Abstract
Background
COVID-19-associated acute respiratory distress syndrome (ARDS) is associated with significant morbidity and high levels of mortality. This paper describes the processes involved in the pathophysiology of COVID-19 from the initial infection and subsequent destruction of type II alveolar epithelial cells by SARS-CoV-2 and culminating in the development of ARDS.
Main body
The activation of alveolar cells and alveolar macrophages leads to the release of large quantities of proinflammatory cytokines and chemokines and their translocation into the pulmonary vasculature. The presence of these inflammatory mediators in the vascular compartment leads to the activation of vascular endothelial cells platelets and neutrophils and the subsequent formation of platelet neutrophil complexes. These complexes in concert with activated endothelial cells interact to create a state of immunothrombosis. The consequence of immunothrombosis include hypercoagulation, accelerating inflammation, fibrin deposition, migration of neutrophil extracellular traps (NETs) producing neutrophils into the alveolar apace, activation of the NLRP3 inflammazome, increased alveolar macrophage destruction and massive tissue damage by pyroptosis and necroptosis Therapeutic combinations aimed at ameliorating immunothrombosis and preventing the development of severe COVID-19 are discussed in detail.
Keywords: COVID-19, SARS-CoV-2, Respiratory infection, Treatment
Graphical abstract
1. Background
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has been classified as a zoonotic β-coronavirus which has entered the human population from an unknown animal host in a similar manner to the closely related SARS-CoV [1,2]. This virus is the cause of COVID-19. This is an illness which for the most part is associated with mild symptoms [3]. However, the illness may progress to severe pneumonia and acute respiratory distress syndrome (ARDS), leading to high levels of morbidity and mortality. Currently, the weight of evidence suggests that approximately 20% of patients infected with SARS-CoV-2 progress to severe disease requiring hospitalisation. Of those individuals admitted 20% develop pneumonia and ARDS with the latter group of patients requiring protracted ventilation [4,5]. Sadly, several authors have reported that the death rate in mechanically ventilated patients approaches 50% [4,5].
2. Immune, structural, physiological and biochemical abnormalities reported in COVID-19 ARDS
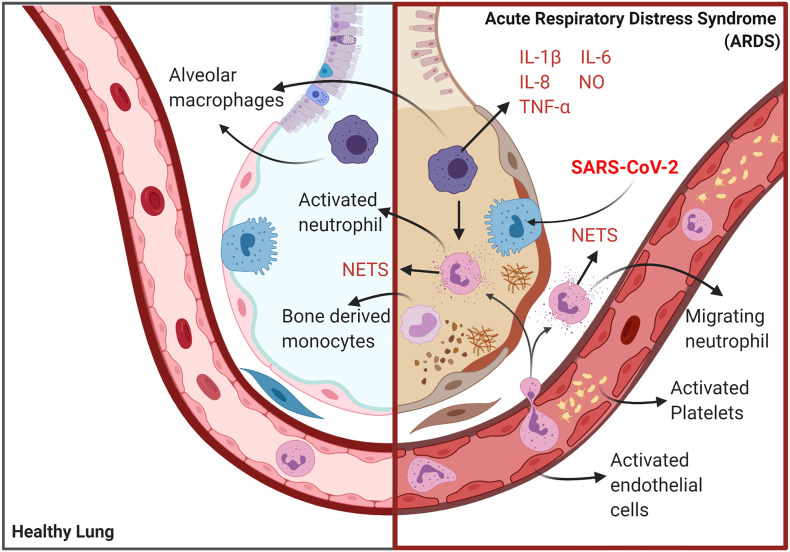
COVID-19 ARDS is exemplified by the existence of diffuse alveolar damage, increased epithelial and endothelial cell permeability, fibrin-rich hyaline membranes, leakage of fluid into the pulmonary interstitium and significant disruption of gas exchange, ultimately leading to the development of hypoxia and, in many cases, respiratory failure [4,[6], [7], [8]] reviewed in Ranucci et al. [9]. These features are typical findings in patients with ARDS secondary to sepsis or severe viral infections in these respects COVID-19 ARDS is unremarkable [10].
However there is evidence to suggest that there may be greater levels of coagulation cascade activation and greater decreases in the activities of the anticoagulant and fibrinolytic system in patients suffering from COVID-19 ARDS compared to those with ARDS secondary to other conditions or illnesses [9,[11], [12], [13]]. The relative importance of excessive hypercoagulation in the pathogenesis of severe COVID-19 is further highlighted by recent evidence which suggests that 70% of individuals who died as a result of SARS-CoV-2 infection satisfied the diagnostic criteria for disseminated intravascular coagulation (DIC), while that was only the case in 1% of survivors [14]. There is also extensive evidence of vascular endothelial cell activation and damage in patients with severe COVID-19 [[15], [16], [17]]. In addition it would appear that one source of such activation and damage is via direct infection by SARS-CoV-2 [17].
Several authors have reported the existence of significant levels of immune dysregulation in the lungs of patients with severe COVID-19 pneumonia or ARDS. For example, there is copious evidence of activated alveolar macrophages [6,[18], [19], [20]]. In addition, the absolute numbers these immune cells are depleted due to excessive levels of pyroptotic programmed death [18,21,22]. Other research teams have reported massively increased levels of activated neutrophils in lung interstitial tissue I and alveoli [7,23] review [24]. These neutrophils also appear to secrete significant levels of highly cytotoxic neutrophil extracellular traps (NETs) [7,25]. High levels of tumour necrosis factor (TNF) and influx of interleukin (IL)-1 secreting bone marrow derived monocytes of peripheral origin is also a common finding in these patients [26]. From a biochemical perspective it is interesting to note that these monocytes also secrete high levels of lactate dehydrogenase which is an accepted marker of immune cells undergoing death by pyroptosis [26]. The presence of hypercytokinemia in the lungs [18,27,28] and in the periphery [18,24,[29], [30], [31], [32]] of patients with severe COVID-19 is another replicated finding. The pattern and intensity of cytokine and chemokine abnormalities is characteristic of cytokine release syndrome and a “cytokine storm” and many authors have suggested that this illness may be viewed as a virally induced sepsis [24,27,29,[33], [34], [35]].
To date the pathophysiology of COVID-19 ARDS has not been proposed and treatment options are limited. Accordingly, this paper proposes a model of the illness based on the observations cited above and extensive research into ARDS secondary to sepsis and or severe viral infections and discusses potential treatments targeting the proposed drivers of pathology.
3. The pathophysiology of COVID-19 ARDS
There is accumulating evidence that SARS-CoV-2 infects and activates type 2 alveolar epithelial cells resulting in the development of endoplasmic reticulum stress, NLP3 inflammasome activation and in many cases the death of these cells by apoptosis and pyroptosis [7,8,19,22,23,36,37]. Moreover, the weight of evidence suggests that SARS-CoV-2 may also infect and activate alveolar macrophages (AM). SARS-CoV-2 induced pyroptosis of type II alveolar cells may be a deterministic event in the development of COVID-19 ARDS as there is a growing body of evidence establishing a causative association between the initial pyroptosis of alveolar epithelial and endothelial cells and the ultimate development and progression of ARDS from other causes [[38], [39], [40]]. This association is due in part to the release of high mobility group box 1 (HMBG1) and other inflammatory DAMPS directly contributing to an escalating pattern of tissue damage [[38], [39], [40]].
The activation of AMs leads to their polarisation into a proinflammatory “M1” phenotype secreting high levels of proinflammatory cytokines (PICs) and chemokines such as TNF-alpha IL-1 beta, IL-6, and IL-8 [[41], [42], [43]]. Crucially, the subsequent release of these inflammatory mediators into the pulmonary vasculature plays an indispensable role in the development of ARDS by initiating a series of events which results in the activation of vascular endothelial cells [[44], [45], [46]], platelets [[47], [48], [49]], neutrophils [50,51], ultimately resulting in the formation of platelet neutrophil complexes at the surface of the endothelium [[52], [53], [54]]. The formation of these complexes encourages and enables the recruitment of highly cytotoxic neutrophils and inflammatory activated platelets into the alveolar space and pulmonary intestinal resulting in a host of pathogenic consequences, as discussed below [[44], [45], [46]].
One such consequence is the development of a highly inflammatory and pro-coagulant state typified by hyperactivation of the coagulation cascade and relative exhaustion of the fibrinolytic system with excessive production of PICs, DAMPS and fibrin deposition [44,55,56]. This is described as immuno-thrombosis [44,55,56]. This state has a major pathophysiological role in the development and exacerbation of systemic sepsis as it generates the formation of vascular microthrombi, and is responsible the development of DIC and subsequent multi-organ damage or failure [[57], [58], [59]].
The sequestration of platelet neutrophil complexes in the pulmonary vasculature and the subsequent development of immunothrombosis is also the ultimate cause of micro-thrombi and micro-emboli in the alveocapilary circulation [60,61], intra alveolar fibrin deposits [44,[62], [63], [64], [65]] and in some circumstances the development of DIC. Readers interested in further details are referred to Gando Otomo [66] and Ito [67]. The translocation of NET producing neutrophils into alveoli and interstitial lung tissue also plays a dominant role in the development of an intrapulmonary cytokine storm ultimately leading to the massive levels of lung tissue damage characteristic of COVID-19 ARDS.
In the context of ARDS accumulating data suggests that the self-amplifying cascade of PIC ROS and DAMP production leads to the activation of the NLRP3 inflammasome and the release of IL-1 and IL-18 [68,69]. This is increasingly considered to be an irrevocable step in the development of ARDS and high levels of NLRP3 inflammasome activity associated with a grave prognosis [[70], [71], [72]]. It is however encouraging to note that the inhibition of NLRP3 assembly is associated with increased rates of survival [73,74] (reviewed by Danielski et al. [75]).
Increased levels of IL-1 and IL-18 appear to make independent contributions to the development of ARDS [[70], [71], [72]] but perhaps the most important consequences of unrestrained NLRP3 activation is excessive levels of alveolar macrophage pyroptosis [[76], [77], [78], [79]] which is another important marker of mortality in ARDS [80].
This association is explained by the release of large quantities of DAMPS, PICs and ROS and the recruitment of PIC and ROS producing bone derived monocytes from the periphery [81,82] leading to extensive RIPK mediated necroptosis [38,43]. Importantly, this mode of programmed cell death releases massive amounts of HGMB1, mtDNA, PICs ROS and chemokines forming an autoinflammatory loop described as necroinflammation [83,84] and irreversible lung failure [85]. Crucially, the existence of widespread necroptosis is an acknowledged cause of irreversible lung failure [85] and is predictive of non-resolving ARDS and mortality in patients on mechanical ventilation [38,43]. The processes described above are summarised in Fig. 1 .
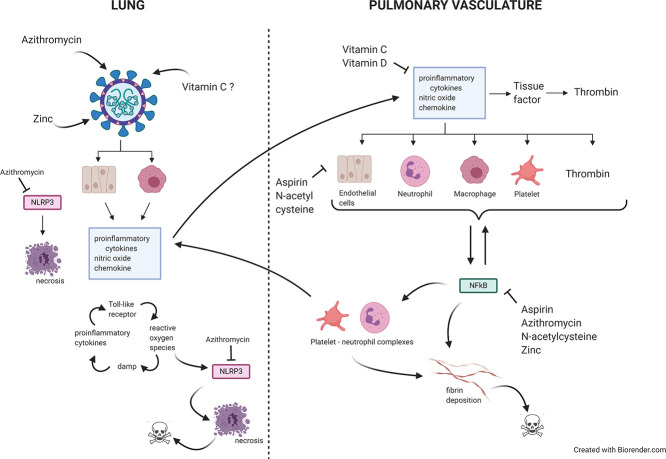
Fig. 1.
The pathophysiology of COVID-19 ARDS. Initial infection and activation of type 2 alveolar cells and alveolar macrophages by SARS-CoV-2 results in the secretion of IL-6, PICs, NO and several chemokines which activate vascular endothelial cells platelets and neutrophils ultimately forming a platelet neutrophil complex. The interplay between vascular endothelial cells activated platelets and activated primed neutrophils produces a highly coagulative and inflammatory state described as immunothrombosis. The translocation of neutrophils and platelets into the pulmonary microvasculature results in severe epithelial layer damage alveolar fibrin deposition and the formation of microthrombi. The translocation of NET producing neutrophils into alveoli and lung interstitium coupled with their delayed apoptosis results in the development cytokine storm producing extreme tissue damage and often fatal lung dysfunction created by several feedforward loops involving interplay between PICS DAMPS ROS, activation of the NLRP3 inflammasome activation, alveolar macrophage pyroptosis, influx of inflammatory bone derived monocytes and necroptosis.
4. Suggestions for therapeutic intervention
4.1. Zinc
Patients with severe infections and/or experiencing systemic inflammatory response syndrome (SIRS) often present with up to a five-fold reduction in plasma Zn compared to healthy controls due to a redistribution of the ions into the liver [[86], [87], [88], [89]]. Importantly, such a reduction in Zn levels is predictive of increased mortality and the extent of reduction correlates negatively with levels of inflammation, increased severity of symptoms and damage to the heart and other organs [86,90,91]. Due to this, Zn levels have been suggested as a diagnostic marker of SIRS/sepsis and likelihood of mortality [90].
Mechanistically, evidence suggests that this state of affairs seen in an environment of excessive systemic inflammation, stems from increased activity and levels of ZIP-14 and ZIP-8, two promiscuous members of the SLC-39A family of Zn transporters, which stimulate the uptake of Zn from the plasma into the liver [[92], [93], [94]]. This phenomenon, commonly observed in a state of excessive inflammation, is driven by elevated levels of IL-6, lipopolysaccharide (LPS), IL-1 and nitric oxide (NO) which increase the activity of the transcription factors AP-1 and ATF-4, resulting in increased uptake of Zn into the liver resulting in state of hypozincaemia [[92], [93], [94]].
Importantly, grossly reduced levels of plasma Zn results in a compromised and dysfunctional response to pathogen invasion by the innate and humoral branches of the immune system [[95], [96], [97]]. Typically, and perhaps paradoxically, the results of depleted Zn influenced immune dysfunction in sepsis patients [87] manifests as an exacerbated inflammatory response associated with increased activity of NF-κB, excessive levels of PIC production, oxidative damage to tissues and massive levels of leucocyte death [86,[98], [99], [100]]. From a pathophysiological perspective, it is important to note that these factors seen in patients in the early stages of SIRS and sepsis development appear to be associated with significant increases in mortality [91,98].
Data also suggests that another important factor associated with increased mortality in sepsis patients is elevated levels of NF-κB activity, most notably in the lungs [98,101]. In this context, it is encouraging to note that Zn supplementation may reduce NF-κB activity in vivo either directly or by inhibiting the STAT-3-NF-κB signalling pathway [91,101,102]. Data from animal studies also suggests that Zn supplementation may restore immune homeostasis in patients experiencing severe systemic inflammation and inhibit the development of sepsis and ARDS [103,104].
