Abstract
Purpose
To understand how verification computed tomography-quality assurance (CT-QA) scans influenced clinical decision-making to replan patients with head and neck cancer and identify predictors for replanning to guide intensity-modulated proton therapy (IMPT) clinical practice.
Patients and Methods
We performed a quality-improvement study by prospectively collecting data on 160 consecutive patients with head and neck cancer treated using spot-scanning IMPT who underwent weekly verification CT-QA scans. Kaplan-Meier estimates were used to determine the cumulative probability of a replan by week. Predictors for replanning were determined with univariate (UVA) and multivariate (MVA) Cox model hazard ratios (HRs). Logistic regression was used to determine odds ratios (ORs). P < .05 was considered statistically significant.
Results
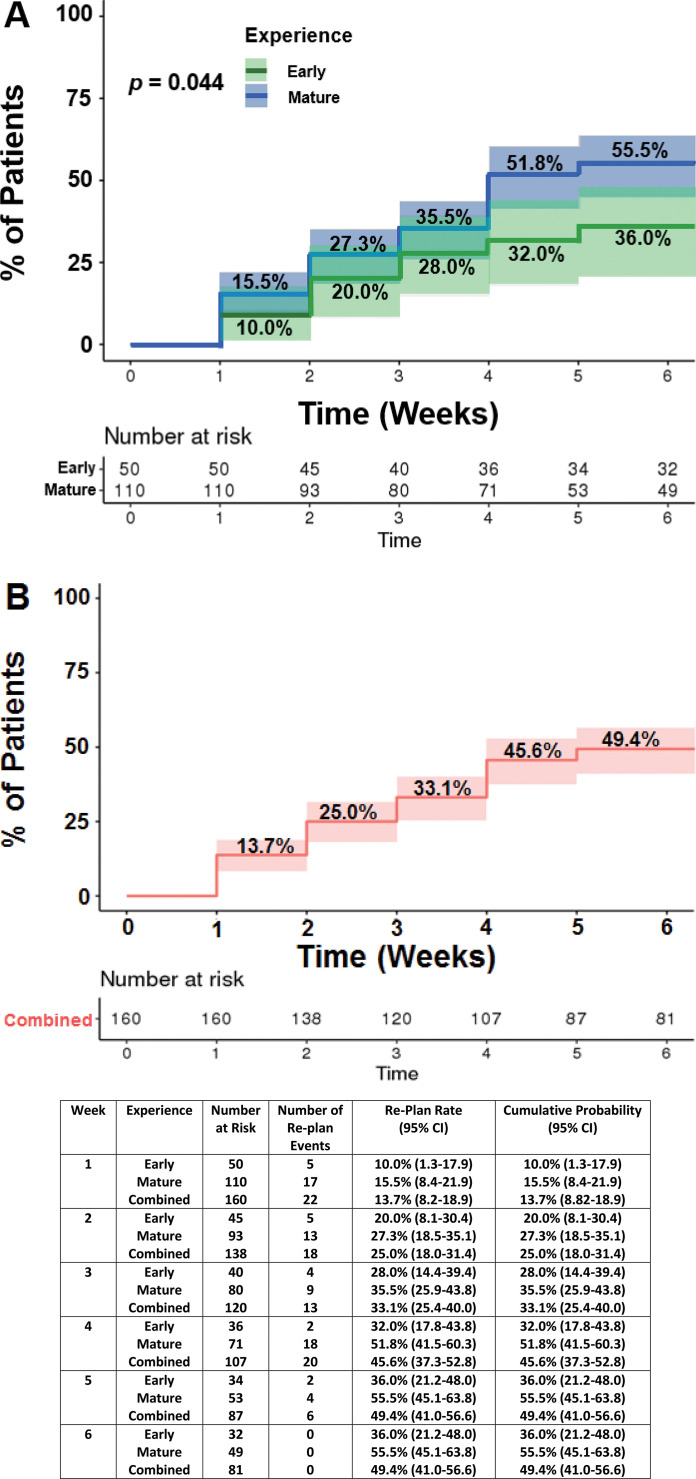
Of the 160 patients, 79 (49.4%) had verification CT-QA scans, which prompted a replan. The cumulative probability of a replan by week 1 was 13.7% (95% confidence interval [CI], 8.82-18.9), week 2, 25.0% (95% CI, 18.0-31.4), week 3, 33.1% (95% CI, 25.4-40.0), week 4, 45.6% (95% CI, 37.3-52.8), and week 5 and 6, 49.4% (95% CI, 41.0-56.6). Predictors for replanning were sinonasal disease site (UVA: HR, 1.82, P = .04; MVA: HR, 3.64, P = .03), advanced stage disease (UVA: HR, 4.68, P < .01; MVA: HR, 3.10, P < .05), dose > 60 Gy equivalent (GyE; relative biologic effectiveness, 1.1) (UVA: HR, 1.99, P < .01; MVA: HR, 2.20, P < .01), primary disease (UVA: HR, 2.00 versus recurrent, P = .01; MVA: HR, 2.46, P = .01), concurrent chemotherapy (UVA: HR, 2.05, P < .01; MVA: not statistically significant [NS]), definitive intent treatment (UVA: HR, 1.70 versus adjuvant, P < .02; MVA: NS), bilateral neck treatment (UVA: HR, 2.07, P = .03; MVA: NS), and greater number of beams (5 beam UVA: HR, 5.55 versus 1 or 2 beams, P < .02; MVA: NS). Maximal weight change from baseline was associated with higher odds of a replan (≥3 kg: OR, 1.97, P = .04; ≥ 5 kg: OR, 2.13, P = .02).
Conclusions
Weekly verification CT-QA scans frequently influenced clinical decision-making to replan. Additional studies that evaluate the practice of monitoring IMPT-treated patients with weekly CT-QA scans and whether that improves clinical outcomes are warranted.
Keywords: CT verification, CT quality assurance, IMPT, proton therapy, head and neck cancer
Introduction
Intensity-modulated proton therapy (IMPT) is a form of radiotherapy that uses a spot-scanning pencil beam to deliver protons to the target spot by spot and layer upon layer. Importantly, IMPT has multiple advantages over other types of photon- and proton-based therapies, including passive-scatter techniques [1–7]. Specifically, IMPT effectively delivers high dose to targets with complex shapes and limits the dose to adjacent critical structures [8, 9]. Moreover, IMPT has been lauded as an ideal tool for certain situations in head and neck (H&N) cancer, which commonly requires the delivery of high doses adjacent to multiple, small critical organs at risk (OARs) [10–12].
As more facilities adopt IMPT into clinical practice, quality assurance in treatment delivery is vitally important for patient safety and the development of any high-quality proton therapy program [13, 14]. Recognizing the sensitivity of IMPT to small changes in patient anatomy and setup, we implemented a quality improvement study to monitor weekly dose distributions using verification computed tomography quality assurance (CT-QA) scans. A verification CT-QA scan's purpose is to evaluate the on-treatment dose distribution to ensure it has not unacceptably deviated from the original plan. If the CT-QA scan reveals an unacceptable dose deviation, a replan may be necessary. However, this practice is resource intensive. Thus, we designed a study to investigate this practice's effect on our H&N IMPT program and whether its continuation was justified. We aimed to investigate the optimal timing of CT-QA scans, whether CT-QA scans influenced decision-making to replan and to determine predictors for replanning to guide clinical practice.
