Abstract
Purpose
During COVID-19, many operating rooms were reserved exclusively for emergent cases. As a result, many elective surgeries for renal cell carcinoma (RCC) were deferred, with an unknown impact on outcomes. Since surveillance is commonplace for small renal masses, we focused on larger, organ-confined RCCs. Our primary endpoint was pT3a upstaging and our secondary endpoint was overall survival.
Materials and methods
We retrospectively abstracted cT1b-T2bN0M0 RCC patients from the National Cancer Database, stratifying them by clinical stage and time from diagnosis to surgery. We selected only those patients who underwent surgery. Patients were grouped by having surgery within 1 month, 1–3 months, or >3 months after diagnosis. Logistic regression models measured pT3a upstaging risk. Kaplan Meier curves and Cox proportional hazards models assessed overall survival.
Results
A total of 29,746 patients underwent partial or radical nephrectomy. Delaying surgery >3 months after diagnosis did not confer pT3a upstaging risk among cT1b (OR = 0.90; 95% CI: 0.77–1.05, P = 0.170), cT2a (OR = 0.90; 95% CI: 0.69–1.19, P = 0.454), or cT2b (OR = 0.96; 95% CI: 0.62–1.51, P = 0.873). In all clinical stage strata, nonclear cell RCCs were significantly less likely to be upstaged (P <0.001). A sensitivity analysis, performed for delays of <1, 1–3, 3–6, and >6 months, also showed no increase in upstaging risk.
Conclusion
Delaying surgery up to, and even beyond, 3 months does not significantly increase risk of tumor progression in clinically localized RCC. However, if deciding to delay surgery due to COVID-19, tumor histology, growth kinetics, patient comorbidities, and hospital capacity/resources, should be considered.
Keywords: Renal cell carcinoma, Kidney cancer, Nephrectomy, Surgical delay, COVID-19
1. Introduction
The emergence and rapid spread of the 2019 Novel Coronavirus (COVID-19) has disrupted healthcare systems and highlighted new perspectives on healthcare delivery. As a result of the pandemic's exponential growth, hospitals, in conjunction with professional body recommendations, have deferred nonemergent surgeries [1]. These delayed surgeries include many potentially curative urologic oncologic surgeries [2,3], such as partial and radical nephrectomies for renal cell carcinoma (RCC), which remain the preferred curative treatment for localized kidney cancer [4].
As a result of the COVID-19 pandemic, many patients with known renal masses will experience surgical treatment delays. For oncology patients, delay of definitive treatment may increase the risk of progression to metastatic disease and consequently allow some neoplasms to transform from curable to uncurable [5]. Thus, urologists and kidney cancer patients must understand the effects of treatment delay, both during the COVID-19 pandemic and in the coming months [6].
Active surveillance literature has demonstrated that urologists can safely monitor most cT1a renal masses [4,7,8]. However, for cT1b-cT2b masses this is less clear. The effect of surgical delay on kidney cancer outcomes has not been robustly evaluated [9], [10], [11]. In this current study, we examine oncologic outcomes as a function of time to surgery using the National Cancer Database (NCDB). Specifically, we selected only those patients who underwent surgery to best model the effects of surgical delay on patients scheduled to receive surgery.
2. Materials and methods
2.1. Data collection and source
The NCDB, a national oncology registry, records approximately 70% of the incident cancer cases in the United States. It contains information regarding patient demographics, neoplasm staging (both clinical and pathologic staging), surgical treatment, and survival outcomes [12], [13], [14].
2.2. Study population
Exploratory analysis identified 422,258 patients with kidney cancer between 2004 and 2014. We excluded patients with missing staging data or histology. NCDB captures the first course of treatment for patients. Patients receiving percutaneous ablation, active surveillance, or who never underwent surgery (partial or radical nephrectomy) were excluded. The study population only contains cT1b–T2bN0M0 patients. Lastly, we identified and excluded 23,065 patients whose time from diagnosis of kidney cancer to surgery was coded as “0 days.”
2.3. Data analysis
Patients were stratified into 3 groups by clinical stage (cT1b, cT2a, and cT2b). Within each clinical stage strata, patients were grouped by their time from diagnosis to surgery: <1 month, 1–3 months, and >3 months. Means and medians assessed continuous variables. Frequencies and proportions characterized categorical variables.
We examined how surgical delay may increase risk of pathologic T3a (pT3a) upstaging using logistic regression. We defined upstaging in patients with clinically localized disease (cT1-cT2) who had pT3a disease after receiving partial or radical nephrectomy. For example, we did not classify a patient with a cT1b lesion who is found to have pT2a disease as upstaged. We excluded those upstaged to ≥pT3b tumors as these constituted only 1.7% of the entire cohort.
Survival analyses conducted within each clinical stage strata grouped patients by time from diagnosis to surgery. Only overall survival (OS) was assessed, as the NCDB does not contain information regarding cancer-specific survival (CSS) nor recurrence-free survival. Kaplan-Meier curves illustrated OS among these patient groups and produced survival percentages at 1, 3, 5, and 10 years. Log rank tests quantified statistical differences in survival. Cox proportional hazards modeled how delays in surgery correlated to OS in both univariate and multivariable models. Multivariable models were constructed to adjust for various clinical factors, such as histology, tumor size, insurance status, and ethnicity.
Lastly, we conducted a sensitivity analysis for pT3a upstaging and OS, by sub-stratifying surgical delay into <1 month, 1–3 months, 3–6 months, and >6 months. All analyses were conducted using SAS statistical software package (v. 9.4; SAS Institute Inc., Cary, NC).
3. Results
3.1. Cohort demographics
The final analytical cohort encompassed 29,746 patients with cT1b (N = 22,719), cT2a (N = 4,670), and cT2b (N = 2,357) tumors. Table 1 describes cohort demographics by clinical stage. Median follow-up for cT1b, cT2a, and cT2b tumors was 47.7 (interquartile range [IQR]: 45.1), 33.2 (IQR: 24.8), and 35.2 (IQR: 24.9) months, respectively.
Table 1.
