Abstract
Objective: Fragmented QRS (fQRS) have been reported as a predictor of major adverse cardiac events (MACE) and mortality in several studies on cardiovascular disease. However, most studies have yielded discrepant results. This study aimed to explore the correlation between fQRS and cardiovascular events in patients with acute myocardial infarction (AMI) during their hospital stay and follow-up period, and the predictive value of fQRS in the prognosis of AMI.
Methods: We searched for relevant studies in four databases, Medline, Embase, PubMed, and the Cochrane Library from January 2010 to March 2020. Our initial search yielded 585 articles. Of these, we screened 19 studies, and finally included a total of 6,914 patients in this analysis, comparing death events or MACE in AMI patients with or without fQRS.
Results: Fragmented QRS was significantly associated with a higher risk of in-hospital mortality (OR, 3.97; 95% CI, 2.45–6.44; p < 0.00001), long-term mortality (OR, 2.93; 95% CI, 1.76–4.88; p < 0.0001), in-hospital MACE (OR, 2.48; 95% CI, 1.62–3.80; p < 0.0001), and long-term MACE (OR, 3.81; 95% CI, 2.21–6.57; p < 0.00001). In particular, it demonstrated a higher predictive value for in-hospital cardiovascular mortality and long-term all-cause mortality in AMI patients and in-hospital mortality in patients with ST-segment elevation myocardial infarction (STEMI). Moreover, fQRS was also associated with an increased risk of ventricular arrhythmias (OR, 2.76; 95% CI, 1.72–4.43; p < 0.0001) and heart failure (OR, 1.65; 95% CI, 1.02–2.66; p = 0.04). Fragmented QRS was negatively associated with left ventricular ejection function (LVEF) (MD, −5.47; CI, [−7.03, −3.91]; p < 0.00001) and positively associated with a high incidence of coronary artery triple vessel lesions (OR, 2.14; 95% CI, 1.31–3.51; p = 0.002) in AMI patients.
Conclusion: Fragmented QRS is significantly associated with in-hospital and long-term mortality and MACE in patients with AMI, as well as ventricular arrhythmias and heart failure. Furthermore, it may be a marker of mortality and MACE risk. Moreover, fQRS also indicates a reduced LVEF and a high incidence of coronary artery triple vessel lesions in AMI patients.
Meta-analysis Registration: https://www.crd.york.ac.uk/prospero; ID: CRD42020171668.
Keywords: cardiovascular events, acute myocardial infarction, fragmented QRS, electrocardiogram, mortality
Introduction
Acute myocardial infarction (AMI) is a significant cause of death in patients with coronary heart disease (CHD). Although reperfusion therapy is effective in improving the short- and long-term cardiac prognosis of patients with myocardial infarction(MI), risk stratification after a percutaneous coronary intervention (PCI) is still challenging (Wright et al., 2011; Mozaffarian et al., 2015). Moreover, the comorbidities of AMI are associated with an increased incidence of adverse events. For example, the co-existence of diabetes increases the risk of 1-year mortality and adverse cardiovascular events after infarction (Marfella et al., 2018a,b). Furthermore, myocardial function after infarction is a critical indicator for predicting future cardiovascular events. However, the imaging methods used to evaluate myocardial function are expensive and not easy to acquire (Eitel et al., 2014; Stone et al., 2016).
The purpose of established risk stratification tools is to identify individuals at high risk and require more intensive therapy (Vignoli et al., 2019). However, these tools have some limitations in the assessment of emergency admissions for AMI because more clinical and laboratory data are needed. Moreover, these tools do not incorporate the use of new electrocardiographic parameters that represent disorders in the electrical activity of the myocardial cells and provide accurate prognostic information, thereby promoting risk stratification and the evaluation of prognosis beyond traditionally used variables.
Several studies have evaluated the potential clinical usefulness of fragmented QRS (fQRS) to identify patients with poor outcomes. Das et al. found a correlation between fQRS and the presence of a myocardial scar. They reported that fQRS had a higher diagnostic sensitivity and negative predictive value for old MI than a pathological Q wave (Das et al., 2006). During the past decade, extensive clinical studies have demonstrated that fQRS has a predictive value for mortality and major adverse cardiac events (MACE) in patients with AMI. However, the predictive value of fQRS for in-hospital MACE and long-term mortality is still controversial. This systematic review and meta-analysis provide a framework for assessing the value of fQRS in predicting in-hospital and long-term cardiovascular events in AMI patients.
Methods
Protocol and Registration
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020171668).
Search Strategy
Two researchers independently searched the Embase, Cochrane-Library, Medline, and PubMed databases to retrieve published studies from January 2010 to March 2020, using a search strategy that included the terms “fragmented QRS,” “acute myocardial infarction,” “STEMI,” “NSTEMI,” “MACE,” “cardiovascular events,” and “mortality.”
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (a) cohort studies (prospective or retrospective), cross-sectional studies, or randomized control trials; (b) studies that enrolled patients with recent AMI; (c) studies allocating patients to an fQRS group (experimental group) and a non-fQRS group (control group); (d) the first electrocardiogram recorded on admission or within 48 h of admission; (e) studies reporting in-hospital or long-term mortality/MACE.
The exclusion criteria were as follows: (a) studies including populations with other diseases, such as hypertrophic cardiomyopathy, congenital heart disease, Brugada syndrome, Chagas' disease atrial fibrillation, and renal failure; (b) studies not published in English; (c) studies including patients with unstable angina; (d) publications without full texts or with an unknown date.
Data Extraction and Preset Endpoints
The following information was obtained from each study: title, first author, year of publication, country, the demographic data of participants, the method used to identify cases and controls, the prevalence of fQRS, the technique used to diagnose AMI, and main conclusions. The prespecified outcomes were mortality and MACE in-hospital or during follow-up.
Definition
fQRS was defined as the presence of a new R wave (R0) or notching in the nadir of the S wave, or the presence of >1 R0 (fragmentation) in 2 contiguous leads, corresponding to a major coronary artery territory. AMI was defined as acute STEMI and NSTEMI. Mortality was defined by all-cause mortality or cardiovascular death. MACE were defined as cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke (Wise et al., 2019). Heart failure was defined as Killip ≥ 2 (Cenko et al., 2019), or definitions from each trial were used (Table 3).
Bias Assessment
The internal validity of studies was assessed using the Quality in Prognosis Studies (QUIPS) tool (Hayden et al., 2013). A Funnel plot and the Egger test were used to test for any potential publication bias.
Statistical Analysis
Statistical analysis was performed using Review Manager 5.1, State 16, and Meta-Disc 1.4 software. We extracted the baseline data and end events of the experimental and control groups in each study. For the baseline data, the counting data used the chi-square test, and the continuity data used the t-test after summarizing the data: the difference was statistically significant with p < 0.05. Meta-analysis was performed according to different endpoint events. We also conducted a subgroup analysis to evaluate NSTEMI and STEMI, inclusion criteria, QRS duration, previous MI, and, more importantly, cardiovascular mortality and all-cause mortality, respectively.
The effect size was presented as the odds ratio (OR), likelihood ratio (LR) indicating how many times more (or less) likely a patient experiencing an endpoint is to express fQRS. Since heterogeneity in endpoints from study to study was anticipated, a random effect model was used for the primary analysis, with study as a random variable. Heterogeneity was assessed the I2 statistic test and its 95% confidence intervals (CI) and the random-effects model was used to account for significant statistical variation. The sensitivity analysis was performed by calculating the number of patients in the trial as a percentage of the total number analyzed, to value the weight of the overall results of the meta-analysis for each study. Meta-regression analysis was used to assess the potential impact of baseline characteristics on heterogeneity.