Materials and Methods
Study Design and Patients
The study was a prospectively collected quality improvement project approved by the Mayo Clinic institutional review board. Initial study subjects included 50 consecutive patients with H&N cancer treated with IMPT between June 2015 and October 2016. These patients were referred to as the “early” cohort, referring to our early practice experience using proton therapy for H&N cancer. The data from those first 50 patients were analyzed and used to guide our IMPT practice as it matured. After the initial evaluation of the H&N verification CT-QA scan program, a more comprehensive program was implemented to systematically monitor the CT-QA scans for all disease sites treated with IMPT, including its continuation for H&N cancer. An additional 110 consecutive patients were evaluated through June 2018. Those latter patients were referred to as the “mature” cohort, referring to our more-mature practice experience using IMPT for H&N malignancies. Inclusion criteria included patients with histologic confirmation of H&N malignancy undergoing IMPT treatment and weekly CT-QA scans throughout treatment. Exclusion criteria included hypofractionated or dose de-escalated radiotherapy consisting of <25 fractions.
Spot-Scanning Intensity Modulated Proton Therapy
Treatment position was head first, supine, with arms at side in a 5-point thermoplastic mask and a custom AccuCushion (Klarity Medical Products, Newark, Ohio). Depending on the clinical situation, a fixed mouthpiece, such as Precise Bite (CIVCO, Coralville, Iowa), TruGuard (Bionix, Toledo, Ohio), or a custom oral stent was used. Arms were at the patient's side using indexable hand grips. Spot-scanning pencil-beam IMPT (Hitachi ProBeat, Hitachi Ltd, Tokyo, Japan) was used for all patients. Custom beam arrangements used 1 to 5 beam angles per patient. If a replan was pursued, the beam arrangement and/or the number of fields was, in rare instances, modified, in an effort to improve robustness and planning of objective dosimetry at the discretion of the treating radiation oncologist. Multiple field optimization was used to plan all but one (99.4%; 159 of 160) of the patients; the plan for 1 patient used single-field optimization, and the patient was treated with a single beam. The Eclipse treatment planning system (TPS; Varian, Palo Alto, California) was used for optimization of all plans. In our early IMPT practice, dose calculation was performed with Eclipse TPS version 13 and used an optimization target volume–based strategy that has been previously described [15]. As our IMPT practice matured, we transitioned to a robust optimization strategy using Eclipse TPS version 15 and the nonlinear universal proton optimizer. A graphic processing unit–based Monte Carlo dose-calculation algorithm for verification of the Eclipse plan was also developed and used [16, 17]. Robust optimization parameters routinely used 3%-range and 3-mm setup uncertainties applied to the clinical target volumes (CTVs), which account for conceivable systematic and statistical uncertainties related to treatment delivery. Robust IMPT plans were achieved through a combination of optimization target volumes, which were an expansion of the CTVs to ensure robust coverage of the target volume and robust optimization using the nonlinear universal proton-optimizer system. The degree to which each method was employed varied by dosimetrist, but a typical method would use a 3-mm expansion of the CTV for the optimization target volume and specify 3-mm setup uncertainty and 3% range uncertainty in the optimizer. Regardless of the method employed, the goal was to achieve 97% of the volume receiving 97% of the prescription dose in the presence of a 3-mm shift in any direction or a 3% range of uncertainty. Typically, if any of the target robustness dose-volume histogram (DVH) curves fell below D95%; < 95%, the plan was not considered to be robust, and optimization continued. For image-guided radiation instructions, we mandated more stringent instructions for the head region (within 2-3 mm) than we mandated for the shoulders (within 3-5 mm), and robust optimization planning would reflect those image-guided radiation considerations. None (0%) of the patients in the early cohort were replanned using the Eclipse robust optimization planning strategy, whereas all 110 patients (100%) in the mature cohort were replanned using this method. Planning objectives for CTVs were V100% ≥ 95%, D95% ≥ 100%, and D1% ≤ 110%. Those CTV planning objectives were applied to the nominal plan and checked by the treating radiation oncologist to be maintained in the robustness analysis. Plans were not accepted unless objectives were maintained in the robustness analysis, and the planning process continued until a robust plan was achieved. Dose constraint objectives for OARs were consistent with standard of care practice (Supplemental Table S1).
Verification CT-QA Scans
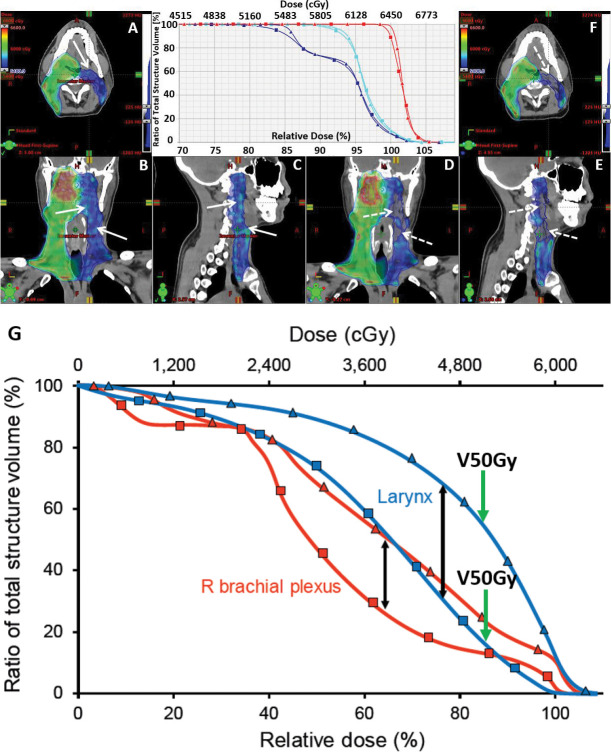
Verification CT-QA scans were performed once weekly throughout each treatment course. The CT-QA scans were performed with a treatment-integrated, diagnostic-quality CT-on-rails system mounted adjacent to the proton gantry (n = 50 patients; 31%) or in a simulation room (n = 86 patients; 54%), or both CT-on-rails and simulation room (n = 24 patients; 15%). Of those patients replanned (n = 79), 39.2% (n = 31) were based on a CT-on-rails verification scan, and 60.8% (n = 48) were based on a simulation room verification scan. A matrix laboratory (MATLAB) topogram-comparison program was used to guide alignment of the patient for CT-QA scans in the simulation room. A simple MATLAB command-line program with plotting graphic user interface was designed and compiled as a Windows (Microsoft, Redmond, Washington) executable program. The program was engineered to work with topogram projection images (ie, Digital Imaging and Communications in Medicine standards, National Electrical Manufacturers Association, Arlington, Virginia) from all the CT scanners. The program established a simple Digital Imaging and Communications in Medicine standard topogram database from the planning CT based on a Windows folder directory tree, which could then be used to guide appropriate adjustments in daily patient setup, including setup for the verification CT-QA scan. The software overlays the new topogram on the original, allowing the therapist to see where the patient setup for the CT-QA scan may differ from the original planning scan. Manual corrections to the patient's setup were made based on examination of the overlaid images. The CT-QA scans were acquired with the same scan parameters as the planning CT. Both rigid and deformable image registration were used to transfer target structures on the verification CT. Dose calculations were performed on a rigid registration of the CT-QA scan to the planning scan. The Eclipse TPS was used to reevaluate proton-dose distributions within each CT-QA scan. The CT-QA dose distributions were compared with the original plan, side-by-side in plan evaluation using the Eclipse TPS. Composite plans taking into account delivered dose to date were also created and certainly influenced clinical decision-making. Dose was modeled with the full prescription on each CT-QA scan, as if each verification CT-QA scan was the patient's CT simulation planning scan. Composite sum plans