The importance of Zn deficiency in the development of ARDS has also been emphasised by the results of a recent paper that reported reduced levels of this ion in intensive care unit patients who went on to develop ARDS compared to those who did not [105]. Moreover, these authors concluded that low levels of Zn predisposed to ventilator induced lung injury [105].
Finally, from the perspective of immune modulation, there is some evidence to suggest that Zn deficiency may exacerbate NLRP3 activity and increase levels of IL-1 beta [106,107].The mechanism underpinning this phenomenon is not completely understood but increased lysosomal permeability in a cellular environment of chronic Zn deficiency may be a contributing factor [107]. Zn is also one of the few supplements with credible evidence of anti-viral actions and given the paucity of such candidates we focus on this area below.
There is evidence that high levels of Zn salts may inhibit the essential RNA dependent RNA polymerase (RdRp) enzyme, which is conserved in all coronaviruses [108]. It appears that this property may extend to the respiratory syncytial virus (RSV), rhinoviruses, hepatitis E, dengue, West Nile and Zika viruses whose RdRp is almost identical to the equivalent enzyme in coronaviruses [[109], [110], [111], [112]]. The inhibitory influence of excess Zn appears to the fact that these RdRp are in effect protein metallothionines dependent on an intricate quantum relationship between bound Zn and magnesium for their activity. This relationship appears to be disturbed in a cellular environment of excess Zn ions [113,114]. In vitro evidence suggesting that Zn chelation may inhibit the RNA dependent RNA polymerase of COVID-19, effectively halting the replication of the virus, has understandably stimulated a great deal of interest in Zn supplementation as a means of inhibiting the replication of COVID-19 in vivo [108].
4.2. Azithromycin
There is a large and accumulating body of evidence demonstrating significant improvements in patients suffering from a range of serious lung diseases following the long term administration of azithromycin (AZM) and other macrolide antibiotics (reviewed by Faverio et al. [115]). Importantly, in the case of AZM, such evidence extends to reducing mortality in patients suffering from ARDS secondary to a range of insults or sepsis [116,117]. For example, Kawamura et al. [116] engaged in a retrospective review of outcomes in 191 ARDS patients and reported a statistically significant improvement in the 60 days survival of patients prescribed AZM compared to those on standard care only. This was also accompanied by reduced time on ventilation [116]. The results of this study echoed earlier work by this team of authors with almost identical outcomes in patients with ARDS induced by sepsis [117]. Importantly, significant reductions in mortality following AZM therapy has also been reported in patients suffering from acute lung injury [118]. In addition, a large meta-analysis of prospective studies involving over 70,000 elderly patients hospitalised with pneumonia also reported a significant improvement in mortality in these individuals prescribed AZM compared to those who had not been given this medicine on admission to hospital [119].
There is a growing appreciation that the benefits accrued by patients following the administration of AZM and other macrolide antibiotics is due to their pleiotropic immunomodulatory properties. A recent meta-analysis concluded that over time the administration of these molecules resulted in reduced levels of chemokines, most notably IL-8, decreasing molecular adhesion (E-selectin, ICAM-1), integrins (CD11b/CD18), reduced levels of PICs, inhibition of neutrophil and eosinophil function, decreased effector T cell activity and reduced levels of matrix metalloproteinase 9 [120].
From the perspective of a potential treatment for ARDS, it should be noted that AZM accumulates in alveolar macrophages and neutrophils to a far greater degree than any other macrolide and levels of the antibiotic within these immune cells may be 2000 times greater than levels found in the plasma following short term or long term administration [121,122]. This is of importance as evidence suggests that AZM is a highly effective NF-κB inhibitor in vivo [123,124]. Hence, AZM offers an option to inhibit NF-κB in a highly targeted manner.
Several human and animal studies have also reported a profound change in the polarisation of activated alveolar macrophages from the highly inflammatory M1 phenotype to the anti-inflammatory M2 phenotype following prolonged treatment with AZM for a range of pulmonary and autoimmune illnesses [[125], [126], [127], [128], [129]]. These changes are characterised by significant changes in the transcriptome of AMs as evidenced by the upregulation of CCL18, fibronectin, arginase 1 and a decrease in the production of Matrix metallopeptidase 9 (MMP-9), inducible nitric oxide synthase 2 (NOS2) and PICs due to the inhibition of STAT-1 and reduced translocation of NF-κB [[125], [126], [127]]. Ultimately, the positive effects of AZM on AM polarisation appears to be via the stimulation of PI3/Akt/mTor signalling, which inhibits the activity of NF-κB and stimulates the expression of genes involved in the transition between the inflammatory and anti-inflammatory phenotypes [[129], [130], [131], [132], [133]]. The activation of the phosphoinositide 3-kinase (PI3K) signalling pathway is also associated with increased AM efferocytosis [134], and reports of improved AM phagocytosis following AZM therapy are unsurprising [[135], [136], [137]].
AZM also appears to induce the recruitment of CD11b+ Gr-1+ myeloid-derived suppressor cells (MDSC) into the lung [126] and this seems to be a general property of macrolides [138]. This is important, as MDSCs are potent suppressors of T cell responses and evidence suggests that they play an important role in ameliorating damage to lung tissue following severe pneumonia or the advent of septic shock [138,139] (reviewed by Alshetaiwi et al. [140]). The combined effects of AZM on AMs and MDSCs go some distance to explaining the reduction in lung tissue damage and inflammation reported following chronic administration of this antibiotic [[141], [142], [143], [144], [145]].
In addition, there is ample evidence to suggest that chronic AZM therapy results in reduced neutrophil infiltration into the lungs and ameliorates pre-existing neutrophilia [[146], [147], [148], [149]]. This appears to be largely due to decreased IL-8 production by alveolar macrophages and epithelial cells, although there is some suggestion that decreased IL-8 production by alveolar myofibroblasts may also be involved [144,145,149,150].
Prolonged AZM treatment also has profound effects on neutrophil function and survival leading to significant and large decreases in PIC production and survival [[151], [152], [153], [154]]. The anti-inflammatory effect of AZM on neutrophil performance appears to be mainly mediated by PI3K mediated inhibition of NF-kB in a similar manner to the anti-inflammatory effects on AMs. In some respects, this can also be said of increased neutrophil apoptosis as a result of decreased levels of IL-6 and IL-8, which would otherwise increase neutrophil survival. However, decreased neutrophil survival following AZM administration also appears to be mediated by the inhibition of GM-CSF [155,156]. This cytokine regulates many aspects of neutrophil function and decreased levels inhibit neutrophil priming, which is important in the development of ARDS [155]. In addition GM-CSF downregulation appears to be responsible for the neutrophil recognition of chemotactic stimuli, which is important in reducing the recruitment of activated neutrophils into inflamed lung tissue [122]. The downregulation of this cytokine is also an important therapeutic benefit as there is evidence that elevated levels of GM-CSF is a major contributor to extensive lung damage and increased mortality in severe pneumonia [157,158].
Finally, there is evidence to suggest that AZM inhibits the activity of the NLRP3 inflammasome and the subsequent release of IL-1 beta [121,159]. Moreover, there is evidence that AZM decreases the stability of NLRP3 mRNA likely via a direct effect on ribosomes [159] which is a different mechanism than in the case of Zn suggesting that the use of Zn and AZM in combination might produce additive or synergistic results.
4.3. Aspirin
Three recent meta-analyses have reported significantly increased survival in ARDS patients prescribed aspirin compared to those who were aspirin free [[160], [161], [162]]. Furthermore, the effect appear to be greater in critically ill patients and patients with ARDS-related sepsis than a wider group of patients [160,161]. Pertinently, there is also data to suggest that aspirin prophylaxis might also reduce the severity of ARDS and result in a substantial reduction in long term mortality (reviewed by Wang et al. [162]).
Several research teams have also reported reduced mortality in patients over 65 presenting at hospital with severe pneumonia prescribed aspirin compared to those who were aspirin naive [[163], [164], [165]]. For example, Falcone et al. reported a four-fold reduction in mortality in aspirin treated patients suffering from severe pneumonia compared to controls [163]. Importantly, this was a large study containing 390 patients in the aspirin arm and 614 aspirin free [163]. Similar results have been provided from earlier studies where patients ingesting aspirin in the community or administered the drug at the point of hospital admission demonstrated significantly reduced mortality especially in patients with severe symptoms and or those who became critically ill [164,165]. Given that aspirin use in the community may be associated with greater health literacy and other adaptive health behaviours, these confounders may need to be borne in mind.
The presence of activated platelets in patients suffering from viral pneumonia or a severe upper respiratory tract infection is a well-documented [[166], [167], [168]]. In addition, platelet activation is associated with a higher risk of myocardial infarction and cardiovascular related mortality seen in this group of patients [166,169]. Hence it seems reasonable to suggest that at least some of the potential benefits of aspirin to this group of patients may be mediated by the anti-platelet effects of aspirin. These are generally attributed to the irreversible acetylation of cyclooxygenase (COX) 1 and the long-term inhibition of thromboxane A2 [170].
Aspirin also exerts a range of beneficial effects on the coagulation cascade and the prevention of thrombosis in addition to inhibiting COX1 (reviewed by Patrono [171]). Several mechanisms appear to be involved including reducing tissue factor production by platelets and immune cells with the acetylation of thrombin and prothrombin also playing an important role [172] (reviewed by Undas et al. [173]). In addition, high dose aspirin (greater than 300 mg/day) appears to increase the efficiency of fibrinolysis [174,175]. Ingestion of aspirin also leads to the upregulation of lipoxin A4 in humans and animals [[176], [177], [178], [179]] via a mechanism involving the irreversible acetylation of COX2 [180,181] (reviewed by Romano et al. [182]). This is an important consequence of aspirin as these molecules play major role in the resolution of inflammation in conditions such as sepsis and ARDS [65,183]. Importantly, aspirin is the only nonsteroidal anti-inflammatory drug capable of activating lipoxins in immune, endothelial and epithelial cells and the only trigger of lipoxin A4 production by platelets [181].
The role of lipoxin A4 in the resolution of acute lung damage and ARDS are multiple. For example, it is involved in the repair of pulmonary epithelium by stimulating the trans-differentiation of alveolar type 2 cells into alveolar type 1 cells and the recruitment of the former from distal tissue [184,185] (reviewed by Chandrasekharan and Sharma-Walia [186]). In addition, the upregulation of this molecule in neutrophils and macrophages also reduces the activity migration priming of the former and the efficiency of phagocytosis and activity of the latter [185,187]. Upregulation of lipoxin A4 also induces a wide range of anti-inflammatory effects stemming from reductions in TNF-alpha [179], increased SOCS2 [188], decreased translocation of HGMB1 [189] and inhibition of NF-κB [[189], [190], [191]].
Finally, there is evidence that aspirin administration inhibits the activation of the NLP3 inflammasome [[192], [193], [194]]. In addition, this may be a specific property of aspirin and may underpin a significant improvement in endothelial function [192,194]. This data raises questions regarding the appropriate dose of aspirin for consideration in a trial of a treatment for COVID-19. In particular, 75 mg/day would appear to have little or no effect on COX2 inhibition, and significant NF-κB inhibition would appear to require doses in excess of 300 mg per day and would seem to be optimal at 1500 mg per day [195,196]. It is possible that the doses of aspirin required may rival those used in the treatment of rheumatic fever [197].
4.4. N-acetylcysteine (NAC)
Oral and intravenous NAC administration has demonstrated clinical efficacy in patients suffering from a wide range of chronic refractory respiratory illnesses including chronic bronchitis [[198], [199], [200]], chronic obstructive pulmonary disease [[201], [202], [203], [204]], cystic fibrosis, bacterial biofilms [205], asthma, pulmonary fibrosis and allergies [[206], [207], [208]]. There has also been interest in NAC as a treatment for ARDS and two recent meta analyses concluded that NAC reduced the need for ventilation and shortened ventilation time, although no reductions in mortality have been reported [209,210]. However, a reduction in the need for ventilation is a major therapeutic benefit as ARDS patients may suffer long term physical and psychological morbidity which are associated with significant health care costs [211]. In addition, NAC supplementation appears to ameliorate many elements involved in the pathophysiology of ARDS. For example, NAC supplementation decreases neutrophil recruitment and activity while increasing neutrophil apoptosis in an environment of intense lung inflammation [[212], [213], [214], [215], [216]]. The increased levels of neutrophil apoptosis seen following NAC administration appears to be due to increased efferocytosis of AMs which appears to be secondary to improved redox status [214,217]. In addition, evidence suggests that NAC also reduces levels of platelet, monocyte and neutrophil complexes in vivo [218,219]. Furthermore, several studies have reported that NAC supplementation results in a reduction in neutrophil production of NETs [218,220,221].
Evidence from human studies also suggests that NAC reduces levels of platelet monocyte and neutrophil complexes in vivo [218,219]. Several authors have also reported significant reductions in levels of pulmonary inflammation following NAC supplementation at approximately 1200 mg/day as evidenced by reduced levels of PICs, ROS, tissue damage and fibrin deposits in alveoli and interstitial tissue [[222], [223], [224]]. There is also evidence to suggest that NAC improves pulmonary function, an effect which appears to be enhanced when the thiol is inhaled [207,225]. NAC supplementation also exerts several other anti-inflammatory effects relevant to the pathophysiology of ARDS, most notably by decreasing levels of IL-6 [[226], [227], [228]] and NF-κB [[229], [230], [231]].
Finally, authors of a prospective double blind RCT investigating the use of 1200 mg/day in mechanically ventilated patients reported, that compared to placebo, patients administered NAC were less likely to develop ventilator associated pneumonia (VAP), had a significantly reduced stay in intensive care unit and an increased incidence of complete recovery [232]. This may be of significance from the perspective of treating COVID-19 ARDS as VAP may well be a significant cause of long term morbidity and mortality in patients requiring mechanical ventilation despite improvements made by prone positioning [233].
Several research teams have reported reduced endothelial cell activation and improved endothelial function following supplementation with oral NAC [[234], [235], [236]]. Interestingly, the mechanism appears to involve inhibition of the NLRP3 inflammasome [[237], [238], [239]]. In addition significant inhibition of platelet activity has been reported in in patients with type 2 diabetes and in healthy volunteers [[240], [241], [242], [243]], which appears to be secondary to increased levels of intraplatelet glutathione and production of NO with a concomitant reduction in levels of oxidative stress [243].
In addition, evidence suggests that NAC inhibits the development of large vessel thrombosis in inflammatory illnesses such as diabetes [243] and may even reduce thrombus formation to a similar degree to the effect produced by low dose aspirin [218]. Intravenous NAC administration promotes lysis of arterial thrombi in patients who are resistant to antiplatelet thrombin inhibitors and recombinant tissue-type plasminogen activator targeting VWF [244,245]. Such data has increased interest in the use NAC in patients displaying evidence of aspirin resistance despite being prescribed a dose known to inhibit thromboxane A2 [246,247].