Results
Study Selection
Following the above search strategy, initial studies were identified from four online databases. After 188 duplicates were removed, we screened the remaining 395 articles by reading the title and abstract. We excluded 347 articles because they did not meet the inclusion criteria. Out of the remaining 48 articles, 29 were removed after reading the full text. Finally, a total of 19 studies were included in this meta-analysis (Figure 1).
Figure 1.
Flow-diagram for the inclusion of studies in this meta-analysis.
Quality Assessment of the Included Studies
In general, the methodological quality of the included studies was good, without a high risk of bias. Four studies had different inclusion criteria for fQRS, and therefore the conclusions may be biased compared with other studies (Table 1). Three studies did not give a precise reason for the lack of follow-up, and consequently, the relationship between fQRS and the outcome may be different for completing and non-completing participants (Lorgis et al., 2012; Li et al., 2016; Dural et al., 2019). The funnel plot and Egger test did not suggest evidence of publication bias (Figure 2).
Table 1.
Quality in prognosis studies.
| Study | Study participation | Study attrition | Prognostic factor | Outcome measurement | Study confounding | Statistical analysis |
|---|---|---|---|---|---|---|
| measurement | and reporting | |||||
| Ari | Low | Medium | Low | Low | Low | Low |
| Akgul | Low | Medium | Low | Low | Low | Low |
| Attachaipanich | Low | Low | Low | Low | Low | Low |
| Bekler | Low | Medium | Low | Low | Low | Low |
| Bozbeyogl | Low | Low | Low | Low | Low | Low |
| Dural | Low | Medium | Medium | Low | Medium | Low |
| Guo | Low | Medium | Low | Medium | Low | Low |
| Kurtul | Low | Low | Low | Low | Low | Low |
| Li | Low | medium | Low | Low | Low | Low |
| Logis2013 | Low | Low | Low | Low | Low | Low |
| Logis2014 | Low | Low | Low | Low | Low | Low |
| Stavileci | Low | Low | Low | Low | Low | Low |
| Sheng | Low | Low | Medium | Low | Medium | Medium |
| Tanriverdi | Low | Low | Low | Medium | Medium | Medium |
| Umapathy | Medium | Low | Low | Low | Low | Low |
| Ulsu | Low | Low | Low | Low | Low | Low |
| Xia | Low | Low | Low | Low | Low | Low |
| Yildirim | Low | Low | Low | Low | Medium | Medium |
| Zhao | Low | Low | Low | Low | Low | Low |
Figure 2.
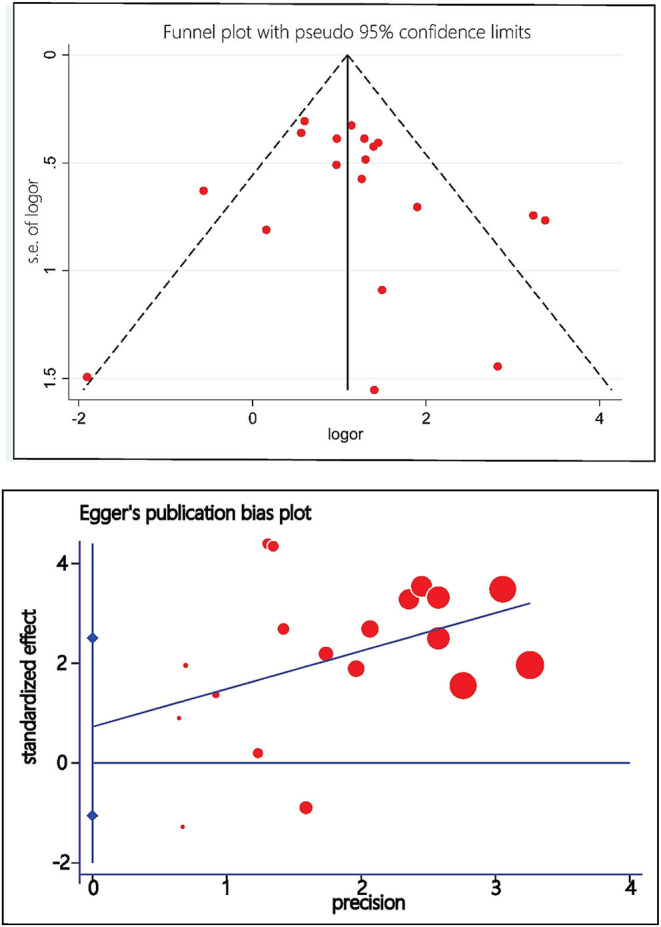
Funnel plot and Egger's test of publication bias.
Inclusion Criteria for fQRS
Four studies had different inclusion criteria (studies by Akgul, Yildirim, Kurtul, and Umapathy). Patients showing fQRS involving the infarct territory within 48 h of admission were included in the “fQRS group.” Patients who did not develop fQRS even after 48 h of admission were included in the “non-fQRS group.”
Of the remaining 15 studies, the first ECG upon admission showing fQRS was included in the “fQRS group” and the first ECG upon admission without fQRS was included in the “non-fQRS group.”
Description of Included Studies
Table 2 shows an overview of studies investigating the role of fQRS in cardiovascular events. In terms of baseline data, the incidence of fQRS was about 40%. Most of the participants were male (76%) and received reperfusion interventional therapy (82%). Gender, history of previous MI, previous PCI, and smoking were statistically different between the fQRS and non-fQRS groups. However, hypertension and diabetes were not statistically different between the fQRS and non-fQRS groups while using statistical analysis (Table 3).
Table 2.
Overview of studies investigating fragmented QRS complex in relation to Cardiovascular events in AMI.