Cohort demographics
| cT1b | cT2a | cT2b | |
|---|---|---|---|
| Variables | Mean / Median / Count (SD / Percentage) | Mean / Median / Count (SD / Percentage) | Mean / Median / Count (SD / Percentage) |
| N | 22,719 | 4,670 | 2,357 |
| Follow up | |||
| Median (IQR) | 47.7 (45.1) | 33.2 (24.8) | 35.2 (24.9) |
| Age | |||
| Mean (SD) | 62 (12.4) | 61.5 (12.1) | 60.1 (11.8) |
| Median (IQR) | 63 (17) | 62 (17) | 61 (16) |
| Sex, n (%) | |||
| Female | 8,622 (38.0) | 1,603 (34.3) | 780 (33.1) |
| Male | 14,097 (62.0) | 3,706 (65.7) | 1,577 (66.9) |
| Race | |||
| White | 19,351 (85.2) | 4,025 (86) | 1,971 (83.6) |
| Black | 2,493 (11.0) | 467 (10.0) | 294 (12.5) |
| Others | 675 (3.0) | 152 (3.2) | 75 (3.2) |
| Unknown | 200 (0.8) | 35 (0.8) | 17 (0.7) |
| Comorbidity index | |||
| 0 | 15,035 (66.2) | 3,202 (68.4) | 1,724 (73.1) |
| 1 | 5,631 (24.8) | 1,118 (23.9) | 495 (21) |
| 2+ | 2,053 (9.0) | 359 (7.7) | 138 (5.9) |
| Histology | |||
| Clear | 18,270 (80.4) | 3,758 (80.3) | 1,626 (69) |
| Non-Clear | 4,331 (19.1) | 873 (18.7) | 680 (28.8) |
| Sarcomatoid | 118 (0.5) | 48 (1.0) | 51 (2.2) |
| Insurance | |||
| None | 741 (3.3) | 200 (4.3) | 135 (5.7) |
| Private | 10,239 (45.1) | 2,175 (46.5) | 1,197 (50.8) |
| Medicaid | 1,206 (5.3) | 254 (5.4) | 154 (6.5) |
| Medicare | 9,978 (43.9) | 1,942 (41.5) | 821 (34.8) |
| Other Gov | 275 (1.2) | 59 (1.3) | 30 (1.3) |
| Unknown | 280 (1.2) | 49 (1.0) | 20 (0.8) |
| Income, n (%) | |||
| <$38,000 | 4,001 (17.7) | 805 (17.3) | 418 (17.8) |
| $38,000–$47,999 | 5,528 (24.5) | 1,115 (23.9) | 568 (24.2) |
| $48,000–$62,999 | 6,204 (27.5) | 1,345 (28.8) | 666 (28.4) |
| $63,000+ | 6,830 (30.3) | 1,402 (30.0) | 695 (29.6) |
| Facility type | |||
| Community Cancer program | 1,668 (7.3) | 397 (8.5) | 183 (7.8) |
| Comprehensive community cancer | 9,263 (40.8) | 1,903 (40.7) | 907 (38.5) |
| Academic/research program | 9,333 (41.1) | 1,843 (39.4) | 1,018 (43.2) |
| Integrated network cancer program | 2,455 (10.8) | 536 (11.5) | 249 (10.6) |
| Distance to facility (miles) | |||
| <12.5 | 11,541 (51.1) | 2,402 (51.5) | 1,160 (49.4) |
| 12.5–49.9 | 7,587 (33.6) | 1,579 (33.8) | 779 (33.2) |
| 50–249.9 | 3,166 (14.0) | 627 (13.4) | 382 (16.3) |
| >250 | 274 (1.2) | 57 (1.2) | 29 (1.2) |
| Time to surgery from Diagnosis (months) | |||
| <1 | 8,303 (37.4) | 2,329 (50.5) | 1,424 (61.5) |
| 1–3 | 10,890 (49.1) | 1,914 (41.5) | 769 (33.2) |
| 3 | 2,995 (13.5) | 370 (8.0) | 123 (5.3) |
| Time to surgery from Diagnosis (months) | |||
| Mean (SD) | 1.8 (1.9) | 1.4 (1.5) | 1.1 (1.2) |
| Median (IQR) | 1.3 (1.4) | 1 (1.2) | 0.8 (0.9) |
| Lymph node surgery | |||
| No | 20,750 (91.3) | 3,797 (81.1) | 1,625 (68.9) |
| Yes | 1,825 (8) | 855 (18.3) | 718 (30.5) |
| Unknown | 144 (0.6) | 27 (0.6) | 14 (0.6) |
| Lymph node status | |||
| Negative | 1,704 (93.4) | 785 (91.8) | 655 (91.2) |
| Positive | 75 (4.1) | 60 (7.0) | 52 (7.3) |
| Unknown | 46(2.5) | 10 (1.2) | 11 (1.5) |
| Systemic therapy | |||
| No systemic therapy | 20,693 (98.0) | 4,521 (96.6) | 2,220 (94.2) |
| Yes | 220 (1.1) | 138 (2.8) | 116 (4.9) |
| Unknown | 193 (0.9) | 30 (0.6) | 21 (0.9) |
| Tumor size (mm) | |||
| Mean (SD) | 52.8 (19.9) | 83.6 (40.7) | 126.8 (84.2) |
| Median (IQR) | 50 (15) | 80 (15) | 115 (22) |
IQR = interquartile range; SD = standard deviation
While median time from diagnosis to surgery was similar between clinical stage groups, we found cT1b (13.5%) had a higher proportion of patients who had surgery >3 months after diagnosis compared to cT2a (8.0%) and cT2b (5.3%) (Table 1).
