Abstract
Analyses of polyphenolic plant extracts have shown significant results when used to control different pathogens. Many of these pathogens are responsible for different infections causing significant public health problems. This work aims basically to determine the efficiency of polyphenolic extract of Pulicaria crispa to prevent biofilm formation by Klebsiella pneumoniae. Strains were identified by their biochemical characters and matrix-assisted laser desorption ionization time-of-flight mass spectrometry. P. crispa is a Saharan plant used to extract polyphenols to assess their inhibitory action against K. pneumoniae development and biofilm forming. High-performance liquid chromatography revealed quercetin as the most important component of the polyphenolic extract. All strains are biofilm forming and are resistant to many antibiotics. The Minimal inhibitory concentrations of biofilm (MICBs) of the extract range from 0.21 mg gallic acid equivalent (GAE) to 3.40 mg GAE. The minimal inhibitory concentrations vary from 0.1 mg GAE to 0.425 mg GAE. Although many plant extracts have already shown their antimicrobial and antibiofilm activities, their application in clinical cases requires a long-term endeavor.
Key words: Antibiofilm, antimicrobial, Klebsiella pneumoniae, plant extract, respiratory pathogens
INTRODUCTION
Besides the many advantages of antibiotics use in clinical practice, many drawbacks are caused by the intensive and the misuse of these powerful therapeutic agents. The emergence of a resistance phenomenon makes antibiotics less effective representing a serious threat to human health.
Bacteria are found in nature either in free-floating planktonic form or as a communicating community called biofilm.[1] Many species are capable of forming biofilms such as Klebsiella pneumoniae.[2]
Many infections that occur during medical care periods are caused by biofilm development on implantable medical devices.[1] The specific structure of biofilm makes bacteria highly resistant to antibiotics and disinfectants.[3] Bacteria included in biofilms seem to be insensitive to biocidal agents compared to their planktonic forms. Concentrations required to inhibit biofilms have been reported to be up to 1000 times more than those inhibiting planktonic forms.[3]
Therapeutic failures and the substantial cost of treating infections caused by resistant bacteria require alternatives to conventional therapeutic agents.[4]
Plant chemicals are among these substitutes, and many studies have shown interesting antibacterial and antibiofilm activities of plant extracts. Due to their important biodiversity, plants are rich in secondary metabolites.[4] In Algeria, a significant number of vegetal species are used for medicinal purposes.[5]
This work aims to evaluate the efficiency of polyphenolic extract of Pulicaria crispa in preventing biofilm formation by K. pneumoniae obtained from respiratory catheters.
MATERIAL AND METHODS
Plant
P. crispa was collected from the Algerian Sahara in El Hoggar region in Tamanrasset. GPS coordinates are as follows: N22, 298106 and E5, 622164. The plant was identified by botanists at Mouloud Mammeri University in Algeria. It was harvested in January 2018 with more interest to flowery tops, cleaned and washed to remove residues, and dried and crushed in fine powder.
Polyphenols
Polyphenols were extracted from 30 G of the powder by maceration during 72 H in a mix of methanol-formic acid 95%–5%, respectively (Sigma-Aldrich). The macerate was filtered, evaporated at 35°C (Rotavapor Stuart), and then stored at 4°C.[6]
Dosage of polyphenols
From a standard solution of gallic acid (1 mg/ml), dilutions were realized: 0.9, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2, and 0.1 mg/ml. Simultaneously, for the polyphenols, dilutions 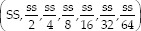 were prepared (SS: stock solution).
were prepared (SS: stock solution).
A volume of 1.58 Ml of distilled water was mixed with 10 μl of calibration range dilution or the sample and 100 μl of the Folin–Ciocalteu reagent (Sigma-Aldrich). At ambient temperature and obscurity, 300 μl of Na2 Co3 were added, and the whole was left 2 H. Absorptions were measured at 765 nm (Biotech Engineering Management Co. Ltd.). The concentration of polyphenols is expressed in equivalent mg of gallic acid.[7]
High-performance liquid chromatography analysis
Phenolic compounds were separated on a C18 (150 mm, 4.6 mm, and 5 μm) column at 25°C. The mobile phase contains water, acetic acid, and methanol in a gradient mode with a rate of flow of 1 ml/min (YLClarity-Céhroatography SW). The obtained chromatograms at 254 nm were compared with those obtained with standards of some well-known phenolic compounds.
