Abstract
Acute kidney injury (AKI) is common among hospitalized patients with Coronavirus Infectious Disease 2019 (COVID-19), with the occurrence of AKI ranging from 0.5% to 80%. The variability in the occurrence of AKI has been attributed to the difference in geographic locations, race/ethnicity, and severity of illness. AKI among hospitalized patients is associated with increased length of stay and in-hospital deaths. Even patients with AKI who survive to hospital discharge are at risk of developing chronic kidney disease or end-stage kidney disease. An improved knowledge of the pathophysiology of AKI in COVID-19 is crucial to mitigate and manage AKI and to improve the survival of patients who developed AKI during COVID-19. The goal of this article is to provide our current understanding of the etiology and the pathophysiology of AKI in the setting of COVID-19.
Key Words: AKI, COVID-19, Collapsing GN, Pathology, Kidney failure
Clinical Summary.
-
•
The most common cause of kidney injury in patients with COVID-19 is acute tubular injury.
-
•
Collapsing glomerulopathy is a recognized glomerular pathology associated with COVID-19 that most commonly affects individuals with high-risk APOL1 polymorphisms.
-
•
Other glomerular pathology associated with COVID-19 might be coincidental or a “second hit” phenomenon.
-
•
It is unclear at this point if SARS-CoV-2 directly infects the kidney.
Acute kidney injury (AKI) has been reported to be a common complication among hospitalized patients with Coronavirus Disease 2019 (COVID-19), with the occurrence of AKI ranging from 0.5% to 80%.1, 2, 3, 4, 5 The understanding of the pathophysiology of AKI in the setting of COVID-19 is rapidly evolving; review of the current literature has demonstrated several etiologies: (1) prerenal azotemia, (2) proximal tubular injury, (3) glomerulopathy, (4) thrombotic microangiopathy, and (5) complications from the treatment of COVID-19. The pathogenesis of kidney injury in patients infected with severe acute respiratory virus-2 (SARS-CoV-2) is multifactorial (Fig 1 ).
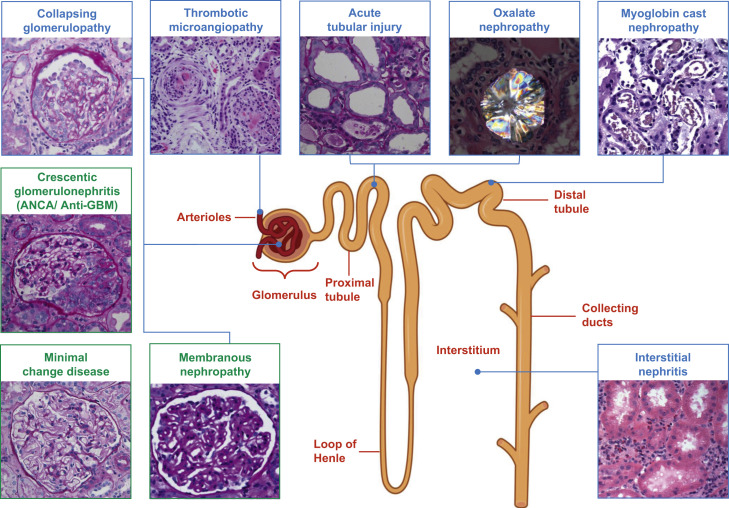
Figure 1.
Various pathophysiological mechanisms associated with COVID-19–related acute kidney injury. Dashed line, the association is less well understood. Dark green box and bolded font, acute tubular injury is the predominant mechanism for acute kidney injury. ACE2, angiotensin-converting enzyme 2; COVID-19, Coronavirus Infectious Disease 2019; SARS-CoV-2, severe acute respiratory virus-2. (Figure 1 was created using Biorender.com.)
Case 1
A 26-year-old man presented with acute shortness of breath and fevers. He was hospitalized and diagnosed with COVID-19. His body mass index on admission was 39 kg/m 2 . On the urinalysis, he had hematuria and proteinuria. The spot urine to protein creatinine ratio was 1.2 g/g of protein/creatinine. His serum creatinine was 2.9 mg/dL (baseline of 0.8 mg/dl). His urine sediment showed muddy brown casts and hyaline casts. He had a prior medical history of obstructive sleep apnea. His clinical condition worsened as his inflammatory markers rose (ferritin, C-reactive protein, D-Dimer). He was intubated and transferred to the intensive care unit. Kidney function deteriorated, and he became anuric. Dialysis was initiated. A kidney sonogram showed no hydronephrosis; right kidney was 13 cm, and left kidney was 14 cm.
Prerenal Azotemia
Patients with COVID-19 often present with fever, volume depletion, and shortness of breath. In addition, around 10% of patients with COVID-19 experienced at least one gastrointestinal symptom (nausea, vomiting, or diarrhea),6 , 7 all of which contributed to fluid losses. In a study from New York, 66% of the patients with AKI had a urine Na of <35 mmol/L, suggestive of a prerenal state or low effective arterial blood volume from heart failure or liver cirrhosis.2 Based on a study by Mohamed and colleagues, prerenal azotemia accounted for 9-10% of AKI in patients with COVID-19.8 While most cases of prerenal AKI were from hypovolemia, some cases of AKI resulted from acute cardio-renal syndrome associated with COVID-19.9
Acute Tubular Injury
Evidence to date shows that the vast majority of AKI cases in patients with COVID-19 were related to acute tubular injury. From a single-center study in the United States (US),8 greater than 60% of AKI cases were attributed to acute tubular injury either from ischemic or toxic tubular injury. Several publications examining native kidney biopsy and autopsy have demonstrated acute tubular injury as the most common pathologic finding in patients with COVID-19 and concomitant AKI.10, 11, 12, 13, 14 Acute tubular injury may occur in the setting of prolonged volume depletion and hemodynamic states that reduce kidney perfusion. In severe COVID-19, viral infection in type II alveolar cells results in immune cell recruitment, which produce an abundance of cytokines that can lead to circulatory collapse.15 From our background knowledge of sepsis-induced AKI, the exact pathophysiology of this illness is not known. However, it is generally accepted that it results from multifactorial injuries. This form of AKI has components of ischemia-reperfusion injury, direct inflammatory injury, coagulation and endothelial cell dysfunction, and apoptosis.16 Table 1 and Figure 2 summarize the kidney biopsy findings in patients with COVID-19 and AKI that have been published at the time of writing this article (September 2020). Based on this series, the median age of 9 patients who developed acute tubular injury was 63 years (interquartile range [IQR], 54 to 69). The median peak serum creatinine was 4.8 mg/dL (IQR, 4.4 to 5.8). Two out of the 9 patients died, and among those who survived, one patient remained on dialysis upon discharge. It is important to note that kidney biopsies are typically not carried out for patients with a clinical diagnosis of acute tubular injury. Thus, the kidney biopsy series that have been published reflect “for-cause” biopsies and may not reflect the full extent of acute tubular injury in COVID-19.