It is important to stress that the anticoagulant and platelet-inhibiting properties of NAC are seen in in patients with serious conditions such as those undergoing major vascular surgery [248]. It is also noteworthy that the anticoagulant effects of NAC extend to decreasing the activity of coagulation factors II, VII and X, thereby producing a significant decrease in prothrombin time [249,250]. These properties have led to an increased focus on an expanded role of NAC in the inhibition of platelet aggregation in the context of reperfusion injury in recent years (reviewed by Nikbakht et al. [251]). Some of the mechanisms underpinning the effects of NAC on the coagulation and fibrinolytic systems ultimately leading to decreased thrombus formation have been discussed above. However a reader interested in an in depth consideration of the area are referred to an excellent review by Gutmann et al. [252].
The weight of evidence suggests that severe vitamin (Vit) D deficiency is a common occurrence in intensive care unit (ICU) patients with sepsis, septic shock and ARDS [253,254]. In addition, several authors have reported positive correlations between Vit D levels and increasing illness severity [[253], [254], [255]]. The extent of Vit D depletion also appears to be predictive of increased mortality reviewed [256]. It is also noteworthy that the level of Vit D depletion on admission to hospital is also predictive of developing severe disease and poor outcomes [257,258].
The relationship between low Vit D levels and increased severity of sepsis, appears to be due, at least in part, to exaggerated levels of inflammation and immune activation compared to patients with adequate levels of this vitamin [[259], [260], [261]]. This phenomenon may well exist in other illnesses whose pathophysiology involves chronic systemic immune activation and inflammation [[259], [260], [261]]. In fact several human studies investigating Vit D levels in conditions such as type 2 diabetes and metabolic syndrome have reported higher activity of NF-κB and increased levels of PICs in patients with Vit D deficiency compared to those whose level of the vitamin is within normal limits [[262], [263], [264], [265], [266]]. These findings are consistent with other lines of evidence suggesting that Vit D plays an indispensable role in modulating inflammation and adequate levels are needed for an optimal anti-inflammatory response to prevent an over exuberant immune response to invading pathogens or sterile insults [[267], [268], [269]]. Unsurprisingly Vit D exerts a wide range of broadly tolerogenic effects on the immune response via engagement with the Vit D receptor and these are summarised in Fig. 2 .
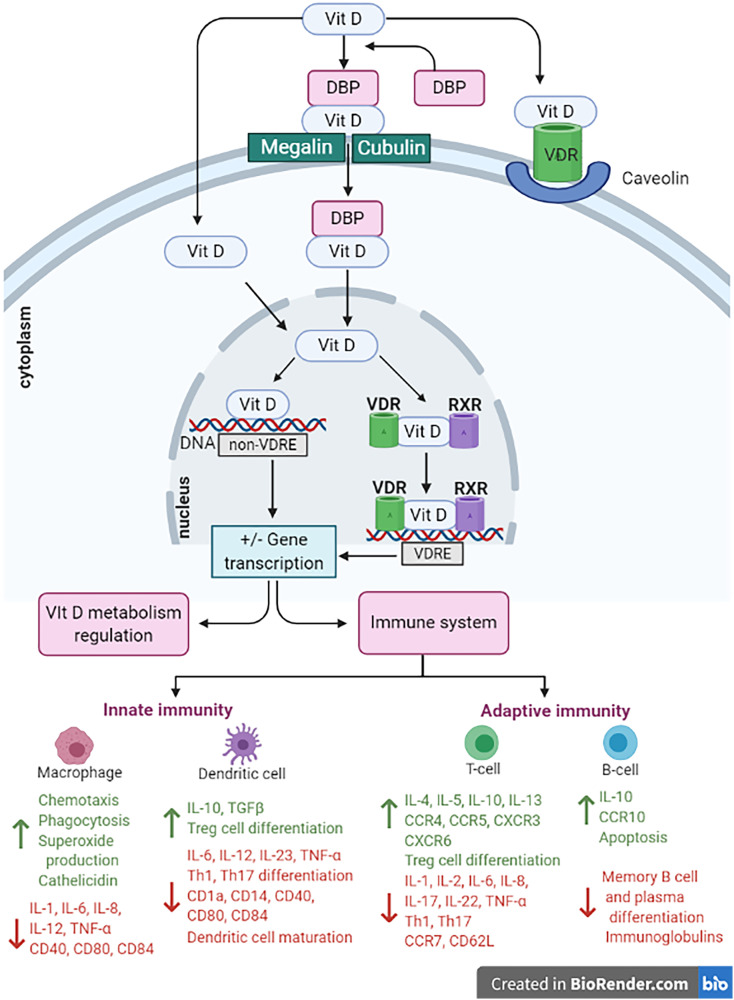
Fig. 2.
The effects of vitamin D on the immune system. Vitamin D inhibits B cell proliferation differentiation and immunoglobulin secretion. Vitamin D also suppresses T cell proliferation, TH17 differentiation increases levels of regulatory T cells and induces a tolerogenic Th2 phenotype. The sum of these effects is reduced levels of interleukin (IL)-17, IL-21 and IL-23 and increased levels of IL-10. Vitamin D also inhibits the maturation of dendritic cells and inhibits the production of pro inflammatory cytokines and chemokines from monocytes and macrophages reducing plasma levels of TNF-alpha, IL-1, IL-6, IL-12 and IL-8. In addition, Vitamin D activity stimulates the production of beta defensins and cathelicidin in monocytes and macrophages following pathogen invasion with forms an essential role in the anti-viral response.
Several human studies confirming a beneficial effect of Vit D on NF-κB activity and levels of PICs most notably TNF-alpha and IL-6 [[262], [263], [264], [265], [266]] reviewed. It is also noteworthy that a recent double blind RCT. Ventilated patients using a dose of Vit D in the range of 250,000 to 500,000 IU (55 ng/ml) over 7 days reported clinically significant reductions hospital stay suggesting that the dose needed to treat COVID-19 ARDS is likely to be of the same order [270].The issue of dosage as also addressed in a recent meta-analysis investigating the use of Vit D in the treatment or prophylaxis of respiratory virus infections which reported significant benefit at levels greater than 40 ng/ml but no evidence of benefit at lower doses [271] reviewed [272]. There is some evidence that Vit D supplementation may improve the immune status in patients with HIV [273] dengue [274] and hepatitis B infections [275].
There is evidence that Vit D supplementation may decrease the replication of rhinoviruses via a mechanism involving the upregulation of the interferon response and cathelicidin (LL-37) in lung epithelial cells [276,277]. LL-37 upregulation appears to be a major element involved in the anti-viral defences of lung epithelial cells and this also appears to be true of macrophages and dendritic cells. Importantly, there is also evidence to suggest that LL-37 upregulation may inhibit the replication of herpes simplex virus type one (HSV-1), respiratory syncytial virus (RSV) vaccinia virus HIV and HTLV-1, H1N1 and Ebola [[278], [279], [280], [281]]. Unsurprisingly, given the information above depleted levels of Vit D are associated with sub optimal LL-37 expression in lung epithelial cells which are a known target for SARS-CoV-2 entry [278]. Hence when considered as a whole this data combined with the multiple lines of evidence discussed above support the use of Vit D as part of a rational treatment approach for COVID-19. Finally, Vit D acts in alliance with many other molecules and systems when exerting its effects on the immune response and hence potential synergies may exist when combined with other molecular players such as melatonin reviewed [282]. Readers interested in a detailed consideration of the role of LL-37 in the pulmonary immune response to infection are referred to the work of [283].
4.5. Vitamin C
Levels of Vit C, otherwise known as ascorbic acid, are grossly depleted in many patients with severe infections sepsis and septic shock despite being administered adequate levels of enteral or parenteral nutritional therapy [[284], [285], [286], [287]]. Mechanistically this state of affairs appears to be due to increased consumption due to high levels of oxidative stress [284,286] and the inhibitory effect of PICs on the sodium dependent Vit C transporter (SVCT) 2 [288,289]. This receptor is expressed in most cells and responsible for the cellular entry of ascorbate [288,289]. Readers interested in a more detailed consideration of this topic are invited to consult a thorough review of the area in Mangin et al. [290].
Ascorbate related hypovitaminosis is associated with increased levels of inflammation and compromised immune function in a similar manner to the scenario of severe Vit D depletion [[291], [292], [293]]. Higher levels of inflammation increased activity of NF-κB increased production of NETs and increased neutrophil netosis [294,295]. Vit C exerts a range of anti-inflammatory effects on the immune system via the modulation of hypoxia inducible factor alpha (HIFalpha) levels and levels of histone acetylation and DNA methylation via regulating the activity of ten eleven translocase (TET) and proteins containing a Jumonyi C (JDHM) domain [284,296,297]. A summary of the phenotypic changes in the immune response induced by the activity of Vit C and the mechanisms involved is included in Fig. 3 .
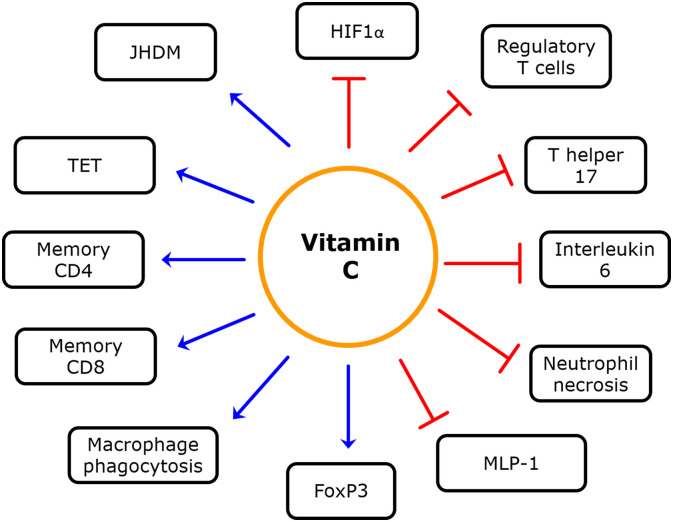
Fig. 3.
The effects of Vitamin C on the immune system. Vitamin C enhances the activity of TET enzymes and Jumonji C domain-containing histone demethylases (JHDMs) thereby decreasing rates of DNA and histone methylation respectively and prevents the hyperactivity of HIF 1 alpha. The net effect of these actions involves increased macrophage phagocytosis decreased neutrophil necrosis and the differentiation of T cells into a TH2 phenotype increases levels of regulatory T cells and inhibits naive T cell differentiation into a Th17 phenotype. Vitamin C is needed for memory CD8 and CD4 T cell formation and optimal T lymphocyte function. Vitamin C supplementation also reduces IL-6 and MCP-1 production by macrophages and dendritic cells while inhibiting dendritic cell maturation.
The bioavailability of oral form of Vit C are limited by the activity of SVCT1 which appears to reach saturation level in the dose range of 500-1000 mg limiting plasma levels to approximately 220 μmol/ml. However, the use of IV administration bypasses the limitations of this receptors leading to plasma levels of Vit C which are some 70 times higher at 15,000 μmol/ml [298,299] suggesting that the IV formulation would be the one of choice in any intervention aiming to treat COVID-19. This point is reinforced by data supplied by the citrus study reporting that IV Vit C administration at 200 mg/kg 4 days resulted in significant reduction in hospital stay and overall mortality in ventilated ARDS patients [300]. In addition the oral doses of Vit C needed to produce evidence of therapeutic benefit in this patient group would appear to be in excess of 6 g/day although at these doses data suggests that ventilation time in ARDS patients may be reduced by 25% review [301]. In addition, there is growing interest in combining Vit C, thiamine and hydrocortisone as a treatment for sepsis and ARDS following the results of a recent study where the use of these preparations in unison resulted in a 25% reduction in mortality [302].
Finally, there is evidence to suggest that high dose IV Vit C inhibits the replication of rhinoviruses [303], H1N1 [304], Chikungunya [305], Zika [305] and seasonal influenza [306]. In addition there is also some evidence that oral supplementation with Vit C (doses over 3 g) may also reduce the risk of infection by prevent respiratory viruses [291].
4.6. Dexamethasone
Dexamethasone exerts its anti-inflammatory effects by upregulating the activity of glucocorticoid receptors (GR) and inhibiting the activity of a number of proinflammatory transcription factors including NF-κB reviewed [307]. This is an important point as the weight of histopathological evidence suggests that non resolving ARDS is associated with increased activity of nuclear NF-κB and decreased activity of nuclear GRs [308,309]. In the light of this data and the potential decrease in mortality in patients suffering from COVID-19 ARDS following dexamethasone administration and its mechanism of action is briefly considered below.
Following diffusion across the cell membrane dexamethasone and indeed other glucocorticoids bind to the cytosolic GR resulting in a conformational change freeing the GR from the constraints of a heat shock chaperone [310]. The unencumbered GR then translocate to the nucleus and bind to glucocorticoid-responsive elements (GREs) located in the promoter regions of proinflammatory and anti-inflammatory genes increasing the transcription of the latter and decreasing the transcription of the former by cis-activation or cis-repression respectively [311,312]. These mechanisms are reviewed in Xavier et al. [313]. GRs may also engage with other transcription factors in the nucleus limiting or modifying their DNA binding activity by various mechanisms such as competing for the same indispensable cofactor and may also act posttranscriptional by modifying the stability of mRNA [312] (reviewed in Laryea et al. [314]).
Dexamethasone upregulated GR activity mediates NF-κB inhibition by increasing the production and inhibiting the degradation IκBα which sequesters NF-κB in the cytoplasm via direct effects on the GRE sequence in the IκBα gene and in the gene responsible for the production of IL-10. The use of dexamethasone also results in the formation of GR-NF-κB complexes in the nucleus resulting in the reduced activity of NF-κB due to restricted access to the indispensable cofactors steroid receptor coactivator-1 (SRC-1) and CREB-binding protein (CBP). Several authors have also reported increased expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in lung epithelial cells following dexamethasone administration at physiological concentrations [315,316] This has the effect of decreasing the activity of MAP kinase which plays an indispensable role in IL-1 and TNF-alpha mediated increases in the activity of NF-κB [315,316].This data is of particular interest as this may be an effect which is unique to this steroid and may underpin significant depression in pulmonary IL-8 levels following its administration [317,318]. Finally, there is also evidence to suggest that dexamethasone ligated GRs form complexes with p65 NF-κB subunits in the cytoplasm which ultimately translocate to the nucleus exhibiting greater anti-inflammatory activity and inducing increased production of anti-inflammatory proteins such as A20 compared to GR receptors acting alone [319,320].
