/CT protocols for BC should integrate forced diuresis (with 20 mg to 40 mg of furosemide intravenously) and oral hyperhydration (1.5–2 L) in everyday clinical practice (10, 16). Supplementary delayed pelvic images may be helpful in selected patients with inconclusive standard images.
Early Dynamic Acquisitions
In an effort to improve tumor conspicuity through an increase of tumor-to-urine SUVmax ratio, few authors have investigated the utility of early dynamic acquisitions, before radioactive urine has had a chance to fill the bladder (13, 67, 68). In these proof-of-concept studies, the authors suggested that such dynamic acquisitions might improve tumor detection and staging; however, the impact on LN staging was not evaluated.
Other Pet Tracers
In an effort to overcome the limitations of excreted urinary 18F-FDG in the setting of BC and pelvic LN evaluation, additional PET tracers have been studied. These studies have not demonstrated sufficient improvement to justify implementation of these tracers in everyday practice.
11C-Acetate PET/CT has been investigated in small cohorts. It has not shown significantly different results compared to MRI and CECT for tumor detection and LN staging (69–71).
In BC patients before cystectomy, 11C-Choline PET/CT detected pelvic LN involvement with an accuracy of 81% (sensitivity of 90% and specificity of 71%) (72). In an intra-patient comparison, 11C-choline PET/CT appeared to have no significant advantage compared to 18F-FDG PET/CT (73). In two studies comparing 11C-Choline PET/CT to conventional imaging for LN staging, sensitivity was of 42–58% and specificity was 66–84% for 11C-Choline PET/CT, compared to 14–75% and 56–90%, respectively, with CECT alone (74, 75). At initial staging, 11C-Choline PET/CT was not able to significantly predict overall survival or cancer-specific death (76). Graziani et al. studied the performance of 11C-Choline PET/CT in detection BC relapse. The authors reported a sensitivity of 67% and a specificity of 85% for local relapse and a sensitivity of 90% and specificity of 92% for distant relapse (77). These results are in line with the performance of 18F-FDG PET/CT.
Regarding the investigation of bone metastases, 18F-sodium fluoride PET/CT has been shown to reveal more bone metastases than standard bone scintigraphy (78) or 18F-FDG PET/CT (79).
Rationale for Pet/Mri
Thanks to its superior contrast resolution, MRI is currently the most effective non-invasive imaging modality for local staging of BC, with an accuracy between 92 and 98% for detection of muscle invasion (18, 80). In spite of this contrast resolution, MRI’s anatomical sequences (T2 weighted, T1 weighted) have not been shown to be superior to CECT in detecting LN involvement. Combining functional sequences such as DWI and dynamic contrast enhancement (DCE) still results in a relatively low sensitivity (80). Thus, MRI and PET complement one another for the staging of BC; the former is useful for local staging, the latter for distant metastasis detection, while together they may be synergistic for revealing pelvic LN involvement.
To date, only small studies have been published investigating the role of PET/MRI in MIBC. In a prospective pilot study including 24 patients, Rosenkrantz et al. highlighted the potential interest of hybrid 18F-FDG PET/MRI over MRI alone including DWI, especially to detect pelvic LN involvement with an accuracy of 95% for PET/MRI versus 76% for MRI alone, as well as non-nodal extravesical pelvic involvement with an accuracy of 100 versus 91%, respectively (81). In contrast, another recently published pilot study investigating PET/MRI in MIBC patients after NAC (N = 18, with LN involvement in only three patients) showed a sensitivity of 80% and a specificity of 56% for detection of the primary tumor, and 0% and 100%, respectively, for detection of LN involvement (82).
Rationale for Artificial Intelligence
Recent advances in artificial intelligence (AI) techniques are able to harness routine PET images into data that can be mined to extract imaging biomarkers for purposes of guiding precision medicine for BC. The term AI encompasses distinct fields such as deep learning, and radiomics, which go beyond the scope of this review. AI can be leveraged on clinical data, molecular and genetic biomarkers, and imaging for several narrow tasks in BC. While there are currently no studies published on the use of AI on 18F-FDG PET images for BC, promising results of its application using CT and MRI images have been reported in terms of predicting the depth of invasion of the primary tumor (83), grade (84), local and systemic staging (85), and assessment of treatment response (86). Additionally, AI based on PET/CT images has been used in other malignancies to predict nodal disease (87), risk stratification (88), treatment response (89), and patient outcomes (90). The main advantages of using AI is its potential reproducibility, as compared to the inherently subjective interpretation of medical images by physicians as well as the ability to harness large quantities of data that may escape the pattern recognition abilities of humans.
