Abstract
Background
During the novel coronavirus disease (COVID-19) pandemic it is crucial for hospitals to implement infection prevention strategies to reduce nosocomial transmission to the lowest possible number. This is all the more important because molecular tests for identifying SARS-CoV-2 infected patients are uncertain, and the resources available for them are limited. In this view, a monocentric, retrospective study with an interventional character was conducted to investigate the extent to which the introduction of a strict hygiene bundle including a general mask requirement and daily screening for suspicious patients has an impact on the SARS-CoV-2 nosocomial rate in the pandemic environment.
Methods
All inpatients from a maximum care hospital in Regensburg (Bavaria) between March 1st and June 10th 2020 were included. Patient with respiratory symptoms were tested for SARS-CoV-2 at admission, patients were managed according to a standard hygiene protocol. At the end of March a strict hygiene bundle was introduced including a general mask obligation and a daily clinical screening of inpatients for respiratory symptoms. Nosocomial infection rate for COVID-19 and the risk for infection transmission estimated by the nosocomial incidence density before and after introduction the hygiene bundle were compared. The infection pressure for the hospital during the entire observational period was characterized by the infection reports in the region in relation to the number of hospitalized COVID-19 patients and the number of infected employees.
Results
In fact, after the introduction of a strict hygiene bundle including a general mouth and nose protection obligation and a daily clinical screening of suspicious patients, a significant reduction of the nosocomial rate from 0.28 to 0.06 (p = 0.026) was observed. Furthermore, the risk of spreading hospital-acquired infections also decreased dramatically from 0.0007 to 0.00018 (p = 0.031; rate ratio after/before 0.25 (95%CI 0.06, 1.07) despite a slow decrease of the hospital COVID 19-prevalence and an increase of infected employees.
Conclusion
The available data underline that a strict hygiene bundle seem to be associated with a decrease of nosocomial SARS-CoV-2 transmission in the pandemic situation.
Keywords: COVID-19, Nosocomial infection, Hygiene bundle, Surgical mask obligation
Background
According to Johns Hopkins University, since its outbreak in November 2019, approximately 30 million cases and more than 940.000 deaths have been recorded worldwide due to the novel coronavirus SARS-CoV-2 [1]. Although most cases are mild or asymptomatic, the novel coronavirus disease (COVID-19) is likely to pose a threat to certain segments of the population. For hospitals, as an indispensable part of the critical infrastructure and health care system, it is crucial to implement infection prevention strategies to reduce nosocomial transmission to the lowest possible number.
Although the infection pathway of SARS-CoV-2 is a droplet or airborne transmissible infection [2,3], the protective effect of surgical masks is unclear. However, the Robert Koch Institute recommends that face masks in health care facilities be worn by all health care professionals [4]. Indeed, there is growing evidence on the protective aspect of infection prevention by surgical masks for hospital staffs [5], but data are sparce with regard on their effect on the reduction of nosocomial infections. Therefore, the question arises whether and to what extent surgical face masks worn by medical staff can contribute to a reduced transmission of SARS-CoV-2 in hospitals.
The present monocentric study with interventional character investigated the effects of bundle measures for the prevention of nosocomial COVID-19 infections and the risk of spread in the clinical setting: an important part of the bundle was the introduction of requirement for wearing mouth and nose protection for hospital staff and inpatients. Furthermore, a daily triage on clinical symptoms was introduced to detect early infections with SARS-CoV-2. The last described measure was chosen since molecular testing of all patients was not possible for a long time due to a lack of test capacities.
Methods
Data collection and characterization of the infectious pressure for the hospital within the pandemic situation
The available retrospectively acquired data were collected in the period from March 1st to June 10th and originate from a maximum care hospital with 905 beds, which provided in-patient care for most of the COVID-19 patients within the Regensburg area (Bavaria, Germany). All inpatients were included within the above period. To better characterize the infection pressure during the course of COVID-19 pandemic within the Regensburg region, daily statistics of all identified COVID-19 cases from the area, hospital prevalence of COVID-10 and infections of hospital employees were obtained.
