Highlights
-
•
Covid-19 infection is associated with elevation of inflammatory markers and coagulopathy.
-
•
Anticoagulation practice for severely ill covid-19 patients is variable among physicians.
-
•
Inflammatory markers levels may impact anticoagulation dosages.
-
•
Anticoagulation does not improve 28-day survival.
Keywords: Covid-19, Sars cov 2, Anticoagulation, Mortality, Practice pattern, Length of stay
Abstract
Covid-19 has affected 16Millions people worldwide with 644 K death as of July 26th, 2020. It is associated with inflammation and microvascular thrombosis—anticoagulation in widely used in these patients especially in patients with elevated d-Dimers. The significance of anticoagulation in these patients is not yet established. We aim to define the anticoagulation pattern and its impact on outcomes (28-day survival, LOSICU, DVT, and PE and bleeding complications. We also observe if levels of d-Dimers affect the anticoagulation prescription.
Methods: We analyzed data of all consecutive patients with Covid-19 ARDS admitted to ICU retrospectively. The primary variable of interest was anticoagulation. The daily dose of anticoagulant medication for each patient was recorded. Survival (28-day survival), Length of stay in ICU (LOSICU), the occurrence of DVT, PE, or bleeding were primary outcome variables. We also recorded confounding factors with potential impact on clinical outcomes. We assign Patients to one of the four groups based on anticoagulant dosing during the ICU (increasing dose, decreasing dose, increase followed by a decrease, multiple changes). We analyze the effect of different anticoagulation dosing strategies on 28-day survival, LOSICU, the occurrence of DVT, PE, and bleeding. We also observe if levels of d-Dimers affect the anticoagulation prescription.
Results: The sample includes 149 patients. The most frequently used medication was subcutaneous Enoxaparin (85.2%). The Enoxaparin mean dose per day for the whole sample was 49.5 mg + 15.7 (mean + SD). There was no significant difference in doses of anticoagulants between survivors and nonsurvivors (62.8 mg + 21.7 mg vs. 61.2 mg + 25.7 mg, p 0.3). Multinomial regression showed no difference in 28-day survival among four-dose modification (increasing dose, decreasing dose, increase followed by a decrease, multiple changes). Logistic regression showed that BMI, d-Dimers, platelets, and the use of mechanical ventilation predict 28-day survival. Kaplan-Meier Survival plots for 4 anticoagulant groups showed no survival advantage for any anticoagulant strategy. Secondary outcome analysis showed that d-dimer levels significantly affect anticoagulants doses.
Conclusion: Prescription of anticoagulation is quite variable in patients admitted to ICU for Covid-19 associated ARDS. Anticoagulation dosing strategy has no significant effect on 28-day survival, LOSICU, the occurrence of DVT, PE, or bleeding.
Introduction
Covid-19 also called novel coronavirus, has affected 16Millions people with 644 K death as of July 26th, 2020, worldwide.1 Covid-19 (SARS CoV-2) spread rapidly via person to person contact and attacks predominantly respiratory system.2 Covid-19 infection is associated with inflammation and microvascular thrombosis,3, 4 as reflected by elevated markers of inflammation (ferritin, CRP) and d-Dimers,5 respectively. It may cause pulmonary micro-thrombosis,6 leading to a ventilation-perfusion (VQ) mismatch. Therefore, many such patients do not respond to lung recruitment maneuvers.7 Studies showed that anticoagulation in patients with Covid-19 infection who manifest elevated d-dimers might be beneficial.8 Data on anticoagulation in Covid-19 positive patients is evolving. The results of the studies are variable, so is the clinical practice. Skeptical physicians used thromboprophylaxis dose. Other physicians use markers (D-Dimer level) as a guide to change the anticoagulation dose. Firm believers use therapeutic dose anticoagulation recommended to treat deep venous thrombosis (DVT) or pulmonary embolism (PE); Enoxaparin 1 mg/kg subcutaneous every 12 h. The results of different strategies on clinical outcomes are not known. We aim to determine anticoagulation practice patterns. We aim to evaluate the impact of varying anticoagulation patterns on clinical outcomes (28-day survival, LOSICU, DVT, and PE) and bleeding complications.
