Abstract
Background/Purpose
To investigate the characteristics of dysosmia and dysgeusia among patients diagnosed with coronavirus disease 2019 (COVID-19) in Taiwan.
Methods
Prospective data collection between January 22, 2020 to May 7, 2020 of nucleic acid confirmed COVID-19 hospitalized patients in northern Taiwan by the Taiwan Centers for Disease Control were analyzed.
Results
Of 217 patients enrolled, 78 (35.9%) reported dysosmia (n = 73, 33.6%) and/or dysgeusia (n = 62, 28.6%). The median duration of COVID-19 associated symptom-onset to development of dysosmia and/or dysgeusia was <1 days (interquartile range [IQR], <1–6 days) and 53 of 78 (67.9%) patients developed dysosmia and/or dysgeusia as one of the initial symptoms of COVID-19. Of 59 closely monitored patients, 41 (69.5%) patients recovered within 3 weeks after symptoms onset and the median time to recovery was 12 days (IQR, 7–20 days). Only 6 of the 59 (10.2%) patients reported persistent dysosmia and/or dysgeusia before discharge from hospitals. Multivariate analysis showed that younger individuals (adjusted hazard ratio [AHR], 0.93 per one-year increase; 95% confidence interval [95% CI], 0.89–0.97; P = 0.001), women (AHR, 2.76; 95% CI, 1.05–7.25; P = 0.04) and travel to North America (AHR, 2.35; 95% CI, 1.05–5.26; P = 0.04) were the significant factors associated with dysosmia and/or dysgeusia.
Conclusion
Dysosmia and/or dysgeusia are common symptoms and clues for the diagnosis of COVID-19, particularly in the early stage of the disease. Physicians should be alerted to these symptoms to make timely diagnosis and management for COVID-19 to limit spread.
Keywords: Olfactory dysfunction, Gustatory dysfunction, Dysosmia, Dysgeusia, COVID-19
Introduction
The coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was declared a pandemic on March 11, 2020, by the World Health Organization. In order to prevent transmission of COVID-19, Taiwan government began onboard quarantine since December 31, 2019. Taiwan Centers for Disease Control (Taiwan CDC) laboratory set up a protocol to test SARS-CoV-2 and announced COVID-19 as a notifiable disease, to be reported to Taiwan CDC within 24 h since January 15, 2020. The first case of SARS-CoV-2 infection in Taiwan was diagnosed on January 21, 2020. Since March 20, 2020, all returned travelers must undergo 14 days of quarantine upon their arrival to Taiwan. By June 30, 2020, a total of 447 COVID-19 confirmed cases with a mortality rate of 1.6% had been identified in Taiwan.1
COVID-19 is characterized by a variety of clinical manifestations. Common symptoms among COVID-19 patients include fever, dry or productive cough, shortness of breath (dyspnea), muscle ache (myalgia), confusion, headache, sore throat, rhinorrhea, chest pain, diarrhea, nausea/vomiting, conjunctival congestion, nasal congestion, fatigue, and generalized malaise.1 Fever, cough, and fatigue were the most common symptoms reportedly associated with COVID-19 during initial outbreaks in China.2, 3, 4, 5 In those early reports from China, dysosmia (include anosmia and hyposmia) and dysgeusia (include ageusia and hypogeusia) were not considered important symptoms for COVID-19.2, 3, 4, 5 For example, Chen et al. reported four patients with rhinorrhea (4%) in their clinical series of 99 patients3 and Guan et al. reported a prevalence of nasal obstruction in 5% of patients in a cohort of 1099 patients.4 However, in the following epidemics in Europe, United States and South Korea, dysosmia and dysgeusia were frequently associated with SARS-CoV-2 infection.6, 7, 8, 9, 10, 11
Acute smell and taste disorders are related to a wide range of upper respiratory tract viral infections.12 , 13 However, absent or diminished ability to smell or taste, resulting from a viral infection targeting the upper respiratory tract, could be easily neglected from history taking by the primary care physician. Transmission of COVID-19 could occur before and immediately after symptom onset.14 Recognition of early signs, might be helpful for earlier diagnosis of COVID-19 and enable rapid quarantine and isolation of patients. Here, we analyzed the data collected by the Taiwan's CDC of laboratory-confirmed COVID-19 cases in northern Taiwan to describe the demographic characteristics, clinical manifestations and outcome of patients with and without dysosmia or dysgeusia.