Indication for reverse transcription polymerase chain reaction (RT-PCR) testing/patients with suspicious symptoms/COVID-19
The indication for a SARS-CoV-2 test was given by the treating physician based on a triage which included the following criteria: respiratory symptoms, elevated body temperature >37.8 °C (according to the official guidelines from the Public Health England [6]), anamnestic data with respiratory symptoms/odour, taste and smell disturbances within the last 7 days or contact with a COVID-19 case. If one of the triage criteria was found to be abnormal, the patient was classified as potentially infectious and separated in an isolation area.
Patients with COVID-19 were characterized by age, gender and comorbidities (diseases of heart/lung (chronic obstructive pulmonary disease (COPD))/kidney/diabetes/tumor diseases) based on the ICD10 coded diagnoses.
Sampling for RT-PCR was performed by naso-/oropharyngeal swabbing recommended by official guidance of the WHO [7], or by oropharyngeal fluid specimen as recently published [8]. For RT-PCR a modified protocol was used recently published by Corman et al. [9].
Definition of nosocomial SARS-CoV-2 infections and risk of spread of infections
A nosocomial infection with SARS-CoV-2 was defined for the case of positive RT-PCR detection in hospitalized patients >6 days after hospitalization. According to data of the official German Health Care Services (Robert Koch Institute), the average incubation period for SARS-CoV-2 infections is 6 days. The nosocomial incidence density as a parameter of the risk of spread was calculated with the number of nosocomial infections per 1000 patient days.
Standard hygiene management for COVID-19
During the observation period COVID-19 patients or suspected cases were placed in a separate isolation area. There, the patients were isolated in single room isolation or even cohorted. They were cared for by physicians/nurses with area reference, who were not allowed to care for non-COVID patients. During medical or nursing care on COVID-19 patients or suspected cases, the staff wore protective clothing, which included respirators (FFP2), safety goggles, protective gowns and gloves. Access to the hospital was restricted until May 29th external visitors were not allowed.
Interventional bundle strategy including mask obligation and clinical screening for COVID-19 infections
After March 26th a strict surgical mask obligation was introduced throughout the hospital for all employees in all areas. This mask obligation included wearing of surgical masks (4 layers/splash proof) for medical staff at ward throughout the shift. Unless medical reasons presented a contraindication (e.g. respiratory insufficiency, cognitive disorders), patients were also instructed to wear masks especially when they wanted to leave the patient room or when medical staff or visitors were in the patient room. Due to non-systematic observations of the medical staff, patient compliance was good.
Furthermore, a daily clinical triage of all inpatients for suspicious symptoms was performed (respiratory symptoms, elevated body temperature >37.8 °C, odour, taste and smell disturbances) (Table 1 ).
Table 1.
Overview of infection prevention measures during the COVID-19 pandemic: at the end of March 2020, a strict hygiene bundle was introduced including a general mask obligation and a daily clinical screening for suspicious respiratory symptoms.
| Standard protocola | Hygiene bundle | |
|---|---|---|
| Triage of all patients at admission to hospital | Yes | Yes |
| Daily triage of all patients for symptoms of respiratory infections | No | Yes |
| Care of COVID-19 patients/and suspected cases in an isolation area | Yes | Yes |
| Cohorting of COVID-19 patients | Yes | No |
| Rapid (molecular) testing for SARS-CoV-2 of patients with respiratory symptoms | Yes | Yes |
| Respirators (FFP2)/protective clothing for nurses and doctors while caring for COVID 19 patients/suspected cases (direct contact) | Yes | Yes |
| Mandatory surgical masks for all employees | No | Yes |
| inpatients were advised to wear surgical masks | No | Yes |
| Restrictions on access to the hospital/ban on visiting patients | Yes | Yes |
Recommendations of the national health care services (KRINKO).
