Abstract
Background
As an ongoing worldwide health issue, Coronavirus disease 2019 (COVID–19) has been causing serious complications, including pneumonia, acute respiratory distress syndrome (ARDS), and multi-organ failure. However, there is no decisive treatment approach available for this disorder, which is primarily attributed to the large amount of inflammatory cytokine production. We aimed to identify the effects of Nano-curcumin on the modulation of inflammatory cytokines in COVID-19 patients.
Method
Forty COVID-19 patients and 40 healthy controls were recruited and evaluated for inflammatory cytokine expression and secretion. Subsequently, COVID-19 patients were divided into two groups: 20 patients receiving Nano-curcumin and 20 patients as the placebo group. The mRNA expression and cytokine secretion levels of IL-1β, IL-6, TNF-α and IL‐18 were assessed by Real‐time PCR and ELISA, respectively.
Result
Our primary results indicated that the mRNA expression and cytokine secretion of IL-1β, IL-6, TNF-α, and IL-18 were increased significantly in COVID-19 patients compared with healthy control group. After treatment with Nano-curcumin, a significant decrease in IL-6 expression and secretion in serum and in supernatant (P = 0.0003, 0.0038, and 0.0001, respectively) and IL-1β gene expression and secretion level in serum and supernatant (P = 0.0017, 0.0082, and 0.0041, respectively) was observed. However, IL-18 mRNA expression and TNF-α concentration were not influenced by Nano-curcumin.
Conclusion
Nano-curcumin, as an anti-inflammatory herbal based agent, may be able to modulate the increased rate of inflammatory cytokines especially IL-1β and IL-6 mRNA expression and cytokine secretion in COVID-19 patients, which may cause an improvement in clinical manifestation and overall recovery.
Keywords: COVID-19, Nano-curcumin, Cytokine storm, IL-1β, IL-6
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, Coronavirus disease 2019; SARS-CoV, severe acute respiratory syndrome-CoV; MERS-CoV, middle east respiratory syndrome-CoV; PBMCs, peripheral blood mononuclear cells; ICU, intensive care unit; IL-1β, Interleukin-1 β; IL-6, Interleukin-6; IL-7, Interleukin_7; IL-8, Interleukin-8; IL-18, Interleukin-18; TNF-α, tumor necrosis factor-alpha; MCP-1, monocyte chemoattractant peptide; G-CSF, granulocyte-colony stimulating factor; TLR, toll-like receptors; CD80, cluster of differentiation 80; CD86, cluster of differentiation 86; ROS, reactive oxygen species; PCR, polymerase chain reaction; PBS, phosphate buffered saline; FCS, fetal calf serum; PMA, phorbol myristate acetate; ELISA, enzyme-linked immunosorbent assay; mRNA, messenger RNA; cDNA, complementary DNA; TMB, tetra methyl benzidine; Th1, T-helper-1; IBD, inflammatory bowel disease; MS, multiple sclerosis
1. Introduction
Coronavirus disease 2019 (COVID-19), as a health emergency and global concern, is caused by coronavirus, belonging to the Coronaviridae family, which has a single and sensitive 26 to 32 kb RNA [1], [2]. Coronaviruses have been identified in several mammalian hosts, especially in camels and bats. Some members of coronaviruses family are not pathogenic to human or may cause mild clinical symptoms, but there are two notable exceptions [3]. One of them caused an outbreak in November 2002 as Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) in Guangdong, southern china, involving 37 countries with 8000 cases of infections and 774 death cases. The other one was Middle East Respiratory Syndrome- Coronavirus (MERS-CoV), appeared in Saudi Arabia in 2012 that affected approximately 2500 cases. The newest member of this viral family, Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) thta is able to cause COVID-19, appeared in late 2019 in Wuhan, China and has been an ongoing pandemic and a health issue until now [4].
There are mild, moderate, and severe forms of infection with SARS-CoV-2, however the common clinical symptoms are predominantly fever, dry cough, and tiredness. Less common symptoms include headache, sore throat, nasal congestion, loss of taste or smell, gastrointestinal symptoms like diarrhea and vomiting are observed in some cases [5]. Additionally, in moderate to severe form of the infection, or in severe pneumonia, patients may exhibit symptoms, such as difficulty breathing or shortness of breath, chest pain or pressure, hypoxia, severe dyspnea, respiratory distress, and tachypnea. The fetal state of COVID-19 is associated with respiratory failure and acute respiratory distress syndrome (ARDS), in which patients need to be admitted to the intensive care unit (ICU) and related mortality rate is higher [6], [7].
