Graphical abstract
Schematic representation of TPC2 role, investigated through genetic ablation and drug blocker, in the inhibition of Coronaviruses release into the cells.
Recently, an interesting review appeared in Pharmacological Research presented a list of candidate drugs against SARS-CoV-2 and COVID-19 [1]. In the present insight, we highlight novel experimental evidence that the flavanone Naringenin, targeting the endo-lysosomal Two-Pore Channels (TPCs), could be added to the list of potential weapons against SARS-CoV-2 infection and COVID-19 disease.
Coronaviruses (CoV) are a large family of enveloped positive-sense single-stranded RNA viruses including the highly pathogenic SARS-CoV-1 and -2, MERS-CoV, and other four human CoV of less recent zoonotic origin (229E, OC43, HKU1, NL63) known to cause illnesses ranging from the common cold to acute respiratory tract infection. The current COVID-19 pandemic is one of the largest challenges in medicine and public health and the management of COVID-19 patients is burdened by the lack of antivirals hampering SARS-CoV-2 replication without side effects. Possible pharmacological strategies for the management of COVID-19 patients include drug repurposing such as in the case of hydroxychloroquine, an anti-malarial drug, which has proved to be very controversial [2]. In this scenario, the availability of well tolerated drugs actively impairing virus replication at the early stages of infection represents a promising option and an urgent medical need. Intriguingly, it has been shown that CoV infection depends on trafficking of virions to lysosomal compartments and processing of the S protein by lysosomal proteases is required for productive entry to occur. In this context, the role played by endo-lysosomal TPCs on CoV biology and the feasibility of blocking the intracellular pathway of the virus by inhibiting these channels was preliminarily inferred by our group [[3], [4], [5]] and confirmed by other authors [[6], [7], [8]]. Genetic ablation of TPCs or TPC blockers have been previously shown to reduce infectivity affecting trafficking through the endo-lysosomal system of Ebola virus and MERS-CoV [9,10]. Finally, it has recently been demonstrated that a two-pore channel 2 (TPC2) is critically important for SARS-CoV-2 entry into cells and tetrandrine, a TPC inhibitor, previously described to block Ebola virus trafficking in a TPC-dependent manner [9], was found to impede the entry process of SARS-CoV-2 pseudoviruses [11].
During the infection process SARS-CoV replication takes place in specific membrane-enclosed cellular compartments, hidden from innate immunity, and membrane fusion events are of pivotal importance for the replication of the virus. Cathepsins B and L, belonging to the papain subfamily of cysteine proteases and located predominantly in endo-lysosomal vesicles, have been described to be fundamental for cleavage of viral S protein allowing the release of the fusogenic peptide S2. Of note, tetrandrine treatment suppressed the replication of common cold related human coronaviruses (HCoV) [12]. These findings support the potential role of TPC-dependent membrane trafficking and translocation of endosomes containing CoVs particles. Moreover, TPCs are known to promote endo-lysosomal trafficking and evidence is emerging of their contributions to trafficking dysfunction [13,14]. Therefore, inhibition of TPCs should both impair the fusogenic potential of the endo-lysosomal system and alter the normal trafficking, limiting viral replication. Noteworthy, our recent evidence has shown that the activity of human TPC channels can be inhibited by the natural flavonoid compound Naringenin (Nar) [4], one of the main flavonoids present in the human diet. In the present study, we focus on Nar and show data pointing to a novel anti-CoV pharmacological strategy, highly promising for efficient and safe prophylaxis and therapy.