Bacterial strains
Strains were obtained from bronchial catheters of patients with low respiratory infections. They were identified by their biochemical characters and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-SM). The K. pneumoniae ATCC 700603 was used as a positive control.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry
From a young culture, a colony was deposited in double on the target plate (Bruker Daltonics TM) and then covered with the prepared matrix solution. The whole was dried and analyzed. The obtained spectra were compared to a database (Maldi Biotyper 3.0. Bruker Daltonics. Germany). A good identification of the species is confirmed if the scores are higher than 1.9.[8]
Preparing bacterial inoculum
Young colonies were emulsified in physiological sterilized water. The obtained suspension had a charge near 108 UFC/ml. Another inoculum was prepared using heart–brain broth (CONDA) instead of physiological water to assess the minimal inhibitory concentration of biofilm CMIBs.[9,10]
Antibacterial activity and minimal inhibitory concentrations
Antimicrobial activity was assessed by depositing 10 μl of the crude extract and dilutions (MICs) on a 6-mm disc which was deposited on an inoculated Petri dish. Gentamicin and ciprofloxacin (Liofilchem) were used as a control following the Clinical and Laboratory Standards Institute method.[9,10]
Biofilm formation and antibiofilm activity
Young colonies were added to 10 ml of brain–heart infusion broth (BHIB) with 2% of saccharose and then incubated. Tubes were cleaned using phosphate-buffered saline (PBS) at pH = 7.3 (Metrohm 620) and then dried. Tubes undergo coloration by crystal violet 0.1% during 15 min and then washed and dried in upside-down position. There is a biofilm forming only when a film covers the walls and the bottom of tubes.[11]
MICB assessment using the phenolic extract of P. crispa was realized in 96-well microplate. We added 100 μl of the inoculum with BHIB supplemented with 2% of saccharose and then incubated the microplate. Floating bacteria were eliminated from wells by aspiration, and wells are then rinsed three times using PBS. After that, 30 μl of every dilution and 70 μl of tryptic soy broth were added according to O'Toole protocol with some modifications.[12] One line of the microplate was used for the positive control and another one for the negative control.
After incubation of the microplate, wells were emptied and rinsed three times with PBS to remove surplus, dried then colored with crystal violet. After that, it was washed again and dried.
Reading was accomplished by ELISA microplate reader at 590 nm (Elisa Plate Analyzer).[12] A very low absorption shows that biofilm formation was inhibited by polyphenolic extract.
The mean absorbance was used to express results, and biofilm inhibition was determined using the following equation:
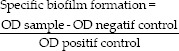
Values of specific biofilm formation (SBF) less than positive control indicate a biofilm inhibition. The lower it is, the more the extract acts to inhibit biofilm formation.[13]
Statistical analysis
Data were represented using arithmetic means and standard deviation of means (Excel 2016 and IBM SPSS Statistics 25.0) and studied using one-way analysis of variance where P ≤ 0.05 was taken as statistically significant.
RESULTS
Polyphenolic extract
The extraction yield was 14.51% ±1.94%, and the concentration was 6.80 ± 0.81 mg gallic acid equivalent (GAE) per G of dry matter. Table 1 provides the polyphenol composition obtained by high-performance liquid chromatography (HPLC).
Table 1.