Methods
We included all patients with confirmed Covid-19 infection admitted to the ICU of Dubai hospital, retrospectively. We excluded patients with age <18 years and patients treated with extracorporeal membrane oxygenation therapy (ECMO). ECMO requires closely monitored larger dose anticoagulation for ECMO circuit patency. The primary variable of interest was anticoagulation. Daily doses of anticoagulant medication for each patient were recorded for all days of ICU stay. We assigned patients to one of the four groups (fixed-dose, increasing amount, decreasing dose, multiple changes in quantities). Primary clinical outcomes were 28-day survival, LOSICU, and DVT, PE, and bleeding. The secondary aim was to observe if d-Dimer levels have any impact on anticoagulant dosing. We also recorded confounding factors that may affect clinical outcomes. Demographics: age, gender, body mass index (BMI), nationality, clinical parameters, swab positivity for viral DNA, number of swabs, days to turn swab test negative, days of symptoms, presence of symptoms, cough, fever, dyspnea, gastric complaints. We also recorded diabetes, hypertension, coronary disease, renal failure, and outpatient dialysis. We recorded inpatient clinical data, including vital signs; fever, tachycardia, Blood Pressure, hypoxia, use of oxygen l/m, mechanical ventilation, vasopressors, or inpatient dialysis. Laboratory parameters: markers of inflammation (CRP, Ferritin, procalcitonin), hematologic indices (WBC, platelets count), chemistries (electrolytes), and organism culture studies, including blood cultures bacteremia, were also recorded since secondary bacterial infection with bacteria impact the clinical outcome. Anticovid-19 medication therapies; chloroquine, antivirals, and Steroids were also recorded as they may be significant confounding factors. We calculated APACHE 2 scores within 24 h of admission to the ICU to assess illness severity.
Statistical analysis
We assess four anticoagulation practice patterns (Fixed-dose, increasing dose, decreasing dose, variable quantity) with Multinomial regression analysis to determine the impact on outcome variables, 28-day survival, and LOSICU adjusting for confounding factors. We constructed Kaplan-Meier plots to detect survival differences among these four groups.
Results
The sample includes 149 patients. The sample characteristics are described in Tables 1 and 2 . The practice pattern of anticoagulation is summarized in Table 3 . The most frequently used medication was subcutaneous Enoxaparin (85.2%). Therefore, all anticoagulation pattern analyses include Enoxaparin only. Mean dose of enoxaparin per 24 h for sample was 49.5 mg + 15.7 (mean + SD). There was no significant difference in dose of anticoagulants between survivors and nonsurvivors (62.8 mg + 21.7 vs. 61.2 mg + 25.7, p 0.3).
Table 1.
Sample characteristics, categorical variables.
| Categorical variables | total N = 149 | alive N = 63 | died N = 86 | p value |
|---|---|---|---|---|
| Gender Male/Female | 129/20 | 53/10 | 76/10 | 0.40 |
| Nationalities Local/Expatriates | 140/8 | 59 | 80 | 0.40 |
| Diabetes | 70 | 31 | 39 | 0.30 |
| Hypertension | 42 | 18 | 24 | 0.50 |
| Coronary artery disease | 10 | 2 | 8 | 0.12 |
| Prior Renal impairment | 23 | 5 | 18 | 0.03 |
| Outpatient dialysis | 11 | 3 | 8 | 0.24 |
| Alcohol user | 1 | 0 | 1 | 0.50 |
| Mechanical Ventilation | 122 | 45 | 77 | 0.01 |
| Vasopressors use | 120 | 42 | 78 | 0.02 |
| Dialysis required in hosp. | 55 | 13 | 42 | 0.01 |
| Bacteremia | 64 | 24 | 40 | 0.20 |
| Central line infection | 58 | 22 | 36 | 0.25 |
| On AntiCovid-19 Treatment | 142 | 62 | 80 | 0.03 |
| Chloroquine | 133 | 60 | 73 | 0.01 |
| Ritonavir | 58 | 18 | 40 | 0.02 |
| Favipiravir | 116 | 55 | 61 | 0.01 |
| Steroids | 108 | 47 | 60 | 0.30 |
| Received GI prophylaxis | 142 | 59 | 83 | 0.60 |
Table 2.