Dexamethasone may have value in COVD-19 characterised by a cytokine storm. Dexamethasone has potent anti-inflammatory effects that are dose related. It inhibits LPS-stimulated ΤΝF-alpha release, as well as levels of IL-6, IL-8 and of IL-10 [321]. In bacterial community acquired pneumonia, dexamethasone compared to placebo significantly lowered levels of f IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1) and TNF-alpha [322]. Timing appears to be a critical factor, as corticosteroids may have value in severe SARS-CoV-2 infection characterised by the presence of the hyperinflammation phase of a cytokine storm [34]. In early and milder disease, viral clearance can be delayed and inhibited, resulting in poorer outcomes. Evidence of this derives from the SARS experience, where a 20.7-fold higher risk of ICU admission was seen with the routine use of steroids [323]. Similarly, during the middle east respiratory syndrome (MERS) epidemic, corticosteroid therapy worsened outcomes due to delayed clearance of MERS coronavirus [324]. The extant data from the COVID-19 pandemic suggests that use of steroids in non-critical patients without a cytokine storm is not advisable [325]. Dexamethasone may have benefit in ventilator induced lung injury [326]. There are case reports of the use of dexamethasone in the treatment of SARS-CoV-2 pneumonia complicated by pulmonary oedema resulting from the pulmonary capillary leak syndrome [327]. Preliminary non-peer reviewed data from the Randomised Evaluation of COVID-19 (RECOVERY) trial suggests a benefit in people with severe COVID-19 infection on ventilators in ICU (2020).
5. Conclusion
A comprehensive and highly plausible model has been proposed in this paper detailing the pathophysiological steps of COVID-19 from the point of initial infection of type II alveolar epithelial cells by SARS-CoV-2 to the ultimate development of ARDS. This model highlights are various several potential control points where targeted therapeutic interventions might produce significant benefits in reducing the severity of disease. Several lines of evidence suggest a role for dexamethasone for the treatment of ARDS. In addition, consideration of data supplied by human an animal studies also highlight the potential efficacy of Zn supplementation, aspirin (acetylsalicylic acid), the macrolide antibiotic azithromycin, oral or intravenous administration of NAC, IV Vit C and oral Vit D. Moreover, when used at appropriate doses, these supplements and drugs generally have an exceptionally good safety record. Hence, based on this evidence, it is recommended that randomised trials of these therapeutic substances in COVID-19 are in order. Given that excessive levels systemic inflammation seen in patients with severe COVID-19 is likely to lead to depleted levels of Vit C, Vit D and Zn in many individuals and significant differences in their mode of action as anti-inflammatory agents, it is suggested that they should be used in combination. Equally, the use of aspirin at a dose in excess of 300 mg, NAC and AZM offers the prospect of localised NF-κB inhibition and reducing the activation of the coagulation cascade characteristic of severe COVID-19.
List of abbreviations
- AM
alveolar macrophages
- ARDS
acute respiratory distress syndrome
- AZM
azithromycin
- CBP
CREB-binding protein
- COX
cyclooxygenase
- DAMPS
damage-associated molecular patterns
- DIC
disseminated intravascular coagulation
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GR
glucocorticoid receptor
- GREs
glucocorticoid-responsive elements
- HIPalpha
hypoxia inducible factor alpha
- HMBG1
high mobility group box 1
- HSV-1
herpes simplex virus type one
- ICU
intensive care unit
- IL
interleukin
- JDHM
Jumonyi C
- LPS
Lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- MDSC
CD11b + Gr-1+ myeloid-derived suppressor cells
- MERS
middle east respiratory syndrome
- MKP-1
mitogen-activated protein kinase phosphatase-1
- MMP-9
matrix metallopeptidase 9
- mtDNA
mitocondrial DNA
- NAC
N-acetylcysteine
- NETs
neutrophil extracellular traps
- NF-kB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- NLRs
NOD-like receptors
- NO
nitric oxide
- NOS2
inducible nitric oxide synthase 2
- PI3K
phosphoinositide 3-kinase
- PICs
proinflammatory cytokines
- RCT
randomised controlled trial
- RdRp
RNA dependent RNA polymerase
- ROS
reactive oxygen species
- RSV
respiratory syncytial virus
- SARS-CoV-2
severe acute respiratory syndrome CoronaVirus 2
- SIRS
systemic inflammatory response syndrome
- SRC-1
steroid receptor coactivator-1
- SVCT
sodium dependent Vit C transporter
- TET
ten eleven translocases
- TLR
Toll-like receptor 9
- TNF
tumour necrosis factor
- VAP
ventilator associated pneumonia
- VIT
vitamin
- Zn
zinc
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
CRediT authorship contribution statement
GM conceptualized the work and was a major contributor in writing the manuscript. CCB created the figures. All authors have drafted part and approved the final manuscript.
Declaration of competing interest
MB is supported by a NHMRC Senior Principal Research Fellowship (1059660 and 1156072). MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Australian Rotary Health, A2 Milk Company, Meat and Livestock Australia, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Astra Zeneca, Lundbeck, Merck, Pfizer, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier – all unrelated to this work. LO is supported by a NHMRC Early Career Fellowship (1158487). WM is currently funded by an Alfred Deakin Postdoctoral Research Fellowship and a Multiple Sclerosis Research Australia early-career fellowship. WM has previously received funding from the Cancer Council Queensland and university grants/fellowships from La Trobe University, Deakin University, University of Queensland, and Bond University. WM has received industry funding and has attended events funded by Cobram Estate Pty. Ltd. WM has received travel funding from Nutrition Society of Australia. WM has received consultancy funding from Nutrition Research Australia. WM has received speaker honoraria from The Cancer Council Queensland and the Princess Alexandra Research Foundation. The Food & Mood Centre has received Grant/Research support from Fernwood Foundation, Wilson Foundation, the A2 Milk Company, and Be Fit Foods. AO is supported by a Future Leader Fellowship (#101160) from the Heart Foundation Australia and Wilson Foundation. She has received research funding from National Health and Medical Research Council, Australian Research Council, University of Melbourne, Deakin University, Sanofi, Meat and Livestock Australia and Woolworths Limited and Honoraria from Novartis.
Acknowledgements
Figures were created with BioRender.com.
References
- 1.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carsana L., Sonzogni A., Nasr A., Rossi R., Pellegrinelli A., Zerbi P. 2020. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv. (2020.04.19.20054262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. 2020. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. medRxiv. (2020.04.06.20050575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. Journal of Thrombosis and Haemostasis: JTH. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzales J.N., Lucas R., Verin A.D. The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches. Austin J Vasc Med. 2015;2:1009. [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang X., Du R., Wang R., Cao T., Guan L., Yang C. Comparison of Hospitalized Patients With ARDS Caused by COVID-19 and H1N1. Chest. 2020;158(1):195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5(10):e137799. doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copin M.C., Parmentier E., Duburcq T., Poissy J., Mathieu D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46:1124–1126. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su H., Yang M., Wan C., Yi L.-X., Tang F., Zhu H.-Y. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J. 2020. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. medRxiv. (2020.02.23.20026690) [Google Scholar]
- 19.Mason R.J. Pathogenesis of COVID-19 from a cell biologic perspective. Eur. Respir. J. 2020;55(4) doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salomé B., Magen A. Dysregulation of lung myeloid cells in COVID-19. Nat. Rev. Immunol. 2020;20(5):277. doi: 10.1038/s41577-020-0303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang M. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV Infection. SSRN Electron. J. 2020 [Google Scholar]
- 23.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 24.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y. 2020. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. medRxiv. (2020.03.23.20039362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y. 2020. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv. (2020.03.24.20042655) [Google Scholar]
- 29.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death & Differentiation. 2020;7(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C., Cai J., Chen R., Shi Z., Bian X., Xie J. Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19. Research Square. 2020;57:102833. doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore B.J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020:eabb8925. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 34.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. The Journal of infection. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. (105954-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao X.-H., He Z.-C., Li T.-Y., Zhang H.-R., Wang Y., Mou H. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020;30(6):541–543. doi: 10.1038/s41422-020-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faust H., Mangalmurti N.S. Collateral damage: necroptosis in the development of lung injury. Am. J. Phys. Lung Cell. Mol. Phys. 2020;318:L215–L225. doi: 10.1152/ajplung.00065.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauler M., Bazan I.S., Lee P.J. Cell death in the lung: the apoptosis-necroptosis axis. Annu. Rev. Physiol. 2019;81:375–402. doi: 10.1146/annurev-physiol-020518-114320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueno H., Matsuda T., Hashimoto S., Amaya F., Kitamura Y., Tanaka M. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. J. Respir. Crit. Care Med. 2004;170:1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 41.Aberdein J.D., Cole J., Bewley M.A., Marriott H.M., Dockrell D.H. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin. Exp. Immunol. 2013;174:193–202. doi: 10.1111/cei.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Losa García J.E., Rodríguez F.M., Martín de Cabo M.R., García Salgado M.J., Losada J.P., Villarón L.G. Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediat. Inflamm. 1999;8:43–51. doi: 10.1080/09629359990711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang C.-Y., Chen C.-S., Yiang G.-T., Cheng Y.-L., Yong S.-B., Wu M.-Y. New insights into the immune molecular regulation of the pathogenesis of acute respiratory distress syndrome. Int. J. Mol. Sci. 2018;19:588. doi: 10.3390/ijms19020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93:212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 45.Han S., Mallampalli R.K. The acute respiratory distress syndrome: from mechanism to translation. Journal of immunology (Baltimore, Md: 1950) 2015;194:855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang X., Xiu H., Zhang S., Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/1264913. (1264913-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Haouari M. Platelet oxidative stress and its relationship with cardiovascular diseases in type 2 diabetes mellitus patients. Curr. Med. Chem. 2019;26:4145–4165. doi: 10.2174/0929867324666171005114456. [DOI] [PubMed] [Google Scholar]
- 48.Freedman J.E. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 2008;28:s11–s16. doi: 10.1161/ATVBAHA.107.159178. [DOI] [PubMed] [Google Scholar]
- 49.Violi F., Pignatelli P., Basili S. Nutrition, supplements, and vitamins in platelet function and bleeding. Circulation. 2010;121:1033–1044. doi: 10.1161/CIRCULATIONAHA.109.880211. [DOI] [PubMed] [Google Scholar]
- 50.Øynebråten I., Barois N., Bergeland T., Küchler A.M., Bakke O., Haraldsen G. Oligomerized, filamentous surface presentation of RANTES/CCL5 on vascular endothelial cells. Sci. Rep. 2015;5:9261. doi: 10.1038/srep09261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonmez O., Sonmez M. Role of platelets in immune system and inflammation. Porto Biomedical Journal. 2017;2:311–314. doi: 10.1016/j.pbj.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finsterbusch M., Schrottmaier W.C., Kral-Pointner J.B., Salzmann M., Assinger A. Measuring and interpreting platelet-leukocyte aggregates. Platelets. 2018;29:677–685. doi: 10.1080/09537104.2018.1430358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sreeramkumar V., Adrover J.M., Ballesteros I., Cuartero M.I., Rossaint J., Bilbao I. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark K. Platelet-neutrophil crosstalk and netosis. HemaSphere. 2019;3:89–91. doi: 10.1097/HS9.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 56.Kimball A.S., Obi A.T., Diaz J.A., Henke P.K. The emerging role of NETs in venous thrombosis and immunothrombosis. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assinger A., Schrottmaier W.C., Salzmann M., Rayes J. Platelets in sepsis: an update on experimental models and clinical data. Front. Immunol. 2019;10:1687. doi: 10.3389/fimmu.2019.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dewitte A., Lepreux S., Villeneuve J., Rigothier C., Combe C., Ouattara A. Blood platelets and sepsis pathophysiology: a new therapeutic prospect in critically [corrected] ill patients? Ann. Intensive Care. 2017;7 doi: 10.1186/s13613-017-0337-7. (115-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nedeva C., Menassa J., Puthalakath H. Sepsis: inflammation is a necessary evil. Frontiers in Cell and Developmental Biology. 2019;7 doi: 10.3389/fcell.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim M.Y., Ataga K.I., Key N.S. Hemostatic abnormalities in sickle cell disease. Curr. Opin. Hematol. 2013;20:472–477. doi: 10.1097/MOH.0b013e328363442f. [DOI] [PubMed] [Google Scholar]