1. Naringenin was very effective in inhibiting human coronaviruses infection
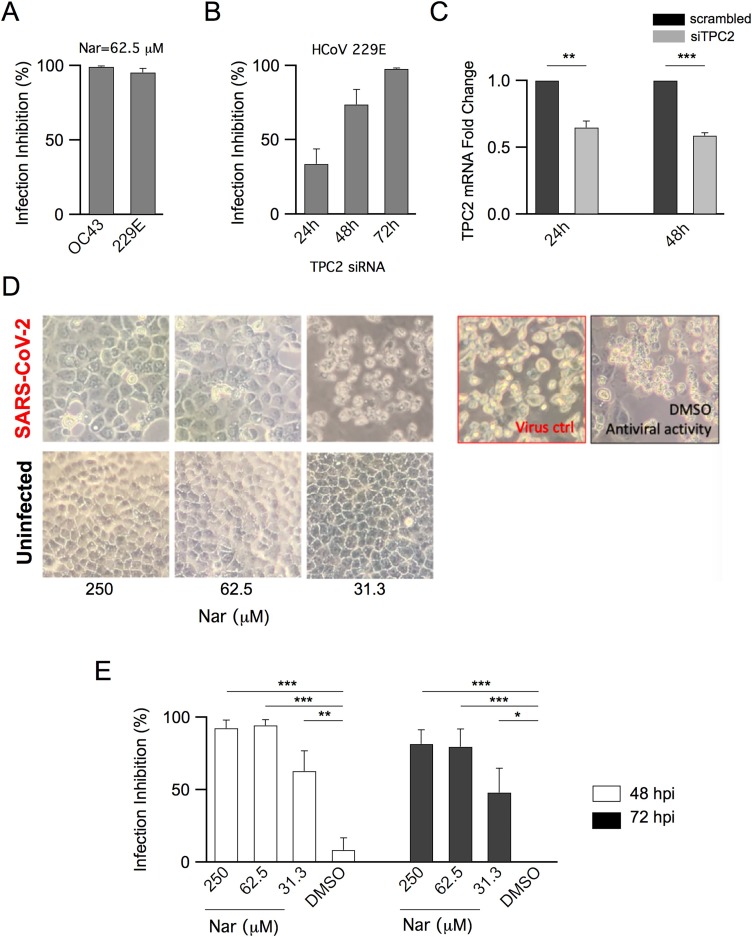
Vero E6 cells were treated adding 62.5 μM Nar 1 h before infection with HCoVOC43 and HCoV229E (MOI:0.01). End-point RT/PCR results indicated a strong inhibition of viral replication as shown in Fig. 1 A.
Fig. 1.
Antiviral Activity of Naringenin and of Endo-Lysosomal Two-Pore Channel 2 Knockdown in CoV Infection.
Antiviral activity of Nar on HCoVOC43 and HCoV229E: A) Inhibition of HCoVOC43 and HCoV 229E was evaluated respectively at 24 hpi and 48 hpi in Vero E6 cells treated with 62.5 μM Nar. Differences between treated samples (n = 3) and controls (untreated infected cells, n = 3, not shown) were significant (P < 0.001).
TPC2 silencing abolishes HCoV229E infection: B) HCoV229E infection inhibition was evaluated at 24-48-72 hpi in Huh7.5 cells pretreated with siRNA to silence TPC2. Differences between treated samples (n = 4, n = 3 at 72 hpi) and controls (untreated infected cells, n = 4, not shown) were significant (P < 0.05 at 24 hpi and P < 0.001 at 48 hpi and 72 hpi); C) Transient inhibition of TPC2 expression after 24 h and 48 h in Huh7.5 cells. Differences between treated samples (siTPC2, n = 3) and controls (scrambled siRNA, n = 3) were significant (P < 0.01 at 24 h and P < 0.001 at 48 h).
Antiviral activity of Nar on SARS-CoV-2: D) CPE on infected Vero E6 cells treated with different concentrations of Nar, as indicated. Bright-field microscopy images (72 hpi) of representative CPE of SARS-CoV-2, hCoV-19/Italy/UniSR1/2020 isolate, detected on both infected and uninfected cells, compared to the untreated infected cells (virus control, red square). DMSO had no effect on virus replication (black square); E) Inhibition of infection calculated on CPE detected at 48 and 72 hpi. Differences between treated samples (n = 9) and controls (untreated infected cells, DMSO, n = 6) were significant (P < 0.001 at 250 and 62.5μM of Nar, P < 0.01 and P < 0.05 at 31.5 μM of Nar, respectively at 48 hpi and 72 hpi).
All data were expressed as mean ± sem. Student’s t-test was used for statistical comparison between means (*P < 0.05; **P < 0.01; ***P < 0.001).