Conclusion and Future Directions
There is strong and still evolving evidence supporting the utility of 18F-FDG PET/CT in the management of MIBC. 18F-FDG PET/CT appears to outperform and/or complement conventional imaging techniques for several tasks. Current data support the idea that 18F-FDG PET/CT may help to select the most efficacious treatment for each patient at each step of MIBC management. There is some agreement among the medical community that 18F-FDG PET/CT is relevant to guide management at initial staging in patients considered as oligometastatic by conventional imaging, such as patients with enlarged pelvic LN. However, to date, prospective studies with high level of evidence are lacking in order to allow the systematic adoption of 18F-FDG PET/CT in structured guidelines. With the advent of AI, the broad range of clinical, biological, anatomical, and metabolic data may be harnessed in order to lead to improved precision medicine.
Author Contributions
AG, LD, and MR designed the review article. AG, HVR, MR, HS, and LD performed the literature review and wrote and edited the manuscript. J-FG, OD, P-YS, and HS critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström P-U, Choi W, et al. Bladder cancer. Lancet. (2016) 388:2796–810. 10.1016/S0140-6736(16)30512-8 [DOI] [PubMed] [Google Scholar]
- 3.Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. (2020) 29:S0302-2838(20)30230-X. 10.1016/j.eururo.2020.03.055 [DOI] [PubMed] [Google Scholar]
- 4.Rouanne M, Roumiguié M, Houédé N, Masson-Lecomte A, Colin P, Pignot G, et al. Development of immunotherapy in bladder cancer: present and future on targeting PD(L)1 and CTLA-4 pathways. World J Urol. (2018) 36:1727–40. 10.1007/s00345-018-2332-5 [DOI] [PubMed] [Google Scholar]
- 5.Feld E, Harton J, Meropol NJ, Adamson BJS, Cohen A, Parikh RB, et al. Effectiveness of first-line immune checkpoint blockade versus carboplatin-based chemotherapy for metastatic urothelial cancer. Eur Urol. (2019) 76:524–32. 10.1016/j.eururo.2019.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siefker-Radtke A, Curti B. Immunotherapy in metastatic urothelial carcinoma: focus on immune checkpoint inhibition. Nat Rev Urol. (2018) 15:112–24. 10.1038/nrurol.2017.190 [DOI] [PubMed] [Google Scholar]
- 7.Whyard T, Waltzer WC, Waltzer D, Romanov V. Metabolic alterations in bladder cancer: applications for cancer imaging. Exp Cell Res. (2016) 341:77–83. 10.1016/j.yexcr.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 8.Wang N, Jiang P, Lu Y. Is fluorine-18 fluorodeoxyglucose positron emission tomography useful for detecting bladder lesions? a meta-analysis of the literature. Urol Int. (2014) 92:143–9. 10.1159/000351964 [DOI] [PubMed] [Google Scholar]
- 9.Fang N, Wang Y, Zeng L, Wu Z, Cui X, Wang Q, et al. Feasible Method to enable clear visualization of suspected bladder cancer with 18F-FDG PET/CT. Clinical Imaging. (2014) 38:704–9. 10.1016/j.clinimag.2014.04.018 [DOI] [PubMed] [Google Scholar]
- 10.Harkirat S, Anand S, Jacob M. Forced diuresis and dual-phase 18 F-fluorodeoxyglucose-PET/CT scan for restaging of urinary bladder cancers. Indian J Radiol Imaging. (2010) 20:13. 10.4103/0971-3026.59746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higashiyama A, Komori T, Juri H, Inada Y, Azuma H, Narumi Y. Detectability of residual invasive bladder cancer in delayed 18F-FDG PET imaging with oral hydration using 500 mL of water and voiding-refilling. Ann Nucl Med. (2018) 32:561–7. 10.1007/s12149-018-1280-x [DOI] [PubMed] [Google Scholar]
- 12.Nayak B, Dogra PN, Naswa N, Kumar R. Diuretic 18F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. Eur J Nucl Med Mol Imaging. (2013) 40:386–93. 10.1007/s00259-012-2294-6 [DOI] [PubMed] [Google Scholar]