| References | Publish | First author | N | Population | Type | Definition of fQRS | Definition of MACE | Male | PCI | Conclusion | Follow-up(M) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ari et al. (2012) | 2011 | Ari | 85 | STEMI | Prospective | The presence of an extra R wave (R1), notched R wave, notched S wave, or more than one R1 wave in 2 contiguous derivations corresponding to the feeding area of one of the major coronary arteries, complete and incomplete BBB were excluded | Cardiac causes mortality, MI, and TVR | 85 | 85 | Fragmented QRS may provide great benefits in the evaluation and follow-up of coronary artery disease | 6.6 ± 2.3 |
| Akgul et al. (2015) | 2014 | Akgul | 414 | STEMI | Prospective | The presence of an extra R wave (R1), notched R wave, notched S wave, or more than one R1 wave in 2 contiguous derivations corresponding to the feeding area of one of the major coronary arteries, complete and incomplete BBB were excluded | Cardiovascular mortality, re-infarction, or TVR (percutaneous or surgical) | 328 | 414 | Fragmented QRS was associated with an increased in-hospital cardiovascular mortality, and 1-year all-cause mortality in patients with STEMI who are under primary PCI | 12 |
| Attachaipanich and Krittayaphong (2019) | 2018 | Attachaipanich | 452 | STEMI | Retrospective | The presence of an extra R wave (R′), notched R wave, notched S wave, or more than one R′, in at least 2 contiguous leads, corresponding to coronary artery territory; a duration of <120 mms, without a typical BBB | 344 | – | Fragmented QRS complex on admission ECG was an independent predictor of in-hospital life-threatening arrhythmic events in STEMI patients | In-hospital | |
| Bekler et al. (2014) | 2014 | Bekler | 149 | NSTEMI | Retrospective | The presence of an extra R' or crochetage wave, notched in the nadir of the S wave, or fragmentation of the RS or QS complexes, in 2 contiguous leads, corresponding to a major coronary artery territory, without a typical BBB pattern (QRS ≥120 ms), or incomplete right BBB | Cardiovascular mortality, re-infarction, or repeat TVR (percutaneous or surgical) | 112 | 39 | Fragmented QRS on admission was associated with increased long-term mortality in patients with NSTEMI | 18 |
| Bozbeyoglu et al. (2016) | 2016 | Bozbeyogl | 433 | NSTEMI | Prospective | The presence of various RSR' patterns, with or without a Q wave and included an additional R wave (R'), notching of the R wave, notching of the downstroke or upstroke of the S wave, or the presence of >1 R', in two contiguous leads corresponding to a major coronary artery territory, no complete or incomplete BBB | 290 | 315 | Fragmented QRS may be seen as a cautionary signal for extensive myocardial damage. Therefore, fQRS increases long-term mortality for patients with NSTEMI | 12 | |
| Dural et al. (2019) | 2019 | Dural | 736 | AMI | Prospective | The presence of an additional R wave (R'), R wave or the S wave notching, or the presence of more than one R' wave in two consecutive leads | 550 | 736 | Fragmented QRS associated with the presence of adverse events in patients with ACS | 12 | |
| Guo et al. (2012) | 2012 | Guo | 179 | NSTEMI | Retrospective | (1) the presence of various QRS complexes with an additional R', crochetage wave, notching in the nadir of the S wave, or fragmentation of the RS or QS complexes in two contiguous leads corresponding to a major coronary artery territory, (2) absence or presence of the Q wave, and (3) without a typical BBB (QRS ≥120 ms), or incomplete right BBB | Recurrent AMI (STEMI and NSTEMI), unstable angina pectoris, or need for revascularization (PCI or CABG) which did not result in death | 113 | 78 | Fragmented QRS was a powerful predictor of decreased survival in NSTEMI. The prognostic importance of fQRS was incremental to clinical and conventional factors | 12 |
| Kurtul and Duran (2017) | 2017 | Kurtul | 895 | STEMI | Retrospective | The presence of various RSR'patterns with or without a Q wave with an additional R wave, notching of the R wave, notching of the downstroke or upstroke of the S wave, or the presence of >1 additional R wave in 2 contiguous leads corresponding to a major coronary artery territory, without a typical BBB | – | 677 | 895 | Fragmented QRS was an independent predictor of postprocedural CIN and in-hospital mortality in STEMI patients | In-hospital |
| Li et al. (2016) | 2016 | Li | 513 | NSTEMI | Retrospective | The presence of an additional R' or crochetage wave, notching in the nadir of the S wave, RS fragmentation, or QS complexes on 2 contiguous leads, without a typical BBB | All-cause mortality, cardiac mortality, recurrent MI, revascularization, recurrent angina and heart failure | 399 | 278 | Fragmented QRS complexes were commonly present and an independent predictor of MACE in NSTEMI patients | 5.66 |
| Lorgis et al. (2012) | 2013 | Logis | 307 | AMI | Retrospective | The presence of various RSR' patterns with or without a Q wave and included an additional R wave, notching of the R wave, notching of the downstroke or upstroke of the S wave, or the presence of >1 additional R in 2 contiguous leads, without complete or incomplete BBB | 216 | 207 | Fragmented QRS was an independent predictor of 1-year all-cause mortality after AMI | 28 | |
| Lorgis et al. (2014) | 2014 | Logis | 209 | AMI | Prospective | The presence of various RSR' patterns with or without a Q wave and included an additional R wave, notching of the R wave, notching of the downstroke or upstroke of the S wave, or the presence of > 1 additional R wave in 2 contiguous leads corresponding to a major coronary artery territory, patients with complete or incomplete BBB were excluded | Cardiovascular death or non-fatal recurrent MI | 171 | 171 | Fragmented QRS was associated with increased IS, myocardial perfusion abnormalities, decreased LVEF, and increased left heart volumes. fQRS was a reliable marker of infarct size and acute ventricular remodeling | 12 |
| Stavileci et al. (2014) | 2014 | Stavileci | 296 | STEMI | Retrospective | The presence of notching in R or S waves or extra R waves in the original QRS complex, in at least 2 contiguous leads related to a major coronary artery, a QRS during <120 ms, without a typical BBB | – | 226 | 99 | Persistent fQRS was associated with poor prognosis and there was a lack of expected mortality benefit of RPT, particularly that of fibrinolytic therapy, in STEMI patients with fQRS | In-hospital |
| Sheng et al. (2014) | 2014 | Sheng | 300 | AMI | Retrospective | (1) narrow-fQRS(n-fQRS) :Various RSR' patterns with different morphologies of the QRS complexes, with or without the Q wave, in at least 2 contiguous leads, corresponding to a myocardial territory, without BBB(2) wide fQRS (f-wQRS): For a wide QRS wave of BBB and wide QRS complex of premature ventricular contractions (PVCs) | – | – | – | Fragmented QRS could lower the incidence of the cardiovascular events | In-hospital |
| Tanriverdi et al. (2017) | 2017 | Tanriverdi | 330 | STEMI | Retrospective | The presence of various RSR' patterns, with or without Q wave, included an additional R wave (R' prime) or notching of the R wave or S wave, or more than one R' (fragmentation), QRS duration <120 mms, without typical BBB | – | 259 | 330 | One or more than one leads with fQRS can be useful when describing the patients under high cardiac risk in acute STEMI | In-hospital |
| Umapathy et al. (2018) | 2018 | Umapathy | 103 | STEMI | Prospective | The presence of an additional R wave (R0) or notching in the nadir of the S wave or the presence of >1 R' (fragmentation) in two contiguous leads, corresponding to a major coronary artery territory, without BBB | All-cause mortality, readmission with ACS or congestive heart failure, or VA | 90 | 102 | Fragmented QRS did not predict MACE and LV dysfunction in STEMI patients belonging to Killip class I and II on short term follow-up of 30 days. However, fQRS independently predicted impaired microvascular myocardial reperfusion as assessed by SigmaSTR | 1 |
| Uslu et al. (2015) | 2014 | Ulsu | 542 | STEMI | Retrospective | The presence of notched R or S waves, or an additional wave like RSR' pattern in the original QRS complex, a duration of <120 ms, without a typical BBB | Cardiovascular mortality, reinfarction, or repeat TVR | 435 | 542 | Fragmented QRS reflected the linking between impairment of regional left ventricular systolic function and the presence of severe myocardial injury in STEMI | 18 |
| Xia and Feng (2018) | 2018 | Xia | 420 | STEMI | Retrospective | Typical RSR' patterns, with or without the Q wave, included a notching of R wave or S wave or an additional R wave, or the presence of more than 1 R prime, a QRS during <120 mms, without a typical BBB | – | 242 | 259 | Fragmented QRS was an independent predictor for the adverse cardiac events in STEMI patients undergoing PCI or thrombolysis | In-hospital |
| Yildirim et al. (2014) | 2014 | Yildirim | 335 | STEMI | Prospective | Presence of an additional R wave, or a notch in the tip of the S wave, or more than 1 large R' wave in 2 consecutive derivations | – | 270 | 335 | Fragmented QRS and number of fQRS derivations were a significant predictor of in-hospital major cardiac events in STEMI patients | In-hospital |
| Zhao et al. (2018) | 2018 | Zhao | 216 | STEMI | Retrospective | (1) The presence of notched R or S, or the existence of an additional wave—like R S R ' pattern in the original QRS complex; (2) waves with or without a Q wave; (3) a duration of <120 ms; and (4) no accompanying typical BBB | Cardiovascular mortality, reinfarction, advanced heart failure, repeat TVR, VA, atrioventricular block or stroke | 161 | 216 | Fragmented QRS was a prognostic marker of impaired regional ventricular systolic function | 12 |
BBB, bundle branch block; AMI, acute myocardial infarction; STEMI, ST-segment Elevation Myocardial Infarction; NSTEMI, non-ST-segment Elevation Myocardial Infarction; ACS, acute coronary syndrome; GABG, coronary artery bypass surgery; TVR, target vessel revascularization; VA, ventricular arrhythmia.