Although the exact mechanism of COVID-19 pathogenesis is not completely understood, however it seems that aberrant immune responses plays a key role in the development of the disease [8]. Replication of virus in the epithelial cells of respiratory tracts results in immune system activation and, subsequently, inflammatory cells infiltrate to the injury cite. Increased secretion of the pro-inflammatory cytokine provokes an acute inflammation and immune hyper-response, leading to pulmonary tissue damages and even sometimes ARDS [9].
According to the recent studies, the secretion of several cytokines and mediators, such as interleukin (IL)-1β, IL-6, IL-7, IL-8, IL-18, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, monocyte chemoattractant peptide (MCP)-1, and granulocyte-colony stimulating factor (G-CSF) is increased in COVID-19 patients. Cytokines, especially pro-inflammatory types, play a pivotal role in the pathogenesis of the disease [10]. IL-1β production occurs after virus attachment to toll-like receptors (TLRs) and subsequent inflammasome activation [11]. IL-6 level elevation is associated with the progression of COVID-19 and monitoring of this cytokine may be a potential biomarker in the prognosis of high risk patients [12], [13]. TNF-α also plays a stimulatory role in the development of inflammation and is increased in both the blood and peripheral tissues of COVID-19 patients [14]. Studies on the inflammatory diseases, such as Multiple sclerosis (MS), Inflammatory bowel disease (IBD), Rheumatoid arthritis (RA), and Ankylosing spondylitis (AS) have shown that increased production of IL-1β, IL-6, IL-18, and TNF-α are directly related to the disease activity [15], [16]. Therefore, it is speculated that the same is true for COVID-19 and disease severity may be associated with production of inflammatory cytokines.
Curcumin (also called diferuloylmethane), as the medicinal part of turmeric, is isolated from a plant called “curcuma longa”. Curcumin is a polyphenol of diacryl heptanoids and the chemical formula of this substance is (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) [17]. For many years, curcumin has been used in health issues for its anti-inflammatory, antioxidant and anti-cancer effects, which is related to the activity of methoxy groups [18]. Recent studies demonstrated that curcumin may be beneficial in different disorders, such as Alzheimer’s disease, cardiovascular disease, MS, RA, and psoriasis due to its anti-inflammatory effect [15], [19], [20]. Indeed, curcumin, as an immunomodulatory agent, is able to prevent the progression of tissue damage and development of inflammatory disease. According to different studies, curcumin may affect the immune system by inhibiting production of reactive oxygen species (ROS) in macrophages, upregulating the CD80 and CD86 expression on dendritic cells (DCs), modulating T cells proliferation and function [21], regulating the secretion of pro-inflammatory cytokines and adhesion molecules, and ultimately regulating the pattern of immune responses [22], [23]. Considering that inflammation is the epicenter of COVID-19 etiopathogenesis, it is contemplated that curcumin might be potential in modulating the inflammatory responses as well as ameliorating the disease symptoms.
So far, curcumin has been available in different forms, including powder, capsules and tablets. In order to facilitate the application of curcumin and improve its stability and solubility, researchers have formulated the curcumin with the aid of nanotechnology. Nano-micells and Nano-particles are among the different formulations for Nano-curcumin preparation [21]. In the current study, we used the Nano-micellar form, in which curcumin is concentrated in lipophilic and the hydrophilic part.
Hence, we aimed to evaluate the concentration of inflammatory cytokines in COVID-19 patients and compare it with healthy controls. In addition, since the pro-inflammatory cytokines are the accurate targets in the treatment of several inflammatory diseases, we decided to evaluate the effect of Nano-curcumin on the modulation or even suppression of these cytokines and hopefully introduce a therapeutic agent for COVID-19 patients.
2. Methods and materials
2.1. Study design and patients’ selection
This study is a randomized, double-blind, placebo-controlled study in which a total of 40 COVID-19 patients (aged 19–69 years) and 40 healthy controls were included, who were referred to Imam Reza Hospital of Tabriz University of Medical Sciences. The patient group was also divided into the groups of Nano-curcumin and placebo, based on the intervention method. The patients were clinically diagnosed to be new cases of COVID-19 on the basis of clinical manifestations and laboratory tests. An informed consent was obtained from all patient and control subjects prior to participation. This study was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1398.1314).
The inclusion criteria of the patients were as follows: willingness to cooperate, 18–80 years of age, diagnosis of COVID-19 infection by a pulmonologist and confirmation with PCR test, and ARDS phase. In case of pregnancy or breastfeeding, auto-immune and other acute disorders, suppression of immune system [like infection with human immunodeficiency virus (HIV) or taking immune-suppressor agents], consumption of nutritional supplements, alpha lipoic acid and antioxidants within a month before the study, subjects were excluded. The information about subject and evaluated clinical and laboratory parameters are summarized in Table 1 .
