Abstract
Hyperlipidemia is generally managed with statin-based drugs. Simvastatin serves as a 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) inhibitor, with prolonged use proven to cause side effects. In the present study, antihyperlipidemic material is tested for its effect in lowering lipid in animals and its proven ability to bind to HMGR. Hyperlipidemia rats were divided into four groups, with different doses of 0, 57, and 114 mg/kg BW of apple peel extract (APE) and simvastatin (3.6 mg/kg BW). The total cholesterol (TC), total triglyceride (TG), low-density lipoprotein cholesterol (LDLc), and high-density lipoprotein cholesterol (HDLc) serum were measured. In silico inhibition test of HMGR activity was conducted by molecular docking using PyRx software. This process places HMGR as a receptor and active compound of apple peels as a ligand. APE treatment with a dose of 114 mg/kg BW could significantly reduce LDLc and increase serum HDLc levels. Docking tests confirmed that quercetin, chlorogenic acid, epicatechin, and catechins depicted HMGR inhibition. Quercetin could bind to HMGR at a similar location to amino acid residues as simvastatin. These material extracts have inhibited cholesterol synthesis through a stronger HMGR inhibition than simvastatin.
Key words: Antihyperlipidemic, apple peels, high-density lipoprotein cholesterol, hydroxy-3-methylglutaryl coenzyme A reductase, low-density lipoprotein cholesterol, triglyceride
INTRODUCTION
Hyperlipidemia is a medical condition characterized by an increase in plasma lipids, including triglycerides, cholesterol, cholesterol esters, phospholipids, and or plasma lipoproteins.[1] Hyperlipidemia is characterized by elevated levels of one or more of the plasma lipids, including triglycerides, total cholesterol (TC) and or very low-density lipoprotein (vLDL) cholesterol and low-density lipoprotein (LDL) followed by reduced high-density lipoprotein (HDL levels.[2] Improved dietary patterns are advised as the first treatment to reduce cholesterol.
A 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase is a regulatory enzyme involved in the biosynthesis of cholesterol in the liver, which catalyzes the synthesis of mevalonate from HMG-CoA. Therefore, HMG-CoA reductase (HMGR) inhibition becomes the target of antihyperlipidemic drugs.[2] Statins act as inhibitors for cholesterol synthesis through inhibition of HMGR.[3] However, the use of statins has several side effects such as digestive disorder and myopathy and teratogenic agents.[4] Thus, the utilization of herbal materials, supported by empirical evidence, provides one of the proper alternatives besides the use of statins.
Apple peels discharged from industrial apple chips contain higher amount of flavonoids. The contents of flavonoids and polyphenols in apple peels are higher than in the apple pulp.[5] The ethanol extract of apple peel contains 52.26% flavonoids (quercetin and its derivates) and 16.14% catechin and its derivates.[6] Previous studies[7] indicated the role of apple reduced levels of TC and LDL and increased levels of HDL in hyperlipidemia patients. Ethanolic extract of apple flesh and peels had lowered TC, LDL, and weight of white adipose tissue in hyperlipidemic mice.[8] Anthocyanins and phenolics content of apple peels may contribute to its antihyperlipidemic activity.[9] The supplementation of apple peel phenol to hamster for 28 days decreased serum LDLc and HDLc.[10]
The present study aimed to examine the effect of apple peel extract (APE) on serum lipid profiles (TC, TG, LDL, and HDL) of high-fat diet-induced hyperlipidemic rats. Besides, whether apple peel active compound can bind to HMGR enzyme and inhibit enzyme activity in synthesizing cholesterol was examined through in silico study.
MATERIALS AND METHODS
In silico analysis
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition prediction
The SMILE of 11 active compounds of APE[11] and simvastatin are downloaded from http://pubchem.ncbi.nlm.nih.gov/[Table 1] and analyzed to obtain prediction with PASS software at http://www. pharmaexpert.ru/passonline.