were then created showing the combined effect of both the originally planned dose distribution and the verification CT-QA dose distribution and accounting for the number of fractions to be applied from each respective plan. Information about how the CT-QA scan was interpreted and influenced clinical decision-making to replan was documented prospectively. Target coverage and OAR DVH parameters were required to be reevaluated by the treating physician for therapy to continue. If the dose distribution unacceptably deviated from the original plan, replanning was initiated. Unacceptable deviations were ultimately based on clinical judgment of the treating radiation oncologist and may have been physician dependent. In surveying the treating radiation oncologists, the consensus agreement was that if < 95% of the prescription dose was being delivered to 100% of the CTV (ie, D95% < 100%), he or she would ask for a replan. As long as 95% or more of the prescription dose was being delivered to 100% of the CTV, it would be accepted. In addition, as part of the CT-QA approval, robustness curves were shown. If the CT-QA dose distribution fell within the predetermined and acceptable robustness of the DVH curves, then the CT-QA verification would be deemed acceptable. If it fell outside the original robustness of the DVH curves, a replan may have been requested. There were individualized exceptions in which coverage in a low-risk area may have been accepted. Replanning was performed at the discretion of the treating radiation oncologist if any of the dose objectives listed in Supplemental Table S1 were not met and the dose distribution was outside the expected dosimetric uncertainties accepted during robust optimization and plan evaluation in the original plan. Priorities were assigned to planning objectives to guide the planning dosimetrist on how to apply the cost function in optimizing the plan. Priority 1 objectives were prioritized over priority 2 objectives, which were prioritized over priority 3 objectives. A general rule observed for replanning because of OAR violations included the following: if a priority 1 or 2 OAR objective deviated by > 5% or a priority 3 OAR objective deviated by > 10%, a replan was often considered given the clinical context of the deviation. General rules observed for replanning because of changes in CTV coverage were due to V100% < 90%, D95% < 95%, or D1% > 115%; however, CTV coverage deficiencies were primarily based on the clinical judgment of the treating physician, given each patient's unique clinical scenario, and more or less stringent rules may have been used. Examples of plan evaluations that prompted the treatment team to replan are shown in Figure 1.
Figure 1.
Illustrates plan evaluation for a patient with oropharyngeal cancer treated postoperatively with 3 dose volumes. Representative colorwash dose distributions are shown in 3 views of the original plan (A–C, triangles) and the verification computed tomography-quality assurance (CT-QA) plan (D–F, squares), respectively. The patient's left neck nodal CTV 5400 (blue) dose distribution shows patchy areas of decreased target coverage (dotted arrows) compared with the original plan (solid arrows) after the patient experienced a 4.5-kg absolute weight change resulting in significant neck soft tissue changes. CTV 5400 coverage was reduced from V100% = 98.7% and D95% = 102.0% to V100% = 89.0% and D95% < 100%, prompting a replan. (G) Another patient's dose-volume histogram data showing an unacceptable increase in dose to the larynx and right brachial plexus on the CT-QA scan (triangles) compared with the original plan (squares), prompting a replan. The larynx V50 Gy% (green arrows) was initially below the goal of < 27% (blue squares) but increased to 52% on CT-QA, significantly above the priority 2 organs-at-risk constraint.
Statistical Analyses
Kaplan-Meier estimates were used to determine cumulative probability of a replan as a function of time. Univariate (UVA) and multivariate (MVA) Cox models were used to determine variables associated with re-planning. Type 3 Wald P-values are shown. Logistic regression models were employed to determine odds ratios in the maximal absolute weight change from baseline analysis. Cumulative incidence, hazard ratios (HRs) and odds ratios (ORs) have been described with the 95% confidence interval (CI). Analyses were performed using R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.3 (SAS Institute, Cary, North Carolina). P < .05 was considered statistically significant.
Results
The study evaluated 160 patients treated with IMPT from June 2015 to June 2018. The early cohort consisted of the first 50 consecutive patients (31%) treated. The mature cohort was comprised of an additional 110 consecutively treated patients (69%). Patient, tumor, and treatment characteristics are presented in Table 1. Multiple variables were predictors for replanning using UVA and MVA Cox models as shown in Table 2. Of the 53 treated oropharynx cases, and 25 (47.2%) were replanned, representing the most commonly treated and replanned disease site. However, sinonasal cases were replanned at a higher relative rate with 70.4% (19 of 27) replanned. The probability of replan was significantly higher for sinonasal cases compared with other disease sites (Table 2; UVA: HR, 1.82, P = .04; MVA: HR, 3.64, P = .03). The median time from verification CT-QA to delivering the replan was 4 days (mean [SD], 3.8 [1.54] days; range, 1-8 days).
Table 1.
Patient, tumor, and treatment characteristics.
|
Characteristics |
No replan (n = 81) |
Replan (n = 79) |
Total (n = 160) |
| Disease site, n (%) | |||
| Oropharynx | 28 (34.6) | 25 (31.6) | 53 (33.1) |
| Sinonasal | 8 (9.9) | 19 (24.1) | 27 (16.9) |
| Cutaneous | 19 (23.5) | 7 (8.9) | 26 (16.3) |
| Nasopharynx | 5 (6.2) | 11 (13.9) | 16 (10.0) |
| Oral cavity | 7 (8.6) | 6 (7.6) | 13 (8.1) |
| Salivary gland | 9 (11.1) | 3 (3.8) | 12 (7.5) |
| Other | 5 (6.2) | 8 (10.1) | 13 (8.1) |
| Experience, n (%) | |||
| Early | 32 (39.5) | 18 (22.8) | 50 (31.3) |
| Mature | 49 (60.5) | 61 (77.2) | 110 (68.8) |
| Primary overall stage, n (%) | |||
| I | 19 (23.5) | 1 (1.3) | 20 (12.5) |
| II | 16 (19.8) | 10 (12.7) | 26 (16.3) |
| III | 5 (6.2) | 15 (19.0) | 20 (12.5) |
| IV | 34 (42.0) | 45 (57.0) | 79 (49.4) |
| X | 7 (8.6) | 8 (10.1) | 15 (9.4) |
| Treatment regimen, median (range) | |||
| Dose in GyE (RBE 1.1) | 60 (50-70) | 66 (50-70) | 60 (50-70) |
| Number of fractions | 30 (25-35) | 33 (25-35) | 30 (25-35) |
| Concurrent chemotherapy, n (%) | |||
| No | 50 (61.7) | 28 (35.4) | 78 (48.8) |
| Yes | 31 (38.3) | 51 (64.6) | 82 (51.2) |
| Definitive versus adjuvant, n (%) | |||
| Adjuvant | 57 (70.4) | 39 (49.4) | 96 (60.0) |
| Definitive | 24 (29.6) | 40 (50.6) | 64 (40.0) |
| Primary versus recurrent, n (%) | |||
| Recurrent | 34 (42.0) | 16 (20.3) | 50 (31.2) |
| Primary | 47 (58.0) | 63 (79.7) | 110 (68.8) |
| Lymph node treatment, n (%) | |||
| Bilateral | 26 (32.1) | 43 (54.4) | 69 (43.1) |
| Ipsilateral | 27 (33.3) | 14 (17.7) | 41 (25.6) |
| None | 28 (34.6) | 22 (27.8) | 50 (31.2) |
| Number of beams, n (%) | |||
| 1 | 1 (1.2) | 0 (0) | 1 (0.6) |
| 2 | 10 (12.3) | 3 (3.8) | 13 (8.1) |
| 3 | 49 (60.5) | 36 (45.6) | 85 (53.1) |
| 4 | 21 (25.9) | 34 (43.0) | 55 (34.4) |
| 5 | 0 (0) | 6 (7.6%) | 6 (3.8%) |
Abbreviations: GyE, Gy equivalents; RBE, relative biologic effectiveness.