Table 3.
Baseline and procedural characteristics according to types of AMI.
| fQRS | Non-fQRS | P-value | ||
|---|---|---|---|---|
| Past history and | Age(avg ± SD) | 56.6 ± 13.7 | 59.0 ± 12.9 | <0.001 |
| clinical features | Male | 1,846 | 3,163 | <0.001 |
| DMa | 781/2,547(31%) | 1,115/3,918(28%) | 0.057 | |
| HPTb | 1,226/2,456(50%) | 1,230/3,595(34%) | 0.333 | |
| Prior MIc History of CHDd Prior PCI/CABGe |
143/539(27%) 272/934 202/1,142 |
155/1,329(12%) 381/1286 174/2,007 |
<0.001 0.797 <0.001 |
|
| Smoking | 875/1,650(53%) | 1749/2,909(60%) | <0.001 |
DM, diabetes mellitus;
HPT, hypertension;
prior MI prior myocardial infraction;
history of CHD history of coronary heart disease;
PCI/GABG percutaneous coronary intervention/ coronary-artery bypass grafting.
Meta-Analysis
Clinical Characteristics
All studies, except those conducted by Sheng et al., Li et al., and Logis et al. reported left ventricular ejection function (LVEF) in study groups. Of the 19 studies, 16 studies reported LVEF, and 13 reported LVEF as mean ± standard deviation. Thirteen studies demonstrated a significant negative correlation between LVEF in patients with fQRS and AMI (MD, −5.47; CI, [−7.03, −3.91]; p < 0.00001) with significant heterogeneity (I2 = 84%). Moreover, seven studies reported an obvious relationship between fQRS and triple vessel lesions (OR, 2.14; 95% CI, 1.31–3.51; p = 0.002) with significant heterogeneity (I2 = 79%).
fQRS and Mortality
fQRS and in-hospital mortality
A total of 4,633 patients from 11 studies were included in the analysis of in-hospital mortality (Figure 3). Fragmented QRS was significantly associated with a higher risk of in-hospital mortality (OR, 3.97; 95% CI, 2.45–6.44; p < 0.00001) with obvious heterogeneity (I2 = 58% p = 0.008). The summary positive LR was 1.94 (95% CI, 1.49–2.52) and the negative LR was 0.55(95% CI, 0.41–0.75). The positive and negative LRs revealed significant heterogeneity (I2 = 91.9%, p < 0.0001) (I2 = 67.2%, p = 0.0007) (Figure 4). The heterogeneity was verified by identifying three studies by Yildirim et al. (2014), Akgul et al. (2015), and Kurtul and Duran (2017), using meta-regression analysis. According to the fQRS criteria, we divided the patients into two subgroups: (1) the fQRS positive group (an arbitrary ECG group), if fQRS appeared in any ECG within 48 h; the OR was 23.32 (95% CI, 6.38–85.20); there was no heterogeneity in the subgroup (I2 = 0%, p = 0.8). (2) the fQRS positive group (specific time group), if fQRS was found in the ECG on admission or at the 48th h; the OR was 2.77 (95% CI. 2.06–3.72); there was no heterogeneity in the subgroup (I2 = 0%, p = 0.64). Meanwhile, the heterogeneity was verified by identifying two studies of Akgul et al. and Kurtul et al. using sensitivity analysis. After removing them in the analysis, the overall pooled estimate of OR (2.82, 95% CI, 2.11-3.78) was reduced but removed any significant heterogeneity (I2 = 0%, p = 0.56).
Figure 3.
Summary of the odds risk of mortality and MACE in patients with fQRS. The size of the square for each study is proportional to the sample size. Weighting refers to the contribution of each study to the pooled result and was determined using the random effects model (the endpoints reported in the forest plot are the odds risk of in-hospital mortality, in-hospital MACE, long-term mortality, and long-term MACE, respectively).
Figure 4.
Summary LRs of fQRS to predict in-hospital mortality, in-hospital MACE, long-term mortality, and long-term MACE.
Six studies reported all-cause in-hospital mortality (OR, 3.77; 95% CI, 2.06–6.91; p < 0.00001), five reported in-hospital cardiovascular mortality (OR, 4.31; 95% CI, 2.45–6.44; p = 0.002), and nine merely calculated the mortality in STEMI patients (OR, 4.92; 95% CI, 2.93–8.28) (Table 4). However, there was significant heterogeneity between these studies before the sensitivity analysis identified three studies by Akgul et al., Kurtul et al., and Yildirim et al. as outliers (Yildirim et al., 2014; Akgul et al., 2015; Kurtul and Duran, 2017). After removing the studies by Kurtul and Duran (2017) and Yildirim et al. (2014) the overall pooled OR (2.32, 95% CI, 1.68–3.21) in the meta-analysis for all-cause in-hospital mortality was reduced, with less heterogeneity between studies (I2 = 13%, p = 0.25). After removing the study by Akgul et al. (2015) alone, the overall pooled OR (2.91, 95% CI 1.73–4.87) in the meta-analysis for in-hospital cardiovascular mortality also somewhat decreased, without any heterogeneity between studies (I2 = 0%, p = 0.60). The pooled OR (3.38 95% CI, 2.40–4.77) in the meta-analysis for the in-hospital mortality in STEMI patients decreased, without any heterogeneity between studies (I2 = 0%, p = 0.86) after removing Akgul et al. and Yildirim et al.'s studies (Yildirim et al., 2014; Akgul et al., 2015). Summary estimates in other subgroups are given in Table 4.
Table 4.
Results of subgroup analysis and other endpoints.