Composition of polyphenols
| Compound | Rt (min) |
|---|---|
| Hydroxytyrosol | 10.8 |
| Gallic acid | 17.3 |
| Salicylic acid | 23.5 |
| Proanthocyanidin dimer | 23.6 |
| Protocatechuic acid | 26.5 |
| Caffeic acid | 27.7 |
| Catechin | 30.3 |
| ρ-coumaric acid | 34.00 |
| Caffeic acid | 35.1 |
| Ferulic acid | 37.9 |
| Myricetin-3-o-glucoside | 41.7 |
| Rutin | 43.4 |
| Isoferulic acid | 45.2 |
| Rosmarinic acid | 45.6 |
| Quercetin | 47.1 |
| Quercetin 3-B-o-galactoside | 47.9 |
| Benzoic acid | 43.4 |
| Apigenin 7-o-glucoside | 58.3 |
| Luteolin | 48.9 |
| Ellagic acid | 50.7 |
Rt: Retention time
Major components include quercetin, caffeic acid, and isoferulic acid. The rest of components were found in moderate or small amounts: rutin, ferulic acid, luteolin, and protocatechuic acid [Figure 1].
Figure 1.
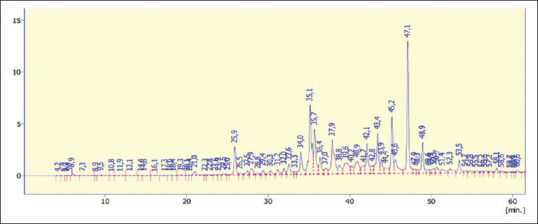
High-performance liquid chromatography analysis of Pulicaria crispa polyphenols
Bacterial identification
The identification of ten strains by MALDI-TOF-MS reveals values higher than 1.9, confirming that the obtained bacteria belong to K. pneumoniae species [Table 2].
Table 2.
Values of the obtained scores by the MALDI-TOF-SM
| Strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Score | 2.157 | 2.323 | 2.036 | 2.131 | 2.18 | 2.222 | 2.172 | 2.125 | 2.086 | 2.2 |
Antimicrobial activity and Minimal Inhibitory Concentrations
Antimicrobial activity of the polyphenolic extract and antibiotics against K. pneumoniae was found important. Inhibition zone values vary between 12.55 ± 0.31 and 24.00 ± 0.02 mm, and MIC values range from 0.1 to 0.425 mg/ml [Table 3].
Table 3.
Inhibition zones and minimal inhibitory concentrations
| Strain | Polyphenols |
Antibiotics IZ (mm) |
||
|---|---|---|---|---|
| IZ (mm) | MICs (mg/Ml) | Gentamycin | Ciprofloxacin | |
| 1 | / | / | S (9.23) | R |
| 2 | 18.55±0.09 | 0.1 | R | R |
| 3 | 12.08±0.31 | 0.21 | R | R |
| 4 | 19.64±0.1 | 0.425 | S (16.00) | R |
| 5 | 23.00±0.05 | 0.1 | S (12.76) | S (11.00) |
| 6 | 18.81±0.22 | 0.425 | R | R |
| 7 | / | / | R | R |
| 8 | 22.1±0.08 | 0.21 | S (18.9) | S (16.08) |
| 9 | 19.5±0.15 | 0.21 | S (19.9) | R |
| 10 | 22.00±0.08 | 0.1 | S (19.00) | S (12.95) |
| C + | 24.00±0.02 | 0.1 | S (23.00) | S (12.00) |
| C− | / | / | / | 0.089±0.0103 |
Values for the IZ are represented by mean±SD of triplicate analysis. SD: Standard deviation, IZ: Inhibition zone, R: Resistant, S: Sensitive, MIC: Minimal inhibitory concentration, /: Not tested
Biofilm formation and antibiofilm activity
From all strains, five are strongly forming biofilms, four are moderately, and one is not forming any biofilms.
Results indicate a high antibiofilm activity for six strains: a moderate one for two strains and a low activity for one strain with CMIBs between 0.21 and 3.4 mg/ml [Table 4].
Table 4.