Table 1.
Summary of Kidney Pathology Findings and Clinical Presentation of AKI Among Patients With COVID-19
| Case # | Pathology | Age | Sex | Race/Ethnicity∗ | Kidney Presentation | SCr† (mg/dL) | Proteinuria (g/g) | KRT | Kidney outcome‡ | Patient Outcome | Other | Risk Alleles | Country | Reference§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute tubular injury | ||||||||||||||
| 1 | ATI | 43 | Female | Black | AKI | 6.7 | NA | Yes | NA | NA | USA | Kudose (JASN)11 | ||
| 2 | ATI | 54 | Female | Hispanic | AKI | 2.9 | 0.2 | No | 2.2 | Alive | Kidney transplant | USA | Kudose (JASN)11 | |
| 3 | ATI | 67 | Male | White | AKI | 5.7 | NA | Yes | Dialysis-dependent | Alive | USA | Kudose (JASN)11 | ||
| 4 | ATI | 51 | Male | Black | AKI | 4.8 | 0.5 | No | 2.5 | Alive | USA | Kudose (JASN)11 | ||
| 5 | ATI | 62 | Male | Hispanic | AKI | 6.3 | NA | Yes | Dialysis-dependent | Died | USA | Sharma (JASN)10 | ||
| 6 | ATI | 69 | Male | Hispanic | AKI, proteinuria | 5.8 | 2.4 | Yes | Dialysis-dependent | Died | USA | Sharma (JASN)10 | ||
| 7 | ATI | 76 | Female | White | AKI | 4.4 | 0.9 | No | 4.4 | Alive | USA | Sharma (JASN)10 | ||
| 8 | ATI | 59 | Male | AA | AKI | 4.5 | 2.8 | No | 5.6 | Alive | USA | Sharma (JASN)10 | ||
| 9 | ATI | 69 | Female | AA | AKI, proteinuria | 1.9 | 7.6 | No | 1.9 | Alive | USA | Sharma (JASN)10 | ||
| Collapsingglomerulopathy with acute tubular injury | ||||||||||||||
| 1 | CG + ATI | 46 | Male | Black | AKI, NS | 12.5 | 5.8 | Yes | Dialysis-dependent | Alive | APOL1+ | G1/G1 | USA | Kudose (JASN)11 |
| 2 | CG + ATI | 62 | Male | Black | AKI, NS | 10.7 | 12.1 | No | 3.8 | Alive | APOL1+ | G1/G1 | USA | Kudose (JASN)11 |
| 3 | CG + ATI | 62 | Male | Black | AKI, Proteinuria | 11.6 | 19.0 | No | 2.3 | Alive | APOL1+ | G1/G2 | USA | Kudose (JASN)11 |
| 4 | CG + ATI | 57 | Male | Black | AKI, proteinuria | 4.9 | 6.2 | No | 4.9 | Alive | USA | Kudose (JASN)11 | ||
| 5 | CG + ATI | 61 | Male | Black | AKI, Proteinuria | 15.0 | 9.0 | Yes | Dialysis-dependent | Alive | USA | Kudose (JASN)11 | ||
| 6 | CG + ATI | 63 | Male | Black | AKI, NS | 4.9 | 12.7 | Yes | Dialysis-dependent | Alive | APOL1+ | G1/G1 | USA | Wu (JASN)31 |
| 7 | CG + ATI | 64 | Female | Black | AKI, NS | 4.2 | 4.6 | No | 3.1 | Alive | APOL1+ | G2/G2 | USA | Wu (JASN)31 |
| 8 | CG + ATI | 65 | Female | Black | AKI, NS | 2.9 | 13.6 | Yes | Dialysis- dependent | Died | APOL1+ | G1/G1 | USA | Wu (JASN)31 |
| 9 | CG + ATI | 44 | Male | Black | AKI, NS | 11.4 | 25.0 | Yes | Dialysis-dependent | Alive | APOL1+ | G1/G1 | USA | Wu (JASN)31 |
| 10 | CG + ATI | 37 | Male | Black | AKI, NS | 9.0 | NA | Yes | Dialysis-dependent | Died | APOL1+ | G1/G2 | USA | Wu (JASN)31 |
| 11 | CG + ATI | 56 | Male | Black | AKI, NS | 6.7 | 3.6 | Yes | 2.8 | Alive | APOL1+ | G1/G1 | USA | Wu (JASN)31 |
| 12 | CG + ATI | 67 | Female | AA | AKI, NS | 2.2 | 3.2 | Yes | Dialysis-dependent | Alive | APOL1+ | G1/G2 | USA | Sharma (KM)30 |
| 13 | CG + ATI | 49 | Male | AA | AKI, NS | 4.8 | 2.5 | Yes | Dialysis-dependent | Alive | APOL1+ | G1/G2 | USA | Sharma (KM)30 |
| 14 | CG + ATI | 56 | Male | African | AKI, proteinuria | 1.8 | 1.8 | No | 1.4 | Alive | APOL1+ | G1/G1 | France | Courturier (CKJ)35 |
| 15 | CG + ATI | 52 | Male | African | AKI, proteinuria | 6.2 | 2.8 | No | 2.2 | Alive | APOL1+ | G1/G2 | France | Courturier (CKJ)35 |
| 16 | CG + ATI | 28 | Female | AA | AKI, NS | 6.5 | 2.0 | Yes | Off dialysis | Alive | APOL1+ | G1/G1 | USA | Magoon (KM)29 |
| 17 | CG + ATI | 56 | Male | AA | AKI, NS | 7.2 | 21.0 | Yes | Off dialysis | Alive | APOL1+ | G1/G1 | USA | Magoon (KM)29 |
| 18 | CG + ATI | 63 | Male | Black | AKI, NS | 4.4 | 4.0 | No | 5.5 | Alive | APOL1+ | G1/G1 | Switzerland | Kissling (KI)32 |
| 19 | CG + ATI | 44 | Female | AA | AKI, NS | 4.0 | 3.9 | Yes | Dialysis-dependent | Alive | USA | Larsen (KIR)34 | ||
| 20 | CG + ATI | 77 | Female | AA | AKI, Proteinuria | 8.1 | 1.5 | Yes | 3.0 | Alive | USA | Sharma (JASN)10 | ||
| 21 | CG + ATI | 71 | Male | Asian | AKI, NS | 4.4 | 18.4 | Yes | Dialysis-dependent | Alive | Initial biopsy MCD, repeat biopsy was CG | USA | Gupta (BMCN)36 | |
| 22 | CG + ATI | 54 | Male | Black | AKI, NS | 4.5 | 16.0 | No | NA | Alive | USA | Gupta (BMCN)36 | ||