Statistics
Preliminary analyses including p-values for possible differences between the two groups—before and after intervention—with regard to age (t-test), gender (Fisher's exact test), pre-existing conditions (Fisher's exact test) was carried out, and COVID-19 prevalence/nosocomial rate (Fisher’s exact test) and nosocomial incidence density (Poisson’s exact test) were analyzed using SPSS, Version 26 software (SPSS, Chicago, USA) and R Version 4.0.0.
Results
Results of the clinical triage for suspicious symptoms and consecutive RT-PCR testing during the observational period
In the course of the clinical triage for COVID-19 suspicious patients, n = 1025 of 6106 patients were classified as suspicious (197/1061 before intervention; 828/5045 after introduction of the hygiene bundle). All suspicious patients were transferred to the isolation ward and tested for SARS-CoV-2; the testing rate was 18.5 (before intervention) and 16.4% after introduction of the hygiene bundle. Of the RT-PCR tested patients at admission, 27 were laboratory-confirmed for SARS-CoV-2 before intervention, and 86 after introducing the bundle measure (Table 2 ).
Table 2.
Results of triage of patients on admission with regard to conspicuous respiratory symptoms and results of RT-PCR for SARS-CoV-2 before and after introducing a strict hygiene bundle.
| N | Standard protocol | Hygiene bundle |
|---|---|---|
| All patients | 1061 | 5045 |
| Patients with conspicuous respiratory symptoms (%) | 197 (18.5) | 828 (16.4) |
| Laboratory confirmed SARS-CoV-2 at admission (%) | 27 (13.7) | 86 (10.3) |
From the remaining 5081 patients which were classified as unremarkable (864 before intervention/4217 during intervention), a total of 10 were identified as nosocomial COVID-19. So, a total of 123 SARS-CoV-2 PCR confirmed patients were included in the study (including the 10 nosocomial cases).
Characteristics of COVID-19
According to Table 3 , COVID-19 patients showed a comparable age and gender distribution both before and after introduction of the hygiene bundle. Likewise, the distribution of comorbidities (risk factors) such as heart, kidney and lung diseases, hypertension, diabetes, tumor diseases was high in both groups with 63 and 72% respectively, but not significantly different. Neither the rate of pneumonia nor the rate of mechanical ventilation was significantly different. The mortality before and during establishing the bundle was comparable with 22% and 16%.
Table 3.
Anthropometric and clinical data of COVID 19 patients before and after introducing a strict hygiene bundle.
| Characteristics | Standard protocol | Hygiene bundle | P |
|---|---|---|---|
| Cases | 27 | 86 | |
| gender n, (f / m) | 10/17 | 37/52 | 0.82 |