- 61.Pfeiler S., Massberg S., Engelmann B. Biological basis and pathological relevance of microvascular thrombosis. Thromb. Res. 2014;133:S35–S37. doi: 10.1016/j.thromres.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Prabhakaran P., Ware L.B., White K.E., Cross M.T., Matthay M.A., Olman M.A. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am. J. Phys. Lung Cell. Mol. Phys. 2003;285:L20–L28. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 63.Sapru A., Curley M.A.Q., Brady S., Matthay M.A., Flori H. Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive Care Med. 2010;36:157–163. doi: 10.1007/s00134-009-1690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xue M., Sun Z., Shao M., Yin J., Deng Z., Zhang J. Diagnostic and prognostic utility of tissue factor for severe sepsis and sepsis-induced acute lung injury. J. Transl. Med. 2015;13:172. doi: 10.1186/s12967-015-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yadav H., Kor D.J. Platelets in the pathogenesis of acute respiratory distress syndrome. American Journal of Physiology Lung Cellular and Molecular Physiology. 2015;309:L915–L923. doi: 10.1152/ajplung.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gando S., Otomo Y. Local hemostasis, immunothrombosis, and systemic disseminated intravascular coagulation in trauma and traumatic shock. Critical care (London, England) 2015;19 doi: 10.1186/s13054-015-0735-x. (72-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito T. PAMPs and DAMPs as triggers for DIC. J. Intensive Care. 2014;2 doi: 10.1186/s40560-014-0065-0. (67-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Long Y., Liu X., Tan X.-Z., Jiang C.-X., Chen S.-W., Liang G.-N. ROS-induced NLRP3 inflammasome priming and activation mediate PCB 118- induced pyroptosis in endothelial cells. Ecotoxicol. Environ. Saf. 2020;189:109937. doi: 10.1016/j.ecoenv.2019.109937. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y., Shi P., Chen Q., Huang Z., Zou D., Zhang J. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019;11:1069–1082. doi: 10.1093/jmcb/mjz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dolinay T., Kim Y.S., Howrylak J., Hunninghake G.M., An C.H., Fredenburgh L. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grailer J.J., Canning B.A., Kalbitz M., Haggadone M.D., Dhond R.M., Andjelkovic A.V. Critical role for the NLRP3 inflammasome during acute lung injury. J. Immunol. 2014;192:5974–5983. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Makabe H., Kojika M., Takahashi G., Matsumoto N., Shibata S., Suzuki Y. Interleukin-18 levels reflect the long-term prognosis of acute lung injury and acute respiratory distress syndrome. J. Anesth. 2012;26:658–663. doi: 10.1007/s00540-012-1409-3. [DOI] [PubMed] [Google Scholar]
- 73.Jin L., Batra S., Jeyaseelan S. Deletion of Nlrp3 augments survival during polymicrobial sepsis by decreasing autophagy and enhancing phagocytosis. J. Immunol. 2017;198:1253–1262. doi: 10.4049/jimmunol.1601745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim M.J., Bae S.H., Ryu J.C., Kwon Y., Oh J.H., Kwon J. SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy. 2016;12:1272–1291. doi: 10.1080/15548627.2016.1183081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Danielski L.G., Giustina A.D., Bonfante S., Barichello T., Petronilho F. The NLRP3 inflammasome and its role in sepsis development. Inflammation. 2020;43:24–31. doi: 10.1007/s10753-019-01124-9. [DOI] [PubMed] [Google Scholar]
- 76.Fan E.K.Y., Fan J. Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res. 2018;19:50. doi: 10.1186/s12931-018-0756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hou L., Yang Z., Wang Z., Zhang X., Zhao Y., Yang H. NLRP3/ASC-mediated alveolar macrophage pyroptosis enhances HMGB1 secretion in acute lung injury induced by cardiopulmonary bypass. Lab. Investig. 2018;98:1052–1064. doi: 10.1038/s41374-018-0073-0. [DOI] [PubMed] [Google Scholar]
- 78.Traeger T., Kessler W., Hilpert A., Mikulcak M., Entleutner M., Koerner P. Selective depletion of alveolar macrophages in polymicrobial sepsis increases lung injury, bacterial load and mortality but does not affect cytokine release. Respiration. 2009;77:203–213. doi: 10.1159/000160953. [DOI] [PubMed] [Google Scholar]
- 79.Wu D.-D., Pan P.-H., Liu B., Su X.-L., Zhang L.-M., Tan H.-Y. Inhibition of alveolar macrophage pyroptosis reduces lipopolysaccharide-induced acute lung injury in mice. Chin. Med. J. 2015;128:2638–2645. doi: 10.4103/0366-6999.166039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang W., Coopersmith C.M. Dying as a pathway to death in sepsis. Anesthesiology. 2018;129:238–240. doi: 10.1097/ALN.0000000000002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McQuattie-Pimentel A.C., Budinger G.R.S., Ballinger M.N. Monocyte-derived alveolar macrophages: the dark side of lung repair? Am. J. Respir. Cell Mol. Biol. 2018;58:5–6. doi: 10.1165/rcmb.2017-0328ED. [DOI] [PubMed] [Google Scholar]
- 82.Schulz D., Severin Y., Zanotelli V.R.T., Bodenmiller B. In-depth characterization of monocyte-derived macrophages using a mass cytometry-based phagocytosis assay. Sci. Rep. 2019;9 doi: 10.1038/s41598-018-38127-9. (1925-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newton K., Manning G. Necroptosis and Inflammation. Annu. Rev. Biochem. 2016;85:743–763. doi: 10.1146/annurev-biochem-060815-014830. [DOI] [PubMed] [Google Scholar]
- 84.Zhu K., Liang W., Ma Z., Xu D., Cao S., Lu X. Necroptosis promotes cell-autonomous activation of proinflammatory cytokine gene expression. Cell Death Dis. 2018;9:500. doi: 10.1038/s41419-018-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang B., Li J., Gao H.-M., Xing Y.-H., Lin Z., Li H.-J. Necroptosis regulated proteins expression is an early prognostic biomarker in patient with sepsis: a prospective observational study. Oncotarget. 2017;8:84066–84073. doi: 10.18632/oncotarget.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Besecker B.Y., Exline M.C., Hollyfield J., Phillips G., Disilvestro R.A., Wewers M.D. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am. J. Clin. Nutr. 2011;93:1356–1364. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cirino Ruocco M.A., Pacheco Cechinatti E.D., Barbosa F., Jr., Navarro A.M. Zinc and selenium status in critically ill patients according to severity stratification. Nutrition (Burbank, Los Angeles County, Calif) 2018;45:85–89. doi: 10.1016/j.nut.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 88.Shanley T.P., Cvijanovich N., Lin R., Allen G.L., Thomas N.J., Doctor A. Genome-level longitudinal expression of signaling pathways and gene networks in pediatric septic shock. Molecular Medicine (Cambridge, Mass) 2007;13:495–508. doi: 10.2119/2007-00065.Shanley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong H.R., Shanley T.P., Sakthivel B., Cvijanovich N., Lin R., Allen G.L. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol. Genomics. 2007;30:146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alker W., Haase H. Zinc and sepsis. Nutrients. 2018;10:976. doi: 10.3390/nu10080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knoell D.L., Liu M.-J. Impact of zinc metabolism on innate immune function in the setting of sepsis. International Journal for Vitamin and Nutrition Research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 2010;80:271–277. doi: 10.1024/0300-9831/a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aydemir T.B., Cousins R.J. The multiple faces of the metal transporter ZIP14 (SLC39A14) J. Nutr. 2018;148:174–184. doi: 10.1093/jn/nxx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lichten L.A., Liuzzi J.P., Cousins R.J. Interleukin-1beta contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G860–G867. doi: 10.1152/ajpgi.90676.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liuzzi J.P., Lichten L.A., Rivera S., Blanchard R.K., Aydemir T.B., Knutson M.D. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fraker P.J., King L.E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 96.Kogan S., Sood A., Garnick M.S. Zinc and wound healing: a review of zinc physiology and clinical applications. Wounds: A Compendium of Clinical Research and Practice. 2017;29:102–106. [PubMed] [Google Scholar]
- 97.Prasad A.S. Zinc: mechanisms of host defense. J. Nutr. 2007;137:1345–1349. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- 98.Abraham E. Alterations in cell signaling in sepsis. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2005;41(Suppl. 7):S459–S464. doi: 10.1086/431997. [DOI] [PubMed] [Google Scholar]
- 99.Hotchkiss R.S., Nicholson D.W. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 100.Jarosz M., Olbert M., Wyszogrodzka G., Młyniec K., Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bao S., Liu M.J., Lee B., Besecker B., Lai J.P., Guttridge D.C. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. American Journal of Physiology Lung Cellular and Molecular Physiology. 2010;298:L744–L754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu M.J., Bao S., Napolitano J.R., Burris D.L., Yu L., Tridandapani S. Zinc regulates the acute phase response and serum amyloid A production in response to sepsis through JAK-STAT3 signaling. PLoS One. 2014;9:e94934. doi: 10.1371/journal.pone.0094934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Souffriau J., Libert C. Mechanistic insights into the protective impact of zinc on sepsis. Cytokine Growth Factor Rev. 2018;39:92–101. doi: 10.1016/j.cytogfr.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Wessels I., Cousins R.J. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309:G768–G778. doi: 10.1152/ajpgi.00179.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boudreault F., Pinilla-Vera M., Englert J.A., Kho A.T., Isabelle C., Arciniegas A.J. Zinc deficiency primes the lung for ventilator-induced injury. JCI Insight. 2017;2 doi: 10.1172/jci.insight.86507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fan Y., Zhang X., Yang L., Wang J., Hu Y., Bian A. Zinc inhibits high glucose-induced NLRP3 inflammasome activation in human peritoneal mesothelial cells. Mol. Med. Rep. 2017;16:5195–5202. doi: 10.3892/mmr.2017.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Summersgill H., England H., Lopez-Castejon G., Lawrence C.B., Luheshi N.M., Pahle J. Zinc depletion regulates the processing and secretion of IL-1β. Cell Death Dis. 2014;5:e1040–e. doi: 10.1038/cddis.2013.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hodge K., Tunghirun C., Kamkaew M., Limjindaporn T., Yenchitsomanus P.-T., Chimnaronk S. Identification of a conserved RNA-dependent RNA polymerase (RdRp)-RNA interface required for flaviviral replication. J. Biol. Chem. 2016;291:17437–17449. doi: 10.1074/jbc.M116.724013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kar M., Khan N.A., Panwar A., Bais S.S., Basak S., Goel R. Zinc chelation specifically inhibits early stages of dengue virus replication by activation of NF-κB and induction of antiviral response in epithelial cells. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaushik N., Subramani C., Anang S., Muthumohan R., Shalimar, Nayak B. Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J. Virol. 2017;91:e00754–17. doi: 10.1128/JVI.00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suara R.O., Crowe J.E., Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 2004;48:783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Godoy A.S., Lima G.M.A., Oliveira K.I.Z., Torres N.U., Maluf F.V., Guido R.V.C. Crystal structure of Zika virus NS5 RNA-dependent RNA polymerase. Nat. Commun. 2017;8 doi: 10.1038/ncomms14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Potapova U., Feranchuk S., Belikov S. 2016. Quantum transition in zinc atoms of flavavirus polymerase triggers the conformation of the polymerase motif F. bioRxiv; p. 063024. [Google Scholar]
- 115.Faverio P., Bini F., Vaghi A., Pesci A. Long-term macrolides in diffuse interstitial lung diseases. Eur. Respir. Rev. 2017;26 doi: 10.1183/16000617.0082-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kawamura K., Ichikado K., Takaki M., Eguchi Y., Anan K., Suga M. Adjunctive therapy with azithromycin for moderate and severe acute respiratory distress syndrome: a retrospective, propensity score-matching analysis of prospectively collected data at a single center. Int. J. Antimicrob. Agents. 2018;51:918–924. doi: 10.1016/j.ijantimicag.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 117.Kawamura K., Ichikado K., Takaki M., Sakata Y., Yasuda Y., Shingu N. Efficacy of azithromycin in sepsis-associated acute respiratory distress syndrome: a retrospective study and propensity score analysis. Springerplus. 2016;5 doi: 10.1186/s40064-016-2866-1. (1193-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Walkey A.J., Wiener R.S. Macrolide antibiotics and survival in patients with acute lung injury. Chest. 2012;141:1153–1159. doi: 10.1378/chest.11-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mortensen E.M., Halm E.A., Pugh M.J., Copeland L.A., Metersky M., Fine M.J. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. Jama. 2014;311:2199–2208. doi: 10.1001/jama.2014.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zimmermann P., Ziesenitz V.C., Curtis N., Ritz N. The immunomodulatory effects of macrolides—a systematic review of the underlying mechanisms. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gualdoni G.A., Lingscheid T., Schmetterer K.G., Hennig A., Steinberger P., Zlabinger G.J. Azithromycin inhibits IL-1 secretion and non-canonical inflammasome activation. Sci. Rep. 2015;5 doi: 10.1038/srep12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Idris S.F., Chilvers E.R., Haworth C., McKeon D., Condliffe A.M. Azithromycin therapy for neutrophilic airways disease: myth or magic? Thorax. 2009;64:186–189. doi: 10.1136/thx.2008.103192. [DOI] [PubMed] [Google Scholar]
- 123.Aghai Z.H., Kode A., Saslow J.G., Nakhla T., Farhath S., Stahl G.E. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr. Res. 2007;62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 124.Stellari F.F., Sala A., Donofrio G., Ruscitti F., Caruso P., Topini T.M. Azithromycin inhibits nuclear factor-κB activation during lung inflammation: an in vivo imaging study. Pharmacol. Res. Perspect. 2014;2:e00058–e. doi: 10.1002/prp2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cory T.J., Birket S.E., Murphy B.S., Hayes D., Jr., Anstead M.I., Kanga J.F. Impact of azithromycin treatment on macrophage gene expression in subjects with cystic fibrosis. J. Cyst. Fibros. 2014;13:164–171. doi: 10.1016/j.jcf.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feola D.J., Garvy B.A., Cory T.J., Birket S.E., Hoy H., Hayes D., Jr. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas. Antimicrob. Agents Chemother. 2010;54:2437–2447. doi: 10.1128/AAC.01424-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haydar D., Cory T.J., Birket S.E., Murphy B.S., Pennypacker K.R., Sinai A.P. Azithromycin polarizes macrophages to an M2 phenotype via inhibition of the STAT1 and NF-κB signaling pathways. J. Immunol. 2019;203:1021–1030. doi: 10.4049/jimmunol.1801228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murphy B.S., Sundareshan V., Cory T.J., Hayes D., Anstead M.I., Feola D.J. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 2008;61:554–560. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- 129.Wang J., Xie L., Wang S., Lin J., Liang J., Xu J. Azithromycin promotes alternatively activated macrophage phenotype in systematic lupus erythematosus via PI3K/Akt signaling pathway. Cell Death Dis. 2018;9:1080. doi: 10.1038/s41419-018-1097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bai D., Ueno L., Vogt P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer. 2009;125:2863–2870. doi: 10.1002/ijc.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hussain A.R., Ahmed S.O., Ahmed M., Khan O.S., Al Abdulmohsen S., Platanias L.C. Cross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis. PLoS One. 2012;7:e39945–e. doi: 10.1371/journal.pone.0039945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Linton M.F., Moslehi J.J., Babaev V.R. Akt signaling in macrophage polarization, survival, and atherosclerosis. Int. J. Mol. Sci. 2019;20:2703. doi: 10.3390/ijms20112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xuejiao S., Lin C., Zhiyi H. PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr. Drug Metab. 2019;20:301–304. doi: 10.2174/1389200220666190227224748. [DOI] [PubMed] [Google Scholar]