Table 2.
Variables that predicted the need for a replan.
|
Variables |
Replan events/total |
Replan rate (95% CI) |
UVA HR (95% CI) |
MVA HR (95% CI) |
UVA
Pa |
MVA
Pa |
| Disease site, n (%) | 0.0443 | 0.0303 | ||||
| Oropharynx | 25/53 | 0.47 (0.32-0.59) | Reference range | Reference range | ||
| Sinonasal | 19/27 | 0.70 (0.47-0.83) | 1.82 (1.00-3.31) | 3.64 (1.56-8.50) | ||
| Cutaneous | 7/26 | 0.27 (0.08-0.42) | 0.53 (0.23-1.22) | 2.86 (0.80-10.23) | ||
| Nasopharynx | 11/16 | 0.69 (0.35-0.85) | 1.79 (0.88-3.65) | 1.47 (0.60-3.65) | ||
| Oral cavity | 6/13 | 0.46 (0.11-0.67) | 1.05 (0.43-2.57) | 2.37 (0.88-6.37) | ||
| Salivary gland | 3/12 | 0.25 (0.00-0.46) | 0.52 (0.16-1.71) | 0.45 (0.12-1.76) | ||
| Other | 8/13 | 0.62 (0.24-0.81) | 1.37 (0.62-3.04) | 2.86 (1.11-7.38) | ||
| 79/160 | 0.49 (0.41-0.57) | |||||
| Experience, n (%) | 0.0537 | — | ||||
| Early | 18/50 | 0.36 (0.21-0.48) | Reference range | — | ||
| Mature | 61/110 | 0.55 (0.45-0.64) | 1.68 (0.99-2.84) | — | ||
| 79/160 | 0.49 (0.41-0.57) | |||||
| Primary overall stage, n (%) | 0.0016 | 0.1464 | ||||
| I or II | 11/46 | 0.24 (0.11-0.35) | Reference range | Reference range | ||
| III | 15/20 | 0.75 (0.47-0.88) | 4.68 (2.14-10.23) | 3.10 (1.19-8.09) | ||
| IV | 45/79 | 0.57 (0.45-0.67) | 2.78 (1.44-5.37) | 2.21 (0.93-5.25) | ||
| X | 8/15 | 0.53 (0.20-0.73) | 2.33 (0.94-5.80) | 1.90 (0.64-5.64) | ||
| 79/160 | 0.49 (0.41-0.57) | |||||
| Radiotherapy dose | 0.0035 | 0.0079 | ||||
| Dose < 60 GyE (RBE 1.1) | 3/13 | 0.23 (0.00-0.43) | 0.54 (0.16-1.78) | 0.41 (0.11-1.44) | ||
| Dose 60 GyE (RBE 1.1) | 25/67 | 0.37 (0.25-0.48) | Reference range | Reference range | ||
| Dose > 60 GyE (RBE 1.1) | 51/80 | 0.64 (0.52-0.73) | 1.99 (1.23-3.22) | 2.20 (1.07-4.49) | ||
| 79/160 | 0.49 (0.41-0.57) | |||||
| Concurrent chemotherapy, n (%) | 0.0023 | 0.4745 | ||||
| No | 28/78 | 0.36 (0.24-0.46) | Reference range | Reference range | ||
| Yes | 51/82 | 0.62 (0.50-0.71) | 2.05 (1.29-3.26) | 1.24 (0.69-2.24) | ||
| 79/160 | 0.49 (0.41-0.57) | |||||
| Definitive versus adjuvant, n (%) | 0.0179 | 0.5724 | ||||
| Adjuvant | 39/96 | 0.41 (0.30-0.50) | Reference range | Reference range | ||
| Definitive | 40/64 | 0.62 (0.49-0.73) | 1.70 (1.10-2.65) | 0.81 (0.38-1.70) | ||
| 79/160 | 0.49 (0.41-0.57) | |||||
| Primary versus recurrent, n (%) | 0.0133 | 0.0138 | ||||
| Recurrent | 16/50 | 0.32 (0.18-0.44) | Reference range | Reference range | ||
| Primary | 63/110 | 0.57 (0.47-0.66) | 2.00 (1.16-3.47) | 2.46 (1.20-5.05) | ||
| 79/160 | 0.49 (0.41-0.57) | |||||
| Primary/recurrent and definitive/adjuvant, n (%) | 0.0041 | — | ||||
| Recurrent adjuvant | 7/28 | 0.25 (0.07-0.39) | Reference range | — | ||
| Primary adjuvant | 32/68 | 0.47 (0.34-0.58) | 2.00 (0.88-4.54) | — | ||
| Recurrent definitive | 9/22 | 0.41 (0.16-0.58) | 1.67 (0.62-4.47) | — | ||
| Primary definitive | 31/42 | 0.74 (0.56-0.84) | 3.69 (1.62-8.40) | — | ||
| 79/160 | 0.49 (0.41-0.57) | |||||
| Lymph node treatment, n (%) | 0.0340 | 0.2409 | ||||
| Bilateral | 43/69 | 0.62 (0.49-0.72) | 2.07 (1.13-3.78) | 0.94 (0.43-2.09) | ||
| Ipsilateral | 14/41 | 0.34 (0.18-0.47) | Reference range | Reference range | ||
| None | 22/50 | 0.44 (0.28-0.56) | 1.31 (0.67-2.56) | 0.51 (0.21-1.24) | ||
| 79/160 | 0.49 (0.41-0.57) | |||||
| Number of beams, n (%) | 0.0157 | 0.8926 | ||||
| 1 or 2 | 3/14 | 0.21 (0.00-0.40) | Reference range | Reference range | ||
| 3 | 36/85 | 0.42 (0.31-0.52) | 2.03 (0.62-6.59) | 1.23 (0.36-4.21) | ||
| 4 | 34/55 | 0.62 (0.47-0.73) | 3.31 (1.02-10.80) | 1.44 (0.40-5.12) | ||
| 5 | 6/6 | 0.00 | 5.55 (1.39-22.21) | 1.18 (0.23-6.07) | ||
| 79/160 | 0.49 (0.41-0.57) |
Abbreviations: GyE, Gy equivalents; RBE, relative biologic effectiveness; UVA, univariate Cox model analysis; MVA, multivariate Cox model analysis; HR, hazard ratio.