| 23 | CG + ATI | 49 | Female | Black | AKI | 8.7 | 20.0 | Yes | 2.8 | Alive | APOL1+ | G1/G1 | France | Izzedine (IMJ)37 |
| 24 | CG + ATI | 38 | Female | Black | AKI | 11.7 | 19.0 | No | 2.9 | Alive | APOL1+ | G1/G1 | France | Izzedine (IMJ)37 |
| Others | ||||||||||||||
| 1 | Myoglobin cast nephropathy | 28 | Male | Black | AKI | 9.0 | NA | Yes | 1.2 | Alive | USA | Kudose (JASN)11 | ||
| 2 | Myoglobin cast nephropathy | 60 | Male | Hispanic | AKI | 8.1 | 4.7 | Yes | Dialysis-dependent | Died | USA | Sharma (JASN)10 | ||
| 3 | Oxalate nephropathy | 50 | Male | White | AKI | 5.8 | 0.2 | Yes | 4.0 | Alive | Italy | Fontana (KIR)61 | ||
| 4 | Oxalate nephropathy | 71 | Male | White | AKI | 6.5 | 1.2 | Yes | 4.4 | Alive | Italy | Fontana (KIR)61 | ||
| 5 | LN Class 4 + 5 | 27 | Female | Asian | AKI, NS | 2.5 | 9.2 | No | NA | Died | Systemic lupus erythematosus | USA | Kudose (JASN)11 | |
| 6 | MN (Plar2+) | 72 | Male | White | NS | 0.8 | 8.8 | No | 1.3 | Alive | USA | Kudose (JASN)11 | ||
| 7 | MN (Plar2-) | 70 | Female | Black | AKI, Proteinuria | 2.9 | 6.8 | No | 2.4 | Alive | Endocrine cancer | USA | Kudose (JASN)11 | |
| 8 | MCD + ATI | 25 | Male | Black | AKI, NS | 2.2 | 21.0 | No | 0.8 | Alive | APOL1+ | G1/G1 | USA | Kudose (JASN)11 |
| 9 | ANCA vasculitis | 64 | Male | AA | AKI, Proteinuria | 7.9 | 5.0 | Yes | 2.4 | Alive | MPO+ | USA | Uppal(KIR)47 | |
| 10 | ANCA vasculitis | 46 | Male | Asian | AKI | 2.9 | NA | No | 1.2 | Alive | PR3+ | USA | Uppal (KIR)47 | |
| 11 | ANCA vasculitis | 25 | Male | White | AKI | 5.5 | NA | No | NA | Alive | PR3+ | Iran | Moeinzadeh (IJKD)46 | |
| 12 | Anti-GBM | 48 | Female | Black | AKI | 20.0 | 3.1 | Yes | Dialysis-dependent | Alive | USA | Kudose (JASN)11 | ||
| 13 | Anti-GBM | 45 | Female | Asian | AKI | 8.2 | NA | No | NA | Alive | UK | Prendicki (KI)48 | ||
| 14 | Anti-GBM | 69 | Female | White | AKI | 32.0 | NA | Yes | NA | Alive | MPO+ | UK | Prendicki (KI)48 | |
| 15 | Anti-GBM | 63 | Female | White | AKI | 15.0 | NA | Yes | 4.2 | Alive | MPO+ | UK | Prendicki (KI)48 | |
| 16 | Anti-GBM | 72 | Female | Black | AKI | 15.5 | NA | Yes | 3.1 | Alive | UK | Prendicki (KI)48 | ||
| 17 | Anti-GBM | 34 | Female | White | AKI | 3.2 | NA | No | 0.9 | Alive | MPO+ | UK | Prendicki (KI)48 | |
| 18 | IgA | 78 | Male | White | AKI, proteinuria | 1.4 | 10.0 | No | 1.4 | Alive | HSP-like skin findings | Spain | Suso (KIR)50 | |
| 19 | TMA | 45 | Female | AA | AKI, proteinuria | 7.4 | 5.7 | Yes | Dialysis- dependent | Died | Recent gemcitabine infusion | USA | Sharma (JASN)10 | |
| 20 | TMA | 69 | Female | White | AKI, proteinuria | 7.8 | 1.4 | Yes | Dialysis-dependent | Died | Atypical complement defect | USA | Jhaveri (KI)54 | |
Abbreviations: AA, African American; AKI, acute kidney injury; ANCA, antineutrophil cytoplasmic autoantibody; anti-GBM, antiglomerular basement membrane; APOL1, apolipoprotein L1; ATI, acute tubular injury; CG, collapsing glomerulopathy; HSP, Henoch-Schonlein purpura; KRT, kidney replacement therapy; LN; lupus nephritis; MCD, minimal change disease; MN, membranous nephropathy; MCD, minimal change disease; MPO, myeloperoxidase; NA, not available; NS, nephrotic syndrome; PR3, proteinase 3; SCr, serum creatinine; TMA, thrombotic microangiopathy.
Abbreviations for the Journals: BMCN; BioMed Central Nephrology; CKJ, Clinical Kidney Journal; IJKD, Iran Journal of Kidney Diseases; IMJ, Internal Medicine Journal; JASN, Journal of American Society of Nephrology; KI, Kidney International; KIR; Kidney International Reports; KM, Kidney Medicine.
Race/ethnicity are cited directly from the source article.
Values refer to the peak serum creatinine (mg/dL).
Values refer to serum creatinine (mg/dL) upon discharge.
Last name of first author, journal name and reference number.
Figure 2.
COVID-19–associated kidney disease: pathology findings. Green boxes, the association between COVID-19 and certain histopathological findings (crescentic glomerulonephritis, minimal change disease, and membranous nephropathy) are still putative. ANCA, antineutrophil cytoplasmic antibody; COVID-19, Coronavirus Infectious Disease 2019; GBM, glomerular basement membrane. (Figure 2 was created using Biorender.com.)