- 134.Sharif O., Brunner J.S., Vogel A., Schabbauer G. Macrophage rewiring by nutrient associated PI3K dependent pathways. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hodge S., Hodge G., Brozyna S., Jersmann H., Holmes M., Reynolds P.N. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 2006;28:486–495. doi: 10.1183/09031936.06.00001506. [DOI] [PubMed] [Google Scholar]
- 136.Hodge S., Hodge G., Jersmann H., Matthews G., Ahern J., Holmes M. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;178:139–148. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 137.Hodge S., Reynolds P.N. Low-dose azithromycin improves phagocytosis of bacteria by both alveolar and monocyte-derived macrophagesin chronic obstructive pulmonary disease subjects. Respirology. 2012;17:802–807. doi: 10.1111/j.1440-1843.2012.02135.x. [DOI] [PubMed] [Google Scholar]
- 138.Namkoong H., Ishii M., Fujii H., Yagi K., Asami T., Asakura T. Clarithromycin expands CD11b+Gr-1+ cells via the STAT3/Bv8 axis to ameliorate lethal endotoxic shock and post-influenza bacterial pneumonia. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hochst B., Mikulec J., Baccega T., Metzger C., Welz M., Peusquens J. Differential induction of Ly6G and Ly6C positive myeloid derived suppressor cells in chronic kidney and liver inflammation and fibrosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alshetaiwi H., Pervolarakis N., McIntyre L.L., Ma D., Nguyen Q., Rath J.A. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Science Immunology. 2020:5. doi: 10.1126/sciimmunol.aay6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Crosbie P.A.J., Woodhead M.A. Long-term macrolide therapy in chronic inflammatory airway diseases. Eur. Respir. J. 2009;33:171–181. doi: 10.1183/09031936.00042208. [DOI] [PubMed] [Google Scholar]
- 142.Shinkai M., Rubin B.K. Macrolides and airway inflammation in children. Paediatr. Respir. Rev. 2005;6:227–235. doi: 10.1016/j.prrv.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 143.Suresh Babu K., Kastelik J., Morjaria J.B. Role of long term antibiotics in chronic respiratory diseases. Respir. Med. 2013;107:800–815. doi: 10.1016/j.rmed.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 144.Vanaudenaerde B.M., Wuyts W.A., Geudens N., Dupont L.J., Schoofs K., Smeets S. Macrolides inhibit IL17-induced IL8 and 8-isoprostane release from human airway smooth muscle cells. Am. J. Transplant. 2007;7:76–82. doi: 10.1111/j.1600-6143.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 145.Yamada T., Fujieda S., Mori S., Yamamoto H., Saito H. Macrolide treatment decreased the size of nasal polyps and IL-8 levels in nasal lavage. Am. J. Rhinol. 2000;14:143–148. doi: 10.2500/105065800782102717. [DOI] [PubMed] [Google Scholar]
- 146.Luu N.V., Yang J., Qu X.J., Guo M., Wang X., Xian Q.Y. Azithromycin inhibits neutrophil accumulation in airways by affecting interleukin-17 downstream signals. Chin. Med. J. 2012;125:491–495. [PubMed] [Google Scholar]
- 147.Piacentini G.L., Peroni D.G., Bodini A., Pigozzi R., Costella S., Loiacono A. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. Allergy and Asthma Proceedings. 2007;28:194–198. doi: 10.2500/aap.2007.28.2958. [DOI] [PubMed] [Google Scholar]
- 148.Tsai W.C., Rodriguez M.L., Young K.S., Deng J.C., Thannickal V.J., Tateda K. Azithromycin blocks neutrophil recruitment inpseudomonasendobronchial infection. Am. J. Respir. Crit. Care Med. 2004;170:1331–1339. doi: 10.1164/rccm.200402-200OC. [DOI] [PubMed] [Google Scholar]
- 149.Verleden G.M., Vanaudenaerde B.M., Dupont L.J., Van Raemdonck D.E. Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 2006;174:566–570. doi: 10.1164/rccm.200601-071OC. [DOI] [PubMed] [Google Scholar]
- 150.Shinkai M., Foster G.H., Rubin B.K. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am. J. Phys. Lung Cell. Mol. Phys. 2006;290:L75–L85. doi: 10.1152/ajplung.00093.2005. [DOI] [PubMed] [Google Scholar]
- 151.Koch C.C. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J. Antimicrob. Chemother. 2000;46:19–26. doi: 10.1093/jac/46.1.19. [DOI] [PubMed] [Google Scholar]
- 152.Legssyer R., Huaux F., Lebacq J., Delos M., Marbaix E., Lebecque P. Azithromycin reduces spontaneous and induced inflammation in ΔF508 cystic fibrosis mice. Respir. Res. 2006;7 doi: 10.1186/1465-9921-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Parnham M.J., Čulić O., Eraković V., Munić V., Popović-Grle S., Barišić K. Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short-term azithromycin treatment. Eur. J. Pharmacol. 2005;517:132–143. doi: 10.1016/j.ejphar.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 154.Silvestri M., Oddera S., Eftimiadi C., Rossi G.A. Azithromycin induces in vitro a time-dependent increase in the intracellular killing of Staphylococcus aureus by human polymorphonuclear leucocytes without damaging phagocytes. J. Antimicrob. Chemother. 1995;36:941–950. doi: 10.1093/jac/36.6.941. [DOI] [PubMed] [Google Scholar]
- 155.Castellani S., D’Oria S., Diana A., Polizzi A.M., Di Gioia S., Mariggiò M.A. G-CSF and GM-CSF modify neutrophil functions at concentrations found in cystic fibrosis. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamasawa H., Oshikawa K., Ohno S., Sugiyama Y. Macrolides inhibit epithelial cell–mediated neutrophil survival by modulating granulocyte macrophage colony–stimulating factor release. Am. J. Respir. Cell Mol. Biol. 2004;30:569–575. doi: 10.1165/rcmb.2003-0105OC. [DOI] [PubMed] [Google Scholar]
- 157.Choi J.C., Jung J.W., Kwak H.W., Song J.H., Jeon E.J., Shin J.W. Granulocyte macrophage-colony stimulating factor (GM-CSF) augments acute lung injury via its neutrophil priming effects. J. Korean Med. Sci. 2008;23:288–295. doi: 10.3346/jkms.2008.23.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Puljic R., Benediktus E., Plater-Zyberk C., Baeuerle P.A., Szelenyi S., Brune K. Lipopolysaccharide-induced lung inflammation is inhibited by neutralization of GM-CSF. Eur. J. Pharmacol. 2007;557:230–235. doi: 10.1016/j.ejphar.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 159.Lendermon E.A., Coon T.A., Bednash J.S., Weathington N.M., McDyer J.F., Mallampalli R.K. Azithromycin decreases NALP3 mRNA stability in monocytes to limit inflammasome-dependent inflammation. Respir. Res. 2017;18 doi: 10.1186/s12931-017-0608-8. (131-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Du F., Jiang P., He S., Song D., Xu F. Antiplatelet therapy for critically ill patients: a pairwise and bayesian network meta-analysis. Shock (Augusta, Ga). 2018;49:616–624. doi: 10.1097/SHK.0000000000001057. [DOI] [PubMed] [Google Scholar]
- 161.Wang L., Li H., Gu X., Wang Z., Liu S., Chen L. Effect of antiplatelet therapy on acute respiratory distress syndrome and mortality in critically ill patients: a meta-analysis. PLoS One. 2016;11:e0154754. doi: 10.1371/journal.pone.0154754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Y., Zhong M., Wang Z., Song J., Wu W., Zhu D. The preventive effect of antiplatelet therapy in acute respiratory distress syndrome: a meta-analysis. Critical Care (London, England) 2018;22:60. doi: 10.1186/s13054-018-1988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Falcone M., Russo A., Cangemi R., Farcomeni A., Calvieri C., Barillà F. Lower mortality rate in elderly patients with community-onset pneumonia on treatment with aspirin. J. Am. Heart Assoc. 2015;4:e001595–e. doi: 10.1161/JAHA.114.001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Winning J., Neumann J., Kohl M., Claus R.A., Reinhart K., Bauer M. Antiplatelet drugs and outcome in mixed admissions to an intensive care unit*. Crit. Care Med. 2010;38:32–37. doi: 10.1097/CCM.0b013e3181b4275c. [DOI] [PubMed] [Google Scholar]
- 165.Winning J., Reichel J., Eisenhut Y., Hamacher J., Kohl M., Deigner H.P. Anti-platelet drugs and outcome in severe infection: clinical impact and underlying mechanisms. Platelets. 2009;20:50–57. doi: 10.1080/09537100802503368. [DOI] [PubMed] [Google Scholar]
- 166.Cangemi R., Casciaro M., Rossi E., Calvieri C., Bucci T., Calabrese C.M. Platelet activation is associated with myocardial infarction in patients with pneumonia. J. Am. Coll. Cardiol. 2014;64:1917–1925. doi: 10.1016/j.jacc.2014.07.985. [DOI] [PubMed] [Google Scholar]
- 167.Kreutz R.P., Tantry U.S., Bliden K.P., Gurbel P.A. Inflammatory changes during the ‘common cold’ are associated with platelet activation and increased reactivity of platelets to agonists. Blood Coagul. Fibrinolysis. 2007;18:713–718. doi: 10.1097/MBC.0b013e328201c77e. [DOI] [PubMed] [Google Scholar]
- 168.Méndez R., Moscardó A., Latorre A., Piró A., Amara-Elori I., Gimeno A. Platelet activation is boosted early in community-acquired pneumonia and sustained until 30 days later. Eur. Respir. J. 2018;52:PA2633. [Google Scholar]
- 169.Violi F., Cangemi R., Falcone M., Taliani G., Pieralli F., Vannucchi V. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2017;64:1486–1493. doi: 10.1093/cid/cix164. [DOI] [PubMed] [Google Scholar]
- 170.Antithrombotic Trialists C., Baigent C., Blackwell L., Collins R., Emberson J., Godwin J. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet (London, England) 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Patrono C. The multifaceted clinical readouts of platelet inhibition by low-dose aspirin. J. Am. Coll. Cardiol. 2015;66:74–85. doi: 10.1016/j.jacc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 172.Undas A., Brummel-Ziedins K.E., Mann K.G. Antithrombotic properties of aspirin and resistance to aspirin: beyond strictly antiplatelet actions. Blood. 2007;109:2285–2292. doi: 10.1182/blood-2006-01-010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Undas A., Brummel-Ziedins K., Mann K.G. Why does aspirin decrease the risk of venous thromboembolism? On old and novel antithrombotic effects of acetyl salicylic acid. Journal of Thrombosis and Haemostasis: JTH. 2014;12:1776–1787. doi: 10.1111/jth.12728. [DOI] [PubMed] [Google Scholar]
- 174.Bailey M.A., Aggarwal R., Bridge K.I., Griffin K.J., Iqbal F., Phoenix F. Aspirin therapy is associated with less compact fibrin networks and enhanced fibrinolysis in patients with abdominal aortic aneurysm. J. Thromb. Haemost. 2015;13:795–801. doi: 10.1111/jth.12872. [DOI] [PubMed] [Google Scholar]
- 175.Tehrani S., Antovic A., Mobarrez F., Mageed K., Lins P.-E., Adamson U. High-dose aspirin is required to influence plasma fibrin network structure in patients with type 1 diabetes. Diabetes Care. 2012;35:404–408. doi: 10.2337/dc11-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chiang N., Bermudez E.A., Ridker P.M., Hurwitz S., Serhan C.N. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Goicoechea M., Sanchez-Niño M.D., Ortiz A., García de Vinuesa S., Quiroga B., Bernis C. Low dose aspirin increases 15-epi-lipoxin A4 levels in diabetic chronic kidney disease patients. Prostaglandins Leukot. Essent. Fat. Acids. 2017;125:8–13. doi: 10.1016/j.plefa.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 178.Morris T., Stables M., Hobbs A., de Souza P., Colville-Nash P., Warner T. Effects of low-dose aspirin on acute inflammatory responses in humans. J. Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 179.Yu S., Xie J., Xiang Y., Dai S., Yu D., Sun H. Downregulation of TNF-α/TNF-R1 signals by AT-lipoxin A4 may be a significant mechanism of attenuation in SAP-associated lung injury. Mediat. Inflamm. 2019;2019 doi: 10.1155/2019/9019404. (9019404-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chiang N., Arita M., Serhan C. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot. Essent. Fat. Acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 181.Colby J., Jaoude J., Liu F., Shureiqi I. Oxygenated lipid signaling in tumor-associated macrophages—focus on colon cancer. Cancer Metastasis Rev. 2018;37 doi: 10.1007/s10555-018-9743-z. [DOI] [PubMed] [Google Scholar]
- 182.Romano M., Cianci E., Simiele F., Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015;760:49–63. doi: 10.1016/j.ejphar.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 183.Toner P., McAuley D.F., Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit. Care. 2015;19:374. doi: 10.1186/s13054-015-1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mishra P., Paital B., Jena S., Swain S.S., Kumar S., Yadav M.K. Possible activation of NRF2 by vitamin E/curcumin against altered thyroid hormone induced oxidative stress via NFkB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 2019;9:7408. doi: 10.1038/s41598-019-43320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zheng S., D’Souza V.K., Bartis D., Dancer R.C.A., Parekh D., Naidu B. Lipoxin A(4) promotes lung epithelial repair whilst inhibiting fibroblast proliferation. ERJ Open Res. 2016;2:00079–02015. doi: 10.1183/23120541.00079-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chandrasekharan J.A., Sharma-Walia N. Lipoxins: nature’s way to resolve inflammation. J. Inflamm. Res. 2015;8:181–192. doi: 10.2147/JIR.S90380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Potey P.M., Rossi A.G., Lucas C.D., Dorward D.A. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J. Pathol. 2019;247:672–685. doi: 10.1002/path.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Machado F.S., Johndrow J.E., Esper L., Dias A., Bafica A., Serhan C.N. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat. Med. 2006;12:330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 189.Liu X., Wang X., Duan X., Poorun D., Xu J., Zhang S. Lipoxin A4 and its analog suppress inflammation by modulating HMGB1 translocation and expression in psoriasis. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-07485-1. (7100-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sham H.P., Walker K.H., Abdulnour R.-E.E., Krishnamoorthy N., Douda D.N., Norris P.C. 15-epi-lipoxin A(4), resolvin D2, and resolvin D3 induce NF-κB regulators in bacterial pneumonia. Journal of Immunology (Baltimore, Md: 1950) 2018;200:2757–2766. doi: 10.4049/jimmunol.1602090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang L.-C., Jiang R.-L., Zhang W., Wei L.-L., Yang R.-H. Effects of aspirin on the expression of nuclear factor-κB in a rat model of acute pulmonary embolism. World J Emerg Med. 2014;5:229–233. doi: 10.5847/wjem.j.issn.1920-8642.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bleda S., De Haro J., Varela C., Ferruelo A., Acin F. Aspirin therapy inhibits NLRP1 (nucleotide-binding domain-like receptor protein 1) inflammasome gene expression in patients with peripheral artery disease. J. Vasc. Surg. 2015;61:1103–1104. doi: 10.1016/j.jvs.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 193.De Haro J., Bleda S., Laime I.V., Carballido B., Uyaguari J., Acin F. Aspirin-dependent platelet inflammatory inhibition in healthy subjects decreases NLRP-1 inflammasome. Ann. Vasc. Surg. 2019;59:244–247. doi: 10.1016/j.avsg.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 194.El-Kharashi O.A., El-Din Aly El-Waseef D.A., Nabih E.S., Mohamed D.I. Targeting NLRP3 inflammasome via acetylsalicylic acid: role in suppressing hepatic dysfunction and insulin resistance induced by atorvastatin in naïve versus alcoholic liver in rats. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2018;107:665–674. doi: 10.1016/j.biopha.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 195.Liao D., Zhong L., Duan T., Zhang R.H., Wang X., Wang G. Aspirin suppresses the growth and metastasis of osteosarcoma through the NF-κB pathway. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2015;21:5349–5359. doi: 10.1158/1078-0432.CCR-15-0198. [DOI] [PubMed] [Google Scholar]