| Factors | Categories | No.of studies | fQRS group | No-fQRS group | OR/MD(95%CI) | Model used | Heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| Events/total | Events/total | I2 | p-value | |||||
| Difference endpoints of mortality | ||||||||
| In-hospital mortality | All-cause | 6 | 153/1,168 | 64/1,564 | 3.77 [2.06, 6.91] | Random | 63 | <0.0001 |
| Cardiovascular | 5 | 40/409 | 29/1,276 | 4.31 [1.75, 10.64] | Random | 58 | 0.002 | |
| Long-term mortality | All-cause | 4 | 61/807 | 38/1,427 | 4.42 [1.68, 11.60] | Random | 75 | 0.003 |
| Cardiovascular | 8 | 116/992 | 79/1,729 | 3.22 [1.79, 5.80] | Random | 66 | <0.0001 | |
| Subgroup for main endpoints | ||||||||
| In-hospital mortality | All | 11 | 185/1,612 | 92/2,607 | 3.32 [2.20, 5.01] | Random | 58 | 0.008 |
| An arbitrary ECG group | 3 | 35/493 | 2/737 | 23.32 [6.38, 85.20] | Random | 0 | 0.8 | |
| Specific time group | 8 | 150/1,119 | 90/1,870 | 2.77 [2.06, 3.72] | Random | 0 | 0.64 | |
| History of MIa | 9 | 154/1,276 | 84/2,132 | 3.30 [2.05–5.29] | Random | 48 | 0.05 | |
| First MI | 3 | 59/518 | 12/1,121 | 12.91 [3.07–54.19] | Random | 75 | 0.02 | |
| QRS duration <120 mms | 9 | 145/1,317 | 75/2,681 | 4.32 [2.57–7.26] | Random | 55 | 0.02 | |
| QRS duration Including≥120 mms | 2 | 54/386 | 19/249 | 3.74 [0.43,32.41] | Random | 61 | 0.11 | |
| Retrospective | 8 | 169/1,310 | 85/2,141 | 3.39 [2.24, 5.14] | Random | 44 | 0.08 | |
| Perspective | 3 | 30/393 | 9/789 | 7.83 [0.80, 76.36] | Random | 77 | 0.01 | |
| NSTEMIb | 1 | 2/85 | 7/348 | 1.17 [0.24, 5.75] | Random | / | / | |
| STEMIc | 8 | 157/1,449 | 68/2,451 | 4.92 [2.93, 8.28] | Random | 53 | 0.03 | |
| Long-term mortality | NSTEMI | 4 | 44/507 | 30/742 | 3.44 [2.04, 5.80] | Random | 0 | 1 |
| STEMI | 2 | 42/244 | 35/712 | 4.91 [0.82, 29.44] | Random | 91 | 0.0001 | |
| History of MI | 7 | 109/1,098 | 82/1,736 | 2.64 [1.92,3.62] | Random | 0 | 0.71 | |
| First MI | 2 | 26/187 | 16/436 | 2.77 [0.13–58.56] | Random | 94 | 0.0001 | |
| QRS duration <120 mms | 8 | 133/1,015 | 98/1,954 | 2.90 [1.71–4.93] | Random | 68 | 0.002 | |
| QRS duration Including≥120 mms | 1 | 2/270 | 0/218 | 4.07 [0.19–85.20] | Random | / | / | |
| Retrospective | 4 | 52/575 | 40/783 | 2.60 [1.64, 4.12] | Random | 0 | 0.67 | |
| Perspective | 4 | 66/417 | 43/946 | 3.24 [1.14, 9.22] | Random | 82 | 0.0007 | |
| In-hospital MACE | QRS duration Including≥120 mms | 1 | 55/217 | 9/118 | 3.32[1.70–6.48] | Random | / | / |
| QRS duration <120 mms | 4 | 103/407 | 87/868 | 2.19[1.39–3.44] | Random | 23 | 0.27 | |
| History of MI | 5 | 165/632 | 87/788 | 2.01 [1.40–2.88] | Random | 41 | 0.15 | |
| First MI | 1 | 15/91 | 20/323 | 2.66 [1.42,4.99] | Random | / | / | |
| Long-term MACE | QRS duration Including≥120 mms | 1 | 57/270 | 29/210 | 1.67 [1.02,2.72] | Random | / | / |
| QRS duration <120 mms | 6 | 262/548 | 131/802 | 4.45 [2.56,7.72] | Random | 75 | 0.001 | |
| History of MI | 5 | 210/582 | 101/527 | 3.12 [1.62–6.02] | Random | 78 | 0.001 | |
| First MI | 2 | 109/236 | 59/485 | 5.85 [3.96–8.64] | Random | 0 | 0.39 | |
| Others endpoints | ||||||||
| VT/VFd | / | 6 | 128/763 | 95/1,455 | 2.76 [1.72,4.43] | Random | 57 | 0.04 |
| Heart failure | / | 5 | 158/839 | 115/1,188 | 1.65 [1.02,2.66] | Random | 64 | 0.03 |
| TVLe | / | 7 | 327/796 | 287/1,188 | 2.14 [1.31–3.51] | Random | 79 | <0.0001 |
| LVEFf | / | 13 | −5.47 [−7.03,−3.91] | Random | 84 | <0.00001 | ||
MI myocardial infarction;
NSTEMI Non-ST-segment elevation myocardial infarction;
STEMI ST-segment elevation myocardial infarction;
VT/VF Ventricular tachycardia/ventricular fibrillation;
TVL Triple vessel lesion;
LVEF Left ventricular ejection function.
fQRS and Long-Term Mortality
Nine studies representing 3,457 patients with fQRS were included in this meta-analysis for long-term mortality (Figure 3). Of 3,457 patients, 233 died during the mean/median follow up of 5.66–28 months. Mortality was more common in patients with fQRS than in controls (135/1285 vs. 98/2172) (OR, 2.93; 95% CI, 1.76–4.88; p < 0.0001), with significant heterogeneity between studies (I2 =66%; p < 0.0001). The pooled positive and negative LRs were 1.77 and 1.66, respectively, with significant heterogeneity in positive LR (I2 = 80.8%, p < 0.0001) and negative LR (I2 =63.3%, p = 0.0053) between studies (Figure 4).
Four studies reported all-cause long-term mortality (OR, 4.22; 95% CI, 1.68–11.60; p < 0.003) and eight reported long-term cardiovascular mortality (OR, 3.22; 95% CI, 1.79–5.80; p < 0.0001), respectively, both with significant heterogeneity between studies (I2 = 75%, p = 0.003, and I2 = 66%, p < 0.0001, respectively). The sensitivity analysis showed that the study by Akgul et al. (2015) was the outlier, which shared the same cause of heterogeneity in in-hospital mortality. The presence of fQRS was still found to be correlated with long-term mortality risk (OR, 2.40; 95% CI, 1.67–3.47; p < 0.00001), all-cause mortality risk (OR, 2.81; 95% CI, 1.40–5.65; p = 0.004), and cardiovascular mortality risk (OR, 2.58; 95% CI, 1.69–3.94; p < 0.0001) after ruling out this study from the analysis, with reduced heterogeneity (I2 = 25, 37%, and 22%; p = 0.23, 0.21, and 0.22, respectively) (Table 4).
The result was not statistically significant (OR, 4.91; 95% CI, 0.82–29.44; p = 0.0006; I2 = 91%) when only patients with STEMI were included (n = 956). The fQRS was still found to be correlated with a higher risk of long-term mortality (OR, 3.44; 95% CI, 2.04–5.80; p < 0.00001), without heterogeneity (I2 = 0%, p = 1.00) when only patients with NSTEMI were considered (n = 1,248). In addition, in the presence of fQRS, whether or not these several studies with obvious heterogeneity are removed. The OR of in-hospital mortality is always higher than that of long-term mortality. In terms of the prediction of in-hospital mortality, the predictive value for in-hospital cardiovascular mortality may be higher than that for all-cause mortality. In terms of long-term mortality, however, the predictive value for all-cause mortality may be higher than that for cardiovascular mortality. Summary estimates in other subgroups are given in Table 4.
fQRS and MACE
A total of 1,610 patients with STEMI in 5 studies were included in the analysis for in-hospital MACE (Figure 3). The incidence of MACE was higher in the fQRS group (158/624 vs. 96/986)(OR, 2.48; 95% CI, 1.62–3.80; p < 0.0001) than in controls, without significant heterogeneity (I2 = 39%,). The summary positive LR was 1.50 (95% CI, 1.29–1.74) (Figure 4) without significant heterogeneity (I2 = 25.6%, p = 0.32). The negative LR was 0.73 (95% CI, 0.48–1.12) with significant heterogeneity (I2 = 86.8%, p < 0.0001) (Figure 4).
Outcomes regarding long-term MACE were available in 7 studies (Figure 3). During a mean/median follow up of 5.66–28 months, 479 out of 1,830 patients died. MACE was more common in patients with fQRS (319/818 vs. 160/1012) (OR, 3.81; 95% CI, 2.21–6.57). Significant heterogeneity existed across studies (I2 = 82%, p < 0.0001). The summary positive LR was 1.99 (95% CI, 1.45–2.72) (Figure 4), and the negative LR was 0.54 (95% CI, 0.44–0.68) with significant heterogeneity in the positive LR (I2 = 89.6%, p < 0.0001) and the negative LR(I2 = 59.2%, p = 0.023). Sensitivity analysis and meta-regression analysis failed to identify a single study to account for heterogeneity (Figure 4).
fQRS and Other Cardiovascular Events
Five studies including 839 patients and 1,188 patients in the fQRS and the non-fQRS group, respectively, reported heart failure (Table 4). There was an association between fQRS and heart failure (OR, 1.65; 95% CI, 1.02–2.66; p = 0.04) with significant heterogeneity (I2 = 64%, p < 0.0001). A total of 6 studies reported VT/VF events (Table 4). Among 2,218 patients, 223 had VT/VF. There was a significant association between fQRS and VT/VF events (OR, 2.76; 95% CI, 1.72–4.43; p < 0.0001) with significant heterogeneity (I2= 57%, p = 0.04). Sensitivity analysis and Meta-regression failed to identify the reason for the heterogeneity of the two studies.