Table 1.
PubChem CID of simvastatin and active compound of apple peel extract
| Compound | PubChem CID |
|---|---|
| Simvastatin | 54454 |
| Pectin | 441476 |
| Catechin | 9064 |
| Epicatechin | 72276 |
| Quercetin | 5280343 |
| Quercetin-3-O-rutinoside (Rutin) | 5280805 |
| Quercetin-3-O-rhamnoside | 5280459 |
| Quercetin-3-O-glucopside | 15959354 |
| Quercetin-3-O-galactoside | 5281643 |
| Chlorogenic acid | 1794427 |
| Phloridzin | 6072 |
| Procyanidin | 107876 |
Ligand structure preparation
The three-dimensional structure of ligands from the active compound of APE was downloaded from PubChem (https://PubChem.ncbi.nlm.nih.gov) and saved in SDF format.[12]
Protein receptors (3-hydroxy-3-methylglutaryl coenzyme A reductase) preparation
The reference protein used was HMGR (ID 1 hwk) obtained from the Protein Data Bank (http://www.rcsb.org/pdb/home/home.do) and saved in PDB format. The separation of proteins from unnecessary molecules was conducted using PyMOL software, (Schrödinger, Inc., New York, USA)[13] and saved in PDB format.
Docking receptors with ligands
The docking process between HMGR receptors and ligands was conducted using AutoDock Vina in PyRx software[13] with the grid box position and Vina Search Space Center X: 2.834 Y: −11.461 Z: 7.220, Dimension (Angstrom) X: 47.334 Y: 50.696 Z: 49.618. Visualization of docking results was used BIOVIA Discovery Studio Software.
In vivo experiment
Materials
BR-1 (Broiler-1) diet was the product of PT Japfa Comfeed Indonesia Tbk. BR-1 diet consists of 21.5-22.5% crude protein; ≤12% moisture content; ≥5% fat; ≤5% crude fiber; ≤7% ash; 0.8%–1.1% calcium; ≥0.5% phosphorus, and 2950–3050 kcal/kg metabolic energy. Butter was from Kimia Farma Ltd. Indonesia. Propylthiouracil (PTU) was from PT Kimia Farma Indonesia. Rats were obtained from Rattus Breeding Centre, Malang, Indonesia.
Research design
Twenty-five Rattus norvegicus male rats (age = 60 days, body weight [BW]= 100–150 g) were randomly divided into five groups: control group (N), hyperlipidemia rat received 3.6 mg/kg BW of simvastatin (Simv), hyperlipidemia rat received APE at different doses: 0 mg/kg BW (APE-0), 57 mg/kg BW (APE-1), and 114 mg/kg BW (APE-2).[14] This research was approved by the Research Ethics Commission Institute of the State Islamic University (UIN) Malang, Indonesia, No. 010/EC/KEP-FST/2018.
Hyperlipidemia induction
The hyperlipidemia was induced by feeding animals with a high-fat diet, containing 3.0% duck egg yolk, 5.5% quail egg yolk, 15.5% butter, 6.0% used cooking oil, 70% BR1, and 0.01% PTU diluted in drinking water.[15] Animals were received PTU ad libitum for 30 days. The administration of APE through gavage was conducted for 30 days.
Preparation of extract
The apple used in this study was Manalagi apple (Malus sylvestris Mill.) variety. The simplicia powder of apple peels was obtained from Balai Materia Medica Batu Malang, East Java. The apple peels were dried using an oven at 45°C, then were milled and sieved using 60 mesh sieves. Extraction was performed using a maceration method with 70% ethanol. The simplicia powder was immersed in ethanol (1:10 (w/v)) for 3 h × 24 h and then filtered using a Buchner funnel. Further, the filtrate was evaporated with a rotary evaporator at 40°C.