Patients and methods
Case definition and data collection
When Taiwan's CDC announced COVID-19 as a notifiable disease on January 15, 2020, the reporting criteria included: fever (≧38 °C) and respiratory symptoms, or cough with tachypnea or respiratory difficulty, or radiologically/pathologically diagnosed pneumonia as well as travel history to a COVID-19 outbreak area within 14 days of disease onset.1 The reporting criteria incorporated rolling updates to include all relevant travel exposure or contact history, and a wider spectrum of clinical illnesses. For example, the reporting clinical criteria was modified as fever or any acute respiratory symptoms, include cough, chest pain, dyspnea, rhinorrhea and nasal stuff since January 25, 2020, and dysfunction of smell (dysosmia) or taste (dysgeusia) were added since March 30, 2020 by Taiwan CDC.1
A confirmed case should meet the reporting criteria for COVID-19 and have a nasopharyngeal swab, throat swab or sputum specimen testing positive for SARS-CoV-2 by real-time reverse transcriptase-polymerase chain reaction (rt-PCR) or virus culture in designated laboratories at hospitals or laboratory of Taiwan CDC. Detailed information including demographics, clinical manifestations, laboratory and image results, should be reported to the National Notifiable Disease Surveillance System (Taiwan CDC). All confirmed cases of COVID-19 were immediately isolated in negative pressure rooms at designated hospitals for medical care until de-isolation. Three consecutive negative samples from respiratory specimens were required for de-isolation. Patients fulfilling the criteria for de-isolation were discharged if there was no need for additional clinical care, and the decision of de-isolation was approved after discussion of the primary care physician with the regional commander of the Infectious Diseases Control Network, which has been established since 2003 by Taiwan CDC.
In this study, we collected the information of confirmed COVID-19 patients derived from 7,500,518 inhabitants-based in northern Taiwan, including Taipei City, New Taipei City, Keelung City and I-Lan County reported to Taiwan CDC during January 22, 2020 to May 7, 2020, accounting for nearly one-third of the total population in Taiwan. Information on clinical presentation, underlying diseases and travel history of patients were collected during case investigation. All information was reconfirmed by Taipei Regional Commander (S.C. Chang) before patients were de-isolated. Laboratory results within 48 h of admission were retrieved from medical records that were uploaded to the National Notifiable Disease Surveillance System. Based on the above information, the investigation team determined the clinical severity of each confirmed patient following the World Health Organization (WHO) interim guidance.15
Statistical analysis
The patients were divided into two groups based on the presence of dysosmia and/or dysgeusia (case group) or not (control group). Categorical variables, such as gender, travel history, clinical features, disease severity, treatment and outcomes, were compared using Pearson's chi-squared test. Continuous variables, such as age and duration of virus shedding, were compared using Mann–Whitney U test. Cox proportional hazards models was used to estimate the unadjusted and adjusted hazard ratios (aHRs) for development of smell or taste dysfunction. Factors with at least borderline significance (P < 0.1) in the univariate analysis were subjected to multivariate analysis. Kaplan–Meier survival curve for the evaluation of factors associated with dysosmia and/or dysgeusia was used among patients with or without pneumonia. All analyses were performed using Stata/SE software, Version 11.0 (https://www.stata.com/).
Ethical approval
Data collection and analysis of cases were determined by the Taiwan Ministry of Health and Welfare to be part of a continuing public health outbreak response and were thus considered exempt from institutional review board approval.