2. TPC2, the molecular target of Naringenin, was critical in regulating HCoV229E infection mechanism
To further assess the involvement of TPC2 in CoV infection we silenced its expression in the human cell line Huh7.5. Cells were pretreated for siRNA-mediated knockdown of TPC2, which was followed by infection with HCoV229E (MOI:0.01). Inhibition of viral replication was determined at 24, 48 and 72 h post-infection (hpi). Partial inhibition of 229E replication was observed at 24 hpi in cells silenced for TPC2 by siRNA while stronger inhibition was observed at 48 and 72 hpi (Fig. 1B). Fig. 1 C shows a significant reduction of TPC2 expression following TPC2 knockdown. These results show that TPC2 indeed plays a crucial role in HCoV infection; as regards the time lag for the stronger effect, we take it as indicating a slow TPC2 turnover rate.
3. Naringenin showed a strong antiviral activity against SARS-CoV-2
Vero E6 cells were infected with SARS-CoV-2 (MOI:0.01) and monitored for the presence of cytopathic effect (CPE) at 48 and 72 hpi. Upon addition of Nar to the external medium, we observed that SARS-CoV-2 infection was inhibited in a time- and concentration-dependent manner (Fig. 1 D-E). Images showed that Nar treatment did not cause toxicity on uninfected cells at any of the tested concentrations and DMSO (vehicle) did not significantly affect SARS-CoV-2 replication (Fig. 1 D-E). Of note, results indicated a strong decrease of CPE (>90 %) at 48 hpi when Vero E6 cells were treated with 250 and 62.5 μM Nar ( Fig. 1E). Marked CPE inhibition was still evident at 72 hpi, showing that both 250 and 62.5 μM concentrations of Nar were very effective in protecting cells from SARS-CoV-2 infection.
On the whole, Nar behaves as a lysosomotropic active natural compound exhibiting human pan-CoV antiviral activity. Interestingly, besides its antiviral power, Nar has anti-inflammatory activity by inhibiting TNF-α and IL-6 secretion [15] which may synergistically enhance its antiviral effect in vivo. The potential of possible Nar-based medical treatment is that it could tackle both viral infection and the cytokine release/cytokine storm syndrome in COVID-19. As regards clinical trials, the therapeutic potential and safety of Nar have been reviewed [16] and a more recent clinical trial on the pharmacokinetics and metabolism of Nar indicates this compound as a very promising candidate for clinical applications [17]. In particular, it has been reported that in healthy humans an oral dose of 600 mg Nar results in a serum Cmax of about 50 μM, whithout relevant toxicity [18]. Interestingly, this dosage could approach the threshold to ameliorate the cytokine storm as well as inhibit the activity of TPCs. The use of Nar, a hydrophobic molecule able to cross biological membranes and to reach intracellular compartments, as a specific inhibitor of TPCs [4,5] provides further support for exploiting TPCs inhibition as a novel antiviral therapy. In our view, optimal Nar therapeutic delivery would require nanotechnological approaches and targeting the drug directly to the upper respiratory airways, a non-invasive method of administration, which would warrant direct and selective access at the sites most susceptible to SARS-CoV-2 infection, given the gradient of infectivity reported in the respiratory tree [19].
In conclusion, data presented in this work point to Nar as a safe anti-SARS-CoV-2 agent endowed with pan-coronavirus inhibitory activity. These findings offer a potential molecular model for CoV infection and a candidate drug target for further, in vivo, experimental trials aimed at improving the management of COVID-19 patients.