3.2. pT3a Upstaging analysis
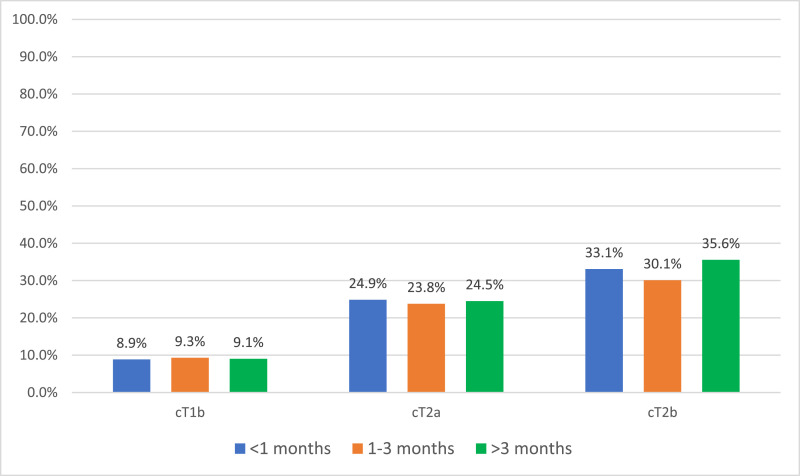
We observed upstaging rates of 9.1%, 24.4%, and 32.3% for cT1b, cT2a, and cT2b tumors, respectively (Supplemental Fig. 1). As clinical stage increases, overall rates of upstaging increase regardless of surgical waiting time (Fig. 1 ). On multivariable logistic regression, surgery occurring 1-3 months after diagnosis did not increase odds of upstaging for any clinical stage compared to surgery <1 month from diagnosis. Similarly, for time to surgery >3 months, multivariable models did not demonstrate increased risk of pT3a upstaging for cT1b (OR = 0.90, 95% CI: 0.77–1.05; P = 0.170), cT2a (OR = 0.90, 95% CI: 0.69–1.19; P = 0.454), or cT2b (OR = 0.96, 95% CI: 0.62–1.51; P = 0.873) masses (Table 2, Table 3, Table 4 ). In each clinical stage strata, we found that age, male sex, Charlson comorbidity index ≥2, and tumor size correlated with upstaging. Non-clear cell histology was inversely associated with upstaging risk in each clinical stage (Table 2, Table 3, Table 4).
Fig. 1.
pT3a Up-staging rates by clinical stage and surgical delay.
Table 2.
Up-staging of cT1b RCC by surgical delay
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Variables | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Surgical Delay | ||||
| <1 mon | Ref | - | Ref | - |
| 1–3 mon | 1.05 [0.95, 1.16] | 0.371 | 0.96 [0.86, 1.07] | 0.447 |
| >3 mon | 1.06 [0.92, 1.23] | 0.422 | 0.90 [0.77, 1.05] | 0.170 |
| Age | ||||
| Per year | 1.03[1.02, 1.03] | <0.001 | 1.03 [1.02, 1.03] | <0.001 |
| Sex | ||||
| Male | Ref | - | Ref | - |
| Female | 0.75 [0.68, 0.82] | <0.001 | 0.71 [0.64, 0.79] | <0.001 |
| Charlson-Deyo | ||||
| 0 | Ref | - | Ref | - |
| 1 | 1.21 [1.09, 1.35] | <0.001 | 1.15 [1.03, 1.29] | 0.010 |
| 2 | 1.40 [1.20, 1.63] | <0.001 | 1.33 [1.14, 1.55] | <0.001 |
| Race | ||||
| White | Ref | - | Ref | - |
| Black | 0.68 [0.57, 0.81] | <0.001 | 0.76 [0.64, 0.91] | 0.004 |
| Others | 1.05 [0.81, 1.37] | 0.705 | 1.07 [0.81, 1.41] | 0.643 |
| Unknown | 0.94 [0.57, 1.57] | 0.823 | 0.95 [0.57, 1.6] | 0.855 |
| Insurance | ||||
| Private | Ref | - | Ref | - |
| Not insured | 1.02 [0.75, 1.32] | 0.983 | 1.14 [0.86, 1.52] | 0.37 |
| Medicaid | 0.86 [0.68, 1.1] | 0.227 | 0.93 [0.73, 1.19] | 0.59 |
| Medicare | 1.40 [1.27, 1.55] | <0.001 | 0.95 [0.84, 1.08] | 0.451 |
| Other Gov | 0.96 [0.61, 1.52] | 0.877 | 0.86 [0.53, 1.38] | 0.528 |
| Unknown | 0.85 [0.51, 1.41] | 0.531 | 0.79 [0.48, 1.32] | 0.375 |
| Income | ||||
| <$38,000 | Ref | - | - | - |
| $38,000–$47,999 | 0.95 [0.82, 1.1] | 0.482 | 0.90 [0.76, 1.05] | 0.179 |
| $48,000–$62,999 | 1.02 [0.89, 1.18] | 0.733 | 0.96 [0.81, 1.14] | 0.654 |
| $63,000+ | 1.06 [0.92, 1.22] | 0.399 | 1.01 [0.83, 1.23] | 0.884 |
| Education (no high school %) | ||||
| >21% or more | Ref | - | Ref | - |
| 13–20.9% | 0.96 [0.83, 1.11] | 0.595 | 0.92 [0.79, 1.08] | 0.313 |
| 7–12.9% | 1.04 [0.9, 1.19] | 0.609 | 0.92 [0.77, 1.09] | 0.342 |
| < 7% | 1.03 [0.89, 1.2] | 0.675 | 0.87 [0.71, 1.07] | 0.191 |
| Facility type | ||||
| Academic | Ref | - | Ref | - |
| Community Cancer Center | 0.61 [0.49, 0.75] | <0.001 | 0.58 [0.46, 0.72] | <0.001 |
| Comprehensive community cancer center | 0.79 [0.71, 0.88] | <0.001 | 0.76 [0.68, 0.86] | <0.001 |
| Integrated Network Cancer program | 1.05 [0.91, 1.22] | 0.511 | 1.12 [0.95, 1.31] | 0.175 |
| Facility location | ||||
| Northeast | Ref | - | Ref | - |
| Midwest | 0.96 [0.84, 1.09] | 0.535 | 0.96 [0.83, 1.1] | 0.527 |
| South | 0.82 [0.72, 0.93] | 0.002 | 0.84 [0.73, 0.96] | 0.012 |
| West | 0.92 [0.78, 1.07] | 0.290 | 0.93 [0.79, 1.1] | 0.411 |
| Distance | ||||
| <12.5 mi | Ref | - | Ref | - |
| 12.5–49.9 mi | 1.13 [1.02, 1.25] | 0.021 | 1.12 [1.01, 1.25] | 0.042 |
| 50–249.9 mi | 1.28 [1.11, 1.46] | <0.001 | 1.19 [1.03, 1.38] | 0.020 |
| 250 mi or more | 1.08 [0.7, 1.66] | 0.732 | 0.97 [0.63, 1.51] | 0.905 |
| Histology | ||||
| Clear cell | Ref | - | Ref | - |
| Non-Clear cell | 0.78 [0.69, 0.88] | <0.001 | 0.75 [0.66, 0.85] | <0.001 |
| Sarcomatoid | 3.74 [2.47, 5.66] | <0.001 | 3.47 [2.27, 5.31] | <0.001 |
| Tumor size | ||||
| Per mm | 1.01 [1.01, 1.01] | <0.001 | 1.01 [1.01, 1.01] | <0.001 |
mm = millimeter; OR = odds ratio; RCC = renal cell carcinoma; 95% CI = 95% confidence interval.