Results
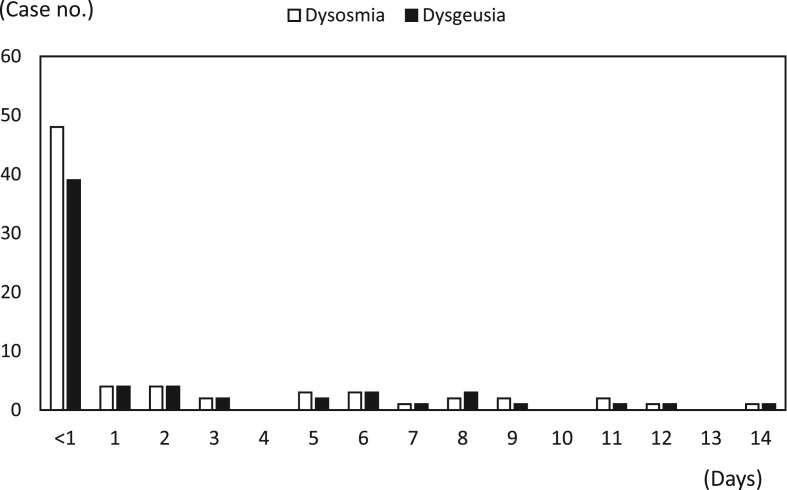
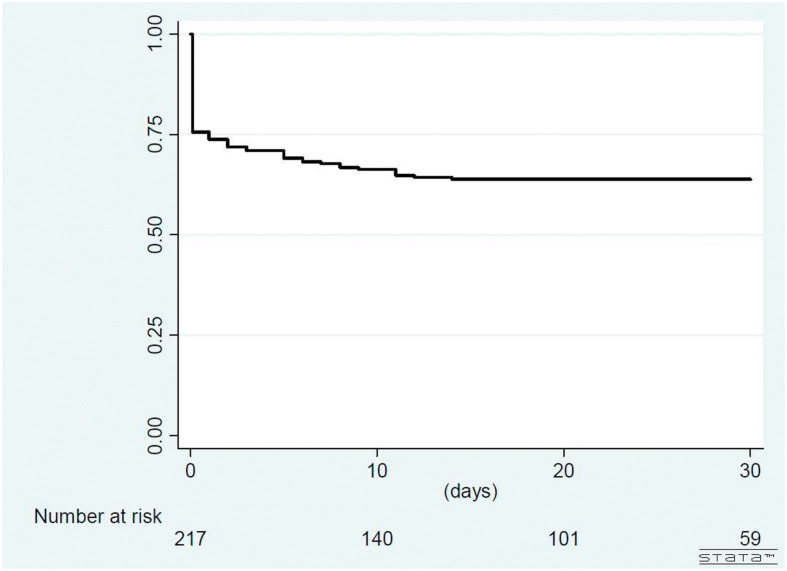
Overall, there were 217 confirmed COVID-19 patients in northern Taiwan. During the study period, 78 (35.9%) patients of them had the symptoms of dysosmia and/or dysgeusia and 139 (64.1%) did not have dysosmia and dysgeusia in their disease courses. Of the 78 case patients, 57 (73.1%) reported both dysosmia and dysgeusia, 73 (93.6%) reported dysosmia and 62 (79.5%) reported dysgeusia, respectively. The prevalence of dysosmia and dysgeusia among overall 217 patients were 33.6% and 28.6%, respectively. The median duration of initial symptom-onset associated with COVID-19 to development of dysosmia and/or dysgeusia was <1 days (range, <1–14 days; 25% and 75% interquartile range [IQR], <1–6 days) and 53 out of 78 case patients (67.9%, 24.4% of overall 217 patients) developed dysosmia and/or dysgeusia at the same day of onset of COVID-19 associated initial symptoms (Figure 1, Figure 2 ). Of 59 closely monitored case patients, 41 (69.5%) recovery from dysosmia and/or dysgeusia dysfunction within 3 weeks and the median time to recovery were 12 days (range, 2–35 days; IQR, 7–20 days). Only 6 of the 59 (10.2%) case patients reported persistent dysosmia and/or dysgeusia before discharge from hospitals.
Figure 1.
Days between symptoms onset and dysosmia and/or dysgeusia among patients with COVID-19.
Figure 2.
Kaplan–Meier survival curve for development of dysosmia and/or dysgeusia among patients with COVID-19.
The demographic characteristics, clinical features, treatment and outcomes of all 217 patients were summarized in Table 1 . The median age of case group was 29 years (IQR, 22–36 years) compared with 37 years (IQR, 26–55 years) of the control group (patients without symptoms of dysosmia and dysgeusia) (P < 0.001) and the proportion of female gender was significantly higher in the case group than that in the control group (66.7% versus 46.0%, P = 0.003). Patients with travel to northern America (p = 0.03), initial clinical symptoms with rhinorrhea (P = 0.01), and patients with upper respiratory tract infection only (mild disease severity) (P = 0.03) had significant higher proportion of dysosmia and/or dysgeusia. The duration of viral shedding between cases and controls was not significantly different. Three patients died and all belonged to the control group. However, there were no significant differences of mortality due to the small number of patients. The causes of death were all severe pneumonia with acute respiratory distress syndrome combined with multiple organ failure.