Type 3 Wald P-value.
More-advanced stage, including stages III and IV, compared with earlier stages I and II (AJCC 7th edition) [18] were more likely to need a replan (UVA stage III: HR, 4.68; stage IV: HR, 2.78, P < .01; MVR stage III: HR, 3.10; 95% CI, 1.19-8.09, P < .05). Higher dose, specifically > 60 GyE (relative biologic effectiveness [RBE], 1.1), was associated with double the rate of replanning compared with a maximum dose of 60 Gy equivalent (GyE; RBE, 1.1) (UVA: HR, 1.99, P < .01; MVA: HR, 2.20, P < .01). The rate of replanning for primary cases was more than double that of recurrent cases, which included recurrent disease after surgery, prior radiotherapy, or both (UVA: HR, 2.00, P = .01; MVA: HR, 2.46, P = .01). The rate of replanning was 62.5% (40 of 64) in patients undergoing definitive IMPT compared with 40.6% (39 of 96) for adjuvant cases (UVA: HR, 1.70, P < .02; MVA, not statistically significant [NS]). Patients receiving concurrent chemotherapy were more than twice as likely to be replanned compared with patients without concurrent chemotherapy (UVA: HR, 2.05, P < .01; MVA, NS). The subgroup of patients with the highest rate of replanning by treatment scenario were the primary definitive-intent cases (HR, 3.69, P < .01). When compared with ipsilateral neck treatment, bilateral neck treatment carried more than twice the rate of replanning (HR, 2.07, P = .03; MVA: NS). Ipsilateral neck cases were replanned in 34.1% (14 of 41), significantly lower than bilateral neck cases (62.3%; 43 of 69) or cases in which no elective nodes were treated (44.0%; 22 of 50). More beams used in the initial IMPT plan were associated with an increased rate of replanning. Compared with plans with 1 or 2 beams, the rate of replanning was approximately 2, 3, or 5 times greater for 3, 4, or 5 beams, respectively (Table 2; UVA and MVA: HR, NS).
In our early experience, the cumulative probability of a replan was 36.0% (95% CI, 21.2-48.0). As our experience with IMPT matured, we found that the cumulative probability of replanning increased to 55.5% (95% CI, 45.1-63.8) (P = .04). For the combined cohort, the cumulative probability of a replan was 49.4% (95% CI, 41.0-56.6). Figure 2 demonstrates the cumulative probability of an IMPT replan as a function of time for the early, mature, and combined experience cohorts.
Figure 2.
Demonstrates the cumulative probability over time that a patient's initial intensity-modulated proton therapy plan would require a replan for the early versus mature (A) and the combined (B) cohorts (95% CI shaded).
Of the 79 patients who required a replan, the most common reason for a replan was decreased target coverage (39 of 79; 49.4%). Replans were also initiated because of increased dose to OARs (19 of 79; 24.1%) or both a decrease in target coverage and increased dose to OARs (9 of 79; 11.4%). There were 12 patients (15.2%) who had another reason for a replan that did not fall into the previous 3 categories. The reasons included issues such as mask alterations, problems with reproducible positioning, chemotherapy port revision in the beam path, and other miscellaneous treatment-team concerns. The most common target-coverage issues were due to inconsistency with shoulder position during setup and soft tissue changes. Soft tissue changes resulted primarily from weight alteration, along with postoperative or treatment-related swelling and contracting. In the 28 patients (35.4%) replanned because of unacceptable dose deviations to OARs, multiple OARs, specifically parotids, larynges, and brachial plexuses, were implicated most commonly (9 of 28; 32.1%), followed by the sinus cavity (6 of 28; 21.4%), brachial plexus (5 of 28; 17.9%), larynx (4 of 28; 14.3%), optic nerve/chiasm (2 of 28; 7.1%), brainstem (1 of 28; 3.6%), and lip (1 of 28, 3.6%). Furthermore, there were 13 (16.5%) of the 79 replanned patients that required a second replan because of additional CT-QA scans revealing dose distributions that unacceptably deviated from the physician-approved adjustments in the first replan. Reasons for a second replan included decreased target coverage (5 of 13; 38.5%), increased dose to OARs (6 of 13; 46.2%), or both (2 of 13; 15.4%).
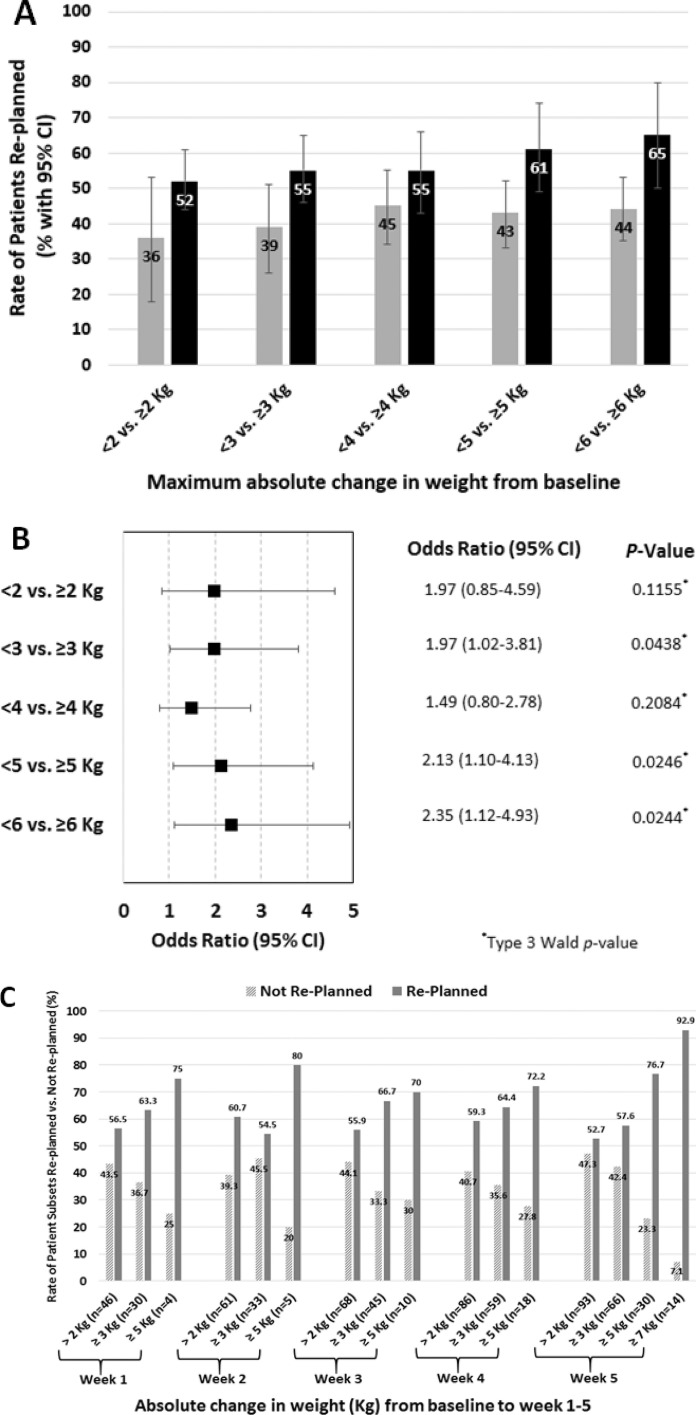
A patient's maximal absolute weight change from baseline at CT simulation was associated with higher odds of a replan. Figure 3a and 3b shows that a maximal change of ≥ 3 kg compared with < 3 kg was associated with nearly double the odds of a replan (OR, 1.97, 95% CI, 1.02-3.81, P = .04), and the odds of a replan more than doubled for patients that had a ≥ 5-kg weight change (OR, 2.13, 95% CI, 1.10-4.13, P = .02). The odds of a replan nearly tripled for patients that had a > 6-kg weight change (OR, 2.95, 95% CI, 1.33-6.51, P < .01 [data not included in Figure 3, which includes results for a change ≥ 6 kg). Figure 3c provides additional data on the rate of patient subsets replanned versus those who were not replanned; subsets include rates for only those patients with absolute weight changes from baseline to weeks 1-5 of > 2, ≥ 3, ≥ 5, and ≥ 7 kg, respectively. The general trend observed is an increased rate of replanning with increased weight changes.
