To the Editor:
We read with great interest the article published by Biquard and colleagues showing that, according to public transcriptomic data, the hepatic expression of angiotensin converting enzyme 2 (ACE2) and the cellular transmembrane protease serine 2 (TMPRSS2) remains unchanged in patients with metabolic-associated fatty liver disease (MAFLD).1 SARS-CoV-2 attaches to cells by binding to its receptor ACE2. TMPRSS2 then cleaves the SARS-CoV-2 spike protein, allowing fusion of cellular and viral membranes.2 Despite this retrospective study, there is growing evidence that patients with MAFLD are at higher risk of COVID-19 disease progression.[3], [4], [5]
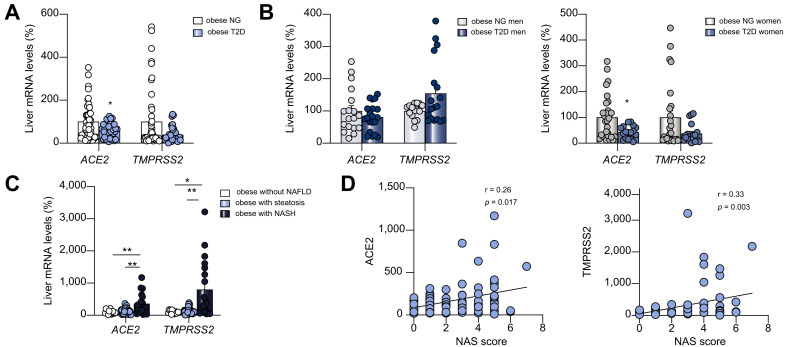
Given the ongoing discussion, we have assessed the expression of SARS-CoV-2 cell entry molecules in the liver of obese patients with non-alcoholic fatty liver disease (NAFLD) and/or type 2 diabetes T2D (see Table S1 for detailed characteristics), since this information seems crucial to understand and prevent cell infection. Considering that T2D has been associated with a worse prognosis in patients with COVID-19 and that well-controlled glycemia was associated with a markedly improved outcome,6 we first focused on patients with T2D. Liver mRNA expression of ACE2 was significantly lower in patients with T2D while TMPRSS2 also tended to decrease but was not statistically significant (Fig. 1 A). Then, we analysed separately men and women. In men, hepatic ACE2 and TMPRSS2 expression remained unchanged between the 2 groups (Fig. 1B). However, in women with T2D, ACE2 was significantly lower while TMPRSS2 gene expression tended to decrease compared to women without T2D (Fig. 1B). These results indicate that while the cell entry machinery of SARS-CoV-2 is not majorly altered in the liver of obese men with T2D, its downregulation in women might indicate a lower susceptibility to liver injury. These findings are in consonance with the well-established protective role of estrogens in dysmetabolism.7
Fig. 1.
ACE2 and TMPRSS2 hepatic mRNA levels in patients with T2D or NAFLD.
(A) ACE2 and TMPRSS2 expression in obese patients with T2D (n = 43) or NG (n = 51); and (B) separately by men and women with T2D or NG. (C) ACE2 and TMPRSS2 expression in obese patients without NAFLD (n = 17), steatosis (n = 57), NASH (n = 20). (D) Correlation between ACE2 and TMPRSS2 with NAS score. ∗p <0.05, ∗∗p <0.01, Mann-Whitney U test (A, B), Krustal-Wallis followed by Dunn post-hoc test (C). ACE2, angiotensin converting enzyme 2; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NG, normoglycemia; T2D, type 2 diabetes; TMPRSS2, transmembrane protease serine 2.