- 13.Sharma A, Mete UK, Sood A, Kakkar N, Gorla AKR, Mittal BR. Utility of early dynamic and delayed post-diuretic 18 F-FDG PET/CT SUV max in predicting tumour grade and T-stage of urinary bladder carcinoma: results from a prospective single centre study. BJR. (2017) 90:20160787. 10.1259/bjr.20160787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan H, Zhou X, Wang X, Li R, Shi Y, Xia Q, et al. Delayed 18 F FDG PET/CT imaging in the assessment of residual tumors after transurethral resection of bladder cancer. Radiology. (2019) 293:144–50. 10.1148/radiol.2019190032 [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Cheng J, Pan L, Hu S, Xu J, Zhang Y, et al. Is whole-body fluorine-18 fluorodeoxyglucose PET/CT plus additional pelvic images (oral hydration–voiding–refilling) useful for detecting recurrent bladder cancer? Ann Nucl Med. (2012) 26:571–7. 10.1007/s12149-012-0614-3 [DOI] [PubMed] [Google Scholar]
- 16.Yildirim-Poyraz N, Ozdemir E, Uzun B, Turkolmez S. Dual phase 18F-fluorodeoxyglucose positron emission tomography/computed tomography with forced diuresis in diagnostic imaging evaluation of bladder cancer. Rev Españo Med Nucl Imagen Mol (Engl Ed). (2013) 32:214–21. 10.1016/j.remnie.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 17.Mertens LS, Bruin NM, Vegt E, de Blok WM, Fioole-Bruining A, van Rhijn BW, et al. Catheter-assisted 18F-FDG-PET/CT imaging of primary bladder cancer: a prospective study. Nucl Med Commun. (2012) 33:1195–201. 10.1097/MNM.0b013e3283567473 [DOI] [PubMed] [Google Scholar]
- 18.van der Pol CB, Sahni VA, Eberhardt SC, Oto A, Akin O, Alexander LF, et al. ACR appropriateness criteria ® pretreatment Staging of muscle-invasive bladder cancer. J Am Coll Radiol. (2018) 15:S150–9. 10.1016/j.jacr.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 19.Ha HK, Koo PJ, Kim S-J. Diagnostic accuracy of F-18 FDG PET/CT for preoperative lymph node staging in newly diagnosed bladder cancer patients: a systematic review and meta-analysis. Oncology. (2018) 95:31–8. 10.1159/000488200 [DOI] [PubMed] [Google Scholar]
- 20.Soubra A, Hayward D, Dahm P, Goldfarb R, Froehlich J, Jha G, et al. The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: a single-institution study and a systematic review with meta-analysis. World J Urol. (2016) 34:1229–37. 10.1007/s00345-016-1772-z [DOI] [PubMed] [Google Scholar]
- 21.Dason S, Wong NC, Donahue TF, Meier A, Zheng J, Mannelli L, et al. Utility of routine preoperative 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET/CT) in identifying pathologic lymph node metastases at radical cystectomy. J Urol. (2020) 204:101097. 10.1097/JU.0000000000001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girard A, Rouanne M, Taconet S, Radulescu C, Neuzillet Y, Girma A, et al. Integrated analysis of 18F-FDG PET/CT improves preoperative lymph node staging for patients with invasive bladder cancer. Eur Radiol. (2019) 29:4286–93. 10.1007/s00330-018-5959-0 [DOI] [PubMed] [Google Scholar]
- 23.Vind-Kezunovic S, Bouchelouche K, Ipsen P, Høyer S, Bell C, Bjerggaard Jensen J. Detection of lymph node metastasis in patients with bladder cancer using maximum standardised uptake value and 18F-fluorodeoxyglucose positron emission tomography/computed tomography: results from a high-volume centre including long-term follow-up. Eur Urol Focus. (2019) 5:90–6. 10.1016/j.euf.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 24.Jensen TK, Holt P, Gerke O, Riehmann M, Svolgaard B, Marcussen N, et al. Preoperative lymph-node staging of invasive urothelial bladder cancer with 18 F-fluorodeoxyglucose positron emission tomography/computed axial tomography and magnetic resonance imaging: correlation with histopathology. Scand J Urol Nephrol. (2011) 45:122–8. 10.3109/00365599.2010.544672 [DOI] [PubMed] [Google Scholar]
- 25.Apolo AB, Riches J, Schöder H, Akin O, Trout A, Milowsky MI, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography in bladder cancer. JCO. (2010) 28:3973–8. 10.1200/JCO.2010.28.7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodfellow H, Viney Z, Hughes P, Rankin S, Rottenberg G, Hughes S, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer: FDG pet in the staging of bladder cancer. BJU Int. (2014) 114:389–95. 10.1111/bju.12608 [DOI] [PubMed] [Google Scholar]