Table 1.
Demographic and baseline clinical features of COVID-19 patients with and without dysosmia or dysgeusia.
| Characteristics | Total (n = 217) | With dysosmia or dysgeusia (Cases, n = 78) | Without dysosmia and dysgeusia (Controls, n = 139) | P-value |
|---|---|---|---|---|
| Demographic | ||||
| Median age (IQR), years | 33 (24–50) | 29 (22–36) | 37 (26–55) | <0.001 |
| Women, n (%) | 116 (53.5) | 52 (66.7) | 64 (46.0) | 0.003 |
| Travel history, n (%) | ||||
| East/South-East Asia | 14 (6.5) | 2 (2.6) | 12 (8.6) | 0.09 |
| North America | 59 (27.2) | 28 (35.9) | 31 (22.3) | 0.03 |
| Europe | 92 (42.4) | 38 (48.7) | 54 (38.9) | 0.16 |
| Africa | 22 (10.1) | 5 (6.4) | 17 (12.2) | 0.17 |
| Other area | 2 (0.9) | 1 (1.3) | 1 (0.7) | 0.99 |
| No travel history | 28 (12.9) | 4 (5.1) | 24 (17.3) | 0.01 |
| Clinical features, n (%) | ||||
| Fever | 98 (45.2) | 33 (42.3) | 65 (46.8) | 0.50 |
| Cough | 115 (53.0) | 43 (55.1) | 72 (51.8) | 0.68 |
| Rhinorrhea | 53 (24.4) | 27 (34.6) | 26 (18.7) | 0.01 |
| Disease severity, n (%) | ||||
| Upper respiratory infection | 129 (59.4) | 54 (69.2) | 75 (54.0) | 0.03 |
| Pneumonia without mechanical ventilatory support | 67 (30.9) | 23 (29.5) | 44 (31.7) | 0.74 |
| Pneumonia with mechanical ventilatory support | 21 (9.7) | 1 (1.3) | 20 (14.4) | 0.002 |
| Treatment, n (%) | ||||
| LPV/r | 6 (2.8) | 2 (2.6) | 4 (2.9) | 0.99 |
| RDV | 5 (2.3) | 1 (1.3) | 4 (2.9) | 0.66 |
| HCQ | 131 (60.4) | 53 (68) | 78 (56.1) | 0.09 |
| Azithromycin | 71 (32.7) | 29 (37.2) | 42 (30.2) | 0.22 |
| Outcome, n (%) | ||||
| Duration of viral shedding (days) | 25 (18–35) | 24 (17–32) | 26 (18–37) | 0.14 |
| Mortality, n (%) | 3 (1.4) | 0 | 3 (2.2) | 0.56 |
Data are presented as number (%) unless otherwise specified.
Abbreviations: IQR, interquartile range; LPV/r, lopinavir/ritonavir; RDV, remdesivir; HCQ, hydroxychloroquine.
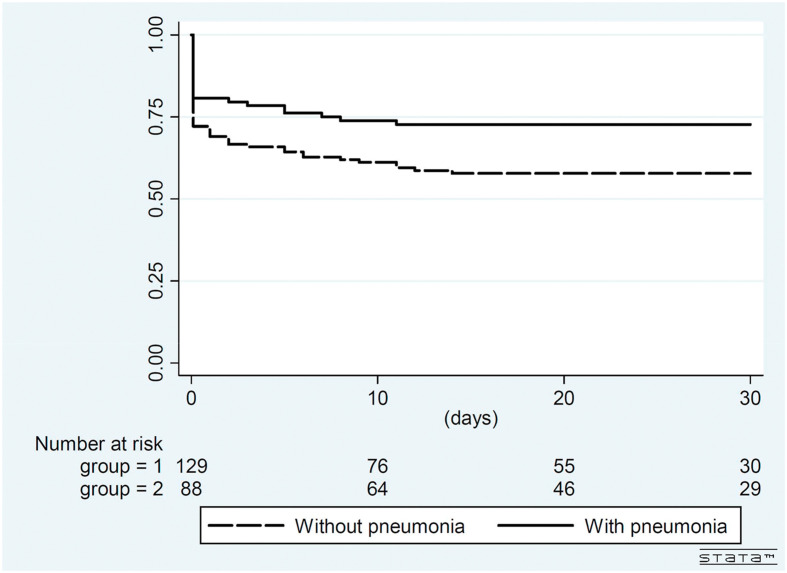
The results of multivariate analysis of factors associated with dysosmia and/or dysgeusia are shown in Table 2 . By multivariate Cox regression analysis, younger individuals (adjusted hazard ratio [AHR], 0.93 per one-year increase; 95% confidence interval [95% CI], 0.89–0.97; P = 0.001), female gender (AHR, 2.76; 95% CI, 1.05–7.25; P = 0.04) and travel to northern America (AHR, 2.35; 95% CI, 1.05–5.26; P = 0.04) were significant independent factors associated with dysosmia and/or dysgeusia after adjustments. Kaplan–Meier survival analysis (Fig. 3 ) suggested that patients without pneumonia had borderline significant higher proportion of dysosmia and/or dysgeusia than those with pneumonia (log-rank test, P = 0.085).