Next, we measured the expression of these genes in the liver according to the presence of NAFLD. Liver mRNA expression of both ACE2 and TMPRSS2 did not show differences between individuals without liver injury and patients with only steatosis, but these genes were upregulated in obese patients with non-alcoholic steatohepatitis (NASH) (Fig. 1C). Moreover, ACE2 and TMPRSS2 were positively correlated with NAFLD activity score (Fig. 1D). Of note, TMPRSS2 was also positively correlated with weight, BMI and cholesterol (data not shown). These results are apparently different to those described in a previous study performing transcriptomics in individuals with and without MAFLD1 , 8 and also in animal models of diet-induced NASH,8 where no changes were detected for either ACE2 or TMPRSS2. However, it is important to highlight that there are important differences in the characteristics of the cohorts. In contrast to the previous report that analyzed lean and obese patients with MAFLD or NASH,1 our cohort is exclusively composed of obese patients. Moreover, whereas in the previous analysis T2D is not mentioned, in our cohort a significant percentage of patients had T2D, which commonly coexists with MAFLD.9 Lastly, methodological differences might also explain the discrepant results, since we used real-time PCR to specifically measure gene expression of ACE2 and TMPRSS2, while in the previous reports8 results were obtained by less quantitative techniques namely microarray or RNA sequencing. Further studies using larger cohorts of patients with liver damage need to be meticulously evaluated to understand whether the SARS-CoV-2 receptor ACE2 and the serine protease TMPRSS2 are indeed affected in advanced stages of NAFLD and to what extent their expression affects the incidence of complications, severity and mortality.
In summary, our results indicate that in the livers of obese patients, SARS-CoV-2 entry factors are differently affected by T2D and NAFLD. While obese women with T2D have unexpectedly lower levels of ACE2 and TMPRSS2 than obese normoglycemic women, obese patients with NASH show markedly higher expression of these genes, suggesting that advanced stages of NAFLD might predispose individuals to COVID-19.
Financial support
This work has been supported by grants from FEDER/Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación (CD: BFU2017-87721; RN: RTI2018-099413-B-I00; MLMC: SAF2017-87301-R; Xunta de Galicia (RN: 2015-CP080 and 2016-PG057) and ED431G 2019/02, Fundación BBVA (RN and MLM), Fundación Atresmedia (RN), and European Foundation for the Study of Diabetes (RN). FIS: PI18/0130. Immunomediatd Nonalcoholic SteaTohepatItis; prevalence and CharacTerization. INSTInCT study (PI: Javier Crespo). Intensificación IDIVAL I-1 (Javier Crespo). The research leading to these results has also received funding from the European Community’s H2020 Framework Programme under the following grant: ERC Synergy Grant-2019-WATCH- 810331 to MS, VP and RN. CIBERobn and CIBERehd are initiatives of the Instituto de Salud Carlos III (ISCIII) of Spain which is supported by FEDER funds. We thank MINECO for the Severo Ochoa Excellence Accreditation to CIC bioGUNE (SEV-2016-0644).
Authors’ contributions
MFF, MMG, AR, MJGR, PI, VV, JE, MS, VP, CD, JC, GF, MLMC, RN contributed to conception and design, acquisition of data, analysis and interpretation of data. PI, MS, VP, CD, JC, GF, MLMC, RN contributed drafting the article and revising it critically for important intellectual content.
Data availability statement
All the data used to support the findings of this study are included within the article. Reagents, resources and protocols are included in Supplementary methods.
Conflict of interest
The authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2020.09.027.
Supplementary data
References
- 1.Biquard L., Valla D., Rautou P.E. No evidence for an increased liver uptake of SARS-CoV-2 in metabolic-associated fatty liver disease. J Hepatol. 2020;73(3):717–718. doi: 10.1016/j.jhep.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y.J., Zheng K.I., Wang X.B., Yan H.D., Sun Q.F., Pan K.H. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: a multicenter preliminary analysis. J Hepatol. 2020;73:719–721. doi: 10.1016/j.jhep.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y.J., Zheng K.I., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020 doi: 10.1111/liv.14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e63. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahrens M., Ammerpohl O., von Schonfels W., Kolarova J., Bens S., Itzel T. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013;18:296–302. doi: 10.1016/j.cmet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data used to support the findings of this study are included within the article. Reagents, resources and protocols are included in Supplementary methods.