Biofilm formation, MICBs, and optic density
| Strain | Biofilm formation | Antibiofilm activity | MIBCs (mg/Ml) | OD 590 nm |
|---|---|---|---|---|
| 1 | +++ | + | 3.40 | 0.108±0.011 |
| 2 | +++ | +++ | 0.85 | 0.288±0.09 |
| 3 | +++ | +++ | 0.425 | 0.32±0.109 |
| 4 | ++ | +++ | 0.21 | 0.31±0.078 |
| 5 | +++ | +++ | 0.425 | 0.309±0.1 |
| 6 | ++ | ++ | 1.7 | 0.254±0.004 |
| 7 | +++ | +++ | 0.21 | 0.3±0.036 |
| 8 | ++ | ++ | 1.7 | 0.259±0.071 |
| 9 | ++ | +++ | 0.85 | 0.288±0.099 |
| 10 | / | / | / | / |
| C + | +++ | / | / | 0.401±0.009 |
| C− | − | / | / | 0.089±0.0103 |
Values for the OD at the CMIBs are represented by mean±SD. OD: Optic density, +++: High formation, ++: Moderate formation, −: Negative, SD: Standard deviation, MIBCs: Minimal Inhibitory Concentration of Biofilm, /: Not tested (the strain is not biofilm forming)
Figure 2 shows the absorbance results. Figure 3 shows the rate of inhibition. SBF values range from 0.004 to 0.077 for the stock solution. They increase with the dilution of the extract until they reach that of the positive control (0.753), where it represents a negative activity.
Figure 2.
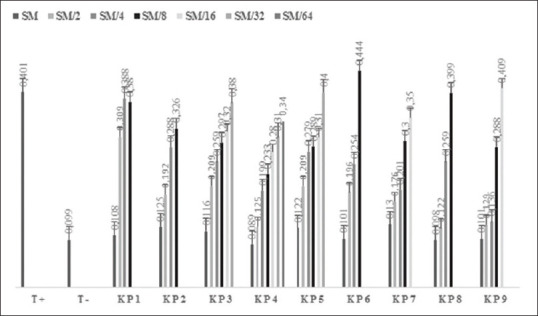
Values of absorbances for each well in the microplate reader. Assays were carried out in triplicate, and the results were expressed as mean values a standard deviation. P < 0.05 was considered statistically significant
Figure 3.
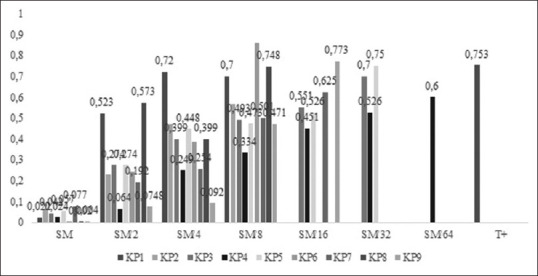
Rate of biofilm inhibition
DISCUSSION
Antimicrobial and antibiofilm activities of plant extracts have been highlighted in several studies. Exploiting these encouraging results could lead to resolve the bacterial resistance and biofilm formation.[4] The formation of biofilms on medical devices is a serious public health problem.[1]
Few studies on the assessment of the inhibitory action of polyphenols on biofilm formation are available in literature. Moreover, no studies have been carried out on the antimicrobial and antibiofilm activities of P. crispa in the Saharan species. This endemic species is used by the local population for antimicrobial and antiseptic properties. Furthermore, the arid zone exposes the plant to stress leading it to synthesize more secondary metabolites responsible of different biological activities.[14]
The present work focuses on strains of K. pneumoniae, currently found in respiratory infections and having a high ability to form biofilms. It should be noticed that 82% of nosocomial infections are caused by biofilm-forming bacterial species on medical devices.[1]
Strains of K. pneumoniae were identified by MALDI-TOF-MS, reported as the best method to identify bacteria by determination of peptide fingerprints.[8] Any natural or artificial surface may be the site of bacterial colonization and formation of biofilms. This is the case for all varieties of biomedical implants.
The transition from a planktonic to biofilm form is regulated by many factors. The authors highlighted that biofilms have mechanisms acting against antimicrobial agents and the defense means of the host.[3]
The qualitative tube method was used to test the ability to form biofilms by strains. This method seems to be easy, despite some difficulties in reading results.[11]
The results of this study are in accordance with another work using the same bacteria isolated from urinary catheters. All strains were biofilm forming.[2] This shows that K. pneumoniae is an extremely biofilm-forming bacterium under different conditions.