| age, years (std) | 66.7 (16.3) | 62.02 (15.4) | 0.19 |
| hospital stay, days (std) | 15.8 (16.0) | 14.8 (11.8) | 0.77 |
| any risk factor n (%) | 17 (63) | 64 (72) | 0.34 |
| - heart diseases | |||
| - hypertension | |||
| - kidney diseases | |||
| - diabetes | |||
| - lung diseases | |||
| - cancer | |||
| Pneumonia n (%) | 21 (78) | 65 (73) | 0.79 |
| Mechanical ventilation n (%) | 4 (15) | 16 (18) | 0.78 |
| Letality n (%) | 6 (22) | 14 (16) | 0.56 |
Infectious pressure for the hospital in the pandemic situation
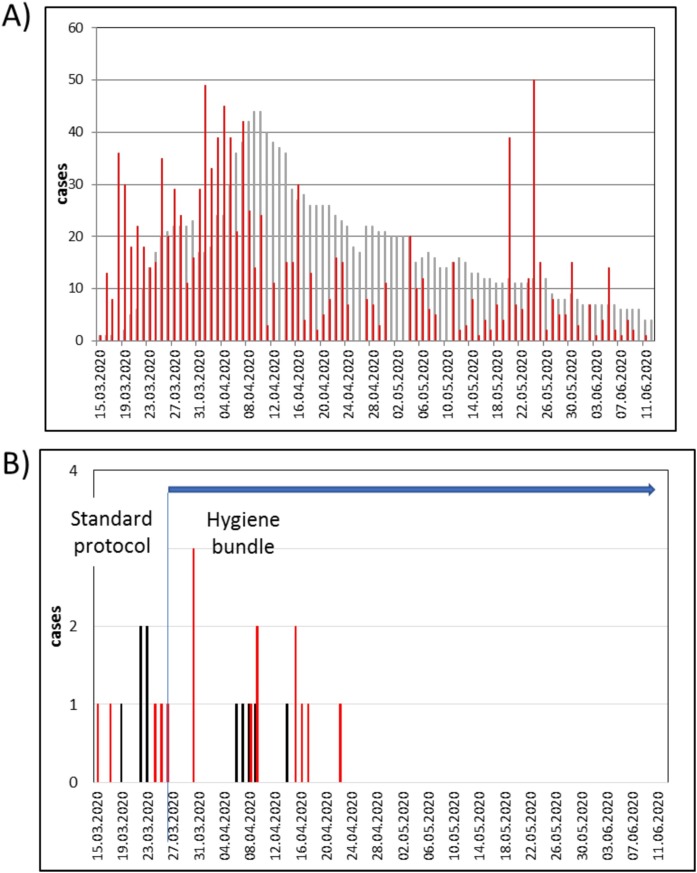
Fig. 1 A shows the daily statistics of COVID-19 inpatients over time demonstrating a rapid increase of patients from mid of March which peaked at April 10th. The epidemic curve of all reported cases of COVID-19 in the Regensburg area according to the date of laboratory confirmation (red bars) have shown also a parallel and rapid increase within the first two weeks of pandemic, followed by a slow decrease until June. Peak numbers in May show single outbreaks in facilities for asylum seekers without consequences for the hospitalization rate.
Fig. 1.
A) Time course of the total daily number of COVID-19 inpatients (grey bars) and course of the reported new infections in the region of Regensburg (red bars) (infections in the region Regensburg are based on the date of laboratory confirmation). B) Time course of SARS-CoV-2 infections in employees (red bars) and nosocomial infections in patients (black bars) before and during the introduction of a strict hygiene bundle including general obligation for hospital staff and inpatients to wear surgical masks (strict hygiene bundle was introduced at March 26th (blue horizontal) and lasted until June (blue vertical)).
Nosocomial rate before and after introduction of a hygiene bundle strategy
During the observational period a total of 10 nosocomial cases were identified, 5 cases in March (before intervention), and 5 cases in April (time course of nosocomial cases Fig. 1B). Before testing positive, nosocomial cases were hospitalized for an average of 13.7 days (range 12–82 days), and 3/10 patients had a history of negative SARS-CoV-2 RT-PCR prior day 6 of hospitalization. Suspicious symptoms at the daily triage were the indication for testing of 9/10 patients, one patient was tested positive before admission. Three of the nosocomial cases died from acute respiratory distress syndrome (ARDS).
With regard to SARS-CoV-2 infected employees (total, n = 18), 5 were found within March, and 13 by the end of April. Three employees who were tested positive on March 30th are likely to have been infected before March 26th (the date of introduction of the hygiene bundle). After that, no further infected employees were obvious.