Table 3.
Up-staging of cT2a RCC by surgical delay
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Variables | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Surgical Delays | ||||
| <1 mon | Ref | - | Ref | - |
| 1–3 mon | 1.01 [0.87, 1.16] | 0.983 | 0.93 [0.8, 1.09] | 0.379 |
| >3 mon | 0.98 [0.75, 1.27] | 0.866 | 0.90 [0.69, 1.19] | 0.454 |
| Age | ||||
| Per year | 1.02 [1.02, 1.03] | <0.001 | 1.02 [1.02, 1.03] | <0.001 |
| Sex | ||||
| Male | Ref | - | Ref | - |
| Female | 0.75 [0.65, 0.87] | 0.002 | 0.74 [0.63, 0.86] | <0.001 |
| Charlson-Deyo | ||||
| 0 | Ref | - | Ref | - |
| 1 | 1.17 [1, 1.38] | 0.056 | 1.11 [0.94, 1.32] | 0.207 |
| 2 | 1.63 [1.27, 2.08] | <0.001 | 1.49 [1.15, 1.93] | 0.002 |
| Race | ||||
| White | Ref | - | Ref | - |
| Black | 0.52 [0.4, 0.68] | <0.001 | 0.62 [0.46, 0.82] | 0.0001 |
| Others | 1.19 [0.81, 1.74] | 0.375 | 1.22 [0.82, 1.82] | 0.326 |
| Unknown | 0.69 [0.29, 1.65] | 0.405 | 0.71 [0.29, 1.73] | 0.448 |
| Insurance | ||||
| Private | Ref | - | Ref | - |
| Not insured | 0.97 [0.68, 1.39] | 0.881 | 1.07 [0.73, 1.55] | 0.740 |
| Medicaid | 0.74 [0.52, 1.04] | 0.087 | 0.81 [0.56, 1.16] | 0.242 |
| Medicare | 1.35 [1.17, 1.56] | <0.001 | 1.02 [0.82, 1.22] | 0.984 |
| Other Gov | 1.20 [0.54, 1.87] | 0.991 | 0.89 [0.46, 1.7] | 0.721 |
| Unknown | 0.63 [0.28, 1.39] | 0.252 | 0.68 [0.3, 1.53] | 0.354 |
| Income | ||||
| <$38,000 | Ref | - | Ref | - |
| $38,000–$47,999 | 1.22 [0.98, 1.52] | 0.075 | 1.04 [0.82, 1.33] | 0.746 |
| $48,000–$62,999 | 1.06 [0.86, 1.32] | 0.581 | 0.86 [0.66, 1.12] | 0.256 |
| $63,000+ | 1.17 [0.95, 1.45] | 0.136 | 0.92 [0.68, 1.25] | 0.604 |
| Education (no high school %) | ||||
| >21% or more | Ref | - | Ref | - |
| 13–20.9% | 1.12 [0.89, 1.39] | 0.329 | 1.04 [0.81, 1.32] | 0.771 |
| 7–12.9% | 1.23 [1, 1.53] | 0.051 | 1.18 [0.91, 1.54] | 0.212 |
| < 7% | 1.14 [0.91, 1.43] | 0.244 | 1.02 [0.75, 1.4] | 0.877 |
| Facility type | ||||
| Academic | Ref | - | Ref | - |
| Community Cancer Center | 0.45 [0.33, 0.61] | <0.001 | 0.41 [0.3, 0.57] | <0.001 |
| Comprehensive community cancer center | 0.72 [0.62, 0.84] | <0.001 | 0.68 [0.57, 0.81] | <0.001 |
| Integrated Network Cancer program | 1.05 [0.85, 1.31] | 0.652 | 1.11 [0.88, 1.41] | 0.370 |
| Facility location | ||||
| Northeast | Ref | - | Ref | - |
| Midwest | 0.95 [0.78, 1.16] | 0.610 | 0.90 [0.72, 1.11] | 0.329 |
| South | 0.80 [0.66, 0.98] | 0.030 | 0.83 [0.67, 1.03] | 0.093 |
| West | 0.76 [0.59, 0.97] | 0.027 | 0.77 [0.59, 0.997] | 0.047 |
| Distance | ||||
| <12.5 mi | Ref | - | Ref | |
| 12.5–49.9 mi | 1.27 [1.09, 1.47] | 0.002 | 1.15 [0.98, 1.35] | 0.087 |
| 50–249.9 mi | 1.23 [1, 1.52] | 0.051 | 1.02 [0.79, 1.26] | 0.171 |
| 250 mi or more | 1.88 [1.08, 3.27] | 0.026 | 1.51 [0.84, 2.71] | 0.990 |
| Histology | ||||
| Clear cell | Ref | - | Ref | - |
| Non-Clear cell | 0.51 [0.42, 0.62] | <0.001 | 0.51 [0.41, 0.62] | <0.001 |
| Sarcomatoid | 1.88 [0.99, 3.57] | 0.054 | 1.75 [0.89, 3.43] | 0.105 |
| Tumor size | ||||
| Per mm | 1.00 [1.00, 1.00] | 0.052 | 1.00 [1.00, 1.00] | 0.055 |
mm = millimeter; OR = odds ratio; RCC = renal cell carcinoma; 95% CI = 95% confidence interval.
Table 4.