In addition, methanol was used since it is the best solvent to extract antimicrobial substances. Its polarity allows extracting polar and moderately polar antimicrobial molecules.[4]
The inhibition of nine strains of K. pneumoniae biofilm formation was demonstrated by the methanolic extract of P. crispa with contractions varying from 0.21 mg GAE to 3.40 mg GAE. Inhibition concentrations of planktonic forms vary between 0.1 and 0.425 mg GAE. These results are encouraging because biofilms of the same bacteria were inhibited by gentamicin at 10 mg/ml, while their planktonic form was inhibited at 0.512 mg/ml.[2] For the same antibiotics, some strains used in this work are resistant while they are sensitive to the action of the polyphenolic extract at lower concentration (0.1 mg/ml). These activities could be explained mainly by the chemical composition of the methanolic extract.
HPLC showed the presence of proanthocyanidin dimer which reduces to small concentration the slim production and thus inhibits the formation of biofilm.[15] Ellagic acid is also present, and it inhibits bacteria and biofilms by destructing the cell membrane.[16]
Gallic acid is revealed by HPLC, and it inhibits glucosyltransferase and fructosyltransferase leading to biofilm inhibition.[17] Rosmarinic acid acts against planktonic forms and young stages of biofilms.[18] Catechins infiltrate cells by destructing the lipidic bilayers.[19]
Flavonoids such as apigenin, luteolin, quercetin, and rutin act by inhibiting nucleic acid synthesis[20] and energy metabolism[21] or other mechanisms such as inhibiting of autoinducer mediated cell–cell signaling.[22]
Furthermore, it should be noticed that some flavonoids such as rutin can inhibit the formation of biofilm without inhibiting the planktonic form,[23] such as the case of strains 1 and 7.
CONCLUSION
This study examines some bacterial strains of K. pneumoniae isolated from respiratory infections in patients with bronchial intubation. This bacterium has a high capacity to form biofilms causing spread of nosocomial infections.
Thus, the study aimed to evaluate the capacity of biofilm forming by some strains of K. pneumoniae and the efficiency of the polyphenolic extract of P. crispa in preventing biofilm formation. These strains showed the ability to form biofilms on intubation probes and resistance to many antibiotics.
Results show that the polyphenolic extract contains quercetin as a major component. It prevents the formation of biofilms in all studied strains and inhibits 80% of planktonic forms.
Finally, although many plant extracts have already shown their antimicrobial and antibiofilm activities, their application in clinical cases may require a long process. Other tests such as toxicity testing, pharmacokinetic and pharmacodynamic studies, drug interactions, and side effects should be accomplished in future researches.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge Mrs. Sadoune-Smail N. and Mr. Benghanem A., professors at the Mouloud Mammeri University of Tizi Ouzou (Algeria), for the identification of the plant studied. In addition, the authors would like to express their gratitude to CRAPC research center team in Bousmail (Algeria) for the HPLC analysis.