Figure 3.
Demonstrates the percentage of patients replanned and grouped by maximal weight change from baseline throughout the patient's intensity-modulated proton therapy course (A). Gray bars show the rate of patients replanned when maximal absolute weight change from the time of simulation (baseline) to the weekly management visit with the greatest absolute change in weight was < 2, < 3, < 4, < 5, or < 6 kg, respectively. These are compared with black bars, which show the rate of patients replanned when the absolute change in weight was ≥ 2, ≥ 3, ≥ 4, ≥ 5, and ≥ 6 kg, respectively. This illustrates the concept that patients with greater absolute-weight changes throughout treatment experienced more replans. (B) The odds ratio (95% CI) for each incremental kilogram change from ≥ 2, ≥ 3, ≥ 4, ≥ 5, and ≥ 6 kg versus the patients that did not experience that level of absolute weight change. (C) The rates of patient subsets replanned versus not replanned at any point during the first 6 weeks; patient subsets include only those patients who experienced a weight change from baseline to weeks 1 to 5 of > 2, ≥ 3, ≥ 5, and ≥ 7 kg, respectively. Subset sample sizes (n) are indicated.
There were 16 categories of beam angles used to treat all patients. Beam information is outlined in Table 3. The frequency of beam arrangements is shown in Supplemental Table S2. By UVA and MVA Cox models, no particular full-beam arrangement significantly increased the rate of a replan. In the UVA model, the left posterior oblique beam was associated with an increased statistical rate of replanning (HR, 1.56, 95% CI, 1.00-2.43, P < 0.05) and persisted in 1 of the 2 beam multivariate models of left posterior oblique and left anterior oblique (HR, 1.58, 95% CI, 1.01-2.46, P = .04). Supplemental Table S3 shows MVA Cox model results for those with at least 20 patients treated with that 2-beam combination as part of their full IMPT beam arrangement.
Table 3.
Intensity-modulated proton therapy beam angle information.
|
Beam No., angle |
Replan events/total |
Hazard ratio (95% CI) |
Replan rate (95% CI) |
Pa |
| Beam 1 ANT | 0.9747 | |||
| No | 56/113 | Reference range | 0.50 (0.39-0.58) | |
| Yes | 23/47 | 1.01 (0.62-1.64) | 0.49 (0.32-0.61) | |
| Beam 2 LAO | 0.1936 | |||
| No | 29/68 | Reference range | 0.42 (0.30-0.53) | |
| Yes | 50/92 | 1.35 (0.86-2.14) | 0.54 (0.43-0.63) | |
| Beam 3 LLAT | 0.1563 | |||
| No | 72/138 | Reference range | 0.52 (0.43-0.60) | |
| Yes | 7/22 | 0.57 (0.26-1.24) | 0.32 (0.09-0.49) | |
| Beam 4 LPO | 0.0497 | |||
| No | 44/103 | Reference range | 0.43 (0.32-0.52) | |
| Yes | 35/57 | 1.56 (1.00-2.43) | 0.61 (0.46-0.72) | |
| Beam 5 POST | 0.3288 | |||
| No | 41/89 | Reference range | 0.46 (0.35-0.55) | |
| Yes | 38/71 | 1.25 (0.80-1.94) | 0.54 (0.40-0.64) | |
| Beam 6 RPO | 0.2332 | |||
| No | 50/110 | Reference range | 0.45 (0.35-0.54) | |
| Yes | 29/50 | 1.32 (0.84-2.09) | 0.58 (0.42-0.70) | |
| Beam 7 RLAT | 0.9259 | |||
| No | 67/137 | Reference range | 0.48 (0.40-0.57) | |
| Yes | 12/23 | 1.03 (0.56-1.90) | 0.52 (0.27-0.69) | |
| Beam 8 RAO | 0.8708 | |||
| No | 32/67 | Reference range | 0.48 (0.34-0.58) | |
| Yes | 47/93 | 1.04 (0.66-1.63) | 0.51 (0.39-0.60) | |
| Beam 9 ASO | 0.4819 | |||
| No | 63/133 | Reference range | 0.47 (0.38-0.55) | |
| Yes | 16/27 | 1.22 (0.70-2.11) | 0.59 (0.36-0.74) | |
| Beam 10 LASO | 0.6776 | |||
| No | 76/153 | Reference range | 0.49 (0.41-0.57) | |
| Yes | 3/7 | 0.78 (0.25-2.48) | 0.42 (0.0-0.70) | |
| Beam 11 RASO | 0.7429 | |||
| No | 72/147 | Reference range | 0.49 (0.40-0.56) | |
| Yes | 7/13 | 1.14 (0.52-2.47) | 0.54 (0.17-0.74) | |
| Beam 12 LPSO | 0.8574 | |||
| No | 78/158 | Reference range | 0.49 (0.41-0.57) | |
| Yes | 1/2 | 0.83 (0.12-6.00) | 0.50 (0.00-0.87) | |
| Beam 13 RPSO | 0.8574 | |||
| No | 78/158 | Reference range | 0.49 (0.41-0.57) | |
| Yes | 1/2 | 0.83 (0.12-6.00) | 0.50 (0.00-0.87) | |
| Beam 14 RSO | — | |||
| No | 78/159 | Reference range | 0.49 (0.41-0.56) | |
| Yes | 1/1 | 7.58 (1.02-56.29) | 0.00 (0.00-0.00) | |
| Beam 15 LSO | — | |||
| No | 78/159 | Reference range | 0.49 (0.41-0.56) | |
| Yes | 1/1 | 7.58 (1.02-56.29) | 0.00 (0.00-0.00) | |
| Beam 16 PSO | 0.8200 | |||
| Nob | 77/157 | Reference range | 0.49 (0.41-0.56) | |
| Yesc | 1/2 | 0.80 (0.11-5.72) | 0.50 (0.00-0.87) |
Abbreviations: ANT, anterior; LAO, left anterior oblique; LLAT, left lateral; LPO, left posterior oblique; POST, posterior; RPO, right posterior oblique; RLAT, right lateral; RAO, right anterior oblique; ASO, anterior superior oblique; LASO, left anterior superior oblique; RASO, right anterior superior oblique; LPSO, left posterior superior oblique; RPSO, right posterior superior oblique; RSO, right superior oblique; LSO, left superior oblique; PSO, posterior superior oblique.