Preparation of rat's blood serum
At the end of the treatment, fasting rats were sacrificed by neck dislocation and blood was taken from the left ventricle. Rat blood was centrifuged at 3000 rpm for 15 min. The serum yielded was stored at −20°C for the measurement of lipoprotein level.
Measurement of lipid serum
Lipid serum measurement was performed using an enzymatic colorimetric method.[16] Serum TC and TG were measured according to the kit protocol (Assay Kit from Elabscience, China). Serum LDL cholesterol (LDLc) measurement used the LDLc Reagent DiaSys, Germany, and HDL cholesterol (HDLc) Reagent from Glory Diagnostic, Spanyol.
Data analysis
Data about the levels of TC, TG, LDLc, and HDLc were analyzed by one-way ANOVA using SPSS software (ver. 16.0). Values of P < 0.05 were considered significantly different.
RESULTS
In silico analysis
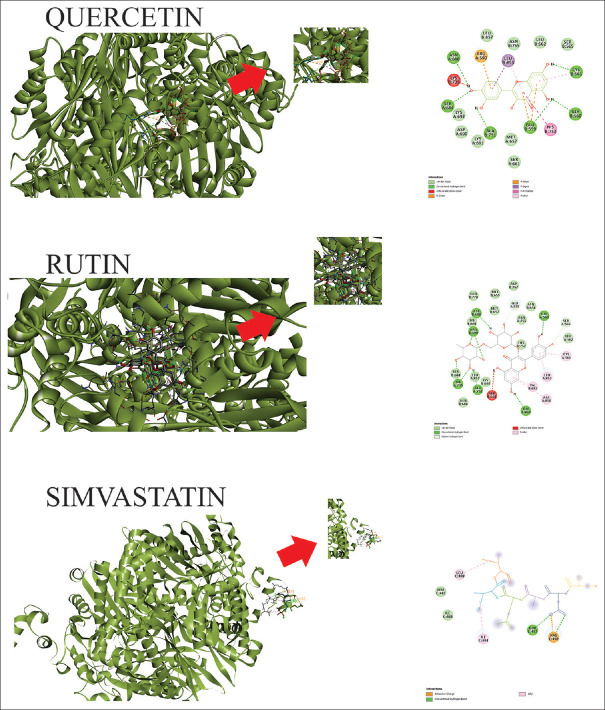
Lipinski Ro5 test showed that three of eight active compounds in APE could pass the membrane such as pectin, phlorizin, and procyanidin [Table 2]. The activity prediction test of the APE phenolic compound confirmed that all compounds play as antihypercholesterol [Pa > Pi, Table 3] which were necessary to inhibit HMG-CoA synthase and HMGR enzymes [Pa > Pi, Table 4]. The docking results indicated that all APE compounds had a lower binding affinity than statin (ΔG-4.5 kcal/mol), emphasizing that quercetin had the most negative affinity value (ΔG-7.4 kcal/mol) [Figure 1].
Table 2.
The prerequisites of Lipinski Ro5 tested compound
| Compound | Lipinski requirement |
Result | ||||
|---|---|---|---|---|---|---|
| Mass <500 Dalton | Hydrogen bond donor ≤5 | Hydrogen bond acceptors ≤10 | Lipophilicity (LogP) ≤5 | Molar refractivity 40-130 | ||
| Simvastatin | 1584.0000 | 0* | 24 | 0* | 0 | No |
| Quercetin-3-O-rutinoside | 610.0000 | 10 | 16 | −1.8788* | 137.4954 | No |
| Quercetin-3-O-rhamnoside | 448.0000* | 7 | 11 | 0.29700* | 104.862045* | Yes |
| quercetin-3-O-galactoside | 464.0000* | 8 | 12 | −0.73060* | 106.273842* | Yes |
| quercetin-3-O-glucoside | 462.0000* | 8 | 11 | 0.06700* | 109.507835* | Yes |
| Quercetin | 302.0000* | 5* | 7* | 2.0109* | 74.0505* | Yes |
| Catechin | 290.0000* | 5* | 6* | 1.5461* | 72.6230* | Yes |
| Epicatechin | 290.0000* | 5* | 6* | 1.5461* | 72.6230* | Yes |
| Procyanidin | 594.0000 | 10 | 13 | 2.7327* | 144.3050 | No |
| Phloridzin | 436.0000* | 7 | 10* | −0.2024* | 104.9250* | Yes |
| Chlorogenic Acid | 353.0000* | 5* | 9* | −1.9806* | 79.8900* | Yes |
| Pectin | 193.0000* | 4* | 7* | −4.4638* | 33.9072 | Yes |
*Fulfilling Lipinski Ro5 Requirements. LogP: Lipophilicity
Table 3.