Table 2.
Results of univariable and multivariable analyses of factors associated with dysosmia and/or dysgeusia among patients with COVID-19.
| Variable | Univariable |
Multivariable |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| Age, per 1-year increase | 0.94 (0.9–0.97) | <0.001 | 0.93 (0.89–0.97) | 0.001 |
| Female gender | 3.34 (1.33–8.35) | 0.01 | 2.76 (1.05–7.25) | 0.04 |
| Travel history to North America | 2.92 (1.33–6.41) | 0.01 | 2.35 (1.05–5.26) | 0.04 |
| Rhinorrhea | 2.2 (0.97–4.97) | 0.06 | 1.92 (0.83–4.45) | 0.13 |
| Cough | 1.83 (0.79–4.24) | 0.16 | – | – |
| Pneumonia | 0.47 (0.2–1.14) | 0.09 | 0.77 (0.31–1.87) | 0.56 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Figure 3.
Kaplan–Meier survival curve for development of dysosmia and/or dysgeusia among patients with COVID-19 with or without pneumonia. (Log-rank test P = 0.085).
Discussion
In this study, we report that dysosmia and/or dysgeusia manifest early in the disease process of COVID-19 in Taiwanese patients and these symptoms usually resolved within 3 weeks. These symptoms were more commonly reported in young female patients and those who had travelled to Northern America. Clinicians should seek to evaluate patients for acute-onset of dysosmia and/or dysgeusia, particularly in the context of suspicion for SARS-CoV-2 infection.
The presentations of COVID-19 overlap with symptoms of a common cold and influenza. Dysosmia and/or dysgeusia have been reportedly associated with initial presentations of COVID-19. Lee et al. reported approximately 15% patients had dysosmia and/or dysgeusia in the early stage of COVID-19 and the median duration to recovery was 7 days for both symptoms.10 Tong et al. conducted a systematic review and meta-analysis of confirmed COVID-19 cases, which showed 52.7% of the patients had dysosmia and 43.9% of the patients had dysgeusia, respectively.16 Our study echoes these findings with the prevalence of dysosmia and dysgeusia being 33.6% and 28.6%, respectively. Overall 24.4% patients with COVID-19 in Taiwan developed dysosmia and/or dysgeusia on the same day as initial symptoms onset and recovered after a median duration of 12 days.
In our present study, we found that younger patients and women more commonly experienced dysosmia and/or dysgeusia than elderly and male patients. In an anonymous electronic survey of 145 confirmed COVID-19 patients and 157 patients with negative test results of SARS-CoV-2, smell or taste change, fever, and body ache were associated with COVID-19 patients, and shortness of breath and sore throat were associated with patients of negative test results (all P < 0.05).17 Interestingly, the survey showed that 214 (72%) were female and the participants had a mean age of 39 years.17 Biadsee et al. conducted a web-based questionnaire assessing initial clinical presentation of 140 confirmed COVID-19 patients.18 The common symptoms included cough, weakness, myalgia, fever, headache, dysosmia, dysgeusia, sore throat, rhinorrhea and nasal congestion. Dysosmia and dysgeusia were reported in 38.3% and 32.8% of the patients, respectively. All symptoms were reported more frequently by female patients than male patients.18 Lee et al. prospectively collected data of cases of anosmia or ageusia via telephone interview among 3191 patients from South Korea.10 In all, 68.9% of patients with dysosmia and/or dysgeusia were female, compared with 31.1% were male (P = 0.01). The median age of cases with dysosmia and/or dysgeusia was also younger than those without (median age, 36.5 years versus 46.0 years, P < 0.001).10 A systematic review by da Costa et al. from six studies with enrollment of 1457 patients of different ethnicities were assessed.19 Totally 885 (60.7%) and 822 (56.4%) had dysosmia and/or dysgeusia, respectively, with women being more often affected. Dysosmia and/or dysgeusia may have been noted even without nasal obstruction/rhinorrhea or before the initial symptoms of COVID-19. Dysosmia and/or dysgeusia usually resolved within the first two weeks after COVID-19.19 Lechien et al. reported 417 mild-to-moderate COVID-19 patients who were recruited from 12 European hospitals.6 They found dysosmia and/or dysgeusia were more frequently reported in female patients than in male patients (P = 0.001).6 In line with the above reports, our results suggest that dysosmia and/or dysgeusia were more common among females and young individuals.