Hyperinflammation has been associated with COVID-19.15 The type of hyperinflammation observed in patients with severe COVID-19 is similar to hemophagocytic syndrome-related cytokine release. Secondary hemophagocytic lymphohistiocytosis is an underrecognized, hyperinflammatory syndrome characterized by a fulminant and fatal hypercytokinemia with multiorgan failure. In adults, secondary hemophagocytic lymphohistiocytosis is most commonly triggered by viral infections and occurs in 3.7–4.3% of patients with sepsis and is sometimes referred to as macrophage activation syndrome.17 , 18 Cardinal features of this syndrome include unremitting fever, cytopenias, and hyperferritinemia. Pulmonary involvement such as acute respiratory distress syndrome (ARDS) occurs in approximately 50% of patients with secondary hemophagocytic lymphohistiocytosis.19 A cytokine profile resembling this hemophagocytic syndrome is associated with COVID-19 severity, characterized by increased interleukin (IL)-2, 6, 7, granulocyte colony–stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α. Many studies have now shown high ferritin levels as a predictor of mortality.20 The incidence of AKI during hemophagocytic lymphohistiocytosis is high (62% [59/95]), of whom 59% (35/59) require kidney replacement therapy. The main causes of AKI in patients with hemophagocytic lymphohistiocytosis are acute tubular injury (49%), hypoperfusion (46%), tumor lysis syndrome (29%), or hemophagocytic lymphohistiocytosis–associated glomerulopathies (17%).21 , 22 Similar pathophysiology of hyperinflammation could lead to acute tubular injury seen with COVID-19. The proinflammatory IL-6 is thought to be the principal cytokine that drives hyperinflammation in many of these syndromes. Among patients with COVID-19, the plasma concentration of IL-6 has been shown to be increased in those with ARDS.23 Cytokine overproduction is also thought to mediate a crosstalk between the lung and kidney leading to bidirectional damage.24 Injured kidney tubular epithelium leads to an upregulation of IL-6. Human and animal studies have shown increased levels of serum IL-6 in patients with AKI, and this was associated with higher alveolar-capillary permeability and pulmonary hemorrhage.25 , 26 ARDS has been shown to induce kidney medullary hypoxia, which may act as an additional insult to tubular epithelial cells.24
Tubular injury from rhabdomyolysis10 and a severe form of hyperinflammation27 should be considered in the differential diagnosis of AKI in patients with COVID-19. Patel and colleagues.27 reported a series of patients with hypermetabolism-related AKI, with striking elevations in uric acid, phosphorus, and potassium levels; lactate-negative anion gap; metabolic acidosis; and drastic decreases in serum albumin levels. There was no evidence of tumor lysis syndrome or rhabdomyolysis. While severe acute tubular injury associated with sepsis can present with rapid AKI, such hypercatabolic states in COVID-19 can also be the cause of severe prerenal azotemia and tubular injury.
Proximal tubular damage leading to AKI along with several electrolyte disorders has been described as well. In a cohort of 49 patients requiring hospitalization in a large academic hospital in Belgium, the investigators found evidence of proximal tubule dysfunction in a subset of patients with COVID-19, as attested by low-molecular-weight proteinuria (70-80%), neutral aminoaciduria (46%), and defective handling of uric acid (46%) or phosphate (19%).28 Among these 49 patients, 22% developed AKI. At the structural level, 6 kidney autopsy samples from patients with COVID-19 showed prominent tubular injury, including in the initial part of the proximal tubule, with loss of brush borders, epithelial cell necrosis, collections of intraluminal debris, and a marked decrease in the expression of megalin in the brush border.28 Interstitial disease was not as common as tubular injury, observed only in 2 out of the 6 patients.28
Case 2
A 67-year-old man presented with acute shortness of breath and fever. He was hospitalized and diagnosed with COVID-19. On the urinalysis, he had hematuria (moderate with 10-20 RBCs) and proteinuria (4+). The 24-hour urine showed 19 g of proteinuria. His serum creatinine was 2.0 mg/dL (baseline of 1.1 mg/dL). He had a prior medical history of diabetes mellitus type 2, hypertension, and gout. His clinical condition worsened as his ferritin, C-reactive protein, and D-Dimer increased. He was intubated and transferred to the ICU. His kidney function deteriorated, and he became anuric. Dialysis was initiated. A kidney sonogram revealed no evidence of hydronephrosis; right kidney was 14.5 cm, and left kidney was 16 cm.
Glomerular Diseases
Glomerular diseases have been reported in association with COVID-19. Collapsing glomerulopathy (CG) is the most common form of glomerular disease, but other forms have been reported with variable frequencies.10 , 11 , 29, 30, 31, 32, 33, 34, 35, 36, 37 CG has emerged as a distinct pathology associated with SARS-CoV-2 infection, which seems to specifically affect individuals of African ancestry who have high-risk APOL1 genotypes (G1/G1, G1/G2, or G2/G2). To date, 24 cases of CG have been published in patients with COVID-19. A total of 18 of 22 of the cases mentioned had APOL1 polymorphisms (12/18 with homozygous G1/G1 and 6/18 with heterozygous G1/G2). All cases presented with AKI or nephrotic syndrome; 23/24 (96%) patients were of Black race or of African or African-American heritage, and 1 patient was of Asian (Indian) origin. Most of the cases, 19/24 (79%), were from the United States and remaining from Europe, whereby 10/24 (42%) patients remained dialysis-dependent, and 20/24 (83%) patients were alive at the time of this writing (September 2020). Among those who were off of dialysis, the median serum creatinine upon discharge was 2.9 mg/dL (IQR, 2.6 to 3.6).
The pathogenesis of COVID-19–associated CG is unclear. CG is a histopathological feature that has been associated with other viral infections; most characteristically seen in HIV-associated nephropathy (HIVAN), but also seen in Epstein-Barr virus, cytomegalovirus, and parvovirus B19.38 How different viral infections would result in similar histopathological findings is worth exploring. Some have proposed a direct viral involvement in CG, particularly in HIVAN39 and parvovirus B19,40 and others suggest that this may result from the systemic response to infection and hyperinflammation.41 In COVID-19, the inflammatory milieu may trigger or exacerbate immune-mediated diseases in predisposed patients.6 , 10 , 11 Virus-induced hyperinflammation causes massive release of granulocyte colony–stimulating factor, various ILs, and interferons.20 , 42 , 43
APOL1 expression is upregulated by viral infections and other inflammatory diseases that activate interferons and toll-like receptor-3.44 Viral infections stimulate host interferon production, and interferon is a potent stimulus to APOL1 gene expression.44 Thus, in individuals with high-risk APOL1 genotypes (which are most common in persons of West African ancestry), SARS-CoV-2 infection and the resulting hyperinflammation may act as a “second hit” that leads to podocyte dysregulation, injury, and CG.45
Other glomerular diseases, such as antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis,10 , 46 , 47 anti-glomerular basement membrane (GBM) disease,48 and immunoglobulin A vasculitis without nephropathy49 have been reported in patients with COVID-19. A total of 9 cases of ANCA vasculitis and anti-GBM disease have been reported, and 5 of 9 required dialysis. All 9 patients received treatment with various immunosuppressive therapy and are alive off dialysis at the time of discharge. A single case of immunoglobulin A nephritis has been reported.50 Other glomerular diseases including membranous nephropathy and minimal change disease have been reported as well.11 There are 2 possible explanations for seeing the variety of glomerular diseases in patients with COVID-19. One can postulate a predilection for a specific glomerular pathology for these patients and SARS-CoV-2 acted as a “second hit”. Alternately, these processes could have been unrelated to SARS-CoV-2 and could represent incidental findings.