Antihypercholesterolemic, 3-Hydroxy-3-methylglutaryl coenzyme A synthase, and 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibition activity prediction using Pass program
| Compound | Antihypercholesterol | HMGS-Inh | HMGR-Inh | |||
|---|---|---|---|---|---|---|
| Pa | Pi | Pa | Pi | Pa | Pi | |
| Simvastatine | 0.533 | 0.027 | 0.356 | 0.040 | 0.881 | 0.001 |
| Quercetin-3-O-rutinoside | 0.9 | 0.003 | - | - | - | - |
| Quercetin-3-O-rhamnoside | 0.804 | 0.005 | 0.225 | 0.041 | - | - |
| Quercetin-3-O-galactoside | 0.871 | 0.004 | 0.125 | 0.112 | - | - |
| Quercetin-3-O-glucoside | 0.444 | 0.030 | 0.198 | 0.032 | - | - |
| Quercetin | 0.516 | 0.020 | - | - | 0.516 | 0.020 |
| Catechin | 0.631 | 0.012 | - | - | 0.039 | 0.037 |
| Epicatechin | 0.631 | 0.012 | - | - | 0.039 | 0.037 |
| Procyanidin | 0.357 | 0.045 | - | - | - | - |
| Phloridzin | 0.722 | 0.007 | 0.163 | 0.067 | - | - |
| Chlorogenic acid | 0.423 | 0.033 | 0.198 | 0.032 | 0.042 | 0.026 |
| Pectin | 0.627 | 0.012 | 0.186 | 0.038 | - | - |
HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A, HMGS-Inh: HMG-CoA synthase inhibition, HMGR-Inh: HMG-CoA reductase inhibition, Pa: probable activity, Pi: probable inactivity, -: Pa, Pi value is 0
Table 4.
Binding affinity value of active compound apple peel with 3-hydroxy-3-methylglutaryl coenzyme A reductase
| Compound | ΔG | Hydrophobic bond | Hydrogen bond |
|---|---|---|---|
| Simvastatin | −7.3 | His752, Ser565, Ala856, Cys561, Val683, Leu853, Glu559, Leu857 | Asn755, Lys691, Asp690, Arg590 |
| Quercetin 3-O-Rutinoside* | −9.5 | Asn755, Met657, Lys691, Leu562, Cys561, Ser565, His752, Glu559, Val683, Leu853, Leu857, Ser684 | Lys735, Arg590, Lys692, Ala751, Asp690, Asn658, Ala856, Gly860 |
| Quercetin-3-O-rhamnoside* | −8.2 | Ser684, Val683, Arg590, Ser661, Asp690, Lys691, Leu853, Glu559, Asn658, Cys561 | Lys753, Lys692, Ala751, Glu665 |
| Quercetin-3-O-galactoside* | −7.6 | Ser684, Lys691, Leu853, Asp690, Gly560, Glu559, Cys561, Asn658, Ser661, Arg590, Val683. | Lys735, Lys692, Ala751, Glu665 |
| Quercetin-3-O-glucoside* | −8.5 | Ala783, Ile729, Asn788, Glu782, Glu726, Thr725, Ile729, Ile733 | Glu789, Glu730, Glu726, Asn788 |
| Quercetin | −7.4 | His752, Ser565, Ala856, Cys561, Val683, Leu853, Leu857, Glu 559 | Asn755, Arg590, Asp690, Lys691 |
| Catechin | −8.1 | Leu562, Cys561, Gly560, Ser565, His752, Glu559, Asp690, Leu853, Ala751 | Arg590, Lys692, Ser684, Lys735 |
| Epicatechin | −8.1 | Lys633, Glu610, Leu584, His635, Glu700, Ser637, Ile699, Pro798, Ser705, Ala585. | Lys606 |
| Procyanidin* | −9.5 | Gly860, Leu857, Cys561, Asn658, Gly560, Glu559, Arg590, His752,Leu853, Val683, Ser684, Asp690 | Ala856, Glu665, Lys692, Ala751, Lys735 |
| Phloridzin* | −8.9 | Gln632, Pro798, Ile699, Ala585, Lys633, Asp586, Leu584, Met782, Ser637, His635, Glu610 | Glu700, Gln648, Ile638, Lys606, Lys633, Leu634, Thr636 |
| Chlorogenic acid | −8,3 | Glu700, Glu 610, Ser 705, Lys 606, Ser 637, Ile 699, Ala 585, Leu 584 | Lys 633, Leu 634, His 635, Ile638 |