Travel history to northern America was also one of the significant factors associated with dysosmia and/or dysgeusia in our study. Tsou et al. described the epidemiology and outcome of the first 100 COVID-19 cases in Taiwan, and most of them were from China, other Asian countries and Europe.20 They found that only 8% of the patients were reported dysosmia and/or dysgeusia. Different viral clades have a likely impact on COVID-19 pathogenesis and spread. The affinity of viruses for tissues and individuals might explain the potential clinical differences between patients from different world regions. Sequence analysis from 2310 viral isolates reveals that point mutations of the SARS-CoV-2 S protein spike can result in increased virulence through instability of the viral machinery and altered viral to cell membrane fusion.21 Other possible mechanism is the variable presentations of host susceptibilities. The angiotensin-converting enzyme 2 receptor (ACE2), which is the receptor of SARS-CoV-2, could be specific to certain populations and ACE2 gene polymorphism, human ACE2 mRNA expression and human ACE2 protein polymorphism might influence SARS-CoV-2 susceptibility and COVID-19 disease outcome.22 Li et al. demonstrated that some ACE2 variants could reduce the association between human ACE2 and SARS-CoV S-protein.23 The impact of the evolution among different viral clades and the expression level of ACE2 in different ethnic population on symptoms and outcomes of COVID-19 infection warrant further study.
The pathophysiological mechanism that causes dysosmia and/or dysgeusia is still uncertain. Dysosmia and/or dysgeusia are related to a wide range of viral infections.13 Infection of the upper respiratory tract can cause dysosmia and/or dysgeusia because of viral damage to the olfactory epithelium.13 , 24 However, traditional nasal cavity manifestations of upper respiratory viral infections (such as rhinovirus, influenza, and adenovirus), such as nasal congestion, rhinorrhea, post-nasal drip and nasal stuff have been reported not common in patients with COVID-19.11 Coronaviruses were known to be neurotropic with invasive neural spread into the neuroepithelium and the olfactory bulb.25, 26, 27 By taking biopsies of the olfactory epithelium from patients with confirmed COVID-19 compared with uninfected controls, elevated levels of the pro-inflammatory cytokine tumor necrosis factor α (TNF-α) were shown to be significantly increased in the olfactory epithelium of the COVID-19 group compared to the control group.28 The ACE2 receptor, which is the main host cell receptor of SARS-CoV-2 for binding and penetrating cells, is widely expressed on epithelial cells of the nasal29 and oral mucosa.30 This suggests that direct inflammation of the olfactory epithelium and damage of mucosal epithelial cells of the oral cavity may explain dysosmia and/or dysgeusia observed in the early stage of COVID-19. In our patients, 34.6% of case group had reported simultaneously symptoms of rhinorrhea and nasal congestion. Hence both local inflammation and neuropathic mechanisms could be occurring in COVID19 to potentiate dysosmia and/or dysgeusia.
Our study had limitations. First, as the data were provided by subjective description by patients, we did not use validated instruments such as standard questionnaires or objective tests in this study. Consequently, self-reporting may underestimate the prevalence of olfactory/gustatory impairment. However, our study with a relatively large population-based in northern Taiwan provide important clinical information. On the other hand, all patients diagnosed with COVID-19 in Taiwan are mandated to be hospitalized until they no longer need to be under airborne isolation, even if they have mild or no symptoms. Hence, our data of dysosmia and/or dysgeusia could more comprehensively cover all patients with COVID-19. With increasing awareness of dysosmia and/or dysgeusia in patients with COVID-19, clinicians may more routinely inquire about these symptoms, thereby improving our understanding of the true prevalence. Second, patients with COVID-19 in Taiwan mostly consisted of young and mild to moderate COVID-19 patients with little comorbidities. Therefore, the results might not be applicable for those patients with elderly age or severe morbidities. However, it is difficult to investigate olfactory and gustatory function in patients in life-threatening condition, such as patients receiving mechanical ventilation at intensive care units.