Up-staging of cT2b RCC by surgical delay
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Variables | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Surgical Delays | ||||
| <1 mon | Ref | - | Ref | - |
| 1–3 mon | 0.83 [0.68, 1.01] | 0.066 | 0.87 [0.7, 1.07] | 0.186 |
| >3 mon | 0.93 [0.62, 1.42] | 0.752 | 0.96 [0.62, 1.51] | 0.873 |
| Age | ||||
| Per year | 1.00 [0.99, 1.01] | 0.557 | 1.01 [0.99, 1.02] | 0.261 |
| Sex | ||||
| Male | Ref | - | Ref | - |
| Female | 0.84 [0.69, 1.02] | 0.080 | 0.79 [0.65, 0.98] | 0.029 |
| Charlson-Deyo | ||||
| 0 | Ref | - | Ref | - |
| 1 | 1.18 [0.94, 1.47] | 0.149 | 1.15 [0.91, 1.44] | 0.252 |
| 2 | 0.66 [0.43, 1.03] | 0.065 | 0.71 [0.45, 1.11] | 0.137 |
| Race | ||||
| White | Ref | - | Ref | - |
| Black | 0.63 [0.47, 0.85] | 0.023 | 0.93 [0.67, 1.29] | 0.659 |
| Others | 0.91 [0.54, 1.54] | 0.732 | 0.80 [0.45, 1.40] | 0.431 |
| Unknown | 0.37 [0.09, 1.51] | 0.167 | 0.32 [0.08, 1.31] | 0.113 |
| Insurance | ||||
| Private | Ref | - | Ref | - |
| Not insured | 0.80 [0.53, 1.23] | 0.308 | 0.83 [0.53, 1.31] | 0.426 |
| Medicaid | 0.80 [0.54, 1.19] | 0.267 | 0.91 [0.6, 1.38] | 0.665 |
| Medicare | 0.86 [0.71, 1.06] | 0.152 | 0.83 [0.64, 1.08] | 0.159 |
| Other Gov | 0.34 [0.11, 1.08] | 0.068 | 0.31 [0.09, 1] | 0.050 |
| Unknown | 1.41 [0.53, 3.73] | 0.486 | 1.39 [0.51, 3.76] | 0.522 |
| Income | ||||
| <$38,000 | Ref | - | Ref | - |
| $38,000–$47,999 | 0.88 [0.66, 1.19] | 0.411 | 0.73 [0.52, 1.03] | 0.072 |
| $48,000–$62,999 | 1.15 [0.87, 1.52] | 0.317 | 0.92 [0.65, 1.31] | 0.661 |
| $63,000+ | 1.16 [0.88, 1.53] | 0.284 | 0.95 [0.64, 1.42] | 0.803 |
| Education (no high school %) | ||||
| >21% or more | Ref | - | Ref | - |
| 13–20.9% | 1.31 [0.97, 1.76] | 0.08 | 1.32 [0.93, 1.86] | 0.116 |
| 7–12.9% | 1.43 [1.07, 1.9] | 0.015 | 1.31 [0.91, 1.9] | 0.153 |
| < 7% | 1.49 [1.1, 2.01] | <0.001 | 1.26 [0.82, 1.93] | 0.290 |
| Facility type | ||||
| Academic | Ref | - | Ref | - |
| Community Cancer Center | 0.61 [0.42, 0.89] | 0.010 | 0.58 [0.39, 0.88] | 0.010 |
| Comprehensive community cancer center | 0.71 [0.58, 0.87] | 0.001 | 0.70 [0.55, 0.88] | 0.002 |
| Integrated Network Cancer program | 0.69 [0.51, 0.95] | 0.022 | 0.70 [0.50, 0.98] | 0.040 |
| Facility location | ||||
| Northeast | Ref | - | Ref | |
| Midwest | 1.24 [0.95, 1.61] | 0.112 | 1.31 [0.99, 1.75] | 0.059 |
| South | 0.76 [0.59, 0.99] | 0.040 | 0.93 [0.70, 1.25] | 0.644 |
| West | 1.01 [0.75, 1.38] | 0.923 | 1.13 [0.81, 1.59] | 0.469 |
| Distance | ||||
| <12.5 mi | Ref | - | Ref | - |
| 12.5–49.9 mi | 1.16 [0.94, 1.42] | 0.161 | 1.07 [0.86, 1.34] | 0.536 |
| 50–249.9 mi | 1.54 [1.19, 1.99] | <0.001 | 1.37 [1.03, 1.82] | 0.032 |
| 250 mi or more | 1.41 [0.62, 3.22] | 0.834 | 1.16 [0.49, 2.78] | 0.733 |
| Histology | ||||
| Clear cell | Ref | - | Ref | - |
| Non-Clear cell | 0.4 [0.32, 0.50] | <0.001 | 0.39 [0.31, 0.49] | <0.001 |
| Sarcomatoid | 1.63 [0.87, 3.03] | 0.124 | 1.78 [0.93, 3.38] | 0.080 |
| Tumor size | ||||
| Per mm | 1.00 [1.00, 1.00] | 0.791 | 1.00 [1.00, 1.00] | 0.757 |
mm = millimeter; OR = odds ratio; RCC = renal cell carcinoma; 95% CI = 95% confidence interval.
3.3. Survival analysis
Table 5 describes the univariate and multivariable Cox regressions for patients with cT1b–T2b RCC tumors, stratified by surgical waiting time. Among cT1b lesions, surgical delay of 1-3 months (Multivariable HR = 1.13, 95% CI: 1.04–1.22; P < 0.001) and >3 months (Multivariable HR = 1.55, 95% CI: 1.40–1.73; P < 0.001) correlated with worse OS. Surgical delay was not associated with worse OS among cT2a (Multivariable HR = 1.33, 95% CI: 0.95–1.85; P = 0.091) or cT2b lesions (Multivariable HR = 0.95, 95% CI: 0.56–1.63; P = 0.862). (Table 5). Across all clinical stages male sex, Charlson comorbidity index, and age were associated with worse OS (Supplemental Tables 1–3).
Table 5.