- 27.Öztürk H. Detecting metastatic bladder cancer using 18F-fluorodeoxyglucose positron-emission tomography/computed tomography. Cancer Res Treat. (2015) 47:834–43. 10.4143/crt.2014.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mertens LS, Mir MC, Scott AM, Lee ST, Fioole-Bruining A, Vegt E, et al. 18F-fluorodeoxyglucose–positron emission tomography/computed tomography aids staging and predicts mortality in patients with muscle-invasive bladder cancer. Urology. (2014) 83:393–9. 10.1016/j.urology.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 29.Mertens LS, Fioole-Bruining A, Vegt E, Vogel WV, van Rhijn BW, Horenblas S. Impact of 18 F-fluorodeoxyglucose (FDG)-positron-emission tomography/computed tomography (PET/CT) on management of patients with carcinoma invading bladder muscle: FDG-PET/CT in carcinoma invading bladder muscle. BJU Int. (2013) 112:729–34. 10.1111/bju.12109 [DOI] [PubMed] [Google Scholar]
- 30.Rouanne M, Alhammadi A, Vilain D, Radulescu C, Lebret T. Value of positron emission tomography in diagnosing synchronous penile metastasis from urothelial bladder cancer. World J Surg Onc. (2015) 13:276. 10.1186/s12957-015-0696-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guney IB, Küçüker KA, Izol V, Kibar M. The role and effect of FDG-PET/CT on patient management and restaging of bladder carcinoma. Turkish J Urol. (2019) 45:423–30. 10.5152/tud.2019.84453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z, Pan L, Cheng J, Hu S, Xu J, Ye D, et al. Clinical value of whole body fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in the detection of metastatic bladder cancer: FDG-PET metastatic bladder cancer. Int J Urol. (2012) 19:639–44. 10.1111/j.1442-2042.2012.02989.x [DOI] [PubMed] [Google Scholar]
- 33.Kibel AS, Dehdashti F, Katz MD, Klim AP, Grubb RL, Humphrey PA, et al. Prospective Study of [18 F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. JCO. (2009) 27:4314–20. 10.1200/JCO.2008.20.6722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huo J, Chu Y, Chamie K, Smaldone MC, Boorjian SA, Baillargeon JG, et al. Increased utilization of positron emission tomography/computed tomography (PET/CT) imaging and its economic impact for patients diagnosed with bladder cancer. Clin Genitour Cancer. (2018) 16:e99–111. 10.1016/j.clgc.2017.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. NCCN guidelines insights: bladder cancer, version 5.2018. J Natl Compr Canc Netw. (2018) 16:1041–53. 10.6004/jnccn.2018.0072 [DOI] [PubMed] [Google Scholar]
- 36.Salaün P-Y, Abgral R, Malard O, Querellou-Lefranc S, Quere G, Wartski M, et al. Good clinical practice recommendations for the use of PET/CT in oncology. Eur J Nucl Med Mol Imaging. (2020) 47:28–50. 10.1007/s00259-019-04553-8 [DOI] [PubMed] [Google Scholar]
- 37.Witjes JA, Babjuk M, Bellmunt J, Bruins HM, De Reijke TM, De Santis M, et al. EAU-ESMO consensus statements on the management of advanced and variant bladder cancer—an international collaborative multistakeholder effort†. Eur Urol. (2020) 77:223–50. 10.1016/j.eururo.2019.09.035 [DOI] [PubMed] [Google Scholar]
- 38.Pontoizeau C, Girard A, Mesbah H, Haumont L-A, Devillers A, Tempescul A, et al. Prognostic value of baseline total metabolic tumor volume measured on FDG PET in patients with richter syndrome. Clin Nucl Med. (2020) 45:118–22. 10.1097/RLU.0000000000002879 [DOI] [PubMed] [Google Scholar]
- 39.Seban R-D, Mezquita L, Berenbaum A, Dercle L, Botticella A, Le Pechoux C, et al. Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur J Nucl Med Mol Imaging. (2020) 47:1147–57. 10.1007/s00259-019-04615-x [DOI] [PubMed] [Google Scholar]
- 40.Seban R-D, Moya-Plana A, Antonios L, Yeh R, Marabelle A, Deutsch E, et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur J Nucl Med Mol Imaging. (2020) 47:2301–12. 10.1007/s00259-020-04757-3 [DOI] [PubMed] [Google Scholar]