Sample Characteristics continuous variables.
| Continuous variables | total N = 149 | alive N = 63 | died N = 86 | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | P value | |
| Age (years) | 50.7 | 11.3 | 48.5 | 10.2 | 52.3 | 11.9 | 0.04 |
| BMI (Kg/m2) | 27.9 | 5.4 | 26.8 | 6.1 | 28.8 | 4.6 | 0.04 |
| Ferritin (ng/ml) | 1806.3 | 3180.8 | 1326.9 | 1030.7 | 2205.7 | 4142.0 | 0.10 |
| D-Dimer (ng/ml) | 4.6 | 9.0 | 3.2 | 6.6 | 5.7 | 10.5 | 0.09 |
| Procalcitonin (ng/ml) | 2.7 | 15.5 | 1.9 | 7.9 | 3.4 | 19.5 | 0.50 |
| CRP (mg/L) | 149.4 | 105.8 | 143.9 | 92.2 | 154.7 | 115.0 | 0.50 |
| Creatinine (mg/dl) | 4.2 | 33.0 | 1.2 | 1.4 | 6.4 | 43.2 | 0.30 |
| CPK (units/L) | 643.8 | 1180.5 | 362.9 | 552.3 | 860.0 | 1460.8 | 0.01 |
| ABG PH | 7.3 | 0.7 | 7.2 | 1.1 | 7.4 | 0.1 | 0.20 |
| PCo2 (Torr) | 38.4 | 14.5 | 37.5 | 11.7 | 39.0 | 16.0 | 0.50 |
| PO2 (Torr) | 82.1 | 67.0 | 68.9 | 49.3 | 89.8 | 74.9 | 0.09 |
| Lactate (mmol/L) | 7.7 | 41.8 | 14.8 | 66.7 | 3.4 | 9.1 | 0.10 |
| Bicarbonate (mEq/L) | 21.0 | 5.1 | 21.0 | 5.5 | 21.1 | 5.0 | 0.90 |
| Calcium (mg/dl) | 7.2 | 2.8 | 7.6 | 2.5 | 6.8 | 3.0 | 0.10 |
| Magnesium (mg/dl) | 2.1 | 0.3 | 2.1 | 0.3 | 2.1 | 0.3 | 0.70 |
| Platelets (103/microliter) | 207.6 | 95.1 | 223.2 | 96.8 | 196.5 | 93.4 | 0.09 |
| LOSICU (days) | 17.4 | 13.7 | 22.7 | 17.1 | 14.1 | 9.9 | 0.01 |
| LOSH (days) | 22.5 | 16.0 | 19.3 | 16.1 | 13.0 | 8.9 | 0.01 |
| APACHE 2 Scores | 17.4 | 7.9 | 15.3 | 6.7 | 18.9 | 8.4 | 0.01 |
BMI-body mass index, CRP-C reactive protein, CPK- creatine phosphokinase.
Table 3.
Anticoagulation dosing patterns.
| Anticoagulation strategy | Total, N | live | died |
|---|---|---|---|
| Preventive dose | 34 | 11 | 23 |
| Therapeutic dose | 40 | 19 | 21 |
| Supratherapeutic | 2 | 0 | 2 |
| Initially preventive then therapeutic | 45 | 20 | 25 |
| Initially preventive then supratherapeutic | 10 | 5 | 5 |
| other patterns | 16 | 6 | 10 |
| Total | 147 | 61 | 86 |
| Medication name | alive | died | |
| Enoxaparin | 127 | 57 | 70 |
| Combination Enoxaparin and heparin | 12 | 3 | 9 |
| others | 6 | 1 | 5 |
| Dose changes | alive | died | |
| Unchanged (fixed dose) | 30 | 14 | 16 |
| Dose increased | 69 | 25 | 44 |
| Dose decreased | 5 | 1 | 4 |