Case 3
A 57-year-old woman presented with fever, cough, and shortness of breath. She was hospitalized and diagnosed with COVID-19. Her laboratory parameters showed a serum creatinine of 3.0 mg/dL (baseline of 0.5 mg/dl). She clinically worsened leading to ICU admission and subsequent intubation. Her platelets decreased, and she developed anemia consistent with hemolysis. Her kidney function deteriorated, and she became anuric. Dialysis was initiated. A blood smear showed several schistocytes. Her coagulation profile was normal. Her serological profile for lupus and antiphospholipid syndrome was negative. After platelet transfusion, a kidney biopsy was performed.
Thrombotic Microangiopathy
The development of coagulopathy and disseminated intravascular coagulation (DIC) is a devastating complication in patients with sepsis that is associated with increased mortality.51 The pathophysiology of DIC is complex and thought to occur by immune overactivation and disordered coagulation leading to pathologic organ dysfunction.52 In patients with severe COVID-19, overall mortality is higher among those who developed DIC; 71.4% of nonsurvivors met the International Society on Thrombosis and Haemostasis diagnostic criteria for DIC, whereas only 0.6% of survivors met criteria for DIC.53 One case of thrombotic microangiopathies (TMA) has been described in patients with COVID-19, with the kidney biopsy showing diffuse cortical necrosis and widespread glomerular microthrombi.53 However, recent evidence suggests that signs and symptoms of severe COVID-19 resemble the pathophysiology and phenotype of complement-mediated TMA,10 , 54 , 55 rather than sepsis-induced coagulopathy or DIC. To our knowledge and at the time of this writing in September 2020, no cases have been reported of kidney vein thrombosis associated with COVID-19; although kidney arterial thrombosis leading to kidney infarction has been reported.56, 57, 58
Treatment-Related AKI
Certain treatment-related causes of AKI in patients with COVID-19 such as the use of antiviral agents leading to tubulointerstitial diseases59 , 60 and 2 cases of biopsy-proven vitamin C–related oxalate nephropathy61 have also been reported. In addition, kidney infarction has been postulated as a cause of AKI.11 , 58 Trials of multiple molecules such as lopinavir/ritonavir, nucleoside analogues, remdesivir, tenofovir, chloroquine phosphate, or hydroxychloroquine sulfate have been used in patients with COVID-19. Moreover, antibiotics, many of which have been implicated in AKI, are commonly given during hospitalization for COVID-19.62 Thus, treatment-related complications need to be considered when determining the etiology of AKI.
Autopsy Findings
In one of the first autopsy series published from China, 26 autopsies were performed for patients with COVID-19.14 These cases were all rapid autopsies with a postmortem interval of 6 or fewer hours, which reduces autolytic artifacts within tissue. All cases in the series showed mild to severe acute tubular injury. Acute tubular injury was characterized by a loss of the proximal tubular brush borders, vacuolar degeneration (nonisometric in most cases), frank epithelial cell necrosis (4 cases), pigmented granules within tubular cytoplasm (4 cases), and pigmented casts in tubular lumens (3 cases). There was evidence of glomerular ischemia in 7 cases, with 3 of the cases showing fibrin thrombi within the glomerular capillary loops. This was rarely associated with an overlying epithelial cell proliferation (pseudocrescent formation). No proliferative changes were identified within the glomeruli, such as endocapillary hypercellularity or true crescents. In another retrospective study of 81 patients in a single center in China, a total of 41 (50.6%) patients experienced AKI. Limited autopsy of 10 patients showed pathologic findings consistent with acute tubular injury.4 More recently, several kidney autopsy series from New York were published. Combined data from Northwell Health and Columbia University Medical Center showed that 72% (31/43) in the autopsies were acute tubular injury with varying degrees of severity.12 There was no evidence of glomerulonephritis, vasculitis, thrombotic microangiopathy, or classic viral nephropathy.
Another autopsy series of 9 patients from NYU was analyzed.63 Other than findings consistent with acute tubular injury, the authors also reported platelet-rich fibrin microthrombi in scattered peritubular capillaries and venules in most cases. In one case, there was thrombotic microangiopathy within the glomeruli with large platelet-rich microthrombi, red cell fragmentation, and mesangiolysis. All the cases exhibited mild to moderate arteriolosclerosis, and one demonstrated arteriolar hyalinosis. No changes to glomerular vessels, foot processes, or GBM were observed on light or electron microscopy. Another autopsy series from the United Kingdom evaluated 10 patients. Interestingly, thrombotic features were observed in at least one major organ in all full autopsies, predominantly in the lung (8/9 [89%] patients), heart (5/9 [56%]), and kidney (4/9 [44%]). Evidence of acute tubular injury was noted in all 9 patients examined.64
Does SARS-CoV-2 Infect the Kidney Parenchyma?
All the aforementioned histopathologic kidney biopsy and autopsy series have tried to identify if SARS-CoV-2 infects the kidney parenchyma. Each study used variable techniques, and the results and interpretation of the findings have led to controversy surrounding this question. In the first autopsy study from China by Su and colleagues,14 ultrastructural analysis by electron microscopy was performed on 9 cases, with putative identification of particles that had the appearance of viral structures in 7 cases. An indirect immunofluorescence staining for SARS-CoV-2 nucleoprotein was performed in 6 cases, 3 of which showed positive staining in tubular epithelial cells. In 2 of these 3 cases, viral particles consistent with coronavirus were also identified. Soon after, Kissling and colleagues described a case of CG with negative reverse transcriptase-polymerase chain reaction for SARS-Co-V-2 in kidney biopsy tissue, but they reported findings suggestive of viral particles on electron microscopy.32 Another autopsy case report by Farkash and colleagues also suggests the presence of viral particles by electron microscopy in the tubular epithelial cells; no immunohistochemistry or in situ hybridization was performed in this case.65 Several articles followed, however, disputing whether the particles identified were virus in origin and suggested multivesicular bodies or clathrin-coated vesicles have an identical appearance.66, 67, 68, 69, 70 Three of the 6 patients in an autopsy series described in the publication by Puelles and colleagues showed a detectable SARS-CoV-2 viral load in all kidney compartments examined by reverse transcriptase-polymerase chain reaction.71 The authors report positive results by in situ hybridization and indirect immunofluorescence with confocal microscopy.71 In another series from Belgium, the authors found transmission electron microscopy identified particles resembling coronaviruses in vacuoles or cisternae of the endoplasmic reticulum in proximal tubule cells. They interpreted the presence of virions in proximal convoluted tubules and rare podocyte virions as the evidence of direct viral infection of the kidney.28 On the other hand, data from Northwell Health and Columbia University Medical Center in New York, describing a total of 52 autopsy cases and 29 kidney biopsies from living patients, report negative results by immunohistochemistry or in situ hybridization in all tested cases.10, 11, 12, 13 In the Columbia series, rare tubular epithelial staining (<1 in 200 cells) with low-intensity dot-like positivity was described in many cases, in the setting of a high background staining, and substantially less intense than the staining in lung specimens from autopsies with COVID-19. The authors conclude that the rare equivocal staining may represent nonspecific staining or low viral abundance. Virus-like particles were commonly identified on electron microscopy, but unequivocal features of coronavirus were not seen, as previously described.67 , 69 An autopsy series from Washington state in the United States included 14 patients, with focally positive immunohistochemistry findings in 2 out of 4 tested kidney samples; they also describe viral-like particles in 3 of 3 cases with electron microscopy examination.72 The various methods used to detect the presence of SARS-CoV-2 as reported by these studies are summarized in Table 2 .