- 41.Dercle L, Seban R-D, Lazarovici J, Schwartz LH, Houot R, Ammari S, et al. 18 F-FDG PET and CT scans detect new imaging patterns of response and progression in patients with hodgkin lymphoma treated by anti–programmed death 1 immune checkpoint inhibitor. J Nucl Med. (2018) 59:15–24. 10.2967/jnumed.117.193011 [DOI] [PubMed] [Google Scholar]
- 42.Seban R-D, Nemer JS, Marabelle A, Yeh R, Deutsch E, Ammari S, et al. Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: association with outcome and transcriptomics. Eur J Nucl Med Mol Imaging. (2019) 46:2298–310. 10.1007/s00259-019-04411-7 [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Zhou X, Liu J, Huang G. Relationship between the expression of PD-1/PD-L1 and 18F-FDG uptake in bladder cancer. Eur J Nucl Med Mol Imaging. (2019) 46:848–54. 10.1007/s00259-018-4208-8 [DOI] [PubMed] [Google Scholar]
- 44.Girard A, Rouanne M. Comment on: relationship between the expression of PD-1/PD-L1 and 18F-FDG uptake in bladder cancer. Eur J Nucl Med Mol Imaging. (2019) 46:1212–3. 10.1007/s00259-019-04296-6 [DOI] [PubMed] [Google Scholar]
- 45.Soubra A, Gencturk M, Froelich J, Balaji P, Gupta S, Jha G, et al. FDG-PET/CT for assessing the response to neoadjuvant chemotherapy in bladder cancer patients. Clin Genitour Cancer. (2018) 16:360–4. 10.1016/j.clgc.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 46.Mertens LS, Fioole-Bruining A, van Rhijn BWG, Kerst JM, Bergman AM, Vogel WV, et al. FDG-positron emission tomography/computerized tomography for monitoring the response of pelvic lymph node metastasis to neoadjuvant chemotherapy for bladder cancer. J Urol. (2013) 189:1687–91. 10.1016/j.juro.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 47.van de Putte EEF, Vegt E, Mertens LS, Bruining A, Hendricksen K, van der Heijden MS, et al. FDG-PET/CT for response evaluation of invasive bladder cancer following neoadjuvant chemotherapy. Int Urol Nephrol. (2017) 49:1585–91. 10.1007/s11255-017-1637-4 [DOI] [PubMed] [Google Scholar]
- 48.Kollberg P, Almquist H, Bläckberg M, Cwikiel M, Gudjonsson S, Lyttkens K, et al. [18 F]Fluorodeoxyglucose-positron emission tomography/computed tomography response evaluation can predict histological response at surgery after induction chemotherapy for oligometastatic bladder cancer. Scand J Urol. (2017) 51:308–13. 10.1080/21681805.2017.1321579 [DOI] [PubMed] [Google Scholar]
- 49.Öztürk H. Comparing RECIST with EORTC criteria in metastatic bladder cancer. J Cancer Res Clin Oncol. (2016) 142:187–94. 10.1007/s00432-015-2022-2 [DOI] [PubMed] [Google Scholar]
- 50.Giannatempo P, Alessi A, Miceli R, Raggi D, Farè E, Nicolai N, et al. Interim fluorine-18 fluorodeoxyglucose positron emission tomography for early metabolic assessment of therapeutic response to chemotherapy for metastatic transitional cell carcinoma. Clin Genitourinary Cancer. (2014) 12:433–9. 10.1016/j.clgc.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 51.Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for assessing tumour response to immunotherapy : report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging. (2019) 46:238–50. 10.1007/s00259-018-4171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiou VL, Burotto M. Pseudoprogression and Immune-related response in solid tumors. JCO. (2015) 33:3541–3. 10.1200/JCO.2015.61.6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dromain C, Beigelman C, Pozzessere C, Duran R, Digklia A. Imaging of tumour response to immunotherapy. Eur Radiol Exp. (2020) 4:2. 10.1186/s41747-019-0134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soria F, Beleni AI, D’Andrea D, Resch I, Gust KM, Gontero P, et al. Pseudoprogression and hyperprogression during immune checkpoint inhibitor therapy for urothelial and kidney cancer. World J Urol. (2018) 36:1703–9. 10.1007/s00345-018-2264-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Humbert O, Cadour N, Paquet M, Schiappa R, Poudenx M, Chardin D, et al. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging. (2020) 47:1158–67. 10.1007/s00259-019-04573-4 [DOI] [PubMed] [Google Scholar]