| Increase followed by decrease | 43 | 21 | 22 |
| Total | 147 |
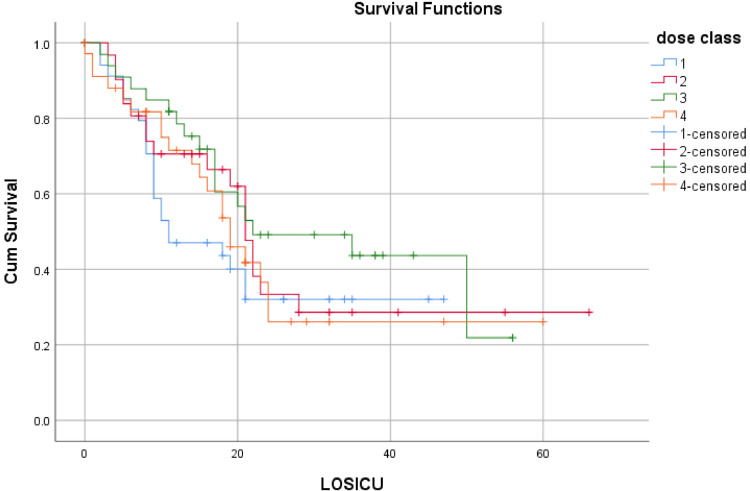
Only 20% of patients have a fixed dose of anticoagulant, while 80% have changes in doses throughout the ICU stay. Logistic regression analysis for anticoagulant dose (set versus changing dosage) showed no impact on 28-day survival (p-value 0.7). Multinomial regression showed no difference in 28-day survival among four strategies of dose modification. Kaplan-Meier Survival plot showed no survival advantage for any group (Fig. 1 ). Only BMI, d-Dimers, platelets, and the use of mechanical ventilation predict 28-day survival (Table 4 ).
Fig. 1.
Kaplan-Meier plot for survival.
Table 4.
Logistic Regression- variables predicting 28-day survival
| Variables | Sig. |
|---|---|
| BMI | 0.002 |
| Steroids | 0.092 |
| D-Dimer | 0.017 |
| Creatinine | 0.062 |
| Platelets | 0.010 |
| Mechanical ventilation | 0.007 |
Variable(s) entered in the model: APACHE 2, age, gender, BMI, nationality, diabetes, hypertension, CAD, renal, dialysis, ventilation, pressers, dialysis, Bacteremia, line infection, chloroquine, Lopinavir/Ritonavir, Favipiravir, Steroids, ferritin, d-dimer, procalcitonin, CRP, creatinine, CPK, ABG PH, PCo2, PO2, lactate, bicarbonate, calcium, magnesium, GI PRO, platelets, Mechanical ventilation.
For LOSICU, multiple regression analyses showed that anticoagulation doses or patterns do not predict LOSICU. Factors predicting LOSICU include mechanical ventilation (P=.01) and Lactate (p=.01).
DVT, PE, and bleeding occurrences: no patients had any documentation of DVT or PE. One patient developed retroperitoneal bleeding, who also had severe thrombocytopenia and renal failure. Another patient developed significant gastrointestinal bleeding requiring gastroscopy and colonoscopies; both patients had treatment with variable anticoagulation doses. Despite the highly variable practice of anticoagulation DVT, PE and bleeding complications were exceptionally low in our sample, not enough to make any comparisons among the four groups.
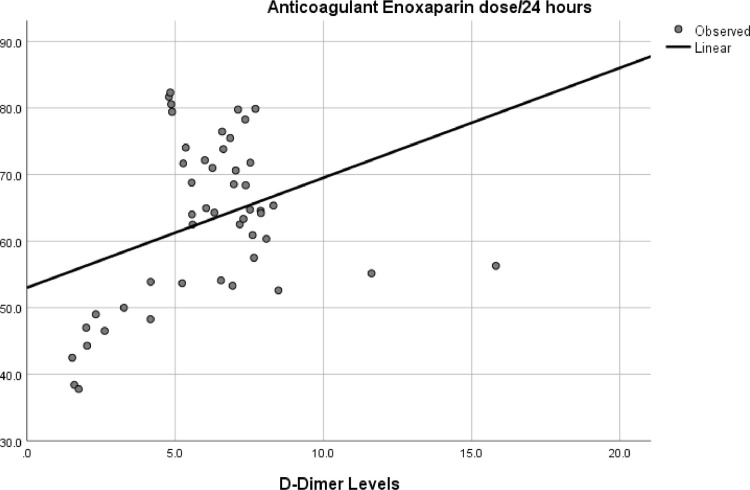
Linear regression analysis between d-Dimer levels and Enoxaparin's average daily dose showed positive interaction (p=.04) (Fig. 2 ).