Subgroup Analysis of Main Endpoints
This meta-analysis conducted a subgroup analysis on the main endpoint events based on whether the QRS duration was included ≥120 mms and whether the patients included in the study had a previous history of MI or PCI. The pooled OR of in-hospital mortality, MACE, and long-term MACE were higher than those in the studies with a previous history of MI. In the subgroup analysis of QRS duration, it was found that the total value of hospital deaths was lower in the group with a QRS duration of included ≥120 mms compared to the group with a QRS duration of <120 mms. The clinical value of other subgroups may be low because there was no statistical significance or small study sample size (Table 4).
Discussion
The main findings of this meta-analysis are: (1) the fQRS on admission ECG was strongly associated with mortality and MACE in AMI patients during the in-hospital stay and follow-up; (2) fQRS was positively correlated with ventricular arrhythmia (VA) and heart failure in in-hospital patients; (3) fQRS also indicated a decreased LVEF and a high incidence of coronary artery triple vessel lesions in AMI patients.
The Clinical Significance of fQRS
In this meta-analysis, we found that the presence of fQRS on admission ECG was associated with a nearly 4-fold risk of in-hospital mortality and long-term MACE, and an almost 3-fold risk of MACE and VT/VF in the hospital, and mortality during the follow-up period. Thus, this meta-analysis suggests that fQRS has a definite predictive value for in-hospital and long-term cardiovascular events. In the subgroup analysis, we found that for patients with no previous history of MI and PCI, the emergence of fQRS suggests that the risk of in-hospital death, in-hospital MACE events, and long-term MACE events may be higher, which has not been reported in previous studies. We speculate that the reason for these opposing findings may be that fQRS mainly develops after MI, so the possibility of hemodynamic and electrophysiological instability is higher. Additionally, meta-analyses of previous studies showed that for ischemic cardiomyopathy, the longer the QRS duration, the greater the risk of death. On the contrary, in our meta-analysis, we calculated a higher OR value for studies with a QRS duration of <120 mms (Rosengarten et al., 2015). Unfortunately, the specific reason behind these inconsistencies is not clear; although, it may be related to the inclusion criteria for fQRS.
The comorbidities of MI negatively affect prognosis, especially in patients with diabetes mellitus, which can increase the incidence of adverse events including MACE and death. State of diabetes could cause downregulation of stem endothelial cells implied in regenerative post-STEMI events, and loss of epigenetic regulators implied in intracoronary vessels thrombosis, and prediabetes increases the inflammatory burden in pericoronary adipose tissue (Marfella et al., 2013, 2018a,b; Sardu et al., 2019; D'onofrio et al., 2020). There are often concerns for patients with these complications. However, our meta-analysis did not show a statistical difference in the incidence of diabetes between the fQRS and non-fQRS groups, possibly due to the different mechanisms of cardiovascular events associated with fQRS and diabetes. Moreover, our study found a negative correlation between fQRS and LVEF in patients with MI, which could be the result of multiple vascular diseases and larger infarct area and might also be one of the reasons for poor prognosis (Gungor et al., 2016).
fQRS and Mortality
Previous studies identified fQRS as a significant predictor of in-hospital mortality in patients with AMI (Das et al., 2007, 2009; Gungor et al., 2016; Attachaipanich and Krittayaphong, 2019). Our meta-analysis suggests that fQRS increases in-hospital and long-term mortality in AMI patients. The underlying mechanism may be related to myocardial scar formation, but there is still a lack of relevant research evidence (Rosengarten et al., 2015). A negative correlation between fQRS and LVEF was demonstrated in our study and previous studies. Moreover, data from this study shows that fQRS increases the risk of triple vessel lesions, indicating patients with advanced coronary artery disease (Thuijs et al., 2019). Previous studies have reported that fQRS is related to the size of the infarct area after MI (Pietrasik et al., 2007; Das et al., 2009; Lorgis et al., 2012), which may be responsible for the risk of mortality and MACE in patients with AMI. The risk of cardiovascular mortality was higher than that of all-cause mortality at the time of hospitalization in our meta-analysis. Bozbeyoglu et al. have suggested that cardiovascular mortality associated with fQRS may be due to VA, sudden cardiac death (SCD), and deterioration of left ventricular systolic function secondary to myocardial scarring (Bozbeyoglu et al., 2016). Fragmented QRS is also a predictor of progression to heart failure after MI (Korhonen et al., 2010; Akgul et al., 2015). This meta-analysis correlates heart failure with fQRS, which may lead to an increase in cardiovascular mortality.
It is noteworthy that fQRS had a higher predictive value for hospital mortality than follow-up mortality. Another study showed that the mortality in the fQRS group mainly occurred in the first month after discharge during the follow-up period (Akgul et al., 2015). Therefore, monitoring cardiac rhythm for longer than 24–48 h may be required in patients with fQRS (Attachaipanich and Krittayaphong, 2019), and MI patients with fQRS need more attention during hospitalization and for 1 month after discharge. In particular, fQRS significantly increased in-hospital mortality in the group with arbitrary ECG, according to the subgroup analysis. These three studies concluded that fQRS significantly increased the risk of in-hospital mortality, a finding consistent with our observation that one should not ignore the warning signs of transient fQRS found within 48 h of admission. Furthermore, virtually all patients developed fQRS within 48 h after primary PCI, especially within 24 to 48 h (Akbarzadeh et al., 2013; Sheng et al., 2014; Zhang et al., 2017). Therefore, an assessment based on the presence of fQRS on admission ECG may underestimate the predictive value of fQRS for mortality.
fQRS and MACE
This is the first meta-analysis on the relationship between fQRS and in-hospital MACE in patients with AMI and is consistent with the results of previous studies that established fQRS as a significant predictor for in-hospital MACE in AMI (Yildirim et al., 2014; Bozbeyoglu et al., 2016). Although it is not as high as the value for mortality, this is a significant conclusion that cannot be ignored. Of the five studies included, four studies found a significant correlation between fQRS and MACE events. Stavileci et al. (2014) reported that persistent fQRS on admission ECG was associated with poor prognosis in patients with STEMI. Lorgis et al. (2012) have reported that persistent fQRS and BNP were associated with decreased survival in univariate analysis. However, these were not significant independent predictors of MACE in multivariate Cox regression analysis. This study found that fQRS was not a predictor of heart failure or VA in univariate analysis. Fragmented QRS did not predict in-hospital MACE in STEMI patients in a study by Umapathy et al. (2018), that included STEMI patients presenting Killip I and II with a lower incidence of triple-vessel disease (1.7%) and fQRS resolving in 26% of the patients, indicating better myocardial revascularization (Umapathy et al., 2018). In contrast, other study cohorts included all patients with fQRS who had a higher incidence of triple-vessel disease, larger infarct size, and worse left ventricular function. However, it cannot be ignored that our study only analyzed in-hospital MACE in patients with STEMI, because we did not find any reports of in-hospital MACE in patients with NSTEMI. Therefore, it is unclear whether our findings represent AMI. In terms of long-term MACE prediction, our meta-analysis found that fQRS is closely related to long-term MACE, but that heterogeneity was high, and no obvious abnormality was found in a single study through sensitivity analysis.
fQRS and Other Cardiovascular Events
Fragmented QRS was an independent predictor of VA in AMI. Attachaipanich et al. showed that the time from admission to the last life-threatening arrhythmia event was longer in the fQRS than in the non-fQRS group (Attachaipanich and Krittayaphong, 2019). This meta-analysis found that fQRS was significantly associated with VT events. The impulse conduction velocity delay, reentrant circuit, and increased risk of automaticity due to fibroblast infiltration form an arrhythmogenic substrate associated with an increased risk of cardiac arrhythmia that originates from the peri-infarct area (Attachaipanich and Krittayaphong, 2019). This could also increase the mortality and the risk of MACE.