- 56.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. (2017) 23:1920–8. 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 57.Seban R-D, Schwartz LH, Bonardel G, Dercle L. Diagnosis of hyper-progressive disease in patients treated with checkpoint inhibitors using 18F-FDG PET/CT. J Nucl Med. (2020) 61:1404–5. 10.2967/jnumed.120.242768 [DOI] [PubMed] [Google Scholar]
- 58.Dercle L, Mokrane F-Z, Schiano de Colella JM, Stamatoullas A, Morschhauser F, Brice P, et al. Unconventional immune-related phenomena observed using 18F-FDG PET/CT in Hodgkin lymphoma treated with anti PD-1 monoclonal antibodies. Eur J Nucl Med Mol Imaging. (2019) 46:1391–2. 10.1007/s00259-019-04310-x [DOI] [PubMed] [Google Scholar]
- 59.Mekki A, Dercle L, Lichtenstein P, Marabelle A, Michot J-M, Lambotte O, et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur J Cancer. (2018) 96:91–104. 10.1016/j.ejca.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 60.Khan Z, Di Nucci F, Kwan A, Hammer C, Mariathasan S, Rouilly V, et al. Polygenic risk for skin autoimmunity impacts immune checkpoint blockade in bladder cancer. Proc Natl Acad Sci USA. (2020) 117:12288–94. 10.1073/pnas.1922867117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alongi P, Caobelli F, Gentile R, Stefano A, Russo G, Albano D, et al. Recurrent bladder carcinoma: clinical and prognostic role of 18 F-FDG PET/CT. Eur J Nucl Med Mol Imaging. (2017) 44:224–33. 10.1007/s00259-016-3500-8 [DOI] [PubMed] [Google Scholar]
- 62.Öztürk H, Karapolat I. Efficacy of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography in restaging muscle-invasive bladder cancer following radical cystectomy. Exp Therap Med. (2015) 9:717–24. 10.3892/etm.2015.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zattoni F, Incerti E, Dal Moro F, Moschini M, Castellucci P, Panareo S, et al. 18F-FDG PET/CT and urothelial carcinoma: impact on management and prognosis—a multicenter retrospective study. Cancers. (2019) 11:700. 10.3390/cancers11050700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hashimoto N, Morita K, Tsutsui Y, Himuro K, Baba S, Sasaki M. Time-of-flight information improved the detectability of subcentimeter spheres using a clinical PET/CT scanner. J Nucl Med Technol. (2018) 46:268–73. 10.2967/jnmt.117.204735 [DOI] [PubMed] [Google Scholar]
- 65.Akamatsu G, Mitsumoto K, Taniguchi T, Tsutsui Y, Baba S, Sasaki M. Influences of point-spread function and time-of-flight reconstructions on standardized uptake value of lymph node metastases in FDG-PET. Eur J Radiol. (2014) 83:226–30. 10.1016/j.ejrad.2013.09.030 [DOI] [PubMed] [Google Scholar]
- 66.Dierickx LO, Dercle L, Chaltiel L, Caselles O, Brillouet S, Zerdoud S, et al. Evaluation of 2 diuretic 18fluorine-fluorodeoxyglucose positron emission tomography/computed tomography imaging protocols for intrapelvic cancer. Q J Nucl Med Mol Imaging. (2019) 63:284–91. 10.23736/S1824-4785.17.02912-0 [DOI] [PubMed] [Google Scholar]
- 67.Belakhlef S, Church C, Jani C, Lakhanpal S. Early dynamic PET/CT and 18F-FDG blood flow imaging in bladder cancer detection: a novel approach. Clin Nucl Med. (2012) 37:366–8. 10.1097/RLU.0b013e3182443110 [DOI] [PubMed] [Google Scholar]
- 68.Yoon H-J, Yoo J, Kim Y, Lee DH, Kim BS. Enhanced application of 18F-FDG PET/CT in bladder cancer by adding early dynamic acquisition to a standard delayed PET protocol. Clin Nucl Med. (2017) 42:749–55. 10.1097/RLU.0000000000001780 [DOI] [PubMed] [Google Scholar]
- 69.Salminen A, Jambor I, Merisaari H, Ettala O, Virtanen J, Koskinen I, et al. 11C-acetate PET/MRI in bladder cancer staging and treatment response evaluation to neoadjuvant chemotherapy: a prospective multicenter study (ACEBIB trial). Cancer Imaging. (2018) 18:25. 10.1186/s40644-018-0158-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schöder H, Ong SC, Reuter VE, Cai S, Burnazi E, Dalbagni G, et al. Initial Results with 11C-Acetate positron emission tomography/computed tomography (PET/CT) in the staging of urinary bladder cancer. Mol Imaging Biol. (2012) 14:245–51. 10.1007/s11307-011-0488-0 [DOI] [PubMed] [Google Scholar]