| Pectin* | −6.5 | His762, Lys691, Leu857 | Arg590, Ser684, Asp690, Ala751, Lys692, Lys753 |
Bold prints indicate the same amino acid as simvastatin. *HMG-CoA reductase inhibition activity prediction using Pass program negative. HMG-CoA: 3-Hydroxy-3-methylglutaryl coenzyme A, HMGR: HMG-CoA reductase
Figure 1.
Interaction between ligand and receptor was visualized by BIOVIA Discovery Studio software. Three-dimensional and two-dimensional images of the binding position of active compounds and binding ligand on 3-hdroxy-3-methylglutaryl coenzyme A reductase
The level of serum total cholesterol, triglyceride, low-density lipoprotein, and high-density lipoprotein
The results of this research indicated that the administration of ethanol extract of apple peels did not significantly (P > 0.05) affect serum TC and TGs. The administration of 114 mg/kg BW of APE significantly decreased (P < 0.05) serum LDL level and increased serum HDL level, which is equal to the effect of simvastatin administered for 30 days to reach the N control level [Table 5].
Table 5.
Serum lipid profile of rats treated with apple peel extract
| Parameter (mg/dl) | Treatment |
ANOVA (P) | ||||
|---|---|---|---|---|---|---|
| N | APE-0 | APE-1 | APE-2 | Simv | ||
| TC | 50.2±8.76 | 51.8±8.11 | 50.8±8.35 | 42.8±4.87 | 46.8±4.21 | 0.289ns |
| TG | 76.4±22.57 | 130±36.57 | 137.4±30.59 | 62.6±29.36 | 64.6±20.01 | 0.064ǂќns |
| LDL | 11.0±1.67ab | 13.8±1.17c | 11.8±1.72bc | 8.4±2.42a | 9.8±1.17ab | 0.003** |
| HDL | 41.2±5.53b | 32.2±1.95a | 35±2.00a | 40.6±3.37b | 45.8±3.37b | 0.000** |
ќKruskal-Wallis, **ANOVA test P<0.01, Different letters in the same line showed significant difference (P<0.05). N: Normal rats, APE-0: Hyperlipidemic rats without treatment, APE-1 and APE-2: Hyperlipidemic rats received 57 mg/kg BW APE and 114 mg/kg BW, Simv: Hyperlipidemic rats received 3.6 mg/kg BW simvastatin, APE: Apple peel extract, BW: Body weight, NS: Not significant, TC: Total cholesterol, TG: Triglyceride, LDL: Low-density lipoprotein, HDL: High-density lipoprotein
DISCUSSION
The administration of APE at a dose of 114 mg/kg BW for 30 days reduced LDLc levels and increased HDLc of hyperlipidemia rats to normal level [Table 2]. The effectiveness of APE in amelioration of both lipoprotein levels is the same as simvastatin treatment. This finding is in line with Poblete et al.'s study[8] who reported a decrease in LDLc and an increase in HDLc after the administration of ethanolic extract of apple peel at a dose of 400 mg/kg BW. Rutin can reduce activities of the acyl-CoA cholesterol transferase enzyme.[2,9] Besides, the administration of APE at a dose of 114 mg/kg BW for 30 days in this study did not significantly reduce TG levels. Thilakarathna et al.[10] explained that the administration of ethanolic extract of apple peels for 30 days could not reduce serum TG levels of hamsters with an atherogenic diet.