Overall, we found that fQRS is significantly associated with mortality and MACE in patients with AMI and other adverse events such as heart failure and VA. However, current research is still unclear whether the presence of fQRS leads to a reduction in LVEF and further development of electrophysiological instability, thereby further increasing mortality and events. It has also not yet been established whether the instability of hemodynamics and electrophysiological activities, caused by severe myocardial vascular lesions and larger infarct area, can lead to fQRS and increased risk of MACE and mortality of MI. However, this does not affect the predictive value of fQRS for patient mortality and MACE. The causality is not yet clear, so a series of trials will be needed to prove the cause of MACE and death by excluding other factors such as demographic data, clinical, and laboratory tests on patients at the same level.
Limitation of This Study
(1) All studies included were observational, and most of them were retrospective. None of the studies evaluated the use of fQRS in a randomized fashion. (2) Long-term cardiovascular follow-up from 5 to 26 months extended over a long time. (3) Fragmented QRS was defined differently among included studies, contributing to the significant heterogeneity found in our meta-analysis. (4) The data extracted were not adjusted for other variables, such as age, past medical history, and ventricular function. Further studies may evaluate whether fQRS can be used as an independent predictor of in-hospital and long-term cardiovascular events in the AMI population. (5) In the exclusion criteria, patients with previous MI and PCI could not be excluded and these patients significantly influenced prognosis and the formation of fQRS.
Conclusion
The presence of fragmented QRS is associated with increased risk of in-hospital mortality, long-term mortality, and MACE in patients with AMI, while transient fQRS cannot be ignored. Moreover, it is related to an increased risk of in-hospital heart failure and VT/VF events. In the presence of fQRS, the OR for in-hospital mortality is higher than that for long-term mortality. The OR for long-term MACE is higher than that for in-hospital MACE. In terms of the prediction of in-hospital mortality, the predictive value for in-hospital cardiovascular mortality may be higher than that for all-cause mortality. In terms of long-term mortality, however, the predictive value for all-cause mortality may be higher than that for cardiovascular mortality. The in-hospital mortality in the fQRS group was significantly higher than that in the non-fQRS group when only patients with STEMI were included. The long-term mortality in the fQRS group was significantly higher than that in the non-fQRS group when only patients with NSTEMI were included. These results indicate that fQRS has a predictive value for both in-hospital and long-term adverse events. Even though the evidence for RCT trails is limited, it is still a predictor of clinical significance.
Data Availability Statement
All datasets generated for this study are included in the article.
Author Contributions
GL and QL participated in the study design, searched databases, extracted, and assessed data. GL drafted the manuscript. YP and ZZ revised the manuscript. GL, QL, and JD extracted data. ZZ designed the study. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.01027/full#supplementary-material
References
- Akbarzadeh F., Pourafkari L., Ghaffari S., Hashemi M., Sadeghi-Bazargani H. (2013). Predictive value of the fragmented QRS complex in 6-month mortality and morbidity following acute coronary syndrome. Int. J. Gen. Med. 6, 399–404. 10.2147/IJGM.S40050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgul O., Uyarel H., Pusuroglu H., Surgit O., Turen S., Erturk M., et al. (2015). Predictive value of a fragmented QRS complex in patients undergoing primary angioplasty for ST elevation myocardial infarction. Ann. Noninvasive Electrocardiol. 20, 263–272. 10.1111/anec.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ari H., Cetinkaya S., Ari S., Koca V., Bozat T. (2012). The prognostic significance of a fragmented QRS complex after primary percutaneous coronary intervention. Heart Vessels 27, 20–28. 10.1007/s00380-011-0121-9 [DOI] [PubMed] [Google Scholar]
- Attachaipanich T., Krittayaphong R. (2019). Fragmented QRS as a predictor of in-hospital life-threatening arrhythmic complications in ST-elevation myocardial infarction patients. Ann. Noninvasive Electrocardiol. 24:e12593 10.1111/anec.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekler A., Gazi E., Erbag G., Peker T., Barutcu A., Altun B., et al. (2014). Relationship between presence of fragmented qrs on admission and long term mortality in patients with non st-elevated myocardial infarction. Am. J. Cardiol. 113, S58–S59. 10.1016/j.amjcard.2014.01.159 [DOI] [PubMed] [Google Scholar]
- Bozbeyoglu E., Yildirimtürk Ö., Yazici S., Ceylan U. S., Erdem A., Kaya A., et al. (2016). Fragmented QRS on admission electrocardiography predicts long-term mortality in patients with non–ST-segment elevation myocardial infarction. Ann. Noninvasive Electrocardiol. 21, 352–357. 10.1111/anec.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenko E., Van Der Schaar M., Yoon J., Manfrini O., Vasiljevic Z., Vavlukis M., et al. (2019). Sex-related differences in heart failure after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol 74, 2379–2389. 10.1016/j.jacc.2019.08.1047 [DOI] [PubMed] [Google Scholar]
- Das M. K., Khan B., Jacob S., Kumar A., Mahenthiran J. (2006). Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 113, 2495–2501. 10.1161/CIRCULATIONAHA.105.595892 [DOI] [PubMed] [Google Scholar]
- Das M. K., Michael M. A., Suradi H., Peng J., Sinha A., Shen C., et al. (2009). Usefulness of fragmented QRS on a 12-lead electrocardiogram in acute coronary syndrome for predicting mortality. Am. J. Cardiol. 104, 1631–1637. 10.1016/j.amjcard.2009.07.046 [DOI] [PubMed] [Google Scholar]
- Das M. K., Saha C., El Masry H., Peng J., Dandamudi G., Mahenthiran J., et al. (2007). Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm 4, 1385–1392. 10.1016/j.hrthm.2007.06.024 [DOI] [PubMed] [Google Scholar]
- D'onofrio N., Sardu C., Paolisso P., Minicucci F., Gragnano F., Ferraraccio F., et al. (2020). MicroRNA-33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J. Cell. Physiol. 235, 1438–1452. 10.1002/jcp.29064 [DOI] [PubMed] [Google Scholar]
- Dural M., Sunman H., Algül E., Hocamguliyev H., Sahan H. F., Çimen T., et al. (2019). Relationship between serum bilirubin levels and presence of fragmented QRS in patients with acute coronary syndrome. Biomark. Med. 14, 65–73. 10.2217/bmm-2018-0493 [DOI] [PubMed] [Google Scholar]
- Eitel I., De Waha S., Wohrle J., Fuernau G., Lurz P., Pauschinger M., et al. (2014). Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 64, 1217–1226. 10.1016/j.jacc.2014.06.1194 [DOI] [PubMed] [Google Scholar]
- Gungor B., Ozcan K. S., Karatas M. B., Sahin I., Ozturk R., Bolca O. (2016). Prognostic value of QRS fragmentation in patients with acute myocardial infarction: a meta-analysis. Ann. Noninvasive Electrocardiol. 21, 604–612. 10.1111/anec.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R., Zhang J., Li Y., Xu Y., Tang K., Li W. (2012). Prognostic significance of fragmented QRS in patients with non-ST elevation myocardial infarction: results of a 1-year, single-center follow-up. Herz 37, 789–795. 10.1007/s00059-012-3603-3 [DOI] [PubMed] [Google Scholar]