Overall survival of cT1b - cT2b RCC by surgical delay
| cT1b | ||||
|---|---|---|---|---|
| Univariable |
Multivariable* |
|||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Time to surgery from Diagnosis (months) | ||||
| 1 | Ref | - | Ref | - |
| 1–3 | 1.13 [1.02, 1.24] | 0.013 | 1.13 [1.04, 1.22] | <0.001 |
| >3 | 1.60 [1.41, 1.82] | <0.001 | 1.55 [1.4, 1.73] | <0.001 |
| cT2a | ||||
|---|---|---|---|---|
| Univariable |
Multivariable* |
|||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Time to surgery from Diagnosis (months) | ||||
| 1 | Ref | - | Ref | - |
| 1–3 | 1.25 [1.05, 1.5] | 0.013 | 1.14 [0.95, 1.37] | 0.158 |
| >3 | 1.56 [1.14, 2.13] | 0.005 | 1.33 [0.95, 1.85] | 0.091 |
| cT2b | ||||
|---|---|---|---|---|
| Univariable |
Multivariable* |
|||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Time to surgery from Diagnosis (months) | ||||
| 1 | Ref | - | Ref | - |
| 1–3 | 1.29 [1.01, 1.64] | 0.039 | 1.14 [0.89, 1.47] | 0.303 |
| >3 | 1.24 [0.74, 2.07] | 0.416 | 0.95 [0.56, 1.63] | 0.862 |
Abbreviations: 95% CI = 95% confidence interval; HR = hazard ratio; RCC = renal cell carcinoma
Adjusted for age, sex, Charlson-Deyo index, race, insurance, income, education, facility type, facility location, distance to facility, tumor size.
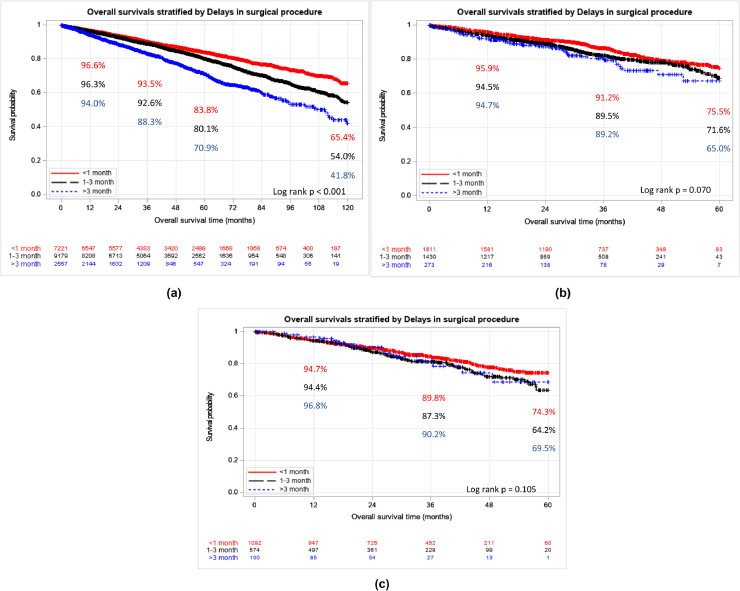
Kaplan Meier estimates illustrate similar findings; 5-year OS for cT1b tumors was 83.8%, 80.1%, 70.9% for delays of <1 month, 1–3 months, >3 months, respectively (P < 0.001). We did not observe statistically different survival in cT2 tumors (Fig. 2 A–C).
Fig. 2.
(A) Overall survival of cT1b RCC by surgical delay. (B) overall survival of cT2a RCC by surgical delay. (C) overall survival of cT2b RCC by surgical delay.
3.4. Sensitivity analysis
Supplemental Tables 4–6 re-examine pT3a upstaging risk by further dividing the surgical delay >3 months category in to 3–6 months and >6 months. Multivariable logistic regression still did not show correlation between surgical delay and upstaging for cT1b-T2b RCC. In the sensitivity analysis for OS, we find again that surgical delay of 1–3, 3–6, and >6 months all portend poorer survival in cT1b tumors (Supplemental Table 7). In cT2a masses, the new stratification finds that patients with a delay of surgery >6 months have worse survival (Multivariable HR = 2.21 (95% CI: 1.33–3.66), P < 0.001). However, delays of 1–3 and 3–6 months had similar survival to those without surgical delay (Supplemental Table 8). Lastly, the expanded stratification still showed no relationship between surgical delay and survival of patients with cT2b tumors (Supplemental Table 9).
4. Discussion
As COVID-19 continues to stress healthcare systems in many countries, hospitals have required the delay of time-sensitive, nonemergent procedures. This has included partial and radial nephrectomy for RCC. Prospective AS data shows that urologists can observe most cT1a tumors for extended periods of time, without detriment to patient survival [7]. However, for larger localized tumors, ≥cT1b, the effects of surgical delay are unclear. Our retrospective analysis using the NCDB is the largest study to date to examine the risks of surgical delay on upstaging and OS in cT1b-T2b RCC. Within each clinical stage strata, surgical delay did not increase the risk of pT3a upstaging for cT1b-T2b RCC (Table 2, Table 3, Table 4). The upstaging rates by clinical stage (cT1b = 9.10%, cT2a = 24.39%, cT2b = 32.26%) seen here are corroborated by prior multi-institutional and population-based studies (Fig. 1, Supplemental Fig. 1) [15,16]. We also find that patients with non-clear cell histology (excluding sarcomatoid) have reduced risk of upstaging – suggesting that histologic information may help surgeons triage patients, as those with nonclear cell histology appear to be less impacted by surgical delay. Regarding OS, we note that surgical delay predicts worse survival for cT1b tumors, but not cT2 tumors (Table 5, Fig. 2).
To date, one systematic review and a few narrative reviews have described how delayed surgery affects oncologic outcomes in localized RCC; there is, however, a relative paucity of data for ≥T1b masses [9,10,17]. Most recently, Shiff et al. examined surgical delay in 1,769 cT1b-cT4 masses in a large multi-institutional Canadian registry. The authors stratified patients by wait times <4 weeks, 4–8 weeks, 8–12 weeks, and 12–24 weeks as well as clinical stage. In no clinical stage strata did surgical delay correlate with worse upstaging, recurrence-free survival, CSS, or OS [18].