- 196.Ornelas A., Zacharias-Millward N., Menter D.G., Davis J.S., Lichtenberger L., Hawke D. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017;36:289–303. doi: 10.1007/s10555-017-9675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ralph A.P., Noonan S., Boardman C., Halkon C., Currie B.J. Prescribing for people with acute rheumatic fever. Aust. Prescr. 2017;40:70–75. doi: 10.18773/austprescr.2017.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Grandjean E.M., Berthet P., Ruffmann R., Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin. Ther. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 199.Reichenberger F., Tamm M. N-acetylcystein in der Therapie der chronischen bronchitis. Pneumologie. 2002;56:793–797. doi: 10.1055/s-2002-36122. [DOI] [PubMed] [Google Scholar]
- 200.Stey C., Steurer J., Bachmann S., Medici T.C., Tramèr M.R. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. Eur. Respir. J. 2000;16:253. doi: 10.1034/j.1399-3003.2000.16b12.x. [DOI] [PubMed] [Google Scholar]
- 201.Dekhuijzen P.N.R., van Beurden W.J.C. The role for N-acetylcysteine in the management of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:99–106. doi: 10.2147/copd.2006.1.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pirabbasi E., Shahar S., Manaf Z.A., Rajab N.F., Manap R.A. Efficacy of ascorbic acid (vitamin C) and/N-acetylcysteine (NAC) supplementation on nutritional and antioxidant status of male chronic obstructive pulmonary disease (COPD) patients. J. Nutr. Sci. Vitaminol. 2016;62:54–61. doi: 10.3177/jnsv.62.54. [DOI] [PubMed] [Google Scholar]
- 203.Sanguinetti C.M. N-acetylcysteine in COPD: why, how, and when? Multidiscip Respir Med. 2016;11 doi: 10.1186/s40248-016-0039-2. (8-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shen Y., Cai W., Lei S., Zhang Z. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD: J. Chron. Obstruct. Pulmon. Dis. 2013;11(3):351–358. doi: 10.3109/15412555.2013.858315. (131230073230003) [DOI] [PubMed] [Google Scholar]
- 205.Olofsson A.-C., Hermansson M., Elwing H. N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl. Environ. Microbiol. 2003;69:4814–4822. doi: 10.1128/AEM.69.8.4814-4822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Conrad C., Lymp J., Thompson V., Dunn C., Davies Z., Chatfield B. Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J. Cyst. Fibros. 2015;14:219–227. doi: 10.1016/j.jcf.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 207.Feng F., Zhang J., Wang Z., Wu Q., Zhou X. Efficacy and safety of N-acetylcysteine therapy for idiopathic pulmonary fibrosis: an updated systematic review and meta-analysis. Exp Ther Med. 2019;18:802–816. doi: 10.3892/etm.2019.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tirouvanziam R., Conrad C.K., Bottiglieri T., Herzenberg L.A., Moss R.B., Herzenberg L.A. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lu X., Ma Y., He J., Li Y., Zhu H., Yu X. N-acetylcysteine for adults with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Hong Kong Journal of Emergency Medicine. 2019;26:288–298. [Google Scholar]
- 210.Zhang Y., Ding S., Li C., Wang Y., Chen Z., Wang Z. Effects of N-acetylcysteine treatment in acute respiratory distress syndrome: a meta-analysis. Exp Ther Med. 2017;14:2863–2868. doi: 10.3892/etm.2017.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rubenfeld G.D., Herridge M.S. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 212.Backer W.A.D., Amsel B., Jorens P.G., Bossaert L., Hiemstra P.S., van Noort P. N-acetylcysteine pretreatment of cardiac surgery patients influences plasma neutrophil elastase and neutrophil influx in bronchoalveolar lavage fluid. Intensive Care Med. 1996;22:900–908. doi: 10.1007/BF02044114. [DOI] [PubMed] [Google Scholar]
- 213.Hasan M.A., Ahn W.-G., Song D.-K. N-acetyl-L-cysteine and cysteine increase intracellular calcium concentration in human neutrophils. Korean J Physiol Pharmacol. 2016;20:449–457. doi: 10.4196/kjpp.2016.20.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Moon C., Lee Y.-J., Park H.-J., Chong Y.H., Kang J.L. N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. Am. J. Respir. Crit. Care Med. 2010;181:374–387. doi: 10.1164/rccm.200907-1061OC. [DOI] [PubMed] [Google Scholar]
- 215.Paranjape S. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature. Faculty Opinions Ltd.; 2006. Faculty opinions recommendation of high-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. [Google Scholar]
- 216.Urban T., Akerlund B., Jarstrand C., Lindeke B. Neutrophil function and glutathione-peroxidase (GSH-px) activity in healthy individuals after treatment with N-acetyl-L-cysteine. Biomed. Pharmacother. 1997;51:388–390. doi: 10.1016/s0753-3322(97)89431-0. [DOI] [PubMed] [Google Scholar]
- 217.Failli P., Palmieri L., D’Alfonso C., Giovannelli L., Generini S., Rosso A.D. Effect of N-acetyl-L-cysteine on peroxynitrite and superoxide anion production of lung alveolar macrophages in systemic sclerosis. Nitric Oxide: Biology and Chemistry. 2002;7:277–282. doi: 10.1016/s1089-8603(02)00120-9. [DOI] [PubMed] [Google Scholar]
- 218.Craver B.M., Ramanathan G., Hoang S., Chang X., Mendez Luque L.F., Brooks S. N-acetylcysteine inhibits thrombosis in a murine model of myeloproliferative neoplasm. Blood Advances. 2020;4:312–321. doi: 10.1182/bloodadvances.2019000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Treweeke A.T., Winterburn T.J., Mackenzie I., Barrett F., Barr C., Rushworth G.F. N-Acetylcysteine inhibits platelet-monocyte conjugation in patients with type 2 diabetes with depleted intraplatelet glutathione: a randomised controlled trial. Diabetologia. 2012;55:2920–2928. doi: 10.1007/s00125-012-2685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Al-Khafaji A.B., Tohme S., Yazdani H.O., Miller D., Huang H., Tsung A. Superoxide induces neutrophil extracellular trap formation in a TLR-4 and NOX-dependent mechanism. Molecular Medicine (Cambridge, Mass) 2016;22:621–631. doi: 10.2119/molmed.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kirchner T., Hermann E., Möller S., Klinger M., Solbach W., Laskay T. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/710239. (710239-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hagiwara S.-I., Ishii Y., Kitamura S. Aerosolized administration ofN-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am. J. Respir. Crit. Care Med. 2000;162:225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- 223.Lai K.Y., Ng W.Y., Osburga Chan P.K., Wong K.F., Cheng F. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann. Intern. Med. 2010;152:687–688. doi: 10.7326/0003-4819-152-10-201005180-00017. [DOI] [PubMed] [Google Scholar]
- 224.Zhang Q., Ju Y., Ma Y., Wang T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: a randomized controlled trial. Medicine (Baltimore) 2018;97:e13087–e. doi: 10.1097/MD.0000000000013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li X., Wei X., Chen C., Zhang Z., Liu D., Hei Z. N-Acetylcysteine inhalation improves pulmonary function in patients received liver transplantation. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nascimento M.M., Suliman M.E., Silva M., Chinaglia T., Marchioro J., Hayashi S.Y. Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study. Perit. Dial. Int. 2010;30:336–342. doi: 10.3747/pdi.2009.00073. [DOI] [PubMed] [Google Scholar]
- 227.Nizomov A. Effect of N-acetylcysteine indicators of proinflammatory cytokines in patients with acute coronary syndrome with st segment elevation. Atherosclerosis. 2015;241:e87. [Google Scholar]
- 228.Saddadi F., Alatab S., Pasha F., Ganji M.R., Soleimanian T. The effect of treatment with N-acetylcysteine on the serum levels of C-reactive protein and interleukin-6 in patients on hemodialysis. Saudi Journal of Kidney Diseases and Transplantation: An Official Publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2014;25:66–72. doi: 10.4103/1319-2442.124489. [DOI] [PubMed] [Google Scholar]
- 229.Farid M., Reid M.B., Li Y.-P., Gerken E., Durham W.J. Effects of dietary curcumin or N-acetylcysteine on NF-κB activity and contractile performance in ambulatory and unloaded murine soleus. Nutrition & Metabolism. 2005;2:20. doi: 10.1186/1743-7075-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Paterson R.L., Galley H.F., Webster N.R. The effect of N-acetylcysteine on nuclear factor-κB activation, interleukin-6, interleukin-8, and intercellular adhesion molecule-1 expression in patients with sepsis*. Crit. Care Med. 2003;31:2574–2578. doi: 10.1097/01.CCM.0000089945.69588.18. [DOI] [PubMed] [Google Scholar]
- 231.Wu X.-Y., Luo A.-Y., Zhou Y.-R., Ren J.-H. N-acetylcysteine reduces oxidative stress, nuclear factor-κB activity and cardiomyocyte apoptosis in heart failure. Mol. Med. Rep. 2014;10:615–624. doi: 10.3892/mmr.2014.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sharafkhah M., Abdolrazaghnejad A., Zarinfar N., Mohammadbeigi A., Massoudifar A., Abaszadeh S. Safety and efficacy of N-acetyl-cysteine for prophylaxis of ventilator-associated pneumonia: a randomized, double blind, placebo-controlled clinical trial. Med Gas Res. 2018:19–23. doi: 10.4103/2045-9912.229599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ayzac L., Girard R., Baboi L., Beuret P., Rabilloud M., Richard J.C. Ventilator-associated pneumonia in ARDS patients: the impact of prone positioning. A secondary analysis of the PROSEVA trial. Intensive Care Med. 2016;42:871–878. doi: 10.1007/s00134-015-4167-5. [DOI] [PubMed] [Google Scholar]
- 234.Martina V., Masha A., Gigliardi V.R., Brocato L., Manzato E., Berchio A. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care. 2008;31:940–944. doi: 10.2337/dc07-2251. [DOI] [PubMed] [Google Scholar]
- 235.Masha A., Brocato L., Dinatale S., Mascia C., Biasi F., Martina V. N-acetylcysteine is able to reduce the oxidation status and the endothelial activation after a high-glucose content meal in patients with type 2 diabetes mellitus. J. Endocrinol. Investig. 2009;32:352–356. doi: 10.1007/BF03345726. [DOI] [PubMed] [Google Scholar]
- 236.Pieper G.M., Siebeneich W. Oral administration of the antioxidant, N-acetylcysteine, abrogates diabetes-induced endothelial dysfunction. J. Cardiovasc. Pharmacol. 1998;32:101–105. doi: 10.1097/00005344-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 237.Kudaravalli J. Improvement in endothelial dysfunction in patients with systemic lupus erythematosus with N-acetylcysteine and atorvastatin. Indian J Pharmacol. 2011;43:311–315. doi: 10.4103/0253-7613.81511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Molnar Z., Szakmany T., Koszegi T. Prophylactic N-acetylcysteine decreases serum CRP but not PCT levels and microalbuminuria following major abdominal surgery. A prospective, randomised, double-blinded, placebo-controlled clinical trial. Intensive Care Med. 2003;29:749–755. doi: 10.1007/s00134-003-1723-1. [DOI] [PubMed] [Google Scholar]
- 239.Zuin R., Palamidese A., Negrin R., Catozzo L., Scarda A., Balbinot M. High-dose N-acetylcysteine in??patients with exacerbations of??chronic obstructive pulmonary disease. Clinical Drug Investigation. 2005;25:401–408. doi: 10.2165/00044011-200525060-00005. [DOI] [PubMed] [Google Scholar]
- 240.Anfossi G., Russo I., Massucco P., Mattiello L., Trovati M. Platelet resistance to the antiaggregating effect of N-acetyl-l-cysteine in obese, insulin-resistant subjects. Thromb. Res. 2003;110:39–46. doi: 10.1016/s0049-3848(03)00284-6. [DOI] [PubMed] [Google Scholar]
- 241.Gibson K.R., Neilson I.L., Barrett F., Winterburn T.J., Sharma S., MacRury S.M. Evaluation of the antioxidant properties of N-acetylcysteine in human platelets: prerequisite for bioconversion to glutathione for antioxidant and antiplatelet activity. J. Cardiovasc. Pharmacol. 2009;54:319–326. doi: 10.1097/FJC.0b013e3181b6e77b. [DOI] [PubMed] [Google Scholar]
- 242.Gibson K.R., Winterburn T.J., Barrett F., Sharma S., MacRury S.M., Megson I.L. Therapeutic potential of N-acetylcysteine as an antiplatelet agent in patients with type-2 diabetes. Cardiovasc. Diabetol. 2011;10:43. doi: 10.1186/1475-2840-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang B., Yee Aw T., Stokes K.Y. N-acetylcysteine attenuates systemic platelet activation and cerebral vessel thrombosis in diabetes. Redox Biol. 2018;14:218–228. doi: 10.1016/j.redox.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chen J., Reheman A., Gushiken F.C., Nolasco L., Fu X., Moake J.L. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J. Clin. Invest. 2011;121:593–603. doi: 10.1172/JCI41062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Martinez de Lizarrondo S., Gakuba C., Herbig B.A., Repessé Y., Ali C., Denis C.V. Potent thrombolytic effect of N-acetylcysteine on arterial thrombi. Circulation. 2017;136:646–660. doi: 10.1161/CIRCULATIONAHA.117.027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.DiNicolantonio J.J., O’Keefe J.H., McCarty M.F. Targeting aspirin resistance with nutraceuticals: a possible strategy for reducing cardiovascular morbidity and mortality. Open Heart. 2017;4:e000642–e. doi: 10.1136/openhrt-2017-000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kreil E., Saldeen T., Nguyenduy T., Greene E., Fitzgibbon C., Robinson D. N-acetyl-l-cysteine attenuates thrombin-induced increases of pulmonary and airway pressures and lung lymph flow in anesthetized sheep. J. Crit. Care. 1990;5:87–94. [Google Scholar]
- 248.Niemi T.T., Munsterhjelm E., Pöyhiä R., Hynninen M.S., Salmenperä M.T. The effect of N-acetylcysteine on blood coagulation and platelet function in patients undergoing open repair of abdominal aortic aneurysm. Blood Coagul. Fibrinolysis. 2006;17:29–34. doi: 10.1097/01.mbc.0000195922.26950.89. [DOI] [PubMed] [Google Scholar]
- 249.Jepsen S., Hansen A.B. The influence of N-acetylcysteine on the measurement of prothrombin time and activated partial thromboplastin time in healthy subjects. Scand. J. Clin. Lab. Invest. 1994;54:543–547. doi: 10.3109/00365519409088566. [DOI] [PubMed] [Google Scholar]
- 250.Thorsen S., Teisner A., Jensen S.A., Philips M., Dalhoff K., Bendtsen F. Effect of N-acetylcysteine on the accuracy of the prothrombin time assay of plasma coagulation factor II+VII+X activity in subjects infused with the drug. Influence of time and temperature. Scand. J. Clin. Lab. Invest. 2009;69:643–650. doi: 10.3109/00365510902943262. [DOI] [PubMed] [Google Scholar]
- 251.Nikbakht M., Ahmadi F., Vaseghi G., Talasaz A.H., Eshraghi A., Naderi J. The role of N-acetylcysteine in platelet aggregation and reperfusion injury in recent years. Curr. Clin. Pharmacol. 2017;12:83–91. doi: 10.2174/1574884712666170704145842. [DOI] [PubMed] [Google Scholar]
- 252.Gutmann C., Siow R., Gwozdz A.M., Saha P., Smith A. Reactive oxygen species in venous thrombosis. Int. J. Mol. Sci. 2020;21:1918. doi: 10.3390/ijms21061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kempker J.A., Han J.E., Tangpricha V., Ziegler T.R., Martin G.S. Vitamin D and sepsis: an emerging relationship. Dermatoendocrinol. 2012;4:101–108. doi: 10.4161/derm.19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nair P., Venkatesh B., Hoechter D.J., Buscher H., Kerr S., Center J.R. Vitamin D status and supplementation in adult patients receiving extracorporeal membrane oxygenation. Anaesth. Intensive Care. 2018;46:589–595. doi: 10.1177/0310057X1804600609. [DOI] [PubMed] [Google Scholar]
- 255.Takeuti F.A.C., Guimaraes F.S.F., Guimaraes P.S.F. Applications of vitamin D in sepsis prevention. Discov. Med. 2018;25:291–297. [PubMed] [Google Scholar]