Type 3 Wald P-value.
No, the beam angle was not used.
Yes, the beam angle was used.
Discussion
We report on a prospectively collected quality-improvement project of 160 patients with H&N cancer who were treated consecutively with spot-scanning IMPT and who underwent weekly verification CT-QA scans. We aimed to understand how CT-QA scans influenced clinical decision-making to replan, and which variables were predictors of the highest rates of replanning to guide clinical practice. This report evaluates the practice of weekly verification CT-QA scans and highlights its clinical utility in the development of any high-quality IMPT program for H&N malignancies. Key findings revealed that the cumulative probability of a replan was 49.4% (79 of 160), suggesting that, at some point during their treatment course, one-half of all patients had an unacceptable dose distribution on CT-QA scan that prompted the treatment team to replan. There was no specific week that bore out as the optimal timing of when CT-QA scans should be performed, but rather, replans were initiated at each weekly time point throughout the treatment course. Multiple factors were associated with replanning on both MVA and UVA Cox models, including sinonasal disease site, advanced stage, doses > 60 GyE (RBE, 1.1), and primary disease. Additional factors associated on UVA Cox models only included concurrent chemotherapy, primary disease, definitive intent treatment, bilateral neck treatment, and 4 or 5 beams used in the plan. A patient's maximal absolute weight change from baseline weight was associated with higher odds of a replan with a ≥ 3-kg weight change nearly doubling the odds, a ≥ 5-kg change more than doubling the odds, and a > 6-kg change nearly tripling the odds. No particular beam arrangement was significantly associated with the need to replan. These data identify patients who may benefit from weekly CT-QA scans. Based on this experience, weekly verification CT-QA scans used for patients with H&N cancer treated with spot-scanning IMPT influenced clinical decision-making to replan, especially when factors associated with replanning were present.
Previous work has shown that patients with H&N cancer are at risk for anatomic changes that can adversely affect radiation dose distribution throughout the treatment course and may require planning [19–22]. Kraan et al [23] showed that treatment uncertainties in patients with oropharyngeal cancer could result in significant differences between planned and delivered IMPT doses, and the investigators recommended repeat diagnostic CT scans during treatment with replanning as indicated. Wu et al [24] demonstrated significant dose differences to CTVs and OARs when verification CT-QA scans were performed during the fourth week of treatment in 10 patients with oropharyngeal cancer treated with IMPT. Wu et al [24] showed that all 10 patients' mean CTV doses were significantly decreased and multiple OAR doses were increased between the simulation and verification CT-QA scans, which prompted replanning. The authors recommended the use of verification CT-QA scans and recognized the need for further prospective evaluation, but gave no guidance on the frequency or timing of CT-QA scans. The present study builds on those recommendations by demonstrating that clinically relevant dose-distribution changes can be appreciated as soon as the first week of treatment and may occur at any week throughout the treatment course. Furthermore, our data demonstrated that 16.5% (13 of 79) of those requiring a replan required a second replan, which emphasized the critical need to follow patients with CT-QA scans longitudinally throughout their entire IMPT course.
Strengths of the present study include its prospectively collected data. Additionally, the intrinsic study design accounted for innumerable plan-evaluation variables and DVH criteria by using real-time clinician, dosimetrist, and physicist judgment to determine whether a replan was indicated. We learned from our early experience and adapted these lessons to our more-mature CT-QA practice. Early on, we recognized positioning as a potential confounder, so a MATLAB program was developed to match external contours between the original simulation CT and the verification CT patient position before scanning the patient. Positioning errors and differences in scans were also minimized by doing CT-QA scans either immediately before or after the actual treatment using a diagnostic-quality CT-on-rails system adjacent to the treatment gantry, which allowed seamless verification in the actual treated position. Additionally, robust optimization planning was routinely used in our mature-practice cohort, which may also have influenced the decreased rate of replanning observed in the mature cohort.
There were limitations with the current study. Although the data were prospectively curated, there were still deficiencies in recording specific details prompting the replan. In those situations, the plans were individually reevaluated by the lead author and discussed with the treating team to clarify details prompting the replan. Additionally, there was significant heterogeneity in the study group because we evaluated all H&N disease sites treated with 25 or more fractions. This contributed to larger 95% CIs—in some instances—when the scrutinized variable(s) suffered from small sample sizes. Furthermore, the assumption that a clinician's decision to replan has any effect on clinical outcomes is not supported by our data, and such analyses were beyond the scope of this designed quality-assurance initiative. Certainly, the long-term effects of replanning in the final weeks of treatment may have little to no clinical effect and warrants further investigation. Additional prospective studies evaluating the efficacy of this practice in improving relevant clinical outcomes measured with longitudinal follow-up is merited before considered standard practice.
Conclusions
In summary, to our knowledge, this study is the first comprehensive evaluation of a prospectively collected QA program aimed at determining the effect on clinical decision-making of weekly verification CT-QA scans in spot-scanning IMPT for patients with H&N cancer. Routine weekly use of CT-QA scans for patients with H&N cancer treated with IMPT influenced clinical decision-making to replan in 49.4% (79 of 160) of the cases in this study. Patients with the following predictive factors had higher rates of replanning: sinonasal disease site, advanced stage III/IV, dose > 60 GyE (RBE, 1.1), primary disease, concurrent chemotherapy, definitive intent treatment, bilateral neck treatment, and those planned with 4 or 5 beams. A patient's maximal absolute weight change from baseline weight was also associated with higher odds of a replan. Additional studies that evaluate the practice of monitoring IMPT-treated patients with weekly CT-QA scans and whether that improves clinical outcomes are warranted.
Supplementary Material
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: Robert L. Foote, MD, declares a patent with royalties from Bionix and a named professorship with Hitachi, and Chris J. Beltran, PhD, declares grant funding from Varian medical; all are outside the submitted work. The authors have no relevant conflicts of interest to disclose.
Funding: This work was supported by the Mayo Clinic.
Equal Contribution: Drs Mundy and Foote contributed equally to the work.
Ethical Approval: All patient data were collected under internal review board–approved protocol.