- 71.Vargas HA, Akin O, Schöder H, Olgac S, Dalbagni G, Hricak H, et al. Prospective evaluation of MRI, 11C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur J Radiol. (2012) 81:4131–7. 10.1016/j.ejrad.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 72.Ceci F, Bianchi L, Graziani T, Castellucci P, Pultrone C, Eugenio B, et al. 11C-Choline PET/CT and bladder cancer: lymph node metastasis assessment with pathological specimens as reference standard. Clin Nucl Med. (2015) 40:e124–8. 10.1097/RLU.0000000000000604 [DOI] [PubMed] [Google Scholar]
- 73.Golan S, Sopov V, Baniel J, Groshar D. Comparison of 11 C-choline With 18 F-FDG in positron emission tomography/computerized tomography for staging urothelial carcinoma: a prospective study. J Urol. (2011) 186:436–41. 10.1016/j.juro.2011.03.121 [DOI] [PubMed] [Google Scholar]
- 74.Brunocilla E, Ceci F, Schiavina R, Castellucci P, Maffione AM, Cevenini M, et al. Diagnostic accuracy of 11C-choline PET/CT in preoperative lymph node staging of bladder cancer: a systematic comparison with contrast-enhanced CT and histologic findings. Clin Nucl Med. (2014) 39:e308–12. 10.1097/RLU.0000000000000342 [DOI] [PubMed] [Google Scholar]
- 75.Maurer T, Souvatzoglou M, Kübler H, Opercan K, Schmidt S, Herrmann K, et al. Diagnostic efficacy of [11C]choline positron emission tomography/computed tomography compared with conventional computed tomography in lymph node staging of patients with bladder cancer prior to radical cystectomy. Eur Urol. (2012) 61:1031–8. 10.1016/j.eururo.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 76.Maurer T, Horn T, Souvatzoglou M, Eiber M, Beer AJ, Heck MM, et al. Prognostic value of 11C-choline PET/CT and CT for predicting survival of bladder cancer patients treated with radical cystectomy. Urol Int. (2014) 93:207–13. 10.1159/000357686 [DOI] [PubMed] [Google Scholar]
- 77.Graziani T, Ceci F, Lopes FL, Chichero J, Castellucci P, Schiavina R, et al. 11C-Choline PET/CT for restaging of bladder cancer. Clin Nucl Med. (2015) 40:e1–5. 10.1097/RLU.0000000000000573 [DOI] [PubMed] [Google Scholar]
- 78.Araz M, Aras G, Küçük ON. The role of 18F–NaF PET/CT in metastatic bone disease. J Bone Oncol. (2015) 4:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mena E, Lin J, Lindenberg ML, Turkbey I, Adler S, McKinney Y, et al. Value of combined 18F-FDG/18F-NaF PET/CT in tumor detection and therapy response in patients with advanced bladder cancer treated with Cabozantinib plus Nivolumab alone or in combination with Ipilimumab. J Nucl Med. (2017) 58:754. [Google Scholar]
- 80.Woo S, Suh CH, Kim SY, Cho JY, Kim SH. The diagnostic performance of MRI for detection of lymph node metastasis in bladder and prostate cancer: an updated systematic review and diagnostic meta-analysis. Am J Roentgenol. (2018) 210:W95–109. 10.2214/AJR.17.18481 [DOI] [PubMed] [Google Scholar]
- 81.Rosenkrantz AB, Friedman KP, Ponzo F, Raad RA, Jackson K, Huang WC, et al. Prospective pilot study to evaluate the incremental value of PET information in patients with bladder cancer undergoing 18F-FDG simultaneous PET/MRI. Clin Nucl Med. (2017) 42:e8–15. 10.1097/RLU.0000000000001432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eulitt PJ, Altun E, Sheikh A, Wong TZ, Woods ME, Rose TL, et al. Pilot study of [18F] fluorodeoxyglucose positron emission tomography (FDG-PET)/magnetic resonance imaging (MRI) for staging of muscle-invasive bladder cancer (MIBC). Clin Genitour Cancer. (2020). 10.1016/j.clgc.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 83.Wang H, Xu X, Zhang X, Liu Y, Ouyang L, Du P, et al. Elaboration of a multisequence MRI-based radiomics signature for the preoperative prediction of the muscle-invasive status of bladder cancer: a double-center study. Eur Radiol. (2020) 30:4816–27. 10.1007/s00330-020-06796-8 [DOI] [PubMed] [Google Scholar]