Fig. 2.
Relationship between d-Dimer levels and Anticoagulant daily dose.
Discussion
Venous thromboembolism frequently occurs in patients treated for ARDS from Covid-19; it is associated with high mortality.9 Patients with Covid-19 suffers from the hypercoagulable state, often resulting in DVT or PE.10, 11 Studies suggest a possible role of lupus anticoagulant and antiphospholipid antibodies.12 The possible mechanism includes activation of the complement cascade and endothelial dysfunction induced by the virus, with or without the antiphospholipid syndrome, leading to Disseminated intravascular coagulation.13, 14 Nadkarni et al. compared prophylactic and therapeutic anticoagulation in 4389 COVID-19 patients and found therapeutic anticoagulation was associated with lower mortality, though not statistically significant.15 Paranjpe et al. documented in their sample of mechanically ventilated Covid-19 patients (n = 395) an in-hospital reduction mortality (29.1% with therapeutic dose anticoagulation versus 62.7% in the control group).8 Studies like this during pandemic peak had a significant impact on anticoagulation practice.
Our clinical observation suggests that anticoagulation prescription is highly variable in patients treated for ARDS secondary to Covid-19 infection. It ranges from fixed preventive dose, fixed therapeutic dose, to changing dosages often daily as a physician taking care of patients also considers a dynamic picture of risk profile as suggested by daily test results. We found none of these differences impact survival or LOSICU in our sample. BMI, d-Dimers, platelets, and the use of mechanical ventilation were the only factors found to affect survival. Only mechanical ventilation and lactate level predicted LOSICU. We observe significant differences between lactate and sodium bicarbonate levels, most likely from the liberal use of sodium bicarbonate infusion in emergency department while awaiting a bed in ICU or CRRT machines for hemodialysis. Therefore, lactate results may not apply to other populations.
The difference in our results and earlier studies8 , 15 may be from a different population, sample size, methodology, and analysis. Studies showing survival improvement did not include several predictors of survival as we did. Therefore, it may also be possible that they may have overestimated the survival benefits. Most such studies recorded anticoagulation on admission day, only assuming it is kept constant throughout the hospital stay, which may not be accurate. We question the survival benefit attributed to anticoagulation when medication may or may not be used subsequently as our data showed a highly variable dosing pattern. Conversely, a variable dose of anticoagulation in our study may be why not translating into a survival benefit. A well-designed prospective study should be considered to address the impact of therapeutic anticoagulation on survival while considering all significant factors that are urgently required.
We found Body mass index, d-Dimers, platelets, and use of mechanical ventilation to be a significant predictor of survival in our study. Others have documented similar results.16, 17, 18, 19
The relationship between MV and an increase in LOSICU that our study documented is apparent and reported by others.20 Similarly, lactate level, reflective of shock, predicts shorter LOSICU. Studies have also demonstrated a similar impact of Lactate on outcomes.21
Despite the variable practice of anticoagulation, occurrences of DVT and PE were low in our sample. Most likely because of the clinician's high threshold for studies requiring visits to the radiology department when study results were thought less likely to change the management. Therefore it is expected that we might have missed DVT and PE. Many patients in our sample eventually died of secondary bacterial infection, multiorgan failure, including respiratory and cardiac failure. Since none of the patients had post mortem studies, We can not exclude coexistent DVT and PE. Llitjos et al. reported a remarkably high incidence of DVT (56%) even with therapeutic dose anticoagulation in severe COVID‐19 patients. They studied only 26 patients with severe disease. Their patients had additional risk factors of higher BMI >30 and had coexisting medical conditions (hypertension). They also actively screened patients for asymptomatic DVT by ultrasonography.9
Bleeding rates were meager in our sample, even in the therapeutic dose anticoagulation group. Others have also reported that bleeding complications, even in patients with disseminated intravascular coagulopathy, are rare in Covid-19 patients.22
We found that d-dimers level effects anticoagulant doses; This suggests that d-dimer may be a significant factor for the physician in the decision for daily dosing of Enoxaparin. Additional factors could play a significant role; planning invasive procedures, platelet counts, renal impairment, prothrombin, or activated partial thromboplastin time. Tang et al. reported that prolong APTT has been reported in patients with Covid-19,23 which was reported24 as a possible reason to avoid the use of anticoagulation at both therapeutic and prophylactic doses. D‐dimer has been suggested to guide anticoagulant treatment in COVID‐19 patients by experts25 although data to support this practice in Covid-19 is lacking.