- Hayden J. A., Van Der Windt D. A., Cartwright J. L., Cote P., Bombardier C. (2013). Assessing bias in studies of prognostic factors. Ann. Intern. Med. 158, 280–286. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- Korhonen P., Husa T., Konttila T., Tierala I., Mäkijärvi M., Väänänen H., et al. (2010). Fragmented QRS in prediction of cardiac deaths and heart failure hospitalizations after myocardial infarction. Ann. Noninvasive Electrocardiol. 15, 130–137. 10.1111/j.1542-474X.2010.00353.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtul A., Duran M. (2017). Fragmented QRS complex predicts contrast-induced nephropathy and in-hospital mortality after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Clin. Cardiol. 40, 235–242. 10.1002/clc.22651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wang X., Mi S. H., Chi Z., Chen Q., Zhao X., et al. (2016). Short-term prognosis of fragmented QRS complex in patients with non-ST elevated acute myocardial infarction. Chin. Med. J. 129, 518–522. 10.4103/0366-6999.176989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorgis L., Cochet A., Chevallier O., Angue M., Gudjoncik A., Lalande A., et al. (2014). Relationship between fragmented QRS and no-reflow, infarct size, and peri-infarct zone assessed using cardiac magnetic resonance in patients with myocardial infarction. Can. J. Cardiol. 30, 204–210. 10.1016/j.cjca.2013.11.026 [DOI] [PubMed] [Google Scholar]
- Lorgis L., Jourda F., Richard C., Zeller M., Gudjoncik A., Buffet P., et al. (2012). Prognostic value of persistent vs. transient fragmented QRS on a 12-lead ECG in patients with acute myocardial infarction. Arch. Cardiovasc. Dis. Suppl. 4:7 10.1016/S1878-6480(12)70416-0 [DOI] [Google Scholar]
- Marfella R., Rizzo M. R., Siniscalchi M., Paolisso P., Barbieri M., Sardu C., et al. (2013). Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: effects on myocardial salvage. Int. J. Cardiol. 168, 3954–3962. 10.1016/j.ijcard.2013.06.053 [DOI] [PubMed] [Google Scholar]
- Marfella R., Sardu C., Balestrieri M. L., Siniscalchi M., Minicucci F., Signoriello G., et al. (2018a). Effects of incretin treatment on cardiovascular outcomes in diabetic STEMI-patients with culprit obstructive and multivessel non obstructive-coronary-stenosis. Diabetol. Metab. Syndr. 10:1 10.1186/s13098-017-0304-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfella R., Sardu C., Calabrò P., Siniscalchi M., Minicucci F., Signoriello G., et al. (2018b). Non-ST-elevation myocardial infarction outcomes in patients with type 2 diabetes with non-obstructive coronary artery stenosis: effects of incretin treatment. Diabetes Obes. Metab. 20, 723–729. 10.1111/dom.13122 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., et al. (2015). Heart disease and stroke statistics−2015 update: a report from the American Heart Association. Circulation 131, e29–e322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- Pietrasik G., Goldenberg I., Zdzienicka J., Moss A. J., Zareba W. (2007). Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q-wave myocardial infarction. Am. J. Cardiol. 100, 583–586. 10.1016/j.amjcard.2007.03.063 [DOI] [PubMed] [Google Scholar]
- Rosengarten J. A., Scott P. A., Morgan J. M. (2015). Fragmented QRS for the prediction of sudden cardiac death: a meta-analysis. Europace 17, 969–977. 10.1093/europace/euu279 [DOI] [PubMed] [Google Scholar]
- Sardu C., D'onofrio N., Torella M., Portoghese M., Loreni F., Mureddu S., et al. (2019). Pericoronary fat inflammation and Major Adverse Cardiac Events (MACE) in prediabetic patients with acute myocardial infarction: effects of metformin. Cardiovasc. Diabetol. 18:126 10.1186/s12933-019-0931-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Q. H., Hsu C. C., Li J. P., Hong T., Huo Y. (2014). Correlation between fragmented QRS and the short-term prognosis of patients with acute myocardial infarction. J. Zhejiang Univ. Sci. B 15, 67–74. 10.1631/jzus.B1300091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavileci B., Cimci M., Ikitimur B., Barman H. A., Ozcan S., Ataoglu E., et al. (2014). Significance and usefulness of narrow fragmented QRS complex on 12-lead electrocardiogram in acute ST-segment elevation myocardial infarction for prediction of early mortality and morbidity. Ann. Noninvasive Electrocardiol. 19, 338–344. 10.1111/anec.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone G. W., Selker H. P., Thiele H., Patel M. R., Udelson J. E., Ohman E. M., et al. (2016). Relationship between infarct size and outcomes following primary pci: patient-level analysis from 10 randomized trials. J. Am. Coll. Cardiol. 67, 1674–1683. 10.1016/j.jacc.2016.01.069 [DOI] [PubMed] [Google Scholar]
- Tanriverdi Z., Dursun H., Colluoglu T., Kaya D. (2017). Single derivation fragmented QRS can predict poor prognosis in successfully revascularized acute STEMI patients. Arq. Bras. Cardiol. 109, 213–221. 10.5935/abc.20170099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuijs D., Kappetein A. P., Serruys P. W., Mohr F. W., Morice M. C., Mack M. J., et al. (2019). Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 394, 1325–1334. 10.1016/S0140-6736(19)31997-X [DOI] [PubMed] [Google Scholar]
- Umapathy S., Yadav R., Goswami K. C., Karthikeyan G., Parakh N., Bahl V. K. (2018). Prognostic significance of fragmented QRS in patients with ST-elevation myocardial infarction undergoing revascularization. Indian Heart J. 70(Suppl. 3), S126–S132. 10.1016/j.ihj.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslu N., Gul M., Cakmak H. A., Atam A., Pusuroglu H., Satilmisoglu H., et al. (2015). The assessment of relationship between fragmented QRS complex and left ventricular wall motion score index in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Ann. Noninvasive Electrocardiol. 20, 148–157. 10.1111/anec.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignoli A., Tenori L., Giusti B., Takis P. G., Valente S., Carrabba N., et al. (2019). NMR-based metabolomics identifies patients at high risk of death within two years after acute myocardial infarction in the AMI-Florence II cohort. BMC Med. 17:3 10.1186/s12916-018-1240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. A., Chapman K. R., Scirica B. M., Bhatt D. L., Daoud S. Z., Zetterstrand S., et al. (2019). Effect of aclidinium bromide on major cardiovascular events and exacerbations in high-risk patients with chronic obstructive pulmonary disease: the ASCENT-COPD randomized clinical trial. JAMA 321, 1693–1701. 10.1001/jama.2019.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. S., Anderson J. L., Adams C. D., Bridges C. R., Casey D. E., Jr, Ettinger S. M., et al. (2011). 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/Non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 57, e215–e367. 10.1016/j.jacc.2011.02.011 [DOI] [PubMed] [Google Scholar]
- Xia W., Feng X. Y. (2018). Fragmented QRS (fQRS) complex predicts adverse cardiac events of ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention and thrombolysis. Med. Sci. Monit. 24, 4634–4640. 10.12659/MSM.908712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim E., Karaçimen D., Özcan K. S., Osmonov D., Türkkan C., Altay S., et al. (2014). The relationship between fragmentation on electrocardiography and in-hospital prognosis of patients with acute myocardial infarction. Med. Sci. Monit. 20, 913–919. 10.12659/MSM.890201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Chen S., Zhao Q., Sun M., Yu B., Hou J. (2017). Fragmented QRS complex is a prognostic marker of microvascular reperfusion and changes in LV function occur in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Exp. Ther. Med. 13, 3231–3238. 10.3892/etm.2017.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Zhang R., Hou J., Yu B. (2018). Relationship between fragmented QRS and NT-proBNP in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Acta Cardiol Sin 34, 13–22. 10.6515/ACS.201801_34(1).20170903A [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article.