- 84.Zhang G, Xu L, Zhao L, Mao Li, Li X, Jin Z, et al. CT-based radiomics to predict the pathological grade of bladder cancer. Eur Radiol. (2020). 10.1007/s00330-020-06893-8. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 85.Wu S, Zheng J, Li Y, Wu Z, Shi S, Huang M, et al. Development and validation of an MRI-based radiomics signature for the preoperative prediction of lymph node metastasis in bladder cancer. EBioMedicine. (2018) 34:76–84. 10.1016/j.ebiom.2018.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cha KH, Hadjiiski LM, Cohan RH, Chan H-P, Caoili EM, Davenport MS, et al. Diagnostic accuracy of CT for prediction of bladder cancer treatment response with and without computerized decision support. Acad Radiol. (2019) 26:1137–45. 10.1016/j.acra.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shaish H, Mutasa S, Makkar J, Chang P, Schwartz L, Ahmed F. Prediction of Lymph node maximum standardized uptake value in patients with cancer using a 3D convolutional neural network: a proof-of-concept study. Am J Roentgenol. (2019) 212:238–44. 10.2214/AJR.18.20094 [DOI] [PubMed] [Google Scholar]
- 88.Cysouw MCF, Jansen BHE, van de Brug T, Oprea-Lager DE, Pfaehler E, de Vries BM, et al. Machine learning-based analysis of [18 F]DCFPyL PET radiomics for risk stratification in primary prostate cancer. Eur J Nucl Med Mol Imaging. (2020). 10.1007/s00259-020-04971-z. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Valentinuzzi D, Vrankar M, Boc N, Ahac V, Zupancic Z, Unk M, et al. [18F]FDG PET immunotherapy radiomics signature (iRADIOMICS) predicts response of non-small-cell lung cancer patients treated with pembrolizumab. Radiol Oncol. (2020) 54:285–94. 10.2478/raon-2020-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nie P, Yang G, Wang N, Yan L, Miao W, Duan Y, et al. Additional value of metabolic parameters to PET/CT-based radiomics nomogram in predicting lymphovascular invasion and outcome in lung adenocarcinoma. Eur J Nucl Med Mol Imaging. (2020). 10.1007/s00259-020-04747-5. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 91.Pichler R, De Zordo T, Fritz J, Kroiss A, Aigner F, Heidegger I, et al. Pelvic lymph node staging by combined 18 F-FDG-PET/CT imaging in bladder cancer prior to radical cystectomy. Clin Genitour Cancer. (2017) 15:e387–95. 10.1016/j.clgc.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 92.Uttam M, Pravin N, Anish B, Nandita K, Arup M. Is [F-18]-fluorodeoxyglucose FDG-PET/CT better than ct alone for the preoperative lymph node staging of muscle invasive bladder cancer? Int braz J Urol. (2016) 42:234–41. 10.1590/S1677-5538.IBJU.2014.0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeong IG, Hong S, You D, Hong JH, Ahn H, Kim C-S. FDG PET–CT for lymph node staging of bladder cancer: a prospective study of patients with extended pelvic lymphadenectomy. Ann Surg Oncol. (2015) 22:3150–6. 10.1245/s10434-015-4369-7 [DOI] [PubMed] [Google Scholar]
- 94.Aljabery F, Lindblom G, Skoog S, Shabo I, Olsson H, Rosell J, et al. PET/CT versus conventional CT for detection of lymph node metastases in patients with locally advanced bladder cancer. BMC Urol. (2015) 15:87. 10.1186/s12894-015-0080-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rouanne M, Girma A, Neuzillet Y, Vilain D, Radulescu C, Letang N, et al. Potential impact of 18F-FDG PET/CT on patients selection for neoadjuvant chemotherapy before radical cystectomy. Eur J Surg Oncol (EJSO). (2014) 40:1724–30. 10.1016/j.ejso.2014.08.479 [DOI] [PubMed] [Google Scholar]
- 96.Hitier-Berthault M, Ansquer C, Branchereau J, Renaudin K, Bodere F, Bouchot O, et al. 18 F-fluorodeoxyglucose positron emission tomography-computed tomography for preoperative lymph node staging in patients undergoing radical cystectomy for bladder cancer: a prospective study: PET scan for bladder cancer. Int J Urol. (2013) 20:788–96. 10.1111/iju.12045 [DOI] [PubMed] [Google Scholar]
- 97.Swinnen G, Maes A, Pottel H, Vanneste A, Billiet I, Lesage K, et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol. (2010) 57:641–7. 10.1016/j.eururo.2009.05.014 [DOI] [PubMed] [Google Scholar]