Table 2.
Method Used to Detect the Presence of SARS-CoV-2 in the Kidney
| Study | Tissue Type | Positive Results/Total # of Cases Tested | Method of Detection |
|---|---|---|---|
| Su and colleagues14 | Autopsy | 3/6 IF | Anti-SARS-CoV nucleoprotein antibody (40,143-T62, Sino Biological, Beijing, China) |
| Puelles and colleagues71 | Autopsy | Unclear number tested and number positive by ISH and IF | RNAscope 2.5 HD Duplex Assay (Advanced Cell Diagnostics, Newark, California, USA) |
| 2 commercially available antibodies for SARS-CoV-2 detection: spike glycoprotein antibody [3A2] (Abcam, ab272420) and SARS-CoV SΔ10 within S2 domain protein (Genetex, GTX632604) | |||
| Santoriello and colleagues13 | Autopsy | 0/10 ISH | RNAscope 2.5 HD Duplex Assay (Advanced Cell Diagnostics, Newark, California, USA) |
| Golmai and colleagues12 | Autopsy | 0/4 ISH | RNAscope 2.5 HD Duplex Assay (Advanced Cell Diagnostics, Newark, California, USA) |
| 0/12 IHC | Mouse anti-SARS-CoV-2 nucleocapsid protein (Clone 1C7, Bioss, Woburn, MA) | ||
| Bradley and colleagues72 | Autopsy | 2/4 IHC | Monoclonal antibody to the SARS-CoV-2 spike protein GeneTex, Irvine, CA |
| Sharma and colleagues10 | Kidney biopsy | 0/10 IHC | Mouse anti-SARS-CoV-2 nucleocapsid protein (Clone 1C7, Bioss, Woburn, MA) |
| Kudose and colleagues11 | Kidney biopsy | 0/16 ISH | RNAscope 2.5 HD Duplex Assay (Advanced Cell Diagnostics, Newark, California, USA) |
| 0/16 IHC | SARS-CoV SΔ10 within S2 domain protein (Genetex, GTX632604) Anti-SARs-CoV-2 nucleocapsid protein clone 001 (40,143-R001; Sino Biologic, Beijing, China) |
||
| Wu and colleagues31 | Kidney biopsy | 0/6 ISH | RNAscope 2.5 HD Duplex Assay (Advanced Cell Diagnostics, Newark, California, USA) |
Abbreviations: IF, immunofluorescence; IHC, immunohistochemistry; ISH, in-situ hybridization.
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) to gain entry to the host cell.73 Access to the cellular ultrastructure needed for viral replication is also dependent on proteases such as transmembrane serine protease 2 (TMPRSS2) and furin.74 ACE2 is expressed in the kidney and is localized to the apical brush border of proximal tubular cells with some expression in the distal tubule, interlobular arteriole endothelium, and vascular smooth muscle.75 , 76 Lower expression of ACE2 is found in the glomerulus and is predominantly seen in podocytes. Some have postulated that the virus could enter the kidney via ACE2. However, SARS-CoV-2 uses TMPRSS2 in addition to ACE2 to be primed to gain entry into host cells. TMPRSS2 is primarily expressed in the distal nephron as opposed to the proximal tubule where ACE2 is expressed.77 This, at least in the basal state, would prove difficult as these 2 proteins are expressed in different tubule compartments. It remains to be determined if ACE2 or TMPRSS2 expression is altered in SARS-CoV-2 infection and thus allow for coexpression in the kidney tubules. An alternative route of entry could be from immune cells such as macrophages or in podocytes. Furthermore, perturbations in components of the renin-angiotensin system could lead to increases in the proinflammatory angiotensin (Ang) II signaling. This is because ACE2 is a critical counter-regulatory enzyme that converts Ang II into Ang-(1-7). Ang-(1-7) functions to attenuate inflammation by signaling through the Mas receptor.78 Preclinical research on SARS-CoV-1 in a mouse model showed reductions in ACE2 protein in the lungs of mice after infection with SARS-CoV-1 and after spike protein enhanced lung injury.79 Furthermore, they showed reduction in lung injury in this model after treatment with an angiotensin-receptor blocker. It is unclear if this occurs in humans and what the clinical significance of these findings are. Moreover, controversy exists as to whether the use of inhibitors of the renin-angiotensin enzyme are helpful or harmful in the context of COVID-19.80 Ongoing clinical trials are currently underway. This is discussed in more detail by Edmonston and colleagues in this issue of ACKD.81
In summary, although direct viral infection of the kidney is possible, it is certainly not a common or even widespread finding reported (Table 2). In fact, SARS-CoV-2 is detected uncommonly by immunohistochemistry or in situ hybridization. Electron microscopy is a suboptimal method of detection as it relies solely on morphology and the particle size, leaving room for confusion with normal cellular structures.