Mano et al. studied 1,278 patients (83% T1b, 17% T2) with renal masses >4cm. Modelling surgical waiting time as a continuous variable, they found no association with surgery delay and pT3a upstaging, 5-year recurrence, or CSS. However, they did note poorer OS (HR = 1.15, P = 0.015). Importantly, older age, high comorbidity index, non-White ethnicity, nonclear cell histology, and partial nephrectomy correlated with being placed in the delayed surgery group [19]. Mehrazin et al. examined a series of 72 ≥cT1b masses initially selected for AS, 23 of which underwent surgery. In this cohort no patient progressed to metastatic disease and linear growth rates were slow (median = 0.34 cm/year), suggesting a delay of several months unlikely precludes these masses from complete resection [20].
Our analysis closely matches these findings, particularly among cT1b RCC. We find no association between surgical delay and pT3a upstaging at 1-3 months of delay or >3 months (Table 2). pT3a upstaging often results in worse oncologic outcomes, compared to those patients with concordant clinical and pathologic staging [13,15,21]. Also as these tumors may remain confined to the renal capsule and exhibit favorable growth rate, complete resection is likely even after a surgical delay [20]. Additionally, we find that surgical delay of 1 month or more (1–3 months or >3 months) confers worse survival among cT1b masses. However, these patients still experience favorable OS (5-year OS: surgical delay 1–3 months=80.1%, >3 months = 70.9%) (Fig. 2A). As surgical delay does not seem to predict upstaging in our study or CSS in prior studies– factors such as comorbidity index (Charlson Index ≥2 Multivariable HR=2.24, 95% CI: 2.02-2.47; p<0.001) and unmeasured confounding, rather than tumor progression, likely drive differential OS. For example, older patients or those with more comorbidities, may require more deliberate coordination and optimization prior to their surgery. Consequently, patients at higher risk for surgery may have delayed operations, reflected in a reduced OS.
In our analysis of cT2 lesions surgical delay does not portend poor OS nor risk of pT3a upstaging. However, substantially fewer patients underwent surgery >3 months after diagnosis for cT2a (N = 370) and cT2b (N = 123) tumors, thus limiting our statistical power. Kim et al. (N = 309) and Shiff et al. (N = 391) examined surgical delay in cT2 masses. Neither retrospective analysis found worse upstaging rates, recurrence-free survival. CSS, or OS [10,18, 22]. We must also consider more deleterious in comorbidities for patients with cT2 renal masses, which likely require careful medical optimization and coordination prior to surgery. Though our data does not show obvious harm from surgical delay, confounding patient variables and tumor growth rates make it difficult to definitively state whether surgical delay in cT2 renal masses is prudent [23].
Our study also has some key differences from prior data, specifically the recent publication by Shiff et al. In addition to a shorter follow-up (median = 22.8 months), the Canada-based population also had longer median wait times compared to our cohort for cT1b (2.6 vs. 1.3 months) and cT2 tumors (1.5 vs. 1.0 months). This likely represents healthcare systems differences between the US-based NCDB population and the previously published Canadian cohort. Our analysis utilized only pT3a upstaging; Shiff et al. employed a more heterogenous upstaging definition – including pT2 for cT1b tumors and pN+ for all patients. While more inclusive, this heterogenous definition requires lymph node dissection among all patients and the survival implications of upstaging from cT1b to pT2 are unclear [18].
Our study has several limitations to consider including those inherent to the NCDB such as its retrospective nature [24]. Our data has relatively short follow-up, especially considering OS was our survival endpoint. Additionally, we could not assess CSS or progression/recurrence-free survival as oncologic endpoints, which is why OS was a secondary endpoint. However, our primary end point was the rate of= pT3a upstaging to gauge the risk of neoplasm progression associated with surgical delay, for which the NCDB is well suited as it captures both clinical and pathologic staging. We did not include those patients upstaged to ≥pT3b disease after surgery. While this represents an important subset of our patients, we focused on pT3a upstaging to be in concordance with prior literature. Furthermore, as only 1.7% were upstaged to ≥pT3b, it is unlikely that including this subset of patients would change our clinical interpretation. We also note that pT3a up-staging may also represent limitations in clinical staging, rather than true oncologic progression––in particular cT2 tumors, of which 20% may be upstaged [16].
Additionally, our grouping points of <1 month, 1–3 months, >3 months were selected based on prior literature and our clinical practice during the COVID-19 pandemic where local institutions delayed most kidney cancer cases by 1–3 months. Furthermore, this selection of surgical delay strata allows us to preserve sample size, particularly for cases delayed >3 months in T2 masses. However, the authors acknowledge that different time points may alter regression results. We have mitigated this limitation by including a sensitivity analysis, dividing the >3 months time to surgery group into 3–6 months, and >6 months––we note similar results. Linear growth rates of renal mass, which are not provided in the NCDB, are often variable and may provide rationale for either prompt surgery or AS. Similarly, we do not have granular tumor pathology data. Thus tumor nephrometry scores are not available to quantify mass complexity, we cannot differentiate hilar, endophytic tumors from polar, exophytic ones. We also are not able to substratify pT3a tumors (i.e., renal vein, sinus fat, perinephric fat invasion) to best understand which patients require more urgent treatment. Additionally, while the NCDB lists Charlson-Deyo comorbidity index, which we controlled for in multivariable analysis, it does not provide detailed information regarding comorbidities which may complicate or delay surgery. Lastly, the analyzed data did not contain granular data regarding why a patient had delayed surgery. Regardless, by focusing on ≥cT1b masses, not typically selected for AS, we reduce this limitation.
Despite these limitations, our study builds significantly on prior work. We recommend that for most patients with cT1b tumors, surgery can be safely delayed up to 3–6 months without significantly reducing survival. Among cT2 tumors, our analysis did not find a significant effect of surgical delay on survival or upstaging. However, we caution providers and patients, that this may represent data limitations. For patients with these larger clinically localized masses, a thorough understanding of a tumor's biology, histology, and patient comorbidities are required when discussing delayed intervention. Although the authors do not recommend delaying resection of tumors, our manuscript aims to guide and reassure providers as they treat kidney cancer during the COVID-19 crisis. Even beyond the current pandemic, urologists, patients, and hospitals must make challenging decisions on how to allocate care and resources while maximizing health outcomes as the healthcare system slowly recovers.