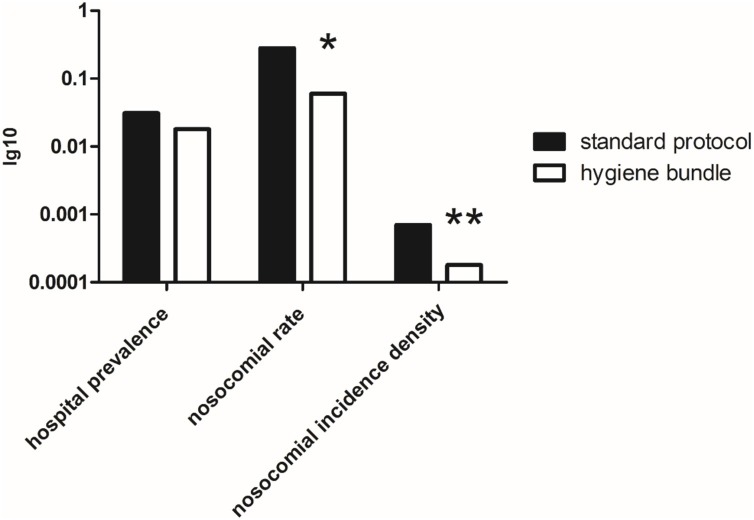
After the introduction of a strict mask requirement on March 26th for all employees during the entire shift and the instruction to wear masks on patients, the rate of nosocomial SARS-CoV-2 infections decreased by almost 80% from 0.28 (5/27 COVID-19) to 0.06 (5/87) (p = 0.026) (Fig. 2 ). Consequently, the nosocomial incidence density of SARS-CoV-2 as a parameter for the nosocomial risk of spread decreased from 0.0007 to 0.00018 by more three fourth (p = 0.031, rate ratio 0.25 (after/before) (95% CI 0.06, 1.07)). The total prevalence of COVID 19 at the hospital (COVID 19 cases / 100 patients) also decreased but not at a significant level (before intervention 0.031, during intervention 0.018 (p = 0.107)).
Fig. 2.
Overview of the hospital COVID-19 prevalence and the indices for nosocomial infections: although the hospital prevalence (COVID-19/all inpatients) did not significantly changed during the course (p = 0.107), the nosocomial rate (nosocomial COVID-19/all COVID-19) and the nosocomial incidence density (nosocomial COVID-19/1000 pd) decreased significantly after introduction of a strict hygiene bundle including a general mask obligation for hospital staff and inpatients and daily screening of suspicious patients (p = 0.026* (Fisher’s exact test) and p = 0.031** (Poisson’s exact test), respectively).
Discussion
Various studies on the transmission risk of SARS-CoV-2 have found high nosocomial rates with up to 15%, which suggested the role of unidentified infectious patients/infectious medical staff members and the lack of protective clothing [[10], [11], [12]]. The reason for rapid transmission is a combination of insufficient protection and a high reproduction index Ro of 2–3, which results from the transmission path (droplet infection/airborne transmission) and a high viral load of infected persons [[13], [14], [15]]. The present data suggest that the introduction of a hygiene bundle including a general obligation for hospital staff and inpatients for wearing surgical mask and a daily screening for suspicious patients is associated with a considerable reduction of the nosocomial rate for COVID 19 of almost 80%. In addition, the risk of a nosocomial SARS-CoV-2 transmission is also remarkable decreased. This is an interesting observation, especially since the infection pressure remained high due to the pandemic situation in the Regensburg area, which was characterized by an increasing number of positively tested employees and a high rate of COVID-19 inpatients during this observation period (Fig. 1A).
When evaluating hygiene measures to reduce nosocomial infections in seasonal (influenza) or pandemic (SARS-CoV-2) situations, factors that characterize infection pressure should always be taken into account: these may include not only information on the prevalence and rate of nosocomial infections, but also the course of infection in the population and the recording of infections among medical staff. The latter one because healthcare workers are a link between the outside world (population) and the inside of the hospital and are often described in studies as triggers for transmission of infections to patients [11,12,16].