HMGR enzyme is attached to the reticulum endoplasmic membrane, so a particular compound must be able to pass the membrane.[17] To pass a membrane and penetrate the cell, a compound must fulfill Lipinski Ro5.[18] The results indicated that 11 flavonoid derivate molecules, besides statin, extracted from apple peels fulfilled Lipinski Ro5 requirements except for procyanidin and rutin [Table 2]. Simvastatin also does not meet the Lipinski Ro5 requirement, due to a large molecule, and more than ten hydrogen bond acceptors made simvastatin a lipophilic compound.[19]
Table 3 demonstrates the activity prediction test of APE content as antihypercholesterol, indicating that all extract compounds exhibit antihypercholesterol activity (Pa > Pi), unlike in simvastatin. Some inhibitors include HMGS activity or HMGR inhibitors or both. Only chlorogenic acid can inhibit the enzyme similar to simvastatin. In addition, the activity prediction test indicated that HMGR inhibitors such as simvastatin, catechin, epicatechin, quercetin, and chlorogenic acid have the highest Pa than Pi, emphasizing HMGR inhibition activity.[19,20] Our results demonstrated that all compounds had better docking scores than simvastatin, except for pectin. Quercetin has similar hydrophobic and hydrogen bonds (eight hydrophobic bonds and six hydrogen bonds) as simvastatin [Table 4]. The eight hydrophobic bonds formed between HMGR and quercetin are likely to occur with C atoms without hydroxyl groups in the cyclic chains A and B quercetin with four bonds.
CONCLUSION
The APE could act as an antihyperlipidemic agent by reducing the LDL level and elevating the HDL level in the hyperlipidemic rat model. The ability of apple peel extract in lowering LDL level was optimal at a dose of 114 mg/kg BW. The molecular docking results clarified the potential of quercetin-3-O-rutinoside, quercetin-3-O-rhamnoside, quercetin-3-O-galactoside, and procyanidin as HMGR inhibitors.
Financial support and sponsorship
This study was financially supported by the Dean of Faculty of Science and Technology of State Islamic University of Maulana Malik Ibrahim Malang, who provided financial support through DIPA in program study empowering research (No. Un. 3.6/HK.00.5/1318/2018).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are very grateful to the Dean of Faculty of Science and Technology of State Islamic University of Maulana Malik Ibrahim Malang, which provided financial support through DIPA in program study empowering research (No. Un. 3.6/HK.00.5/1318/2018).