5. Conclusion
During the current COVID-19 pandemic and subsequent recovery, urologists and their patients can expect delays in radical and partial nephrectomy for clinically localized RCC. In most patients with clinically localized cT1b tumors, surgery may be safely delayed for up to 6 months without significant sacrifices in OS. In patients with cT2 disease, we must carefully weigh tumor characteristics and patient comorbidities when discussing surgical delay. However, our data suggests that most patients experiencing a delay of 3 months due to the COVID-19 pandemic will not experience worse oncological outcomes.
Footnotes
This work is supported by a grant from the National Cancer Institute (P30CA072720). Services, results and/or products in support of the research were generated by Rutgers Cancer Institute of New Jersey Biometrics Shared Resource P30CA072720-5918.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urolonc.2020.10.012.
Appendix. Supplementary materials
References
- 1.American College of Surgeons . 2020. COVID-19 : recommendations for management of elective surgical procedures; p. 2.https://www.facs.org/covid-19/clinical-guidance/elective-surgery [Google Scholar]
- 2.Stensland KD, Morgan TM, Moinzadeh A, et al. Considerations in the triage of urologic surgeries during the covid-19 pandemic. BJU Int. 2020;77:663. doi: 10.1016/j.eururo.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsburg KB, Curtis GL, Timar RE, et al. Delayed radical prostatectomy is not associated with adverse oncological outcomes: implications for men experiencing surgical delay due to the COVID-19 pandemic. J Urol. 2020;204:720. doi: 10.1097/JU.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 4.Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520–529. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 5.Sud A, Jones M, Broggio J, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinder BM, Patel HV, Sterling J, et al. Urologic oncology surgery during COVID-19: a rapid review of current triage guidance documents. Urol Oncol. 2020 doi: 10.1016/j.urolonc.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam R, Patel HD, Osumah T, et al. Comparative effectiveness of management options for patients with small renal masses: a prospective cohort study. BJU Int. 2019;123:42. doi: 10.1111/bju.14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finelli A, Cheung DC, Al-Matar A, et al. Small renal mass surveillance: histology-specific growth rates in a biopsy-characterized cohort. Eur Urol. 2020 doi: 10.1016/j.eururo.2020.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Wallis CJD, Novara G, Marandino L, et al. Risks from deferring treatment for genitourinary cancers: a collaborative review to aid triage and management during the COVID-19 pandemic. Eur Urol. 2020 doi: 10.1016/j.eururo.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katims AB, Razdan S, Eilender BM, et al. Urologic oncology practice during COVID-19 pandemic: a systematic review on what can be Deferrable vs. Nondeferrable. Urol Oncol. 2020 doi: 10.1016/j.urolonc.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stec AA, Coons BJ, Chang SS, et al. Waiting time from initial urological consultation to nephrectomy for renal cell carcinoma–does it affect survival? J Urol. 2008;179:2152. doi: 10.1016/j.juro.2008.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Stewart AK, Winchester DP, et al. The national cancer data base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava A, Rivera-Núñez Z, Kim S, et al. Impact of pathologic lymph node–positive renal cell carcinoma on survival in patients without metastasis: evidence in support of expanding the definition of stage IV kidney cancer. Cancer. 2020 doi: 10.1002/cncr.32912. [DOI] [PubMed] [Google Scholar]
- 14.Sterling J, Rivera-Núñez Z, Patel HV, et al. Factors associated with receipt of partial nephrectomy or minimally invasive surgery for patients with clinical T1a and T1b renal masses: implications for regionalization of care. Clin Genitourin Cancer. 2020 doi: 10.1016/j.clgc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorin MA, Ball MW, Pierorazio PM, et al. Outcomes and predictors of clinical T1 to pathological T3a tumor up-staging after robotic partial nephrectomy: a multi-institutional analysis. J Urol. 1907;190:2013. doi: 10.1016/j.juro.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava A, Patel HD, Joice GA, et al. Incidence of T3a up-staging and survival after partial nephrectomy: size-stratified rates and implications for prognosis. Urol Oncol. 2018;36:12.e7. doi: 10.1016/j.urolonc.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Bourgade V, Drouin SJ, Yates DR, et al. Impact of the length of time between diagnosis and surgical removal of urologic neoplasms on survival. World J Urol. 2014;32:475. doi: 10.1007/s00345-013-1045-z. [DOI] [PubMed] [Google Scholar]
- 18.Shiff B, Breau RH, Patel P, et al. Impact of time-to-surgery and surgical delay on oncologic outcomes for renal cell carcinoma. J Urol. 2020 doi: 10.1097/JU.0000000000001230. 101097ju0000000000001230. [DOI] [PubMed] [Google Scholar]
- 19.Mano R, Vertosick EA, Hakimi AA, et al. The effect of delaying nephrectomy on oncologic outcomes in patients with renal tumors greater than 4cm. Urol Oncol. 2016;34 doi: 10.1016/j.urolonc.2015.12.001. 239.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehrazin R, Smaldone MC, Kutikov A, et al. Growth kinetics and short-term outcomes of cT1b and cT2 renal masses under active surveillance. J Urol. 2014;192:659. doi: 10.1016/j.juro.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell CM, Lebastchi AH, Chipollini J, et al. Multi-institutional survival analysis of incidental pathologic T3a upstaging in clinical T1 renal cell carcinoma following partial nephrectomy. Urology. 2018;117:95. doi: 10.1016/j.urology.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim KH, You D, Jeong IG, et al. The impact of delaying radical nephrectomy for stage II or higher renal cell carcinoma. J Cancer Res Clin Oncol. 2012;138:1561. doi: 10.1007/s00432-012-1230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertolo R, Autorino R, Simone G, et al. Outcomes of robot-assisted partial nephrectomy for clinical T2 renal tumors: a multicenter analysis (ROSULA collaborative group) Eur Urol. 2018;74:226. doi: 10.1016/j.eururo.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Boffa DJ, Rosen JE, Mallin K, et al. Using the national cancer database for outcomes research: a review. JAMA Oncol. 2017;3:1722. doi: 10.1001/jamaoncol.2016.6905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.