The available data show that the increase in hospitalized COVID-19 can be well placed in the context of the development of infection rates in the Region. Furthermore, nosocomial COVID-19 cases occurred particularly in the first half of the pandemic, as did the number of infections among hospital staff. However, the introduction of a hygiene bundle including a mandatory mouth and nose protection for medical staff and inpatients in the first third of the pandemic was significantly associated with a decrease in the nosocomial rate and the risk of nosocomial transmission of infection. In contrast, the number of infected staff was not reduced after the introduction of compulsory masks. In this context, it should be noted that surgical masks are used primarily to protect the environment from the wearer, but most studies have investigated its effect with regard to self-protection [17]. In a recently published meta-analysis on preventive strategies for transmission of SARS-CoV-2, a distance of one meter or more has been shown to be the most effective measure, followed by the wearing of face masks [5]. With regard to influenza, we have already shown that a strict mask policy for hospital employees leads to a 50% reduction in nosocomial infections [18] as it is also hypothesed for the present study on SARS-CoV-2. However, there are contradictory experimental and clinical studies on the functionality of surgical masks with regard to their preventive effect: in the study by Tang et al. [19], it could basically be shown that wearing surgical masks significantly reduced an aerosol cloud in the sagittal direction; theoretical and experimental considerations suggest that surgical masks filter both large and medium droplet sizes and can therefore reduce aerosol SARS-CoV-2 transmission [20]; indeed, in animal studies a reduction of SARS-CoV-2 infection transmission by wearing surgical masks could be demonstrated [21]. In contrast, in the study by Baer et al. [22], wearing surgical masks did not significantly reduce the number of infectious SARS-CoV-2 viruses during coughing. The study situation shows that there are contradictory data on the actual efficacy of masks, and that ultimately randomized, controlled studies are almost completely lacking.
If one focuses on the procedure at admission for patients and the monitoring of inpatients with suspicious symptoms in the available data, it is evident that only few RT-PCR tests had to be performed in the overall process. The focus was always on strict hygiene management and clinical monitoring: tests were only performed if the patient was conspicuous in triage. Nevertheless, only few nosocomial transmissions were counted. In general, the use of PCR testing for screening purposes might be a possible measure particular to identify asymptomatic infections [23,24]. Nevertheless, it must be borne in mind that its use for admission screening involves a considerable consumption of resources and it becomes more effective and with a higher incidence of SARS-CoV-2 in a population [25,26]. Therefore, the combination of thorough clinical screening, RT-PCR testing and consistent use of basic hygiene measures as shown in the present study can effectively reduce rates of nosocomial SARS-CoV-2 transmission.
With regard to the presented clinical data of COVID-19, there was no significant difference between patients admitted before and during the intervention. Overall, a high proportion of patients showed risk factors, and a ventilation frequency between 15 and 18% was found with a total mortality rate of 13 and 16%. In a study on German COVID-19 patients (n = 100) with comparable gender and age distribution, all of the patients (100%) had at least one risk factor for severe COVID-19, whereby a broader spectrum of risk factors was evaluated (adipositas, nicotine abuse, hepatitis, liver failure, cerebral arterial occlusive disease) [27]. The mortality rate in this study was 14%, only slightly lower than in our collective.
It should be noted that there are some limitations of the present study. One is that the present data are from a monocentric study with a relatively small number of COVID-19, which raises statistical problems (bias) and cannot necessarily be considered representative. In addition, there is a before-and-after observational design and a retrospective data evaluation; a randomized controlled trial would certainly have been more meaningful. Due to the triage based on clinical symptoms, asymptomatic infections remain undetected; these are definitely not considered in the present study. On the other hand, in the collected data there are no hints on major in-hospital outbreaks or severe nosocomial cases during the observed period. Furthermore, since no internationally accepted definition of nosocomial cases exists, our definition of nosocomial cases is an internal one, and does not include previous test results. However, due to the long hospitalization period of our nosocomial cases of 13.7 days a mentioned above (Results, chapter 3.4), a nosocomial infection is very likely.
Conclusion
The present study is one of the few that combines SARS-CoV-2 infection data from the population with data from a supplying hospital. Some data are only descriptive, but nevertheless interesting and transferable to the course of the pandemic and the pressure of infection. Despite some uncertainties, we believe that a hygiene bundle including a general mouth and nose protection policy for hospital employees and inpatients is highly suitable for the preventiozn of nosocomial SARS-CoV-2 transmission.
Funding
The present work did not receive any specific grant.
Conflict of interest
None.
References
- 1.https://coronavirus.jhu.edu/map.html.