In conclusion, dysosmia and/or dysgeusia are pertinent clues for the diagnosis of COVID-19, particularly in the early stage of the disease. Particularly among patients with mild disease severity and few symptoms, the presence of dysosmia and/or dysgeusia may important to elicit for the diagnosis of COVID-19. We recommend adding these symptoms to the list of primary screening symptoms for COVID-19 to prevent viral spread in the early stage.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article.
Acknowledgement
The authors would like to thank all participated physicians of Infectious Diseases Control Network who took care of the patients with COVID-19. The authors also appreciate staffs and medical officers of the Taipei Regional Center of Taiwan CDC, who help to do investigation and collection of patient information during the study period. We also thank Dr. Aristine Cheng M.D. (National Taiwan University Hospital, Taipei, Taiwan) for English editing of this manuscript.
References
- 1.Taiwan Centers for Disease Control Coronavirus disease 2019 (COVID-19) https://www.cdc.gov.tw/en/Disease/SubIndex/
- 2.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 Mar 26 doi: 10.1093/cid/ciaa330. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliezer M., Hautefort C., Hamel A.L., Verillaud B., Herman P., Houdart E. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020 Apr 8 doi: 10.1001/jamaoto.2020.0832. (Online) [DOI] [PubMed] [Google Scholar]
- 9.Jang Y., Son H.J., Lee S., Lee E.J., Kim T.H., Park S.Y. Olfactory and taste disorder: the first and only sign in a patient with SARS-CoV-2 pneumonia. Infect Control Hosp Epidemiol. 2020 Apr 20 doi: 10.1017/ice.2020.151. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y., Min P., Lee S., Kim S.W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35:e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 2020 Apr 15 doi: 10.1016/S1473-3099(20)30293-0. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki M., Saito K., Min W.P. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117:272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummel T., Landis B.N., Huttenbrink K.B. Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2011;10:Doc04. doi: 10.3205/cto000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng H.Y., Jian S.W., Liu D.P., Ng T.C., Huang W.T., Lin H.H. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020 May 1 doi: 10.1001/jamainternmed.2020. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. Clinical management of severe acute respiratory infection when COVID-19 is suspected.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Google Scholar]
- 16.Tong J.Y., Wong A., Zhu D., Fastenberg J.H., Tham T. The prevalence of olfactory and gustatory dysfunction in covid-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163:3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 17.Roland L.T., Gurrola J.G., Loftus P.A., Cheung S.W., Chang J.L. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int Forum Allergy Rhinol. 2020;10:832–838. doi: 10.1002/alr.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biadsee A., Biadsee A., Kassem F., Dagan O., Masarwa S., Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms-a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020 Jun 16 doi: 10.1177/0194599820934380. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa K.V.T.D., Carnauba A.T.L., Rocha K.W., Andrade K.C.L., Ferreira S.M.S., Menezes P.L. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020 Jun 9 doi: 10.1016/j.bjorl.2020.05.008. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsou T.P., Chen W.C., Huang S.E., Chang S.C. Epidemiology of the first 100 cases of COVID-19 in Taiwan and its implications on outbreak control. J Formos Med Assoc. 2020;119:1601–1607. doi: 10.1016/j.jfma.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brufsky A. Distinct viral clades of SARS-CoV-2: implications for modeling of viral spread. J Med Virol. 2020 Apr 20 doi: 10.1002/jmv.25902. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devaux C.A., Rolain J.M., Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53:425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Riel D., Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 25.Desforges M., Le Coupanec A., Brison E. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96. doi: 10.1007/978-81-322-1777-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torabi A., Mohammadbagheri E., Dilmaghani N.A., Bayat A.H., Fathi M., Vakili K. Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia. ACS Chem Neurosci. 2020;11:1909–1913. doi: 10.1021/acschemneuro.0c00249. [DOI] [PubMed] [Google Scholar]
- 29.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]