Variability in the anticoagulation pattern has not been documented before in patients with COVID-19, to our knowledge. Existed data included anticoagulation practice on admission day only, without any follow up. Our observation suggests that dose is frequently changed later depending upon clinical conditions (coagulopathy, renal impairment, thrombocytopenia). Our study highlights the importance of anticoagulation, its dose variability, and its impact (or lack of) on clinical outcomes.
We identify the following weakness of our study. A single-center study with a small sample size in a relatively young patient population comprises Asian patients, so the result may not apply to other communities. A small number of patients in each anticoagulation strategy group may lack the power to detect survival benefits, producing false-negative results.
Conclusion
Prescription of anticoagulation is quite variable in patients treated for ARDS from Covid-19 admitted to ICU. Different patterns of anticoagulant medication seem not to impact survival. Well-designed prospective studies are urgently required to define this particularly important therapeutic issue affecting millions of patients.
Financial support
None.
Study was conducted at Dubai Hospital, Dubai, UAE
Declaration of Competing Interest
All authors have no conflicts of interest.
References
- 1.https://www.worldometers.info/coronavirus
- 2.World Health Organization . Vol. 3. World Health Organization; 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief, July 09th 2020. No. WHO/2019-nCoV/Sci_Brief/Transmission_modes/2020. (Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief, July 09th 2020. No. WHO/2019-nCoV/Sci_Brief/Transmission_modes/2020). [Google Scholar]
- 3.Magro C., Justin Mulvey J., Berlin D. "Complement associated microvascular injury and thrombosis in the pathogenesis severe COVID-19 infection: a report of five cases. Translat Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leisman D.E., Deutschman C.S., Legrand M. "Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020:1–4. doi: 10.1007/s00134-020-06059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. “C-reactive protein, procalcitonin, d-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFadyen J.D., Stevens H., Peter K. "The Emerging Threat of (Micro) Thrombosis in COVID-19 and Its Therapeutic Implications. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan C., Chen L., Lu C. Lung recruitability in COVID-19–associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Criti Care Med. 2020;201(10):1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paranjpe I., Fuster V., Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llitjos J.-F., Leclerc M., Chochois C. "High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020 doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicentre prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. April 10th (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harzallah I., Debliquis A., Drénou B. "Lupus anticoagulant is frequent in patients with Covid-19: response to Reply. J Thromb Haemost. 2020 doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uthman I.W., Gharavi A.E. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 14.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadkarni G.N., Lala A., Bagiella E. et al. "Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietz W., Santos-Burgoa C. "Obesity and its Implications for COVID-19 Survival. Obesity. 2020;28(6):1005. doi: 10.1002/oby.22818. -1005. [DOI] [PubMed] [Google Scholar]
- 17.Mahase, E.. "Covid-19: low dose steroid cuts death in ventilated patients by one third, trial finds." (2020). [DOI] [PubMed]
- 18.Cheng Y., Luo R., Wang K. "Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X., Yang Q., Wang Y. "Thrombocytopenia and its association with survival in patients with COVID-19. J Thromb Haemost. 2020;18(6):1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapidus N., Zhou X., Carrat F., Riou B., Zhao Y., Hejblum G. "Biased and unbiased estimation of the average lengths of stay in intensive care units in the COVID-19 pandemic. medRxiv. 2020 doi: 10.1186/s13613-020-00749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin J., Blobner M., Busch R., Moser N., Kochs E., Luppa P.B. Point-of-care testing on admission to the intensive care unit: lactate and glucose independently predict survival. Clin Chem Lab Med (CCLM) 2013;51(2):405–412. doi: 10.1515/cclm-2012-0258. [DOI] [PubMed] [Google Scholar]
- 22.Connors J.M., Levy J.H. "COVID-19 and its implications for thrombosis and anticoagulation. Blood J Am Soc Hematol. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowles L., Platton S., Yartey N. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023‐1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]