- 2.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020:109819. doi: 10.1016/j.envres.2020.109819. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang R., Li Y., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci USA. 2020;117(26):14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Empfehlungen des Robert Koch Institutes; Erweiterte Hygienemaßnahmen im Gesundheitswesen im Rahmen der COVID-10 Pandemie https://www.rki.de/DE/Content/InfAZ/N/NeuartigesCoronavirus/erweiterte/Hygiene.htm.
- 5.Chu D., Akl E.A., Solo K., Yaacoub S., Schünemann H., on behalf of the COVID-19 Systematic Urgent Review Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis Group Effort (SURGE) study authors. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Public Health England: Guidance COVID-19: investigation and initial clinical management of possible cases https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection.
- 7.World Health Organization (WHO) Diagnostic testing for SARS-CoV-2 https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2.
- 8.Guo W., Feng Q., Ye F., Li S., Hong C., Chen L., Li S. Effect of throat washings on detection of 2019 novel coronavirus. Clin Inf Dis. 2020:ciaa416. doi: 10.1093/cid/ciaa416. Published online 2020 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corman V., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. https://pubmed.ncbi.nlm.nih.gov/31992387/ PMDI 31992387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rickman H., Rampling T., Shaw K., Martinez-Garcia G., Hail L., Coen P. Nosocomial transmission of COVID-19: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Inf Dis. 2020 doi: 10.1093/cid/ciaa816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabesch M., Roth S., Brandstetter S., Häusler S., Juraschko E., Weigl M. Successful containment of Covid-19 outbreak in a large maternity and perinatal center while continuing clinical service. Pediatr All Clin Immunol. 2020 doi: 10.1111/pai.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Gayle A.A., Wilder-Smith A., Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27:taaa021. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wölfel R., Corman V., Guggemos W., Seilmaier M., Zange S., Müller M. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 15.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections - more than just the common cold. JAMA. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 16.Voirin N., Barret B., Metzger M.H., Vanhems P. Hospital-acquired influenza: a synthesis using the Outbreak Reports and Intervention Studies of Nosocomial Infection (ORION) statement. J Hospital Inf. 2009;71:1–14. doi: 10.1016/j.jhin.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Long Y., Hu T., Liu L., Chen R., Guo Q., Yang L. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 doi: 10.1111/jebm.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosch A., Rockmann F. Effect of a two step hygiene management on the prevention of nosocomial influenza in a season with high influenza activity. J Hospital Infect. 2016;94:139–143. doi: 10.1016/j.jhin.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Tang J.W., Liebner T.J., Craven B., Settles G.S. A schlieren optical study oft the human cough with and without wearing masks for aerosol infection control. J R Soc Interface. 2009;6(Suppl):S727–S736. doi: 10.1098/rsif.2009.0295.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prather K., Wang C., Scholley R. Reducing transmission of SARS-CoV 2. Science. 2020 doi: 10.1126/j.jiph.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Chan J., Yuan S., Zhang A., Poon V., Chan C., Lee A. Surgical mask partition reduces the risk of noncontact transmission in a golden syrian hamstermodel for coronavirus disease 2019 (COVID-19) Clin Inf Dis. 2020 doi: 10.1093/cid/ciaa644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baer S., Kim M.C., Kim Y. Effectiveness of surgical and cotton masks in locking SARS-CoV 2: a controlled comparison in 4 patients. Ann Int Med. 2020 doi: 10.7326/M20-1342. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S., Hayashi K. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94(May):154–155. doi: 10.1016/j.ijid.2020.03.020. Epub 2020 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Public Health–Seattle and King County and CDC COVID-19 Investigation Team Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucirca L., Lauer S., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 test by time since exposure. Ann Intern Med. 2020 doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundmann H., Donker T., Hengel H., Bürkle H., Hammer T., Wenz F. et al. Switching from algorithm-based to universal admission screening for COVID-19 in hospital seetting. medRxiv 10.1101/2020.08.07.201700001. [DOI]
- 27.Dreher M., Kersten A., Bickenbach J., Balfanz P., Hartmann B., Cornelissen C. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int. 2020;117:271–278. doi: 10.3238/arztebl.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]