References
- 1. .Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, Alahdab F, Altayar O, Nabhan M, Schild SE, Foote RL. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1027–38. doi: 10.1016/S1470-2045(14)70268-2. [DOI] [PubMed] [Google Scholar]
- 2. .Holliday EB, Garden AS, Rosenthal DI, Fuller CD, Morrison WH, Gunn GB, Phan J, Beadle BM, Zhu XR, Zhang X, Hanna E, Glisson BS, Hutcheson KA, El-Naggar AK, Hong JH, Hung TM, Uzel EK, Lewis G, Frank SJ. Proton therapy reduces treatment-related toxicities for patients with nasopharyngeal cancer: a case-match control study of intensity-modulated proton therapy and intensity-modulated photon therapy. Int J Part Ther. 2015;2:19–28. [Google Scholar]
- 3. .Sio TT, Lin HK, Shi Q, Gunn GB, Cleeland CS, Lee JJ, Hernandez M, Blanchard P, Thaker NG, Phan J, Rosenthal DI, Garden AS, Morrison WH, Fuller CD, Mendoza TR, Mohan R, Wang XS, Frank SJ. Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys. 2016;95:1107–14. doi: 10.1016/j.ijrobp.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. .Blanchard P, Garden AS, Gunn GB, Rosenthal DI, Morrison WH, Hernandez M, Crutison J, Lee JJ, Ye R, Fuller CD, Mohamed ASR, Hutcheson KA, Holliday EB, Thaker NG, Sturgis EM, Kies MS, Zhu XR, Mohan R, Frank SJ. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer—a case matched analysis. Radiother Oncol. 2016;120:48–55. doi: 10.1016/j.radonc.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. .McDonald MW, Liu Y, Moore MG, Johnstone PA. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat Oncol. 2016;11:32. doi: 10.1186/s13014-016-0600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. .Romesser PB, Cahlon O, Scher E, Zhou Y, Berry SL, Rybkin A, Sine KM, Tang S, Sherman EJ, Wong R, Lee NY. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118:286–92. doi: 10.1016/j.radonc.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. .Zhang W, Zhang X, Yang P, Blanchard P, Garden AS, Gunn B, Fuller CD, Chambers M, Hutcheson KA, Ye R, Lai SY, Radwan MAS, Zhu XR, Frank SJ. Intensity-modulated proton therapy and osteoradionecrosis in oropharyngeal cancer. Radiother Oncol. 2017;123:401–5. doi: 10.1016/j.radonc.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. .Wang X, Poenisch F, Sahoo N, Zhu RX, Lii MF, Gillin MT, Li J, Grosshans D. Spot scanning proton therapy minimizes neutron dose in the setting of radiation therapy administered during pregnancy. J Appl Clin Med Phys. 2016;17:366–76. doi: 10.1120/jacmp.v17i5.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. .Zhu XR, Poenisch F, Li H, Zhang X, Sahoo N, Wu RY, Li X, Lee AK, Chang EL, Choi S, Pugh T, Frank SJ, Gillin MT, Mahajan A, Grosshans DR. A single-field integrated boost treatment planning technique for spot scanning proton therapy. Radiat Oncol. 2014;9:202. doi: 10.1186/1748-717X-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. .Leeman JE, Romesser PB, Zhou Y, McBride S, Riaz N, Sherman E, Cohen MA, Cahlon O, Lee N. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol. 2017;18:e254–65. doi: 10.1016/S1470-2045(17)30179-1. [DOI] [PubMed] [Google Scholar]
- 11. .Frank SJ, Blanchard P, Lee JJ, Sturgis EM, Kies MS, Machtay M, Vikram B, Garden AS, Rosenthal DI, Gunn GB, Fuller CD, Hutcheson K, Lai S, Busse PM, Lee NY, Lin A, Foote RL. Comparing intensity-modulated proton therapy with intensity-modulated photon therapy for oropharyngeal cancer: the journey from clinical trial concept to activation. Semin Radiat Oncol. 2018;28:108–13. doi: 10.1016/j.semradonc.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. .National Comprehensive Cancer Network. Head and neck cancers (version 12019) https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf Accessed May 10, 2019.
- 13. .Waddle MR, Sio TT, Van Houten HK, Foote RL, Keole SR, Schild SE, Laack N, Daniels TB, Crown W, Shah ND, Miller RC. Photon and proton radiation therapy utilization in a population of more than 100 million commercially insured patients. Int J Radiat Oncol Biol Phys. 2017;99:1078–82. doi: 10.1016/j.ijrobp.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 14. .Particle Therapy Co-Operative Group. Particle therapy facilities in clinical operation. 2020 Feb; https://www.ptcog.ch/index.php/facilities-in-operation. Updated. Accessed February 20, 2020.
- 15. .Ma J, Beltran C, Seum Wan Chan Tseung H, Herman MG. A GPU-accelerated and Monte Carlo-based intensity modulated proton therapy optimization system. Med Phys. 2014;41:121707. doi: 10.1118/1.4901522. [DOI] [PubMed] [Google Scholar]
- 16. .Ma J, Wan Chan Tseung HS, Herman MG, Beltran C. A robust intensity modulated proton therapy optimizer based on Monte Carlo dose calculation. Med Phys. 2018;45:4045–54. doi: 10.1002/mp.13096. [DOI] [PubMed] [Google Scholar]
- 17. .Wan Chan Tseung HS, Ma J, Kreofsky CR, Ma DJ, Beltran C. Clinically applicable Monte Carlo-based biological dose optimization for the treatment of head and neck cancers with spot-scanning proton therapy. Int J Radiat Oncol Biol Phys. 2016;95:1535–43. doi: 10.1016/j.ijrobp.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 18. .Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, editors. American Joint Committee on Cancer AJCC Cancer Staging Manual 7th ed. New York, NY: Springer; 2010. eds; [Google Scholar]
- 19. .Wang W, Yang H, Hu W, Shan G, Ding W, Yu C, Wang B, Wang X, Xu Q. Clinical study of the necessity of replanning before the 25th fraction during the course of intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:617–21. doi: 10.1016/j.ijrobp.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 20. .Barker JL, Jr, Garden AS, Ang KK, O'Daniel JC, Wang H, Court LE, Morrison WH, Rosenthal DI, Chao KSC, Tucker SL, Mohan R, Dong L. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;(59):960–70. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 21. .Hansen EK, Bucci MK, Quivey JM, Weinberg V, Xia P. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64:355–62. doi: 10.1016/j.ijrobp.2005.07.957. [DOI] [PubMed] [Google Scholar]
- 22. .Feng M, Yang C, Chen X, Xu S, Moraru I, Lang J, Schultz C, Li XA. Computed tomography number changes observed during computed tomography-guided radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2015;91:1041–7. doi: 10.1016/j.ijrobp.2014.12.057. [DOI] [PubMed] [Google Scholar]
- 23. .Kraan AC, van de Water S, Teguh DN, Al-Mamgani A, Madden T, Kooy HM, Heijmen BJM, Hoogeman MS. Dose uncertainties in IMPT for oropharyngeal cancer in the presence of anatomical, range, and setup errors. Int J Radiat Oncol Biol Phys. 2013;87:888–96. doi: 10.1016/j.ijrobp.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 24. .Wu RY, Liu AY, Sio TT, Blanchard P, Wages C, Amin MV, Gunn GB, Titt U, Ye R, Suzuki K, Gillin MT, Zhu XR, Mohan R, Frank SJ. Intensity-modulated proton therapy adaptive planning for patients with oropharyngeal cancer. Int J Part Ther. 2017;4:26–34. doi: 10.14338/IJPT-17-00010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.