REFERENCES
- 1.Shattat GF. A review article on hyperlipidemia: Types, treatments and new drug targets. Biomed Pharmacol J. 2014;7:399–409. [Google Scholar]
- 2.Martín-Navarro CM, Lorenzo-Morales J, Machin RP, López-Arencibia A, García-Castellano JM, de Fuentes I, et al. Inhibition of 3-Hydroxy-3-Methylglutaryl–Coenzyme a reductase and application of statins as a novel effective therapeutic approach against acanthamoeba infections. Antimicrob Agents Chemother. 2012;57:375–81. doi: 10.1128/AAC.01426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egom EEA, Hafeez H. Biochemistry of statins. Adv Clin Chem. 2016;73:127–68. doi: 10.1016/bs.acc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Mohammer D, Schaeffeler E, Schwab M, Mörike K. Mechanisms and assessment of statin-related muscular adverse effects. Br J Clin Pharmacol. 2014;78:454–466. doi: 10.1111/bcp.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leontowicz M, Gorinstein S, Leontowicz H, Krzeminski R, Lojek A, Katrich E, et al. Apple and pear peel and pulp and their influence on plasma lipids and antioxidant potentials in rats fed cholesterol-containing diets. J Agric Food Chem. 2003;51:5780–5. doi: 10.1021/jf030137j. [DOI] [PubMed] [Google Scholar]
- 6.Balasuriya N, Rupasinghe HP. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem. 2012;135:2320–5. doi: 10.1016/j.foodchem.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Ravn-Haren G, Dragsted LO, Buch-Andersen T, Jensen EN, Jensen RI, Nemeth-Balogh M, et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur J Nutr. 2013;52:1875–89. doi: 10.1007/s00394-012-0489-z. [DOI] [PubMed] [Google Scholar]
- 8.Poblete M, Neira A, Huilcamán R, Palomo I, Yuri JA, Moore-Carrasco R. Apple extracts present catabolic and hipocolesterolemic effect in mice. Food Nutr Sci. 2015;06:141–50. [Google Scholar]
- 9.Liu C, Sun J, Lu Y, Bo Y. Effects of anthocyanin on serum lipids in dyslipidemia patients: A systematic review and meta-analysis. PLoS One. 2016;11:1–11. doi: 10.1371/journal.pone.0162089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thilakarathna SH, Wang Y, Rupasinghe HP, Ghanam K. Apple peel flavonoid- and triterpene-enriched extracts differentially affect cholesterol homeostasis in hamsters. J Funct Foods. 2012;4:963–71. [Google Scholar]
- 11.He X, Liu RH. Phytochemicals of apple peels: Isolation, structure elucidation, and their antiproliferative and antioxidant activities. J Agric Food Chem. 2008;56:9905–10. doi: 10.1021/jf8015255. [DOI] [PubMed] [Google Scholar]
- 12.Seeliger D, De Groot BL. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des. 2010;24:417–22. doi: 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaziri ND, Liang KH. Acyl-Coenzyme A: Cholesterol acyltransferase inhibition ameliorates proteinuria, hyperlipidemia, lecithin-cholesterol acyltransferase, SRB-1, and low-denisty lipoprotein receptor deficiencies in nephrotic syndrome. Circulation. 2004;110:419–25. doi: 10.1161/01.CIR.0000136023.70841.0F. [DOI] [PubMed] [Google Scholar]
- 14.Kazome YN, Anda TK, Keda MI, Himasaki HS. Serum cholesterol-lowering effect of apple polyphenols. J Oleo Sci. 2005;54:143–51. [Google Scholar]
- 15.Amirabadizadeh A, Foaddodini M, Khatamsaz S, Moktari M. Introduction and optimization of a dietary model for inducing hyperlipidemia in rats. J Babol Univer Med Sci. 2017;19:35–41. [Google Scholar]
- 16.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BM, DeBose-Boyd RA. Underlying mechanisms for sterol-induced ubiquitination and ER-associated degradation of HMG CoA reductase. Semin Cell Dev Biol. 2018;81:121–8. doi: 10.1016/j.semcdb.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;64:4–17. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 19.Menter DG, Ramsauer VP, Harirforoosh S, Chakraborty K, Yang P, Hsi L, et al. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PLoS One. 2011;6:e28813. doi: 10.1371/journal.pone.0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syahputra G, Ambarsari L, Sumaryada T. Docking simulation of enol curcumin, bisdemetoxycurcumin and its analogue as inhibitor for 12-Lipoxygenase enzyme. J Biofisika. 2014;10:55–67. [Google Scholar]