Management
Treatment and management of patients with COVID-19–associated AKI is generally similar to those of patients with AKI associated with septic shock. Conservative management of volume overload, metabolic acidosis, and hyperkalemia can be attempted before considering initiation of dialysis. In cases of CG, TMA and other glomerular diseases, careful management of COVID-19, and specific glomerular pathology might be prudent. Shaikh and colleagues discuss the management of COVID-19-associated AKI in this issue of ACKD.82
Outcomes
Other recent publications have reported on the association of AKI with in-hospital death among those hospitalized with COVID-19.1 , 3 , 83 Early reports from Wuhan, China, found that the risk of death was increased with AKI.84 Based on a recent publication from New York of approximately 9600 hospitalized patients with COVID-19, the risk of in-hospital death was increased with AKI even after adjusting for baseline demographic, comorbid conditions, and illness severity (HR 3.4 for AKI-not requiring dialysis, HR 6.4 for AKI-requiring dialysis).85 The Study of the Treatment and Outcomes in Critically Ill Patients with COVID-19 (STOP-COVID) is a US-based multicenter retrospective cohort study of patients admitted to the ICU. This study found that in 637 patients with AKI requiring dialysis, 55% died within 28 days of ICU admission compared with only 19% of patients without AKI; among patients with Kidney Disease: Improving Global Outcomes (KDIGO) stage 1 and 2 AKI, the 28-day mortality was 34% and 46%, respectively. Among the 216 patients with AKI requiring dialysis who survived to hospital discharge, 73 (34%) remained dependent on dialysis at discharge, and by 60 days of follow-up, 18% remained dialysis-dependent.86 Despite the heterogeneity of published studies in terms of geographical location, patient population, and severity of illness, it is clear that AKI is associated with increased risk of death among patients with COVID-19.
Conclusion
In summary, AKI is not uncommon in patients hospitalized with COVID-19 with an incidence of around 37%. The most common cause of kidney injury in patients with COVID-19 is acute tubular injury. CG is a recognized glomerular pathology associated with COVID-19 that most commonly affects individuals with high-risk APOL1 polymorphisms. Other glomerular pathology associated with COVID-19 might be coincidental or a “second hit” phenomenon. At the time of this writing (September 2020), it is unclear if the SARS-CoV-2 directly infects the kidney.
Acknowledgments
M.E.S. is supported by National Institutes of Health (NIH) K23 DK 117014 and the Claflin Distinguished Scholars Award. The NIH had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication. J.H.N. is supported by the Raggio and Hall Families. Figures 1 and 2 were created using Biorender.com.
Footnotes
Financial Disclosure: M.E.S. reports receiving personal fees and grant support from Massachusetts General Hospital, AbbVie, Gilead Sciences, and Merck and has participated in scientific advisory boards for Gilead and as a scientific consultant to Bioporto, outside the submitted work. K.D.J. serves as a consultant for Astex Pharmaceuticals and Natera and receives fees from Uptodate.com outside of the submitted work. K.D.J. also receives honorarium from the International Society of Nephrology and American Society of Nephrology. V.B. receives honorarium from the International Society of Nephrology. M.A.S. is funded by a grant from Renal Research Institute. The other authors declare that they have no relevant financial interests.
References
- 1.Robbins-Juarez S.Y., Qian L., King K.L. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. 2020;5(8):1149–1160. doi: 10.1016/j.ekir.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher M., Neugarten J., Bellin E. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia P., Wen Y., Duan Y. Clinicopathological features and outcomes of acute kidney injury in critically ill COVID-19 with prolonged disease course: a retrospective cohort. J Am Soc Nephrol. 2020;31(9):2205–2221. doi: 10.1681/ASN.2020040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husain-Syed F., Wilhelm J., Kassoumeh S. Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease-2019. Nephrol Dial Transpl. 2020;35(7):1271–1274. doi: 10.1093/ndt/gfaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronco C., Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16(6):308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inamdar S., Benias P.C., Liu Y., Sejpal D.V., Satapathy S.K., Trindade A.J. Prevalence, risk factors, and outcomes of hospitalized patients with COVID-19 presenting as acute pancreatitis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed M.M.B., Lukitsch I., Torres-Ortiz A.E. Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney360. 2020;1(7):614–622. doi: 10.34067/KID.0002652020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apetrii M., Enache S., Siriopol D. A brand-new cardiorenal syndrome in the COVID-19 setting. Clin Kidney J. 2020;13(3):291–296. doi: 10.1093/ckj/sfaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma P., Uppal N.N., Wanchoo R. COVID-19–Associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31(9):1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudose S., Batal I., Santoriello D. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golmai P., Larsen C.P., DeVita M.V. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31(9):1944–1947. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santoriello D., Khairallah P., Bomback A.S. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez H., Ince C., De Backer D. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos-Casals M., Brito-Zerón P., López-Guillermo A., Khamashta M.A., Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 18.Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seguin A., Galicier L., Boutboul D., Lemiale V., Azoulay E. Pulmonary involvement in patients with hemophagocytic lymphohistiocytosis. Chest. 2016;149(5):1294–1301. doi: 10.1016/j.chest.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bihorac A., Baslanti T.O., Cuenca A.G. Acute kidney injury is associated with early cytokine changes after trauma. J Trauma Acute Care Surg. 2013;74(4):1005–1013. doi: 10.1097/TA.0b013e31828586ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aulagnon F., Lapidus N., Canet E. Acute kidney injury in adults with hemophagocytic lymphohistiocytosis. Am J Kidney Dis. 2015;65(6):851–859. doi: 10.1053/j.ajkd.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husain-Syed F., Slutsky A.S., Ronco C. Lung–kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med. 2016;194(4):402–414. doi: 10.1164/rccm.201602-0420CP. [DOI] [PubMed] [Google Scholar]
- 25.Nechemia-Arbely Y., Barkan D., Pizov G. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19(6):1106–1115. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons E.M., Himmelfarb J., Sezer M.T. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65(4):1357–1365. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 27.Patel N., Rein J.L., Sanchez-Russo L., Winston J., Uribarri J. COVID-19–Associated acute kidney injury: a case series. Kidney Med. 2020;2(5):668–669. doi: 10.1016/j.xkme.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werion A., Belkhir L., Perrot M. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98(5):1296–1307. doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magoon S., Bichu P., Malhotra V. COVID-19-Related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. 2020;2(4):488–492. doi: 10.1016/j.xkme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma Y., Nasr S.H., Larsen C.P., Kemper A., Ormsby A.H., Williamson S.R. COVID-19-Associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. 2020;2(4):493–497. doi: 10.1016/j.xkme.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H., Larsen C.P., Hernandez-Arroyo C.F. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol. 2020;31(8):1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kissling S., Rotman S., Gerber C. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98(1):228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peleg Y., Kudose S., D'Agati V. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5(6):940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.A. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020;5(6):935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couturier A., Ferlicot S., Chevalier K. Indirect effects of severe acute respiratory syndrome coronavirus 2 on the kidney in coronavirus disease patients. Clin Kidney J. 2020;13(3):347–353. doi: 10.1093/ckj/sfaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta R.K., Bhargava R., Shaukat A.-A., Albert E., Leggat J. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol. 2020;21(1):326. doi: 10.1186/s12882-020-01970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izzedine H., Brocheriou I., Arzouk N. COVID-19 associated collapsing glomerulopathy: a report of two cases and a literature review. Int Med J. 2020 doi: 10.1111/imj.15041. In press. [DOI] [PubMed] [Google Scholar]