Declaration of Competing Interest
The authors report no declarations of interest
Acknowledgements
This work was supported by a grant to G.A. from the Italian Ministry of Health: COVID-19 A.F. 2020-2021 N. COVID-2020-12371817.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.phrs.2020.105255.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M., Mösbauer K., Hofmann-Winkler H., Kaul A., Kleine-Weber H., Krüger N., Gassen N.C., Müller M.A., Drosten C., Pöhlmann S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 3.Filippini A., D’Amore A., Palombi F., Carpaneto A. Could the Inhibition of Endo-Lysosomal Two-Pore Channels (TPCs) by the Natural Flavonoid Naringenin Represent an Option to Fight SARS-CoV-2 Infection? Front. Microbiol. 2020;11:970. doi: 10.3389/fmicb.2020.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pafumi I., Festa M., Papacci F., Lagostena L., Giunta C., Gutla V., Cornara L., Favia A., Palombi F., Gambale F., Filippini A., Carpaneto A. Naringenin impairs two-pore channel 2 activity and inhibits VEGF-Induced angiogenesis. Sci. Rep. 2017;7:5121. doi: 10.1038/s41598-017-04974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benkerrou D., Minicozzi V., Gradogna A., Milenkovic S., Bodrenko I.V., Festa M., Lagostena L., Cornara L., D’Amore A., Ceccarelli M., Filippini A., Carpaneto A. A perspective on the modulation of plant and animal two pore channels (TPCs) by the flavonoid naringenin. Biophys. Chem. 2019;254:106246. doi: 10.1016/j.bpc.2019.106246. [DOI] [PubMed] [Google Scholar]
- 6.Grimm C., Tang R. Could an endo-lysosomal ion channel be the Achilles heel of SARS-CoV2? Cell Calcium. 2020;88:102212. doi: 10.1016/j.ceca.2020.102212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen O.H., Gerasimenko O.V., Gerasimenko J.V. Endocytic uptake of SARS-CoV-2: the critical roles of pH, Ca2+ and NAADP. Function. 2020;1 doi: 10.1093/function/zqaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassileva K., Marsh M., Patel S. Two-pore channels as master regulators of membrane trafficking and endocytic well-being. Curr. Opin. Physiol. 2020;17:163–168. doi: 10.1016/j.cophys.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai Y., Kolokoltsov A.A., Chen C.-C., Tidwell M.W., Bauta W.E., Klugbauer N., Grimm C., Wahl-Schott C., Biel M., Davey R.A. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunaratne G.S., Yang Y., Li F., Walseth T.F., Marchant J.S. NAADP-dependent Ca2+ signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium. 2018;75:30–41. doi: 10.1016/j.ceca.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D.E., Min J.S., Jang M.S., Lee J.Y., Shin Y.S., Song J.H., Kim H.R., Kim S., Jin Y.-H., Kwon S. Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, Inhibit Human Coronavirus OC43 Infection of MRC-5 Human Lung Cells. Biomolecules. 2019;9 doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm C., Chen C.-C., Wahl-Schott C., Biel M. Two-Pore Channels: Catalyzers of Endolysosomal Transport and Function. Front. Pharmacol. 2017;8:45. doi: 10.3389/fphar.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchant J.S., Patel S. Two-pore channels at the intersection of endolysosomal membrane traffic. Biochem. Soc. Trans. 2015;43:434–441. doi: 10.1042/BST20140303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin L., Zeng W., Zhang F., Zhang C., Liang W. Naringenin Ameliorates Acute Inflammation by Regulating Intracellular Cytokine Degradation. J. Immunol. Baltim. Md 1950. 2017;199:3466–3477. doi: 10.4049/jimmunol.1602016. [DOI] [PubMed] [Google Scholar]
- 16.Salehi B., Fokou P.V.T., Sharifi-Rad M., Zucca P., Pezzani R., Martins N., Sharifi-Rad J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharm. Basel Switz. 2019;12 doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai Y., Peng W., Yang C., Zou W., Liu M., Wu H., Fan L., Li P., Zeng X., Su W. Pharmacokinetics and Metabolism of Naringin and Active Metabolite Naringenin in Rats, Dogs, Humans, and the Differences Between Species. Front. Pharmacol. 2020;11:364. doi: 10.3389/fphar.2020.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebello C.J., Beyl R.A., Lertora J.J.L., Greenway F.L., Ravussin E., Ribnicky D.M., Poulev A., Kennedy B.J., Castro H.F., Campagna S.R., Coulter A.A., Redman L.M. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020;22:91–98. doi: 10.1111/dom.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., Kato T., Lee R.E., Yount B.L., Mascenik T.M., Chen G., Olivier K.N., Ghio A., Tse L.V., Leist S.R., Gralinski L.E., Schäfer A., Dang H., Gilmore R., Nakano S., Sun L., Fulcher M.L., Livraghi-Butrico A., Nicely N.I., Cameron M., Cameron C., Kelvin D.J., de Silva A., Margolis D.M., Markmann A., Bartelt L., Zumwalt R., Martinez F.J., Salvatore S.P., Borczuk A., Tata P.R., Sontake V., Kimple A., Jaspers I., O’Neal W.K., Randell S.H., Boucher R.C., Baric R.S. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182:429–446. doi: 10.1016/j.cell.2020.05.042. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.