REFERENCES
- 1.Margo K, Black JG. Microbiology: Principles and Explorations. 8th ed. USA: John Wiley & Sons; 2012. Causes and spread of infection; pp. 660–93. [Google Scholar]
- 2.Bellifa S, Hassaine H, Balestrino D, Charbonnel N, M'hamedi I, Terki IK, et al. Evaluation of biofilm formation of Klebsiella pneumoniae isolated from medical devices at the University Hospital of Tlemcen, Algeria. Afr J Microbiol Res. 2013;7:5558–64. [Google Scholar]
- 3.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 4.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borhane EC, Ricardo CC, João CM. Bioactive properties of medicinal plants from the Algerian flora: Selecting the species with the highest potential in view of application purposes. Ind Crops and Prod. 2015;77:582–9. [Google Scholar]
- 6.Revilla E, Garcia-Beneytez E, Gabello F, Martin-Ortega M, Ryan JM. Value of high performance liquid chromatography analysis of anthocyanins in the differentiation of red grape cultivars and red wines made from them. J Chromatogr. 2001;915:53–60. doi: 10.1016/s0021-9673(01)00635-5. [DOI] [PubMed] [Google Scholar]
- 7.Ojeil A, El Darra N, El Hajj Y, Mouncef MB, Rizk T, Maroun R. Identification et caractérisation de composes phénoliques extraits du raisin château ksara. Leban Sci J. 2010;11:117–31. [Google Scholar]
- 8.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, et al. Ongoing revolution in bacteriology: Routine identification of 117 bacteria by matrix-assisted laser desorption ionization time of flight mass spectrometry. Clin Infect Dis. 2009;49:543–51. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 9.Performance Standards for Antimicrobial Disk Susceptibility Tests. 9th ed. USA: Clinical and Laboratory Standards Institute, Wayne; 2006. Clinical and Laboratory Standards Institute National Committee for Clinical Laboratory Standard. [Google Scholar]
- 10.Furtado GL, Medeiros AA. Single disk diffusion testing (KirbyBauer) of susceptibility of Proteus mirabilis to chloramphenicol: Significance of the intermediate category. J Clin Microbiol. 1980;12:550–3. doi: 10.1128/jcm.12.4.550-553.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathur T, Singhal S, Khan S, Upadhyay DJ, Fatma T, Rattan A. Detection of biofilm formation among the clinical isolates of Staphylococci: An evaluation of three different screening methods. Indian J Medic Microbiol. 2006;24:25–29. doi: 10.4103/0255-0857.19890. [DOI] [PubMed] [Google Scholar]
- 12.O'Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;47:2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu C, Gilbert ES. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl Environ Microbiol. 2004;70:6951–6. doi: 10.1128/AEM.70.12.6951-6956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourgaud F. Plantes à parfum, aromatiques et médicinales. Phytomedicine. 2010;17:548–50. [Google Scholar]
- 15.Blanco AR, Roccaro AS, Spoto GC, Nostro A, Rusciano D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob Agents Chemother. 2005;49:4339–43. doi: 10.1128/AAC.49.10.4339-4343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakkiyaraj D, Nandhini JR, Malathy B, Pandian SK. The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling. 2013;29:929–37. doi: 10.1080/08927014.2013.820825. [DOI] [PubMed] [Google Scholar]
- 17.Sendamangalam V, Choi OK, Kim D, Seo Y. The antibiofouling effect of polyphenols against Streptococcus mutans. Biofouling. 2011;27:13–19. doi: 10.1080/08927014.2010.535897. [DOI] [PubMed] [Google Scholar]
- 18.Slobodníková L, Fialová S, Hupková H, Granˇcai D. Rosmarinic acid interaction with planktonic and biofilm Staphylococcus aureus. Nat Prod Commun. 2013;8:1747–50. [PubMed] [Google Scholar]
- 19.Taylor PW, Hamilton-Miller JMT, Stapleton PD. Antimicrobial properties of green tea catechins. Food Sci Techn Bull. 2005;2:71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzoeva O, Grishanin R, Calder P. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol Res. 1997;152:239–46. doi: 10.1016/S0944-5013(97)80034-1. [DOI] [PubMed] [Google Scholar]
- 21.Ávila HP, Smânia Ede F, Monache FD, Smânia A. Structure-activity relationship of antibacterial chalcones. Bioorg Med Chem. 2008;16:9790–4. doi: 10.1016/j.bmc.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 22.Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J App Microbiol. 2010;109:515–27. doi: 10.1111/j.1365-2672.2010.04677.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Regmi SC, Kim JA, Cho MH, Yun H, Lee CS, et al. Apple flavonoid phloretin inhibits Escherichia coli O157: H7 biofilm formation and ameliorates colon inflammation in rats. Infect Immuni. 2011;79:4819–27. doi: 10.1128/IAI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