- 38.Chandra P., Kopp J.B. Viruses and collapsing glomerulopathy: a brief critical review. Clin Kidney J. 2013;6(1):1–5. doi: 10.1093/ckj/sft002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyatt C.M., Klotman P.E., D'Agati V.D. HIV-associated nephropathy: clinical presentation, pathology, and epidemiology in the era of antiretroviral therapy. Semin Nephrol. 2008;28(6):513–522. doi: 10.1016/j.semnephrol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moudgil A., Nast C.C., Bagga A. Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int. 2001;59(6):2126–2133. doi: 10.1046/j.1523-1755.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 41.Albaqumi M., Soos T.J., Barisoni L., Nelson P.J. Collapsing glomerulopathy. J Am Soc Nephrol. 2006;17(10):2854–2863. doi: 10.1681/ASN.2006030225. [DOI] [PubMed] [Google Scholar]
- 42.Jamilloux Y., Henry T., Belot A. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7):102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Z., Ren L., Zhang L. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890.e2. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols B., Jog P., Lee J.H. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int. 2015;87(2):332–342. doi: 10.1038/ki.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David J., Friedman M.R.P. Apolipoprotein L1 and kidney disease in African Americans. Trends Endocrinol Metab. 2016;27(4):204–215. doi: 10.1016/j.tem.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moeinzadeh F., Dezfouli M., Naimi A., Shahidi S., Moradi H. Newly diagnosed glomerulonephritis during COVID-19 infection undergoing immunosuppression therapy, a case report. Iran J Kidney Dis. 2020;14(3):239–242. [PubMed] [Google Scholar]
- 47.Uppal N.N., Kello N., Shah H.H. De Novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. 2020;5(11):2079–2083. doi: 10.1016/j.ekir.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prendecki M., Clarke C., Cairns T. Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020;98(3):780–781. doi: 10.1016/j.kint.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allez M., Denis B., Bouaziz J.-D. Covid-19 related IgA vasculitis. Arthritis Rheumatol. 2020 doi: 10.1002/art.41428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suso A.S., Mon C., Alonso I.O. IgA vasculitis with nephritis (Henoch-Schönlein purpura) in a COVID-19 patient. Kidney Int Rep. 2020;5(11):2074–2078. doi: 10.1016/j.ekir.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iba T., Levy J.H., Raj A., Warkentin T.E. Advance in the management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Clin Med Res. 2019;8(5):728. doi: 10.3390/jcm8050728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor F.B., Jr., Toh C.H., Hoots W.K., Wada H., Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 53.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jhaveri K.D., Meir L.R., Flores Chang B.S. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98(2):509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Izzedine H., Jhaveri K.D., Perazella M.A. Vascular injury and COVID-19-related mortality: what lies below the tip of the iceberg? Clin Nephrol. 2020;94(1):11–13. doi: 10.5414/CN110217. [DOI] [PubMed] [Google Scholar]
- 56.Kashi M., Jacquin A., Dakhil B. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Philipponnet C., Aniort J., Chabrot P., Souweine B., Heng A.-E. Renal artery thrombosis induced by coronavirus disease 2019. Clin Kidney J. 2020;13(4):71. doi: 10.1093/ckj/sfaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Post A., den Deurwaarder E.S.G., Bakker S.J.L. Kidney infarction in patients with COVID-19. Am J Kidney Dis. 2020;76(3):431–435. doi: 10.1053/j.ajkd.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Binois Y., Hachad H., Salem J.-E. Acute kidney injury associated with lopinavir/ritonavir combined therapy in patients with Covid-19. Kidney Int Rep. 2020;5(10):1787–1790. doi: 10.1016/j.ekir.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arrestier R., Stehle T., Letavernier E., Mekontso-Dessap A. Lopinavir-ritonavir associated acute kidney injury is not related to crystalluria in critically-ill COVID-19 patients. Kidney Int Rep. 2020;5(11):2119. doi: 10.1016/j.ekir.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fontana F., Cazzato S., Giovanella S. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int Rep. 2020;5(10):1815–1822. doi: 10.1016/j.ekir.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izzedine H., Jhaveri K.D., Perazella M.A. COVID-19 therapeutic options for patients with kidney disease. Kidney Int. 2020;97(6):1297–1298. doi: 10.1016/j.kint.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rapkiewicz A.V., Mai X., Carsons S.E. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanley B., Naresh K.N., Roufosse C. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farkash E.A., Wilson A.M., Jentzen J.M. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. 2020;31(8):1683–1687. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roufosse C., Curtis E., Moran L. Electron microscopic investigations in COVID-19: not all crowns are coronas. Kidney Int. 2020;98(2):505–506. doi: 10.1016/j.kint.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldsmith C.S., Miller S.E., Martines R.B., Bullock H.A., Zaki S.R. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395(10238):e99. doi: 10.1016/S0140-6736(20)31188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calomeni E., Satoskar A., Ayoub I., Brodsky S., Rovin B.H., Nadasdy T. Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int. 2020;98(1):233–234. doi: 10.1016/j.kint.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller S.E., Brealey J.K. Visualization of putative coronavirus in kidney. Kidney Int. 2020;98(1):231–232. doi: 10.1016/j.kint.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith K.D., Akilesh S., Alpers C.E., Nicosia R.F. Am I a coronavirus? Kidney Int. 2020;98(2):506–507. doi: 10.1016/j.kint.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bradley B.T., Maioli H., Johnston R. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye M., Wysocki J., William J., Soler M.J., Cokic I., Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17(11):3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 76.Soler M.J., Ye M., Wysocki J., William J., Lloveras J., Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Ren Physiol. 2009;296(2):F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 77.Batlle D., Soler M.J., Sparks M.A. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16(6):305–307. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sparks M.A., South A., Welling P. Sound science before quick judgement regarding RAS blockade in COVID-19. Clin J Am Soc Nephrol. 2020;15(5):714–716. doi: 10.2215/CJN.03530320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edmonston D.L., South A.M., Sparks M.A., Cohen J.B. Coronavirus disease 2019 and hypertension: the role of angiotensin-converting enzyme 2 and the renin-angiotensin system. Adv Chronic Kidney Dis. 2020 doi: 10.1053/j.ackd.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaikh S., Umemoto G.M., Vijayan A. Management of acute kidney injury in COVID-19. Adv Chronic Kidney Dis. 2020 doi: 10.1053/j.ackd.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan L., Chaudhary K., Saha A. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2020 doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ng J.H., Hirsch J.S., Hazzan A. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2020 doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gupta S., Coca S.G., Chan L. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Neph. 2020 doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]