- 256.Williams S., Heuberger R. Outcomes of vitamin D supplementation in adults who are deficient and critically ill: a review of the literature. Am. J. Ther. 2016;23:e1890–e1902. doi: 10.1097/MJT.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 257.Bjelakovic G., Gluud L.L., Nikolova D., Whitfield K., Wetterslev J., Simonetti R.G. Vitamin D supplementation for prevention of mortality in adults. The Cochrane database of systematic reviews. 2014:Cd007470. doi: 10.1002/14651858.CD007470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Shojaei M., Sabzeghabaei A., Valaei Barhagh H., Soltani S. The correlation between serum level of vitamin D and outcome of sepsis patients; a cross-sectional study. Arch Acad Emerg Med. 2019;7:e1–e. [PMC free article] [PubMed] [Google Scholar]
- 259.Lucas R.M., Gorman S., Geldenhuys S., Hart P.H. Vitamin D and immunity. F1000prime Reports. 2014;6 doi: 10.12703/P6-118. (118-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Manion M., Hullsiek K.H., Wilson E.M.P., Rhame F., Kojic E., Gibson D. Vitamin D deficiency is associated with IL-6 levels and monocyte activation in HIV-infected persons. PLoS One. 2017;12:e0175517–e. doi: 10.1371/journal.pone.0175517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Shepherd L., Souberbielle J.C., Bastard J.P., Fellahi S., Capeau J., Reekie J. Prognostic value of vitamin D level for all-cause mortality, and association with inflammatory markers, in HIV-infected persons. J. Infect. Dis. 2014;210:234–243. doi: 10.1093/infdis/jiu074. [DOI] [PubMed] [Google Scholar]
- 262.Bergholm R., Leirisalo-Repo M., Vehkavaara S., Mäkimattila S., Taskinen M.-R., Yki-Järvinen H. Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler. Thromb. Vasc. Biol. 2002;22:1637–1641. doi: 10.1161/01.atv.0000033516.73864.4e. [DOI] [PubMed] [Google Scholar]
- 263.Csiszar A., Wang M., Lakatta E.G., Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J. Appl. Physiol. 2008;105(1985):1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Goncalves-Mendes N., Talvas J., Dualé C., Guttmann A., Corbin V., Marceau G. Impact of Vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ishii T., Itoh K., Takahashi S., Sato H., Yanagawa T., Katoh Y. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 266.Ni W., Watts S.W., Ng M., Chen S., Glenn D.J., Gardner D.G. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension (Dallas, Tex: 1979) 2014;64:1290–1298. doi: 10.1161/HYPERTENSIONAHA.114.03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Giulietti A., van Etten E., Overbergh L., Stoffels K., Bouillon R., Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. Diabetes Res. Clin. Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 268.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 269.Zhang Y., Leung D.Y.M., Richers B.N., Liu Y., Remigio L.K., Riches D.W. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. Journal of Immunology (Baltimore, Md: 1950) 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Han J.E., Jones J.L., Tangpricha V., Brown M.A., Brown L.A.S., Hao L. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. Journal of Clinical & Translational Endocrinology. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. Bmj. 2017;i6583:356. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Grant W.B., Al Anouti F., Moukayed M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D3 supplementation can have important patient and public health benefits. Eur. J. Clin. Nutr. 2020;74:366–376. doi: 10.1038/s41430-020-0564-0. [DOI] [PubMed] [Google Scholar]
- 273.Alvarez N., Aguilar-Jimenez W., Rugeles M.T. The potential protective role of vitamin D supplementation on HIV-1 infection. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fatima H., Riaz M., Mahmood Z., Yousaf F., Shahid M. Dengue viral infection deteriorates vitamin D3, K, thrombopoietin, and angiotensinogen levels in humans. European Journal of Inflammation. 2018;16 [Google Scholar]
- 275.Hu Y.-C., Wang W.-W., Jiang W.-Y., Li C.-Q., Guo J.-C., Xun Y.-H. Low vitamin D levels are associated with high viral loads in patients with chronic hepatitis B: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:84. doi: 10.1186/s12876-019-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Schögler A., Muster R.J., Kieninger E., Casaulta C., Tapparel C., Jung A. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur. Respir. J. 2016;47:520–530. doi: 10.1183/13993003.00665-2015. [DOI] [PubMed] [Google Scholar]
- 277.Telcian A.G., Zdrenghea M.T., Edwards M.R., Laza-Stanca V., Mallia P., Johnston S.L. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antivir. Res. 2017;137:93–101. doi: 10.1016/j.antiviral.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 278.Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011;50:194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Harcourt J.L., McDonald M., Svoboda P., Pohl J., Tatti K., Haynes L.M. Human cathelicidin, LL-37, inhibits respiratory syncytial virus infection in polarized airway epithelial cells. BMC Research Notes. 2016;9:11. doi: 10.1186/s13104-015-1836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hsieh I.N., Hartshorn K.L. The role of antimicrobial peptides in influenza virus infection and their potential as antiviral and immunomodulatory therapy. Pharmaceuticals (Basel, Switzerland) 2016;9:53. doi: 10.3390/ph9030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yu Y., Cooper C.L., Wang G., Morwitzer M.J., Kota K., Tran J.P. Engineered human cathelicidin antimicrobial peptides inhibit Ebola virus infection. iScience. 2020;23 doi: 10.1016/j.isci.2020.100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Martín Giménez V.M., Inserra F., Tajer C.D., Mariani J., Ferder L., Reiter R.J. Lungs as target of COVID-19 infection: protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tecle T., Tripathi S., Hartshorn K.L. Review: defensins and cathelicidins in lung immunity. Innate Immun. 2010;16:151–159. doi: 10.1177/1753425910365734. [DOI] [PubMed] [Google Scholar]
- 284.Ang A., Pullar J.M., Currie M.J., Vissers M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 2018;46:1147–1159. doi: 10.1042/BST20180169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Carr A.C., Rosengrave P.C., Bayer S., Chambers S., Mehrtens J., Shaw G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care. 2017;21:300. doi: 10.1186/s13054-017-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kuhn S.-O., Meissner K., Mayes L.M., Bartels K. Vitamin C in sepsis. Curr. Opin. Anaesthesiol. 2018;31:55–60. doi: 10.1097/ACO.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Marik P.E. Vitamin C for the treatment of sepsis: the scientific rationale. Pharmacol. Ther. 2018;189:63–70. doi: 10.1016/j.pharmthera.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 288.Fowler A.A., 3rd, Syed A.A., Knowlson S., Sculthorpe R., Farthing D., DeWilde C. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014;12 doi: 10.1186/1479-5876-12-32. (32-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Oudemans-van Straaten H.M., Spoelstra-de Man A.M., de Waard M.C. Vitamin C revisited. Critical Care (London, England) 2014;18 doi: 10.1186/s13054-014-0460-x. (460-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mangin M., Sinha R., Fincher K. Inflammation and vitamin D: the infection connection. Inflammation Research: Official Journal of the European Histamine Research Society. 2014;63:803–819. doi: 10.1007/s00011-014-0755-z. [et al] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sánchez-Moreno C., Dashe J.F., Scott T., Thaler D., Folstein M.F., Martin A. Decreased levels of plasma vitamin C and increased concentrations of inflammatory and oxidative stress markers after stroke. Stroke. 2004;35:163–168. doi: 10.1161/01.STR.0000105391.62306.2E. [DOI] [PubMed] [Google Scholar]
- 293.Traber M.G., Buettner G.R., Bruno R.S. The relationship between vitamin C status, the gut-liver axis, and metabolic syndrome. Redox Biol. 2019;21 doi: 10.1016/j.redox.2018.101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bozonet S.M., Carr A.C. The role of physiological vitamin C concentrations on key functions of neutrophils isolated from healthy individuals. Nutrients. 2019;11(6):1363. doi: 10.3390/nu11061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mohammed B.M., Fisher B.J., Kraskauskas D., Farkas D., Brophy D.F., Fowler A.A., 3rd Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. 2013;5:3131–3151. doi: 10.3390/nu5083131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Boretti A., Banik B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020;12 doi: 10.1016/j.phanu.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Fenech M., Amaya I., Valpuesta V., Botella M.A. Vitamin C content in fruits: biosynthesis and regulation. Front. Plant Sci. 2019;9 doi: 10.3389/fpls.2018.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Levine M., Conry-Cantilena C., Wang Y., Welch R.W., Washko P.W., Dhariwal K.R. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Padayatty S.J., Sun H., Wang Y., Riordan H.D., Hewitt S.M., Katz A. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 2004;140:533. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 300.Fowler A.A., 3rd, Truwit J.D., Hite R.D., Morris P.E., DeWilde C., Priday A. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hemilä H., Chalker E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J. Intensive Care. 2020;8:15. doi: 10.1186/s40560-020-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Marik P.E., Khangoora V., Rivera R., Hooper M.H., Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock. Chest. 2017;151:1229–1238. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 303.Hemilä H., Herman Z.S. Vitamin C and the common cold: a retrospective analysis of Chalmers’ review. J. Am. Coll. Nutr. 1995;14:116–123. doi: 10.1080/07315724.1995.10718483. [DOI] [PubMed] [Google Scholar]
- 304.Cai Y., Li Y.-F., Tang L.-P., Tsoi B., Chen M., Chen H. A new mechanism of vitamin C effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/675149. (675149-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gonzalez M., Berdiel M., Miranda-Massari J., Duconge J., Adrover-López P. High dose intravenous vitamin C treatment for Zika fever. J Orthomolec Med. 2016;31 [PMC free article] [PubMed] [Google Scholar]
- 306.Zarubaev V.V., Slita A.V., Lavrentyeva I.N., Smirnov V.S. Protective activity of ascorbic acid at influenza infection. Russian Journal of Infection and Immunity. 2017;7:319–326. [Google Scholar]
- 307.Morris G., Berk M., Maes M., Carvalho A.F., Puri B.K. Socioeconomic deprivation, adverse childhood experiences and medical disorders in adulthood: mechanisms and associations. Mol. Neurobiol. 2019;56:5866–5890. doi: 10.1007/s12035-019-1498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Schwingshackl A., Meduri G.U. Rationale for prolonged glucocorticoid use in pediatric ARDS: what the adults can teach us. Front. Pediatr. 2016;4 doi: 10.3389/fped.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Umberto Meduri G., Bell W., Sinclair S., Annane D. Pathophysiology of acute respiratory distress syndrome. Glucocorticoid receptor-mediated regulation of inflammation and response to prolonged glucocorticoid treatment. Presse Medicale (Paris, France: 1983) 2011;40:e543–e560. doi: 10.1016/j.lpm.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Revollo J.R., Cidlowski J.A. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann. N. Y. Acad. Sci. 2009;1179:167–178. doi: 10.1111/j.1749-6632.2009.04986.x. [DOI] [PubMed] [Google Scholar]
- 311.Baschant U., Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J. Steroid Biochem. Mol. Biol. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 312.Liberman A.C., Budziñski M.L., Sokn C., Gobbini R.P., Steininger A., Arzt E. Regulatory and mechanistic actions of glucocorticoids on T and inflammatory cells. Front. Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Xavier A.M., Anunciato A.K.O., Rosenstock T.R., Glezer I. Gene expression control by glucocorticoid receptors during innate immune responses. Front. Endocrinol. 2016;7 doi: 10.3389/fendo.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Laryea G., Muglia L., Arnett M., Muglia L.J. Dissection of glucocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis by gene targeting in mice. Front. Neuroendocrinol. 2015;0:150–164. doi: 10.1016/j.yfrne.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Jang B.C., Lim K.J., Suh M.H., Park J.G., Suh S.I. Dexamethasone suppresses interleukin-1beta-induced human beta-defensin 2 mRNA expression: involvement of p38 MAPK, JNK, MKP-1, and NF-kappaB transcriptional factor in A549 cells. FEMS Immunol. Med. Microbiol. 2007;51:171–184. doi: 10.1111/j.1574-695X.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 316.King E.M., Holden N.S., Gong W., Rider C.F., Newton R. Inhibition of NF-κB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occurring via p38 MAPK. J. Biol. Chem. 2009;284:26803–26815. doi: 10.1074/jbc.M109.028381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Dauletbaev N., Eklove D., Mawji N., Iskandar M., Di Marco S., Gallouzi I.-E. Down-regulation of cytokine-induced interleukin-8 requires inhibition of p38 mitogen-activated protein kinase (MAPK) via MAPK phosphatase 1-dependent and -independent mechanisms. J. Biol. Chem. 2011;286:15998–16007. doi: 10.1074/jbc.M110.205724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Shah S., King E., Newton R. Inhibition of inflammatory gene expression by dexamethasone partly depends on the phosphatase, MKP-1 (DUSP1) Eur. Respir. J. 2013;42:P730. [Google Scholar]
- 319.Altonsy M.O., Sasse S.K., Phang T.L., Gerber A.N. Context-dependent cooperation between NF-kB and the glucocorticoid receptor at a TNFAIP3 enhancer: a mechanism to maintain negative feedback control of inflammation. J. Biol. Chem. 2014;289(12):8231–8239. doi: 10.1074/jbc.M113.545178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Kadiyala V., Sasse S.K., Altonsy M.O., Berman R., Chu H.W., Phang T.L. Cistrome-based cooperation between airway epithelial glucocorticoid receptor and NF-κB orchestrates anti-inflammatory effects. J. Biol. Chem. 2016;291:12673–12687. doi: 10.1074/jbc.M116.721217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Remmelts H.H.F., Meijvis S.C.A., Biesma D.H., van Velzen-Blad H., Voorn G.P., Grutters J.C. Dexamethasone downregulates the systemic cytokine response in patients with community-acquired pneumonia. Clin. Vaccine Immunol. 2012;19:1532–1538. doi: 10.1128/CVI.00423-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Auyeung T.W., Lee J.S.W., Lai W.K., Choi C.H., Lee H.K., Lee J.S. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. The Journal of Infection. 2005;51:98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 325.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet (London, England) 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hegeman M.A., Cobelens P.M., Kamps J., Hennus M.P., Jansen N.J.G., Schultz M.J. Liposome-encapsulated dexamethasone attenuates ventilator-induced lung inflammation. Br. J. Pharmacol. 2011;163:1048–1058. doi: 10.1111/j.1476-5381.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Bahloul M., Ketata W., Lahyeni D., Mayoufi H., Kotti A., Smaoui F. Pulmonary capillary leak syndrome following COVID-19 virus infection. J. Med. Virol. 2020 doi: 10.1002/jmv.26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.