Table 1.
Subjects clinical and laboratory information.
| Nano-curcumin group (n = 20) | Placebo group (n = 20) | P value | Healthy Control (n = 40) | |
|---|---|---|---|---|
| Age, Years | 19–69 (53.3 ± 8.4) | 21–67 (51.4 ± 7.9) | 22–65 (49.8 ± 8.3) | |
| Sex | ||||
| Men | 15 (77%) | (80%)16 | NS | 30 (75%) |
| Women | 5 (22%) | (20%)4 | 10 (25%) | |
| Current smoking | 4 (20%) | (15%)3 | NS | 9 (18%) |
| Diabetes | 2 (10%) | (5%)1 | NS | 0 |
| Hypertension | 1 (5%) | (5%)1 | NS | 0 |
| Cardiovascular disease | 2 (10%) | (15%)3 | NS | 0 |
| Chronic kidney disease | 0 | (5%)1 | _ | |
| Fever | ||||
| <37.3 °C | 1 (5%) | 0 | NS | 0 |
| 37.3–38.0 °C | 9 (45%) | 8 (40%) | ||
| 38.1–39.0 °C | 7 (37%) | 7 (35%) | ||
| >39.0 °C | 4 (20%) | 5 (25%) | ||
| Cough | 12 (60%) | 11 (55%) | NS | 0 |
| Headache | 1 (5%) | 2 (10%) | NS | 0 |
| Dyspnea | 5 (25%) | 4 (20%) | NS | 0 |
| White blood cell count, × 109/L | ||||
| <4 | 5 (25%) | (25%)5 | NS | 0 |
| 4–10 | 9 (45%) | (40%)8 | ||
| >10 | 6 (30%) | (35%)7 | ||
| Lymphocyte count, × 109/L | ||||
| <1·0 | 13 (65%) | 11 (55%) | NS | 0 |
| ≥1·0 | 7 (35%) | 9 (45%) | ||
| Platelet count, × 109/L | ||||
| <100 | 11 (55%) | 11 (55%) | NS | 0 |
| ≥100 | 9 (45%) | 8 (40%) | ||
| Creatinine, μmol/L | ||||
| ≤133 | 18 (90%) | 17 (85%) | NS | 0 |
| >133 | 2 (10%) | 3 (15%) | ||
| Lactate dehydrogenase, U/L | ||||
| ≤245 | 13 (65%) | 13 (65%) | NS | 0 |
| >245 | 7 (35%) | 7 (35%) | ||
| Bilateral involvement of chest radiographs | 19 (95%) | 19 (95%) | NS | 0 |
The treatment group received 160 mg of Nano-curcumin in four 40 mg capsules daily for 14 days and the control group received the placebo capsule. The oral Nano-curcumin capsules used in this study were SinaCurcumin capsules, which are registered product from curcuminoids in Iran (IRC:1228225765) and are industrialized in Nanotechnology Research Center of Mashhad University of Medical Sciences, marketed by Exir Nano Sina Company [24].
Additionally, all COVID-19 subjects in both Nano-curcumin and placebo groups, received Betaferon 300 μg subcutaneously every other day until 5 days, Bromhexine 8 mg tablets every 8 h, and Atrovastatin 40 mg daily.
2.2. Blood sampling
Peripheral blood samples of all subject were collected twice; first, prior to the intervention, 8 ml of fasting blood samples were collected in EDTA tubes and the second sampling was conducted at the end of the study in the same situation.
Standard Ficoll (lymphosep) 1.077 g/ml (Biosera, Heath field, East Sussex, UK) centrifugation (25 min, 450 × g) method was used to isolate peripheral blood mononuclear cells (PBMCs) [25]. We washed the isolated cells twice with phosphate buffered saline (PBS) (Sigma-Aldrich, Schnelldorf, Germany). The mean number of 1.18 × 106 ± 0.12 PBMCs were isolated per ml of whole blood. The number of 5 × 106 of isolated PBMCs were cultured in 5 ml Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% heat-inactivated fetal calf serum (FCS), and subsequently, 100 U/ml of penicillin and 200 mML of glutamine were added to the medium; following by addition of 10 ng/ml of phorbol myristate acetate (PMA) (eBioscience, San Diego, CA, USA) and incubation for 48 h at 37 °C and 5% CO2.
2.3. Analysis of IL-1β, IL-6, IL-18 and TNF-α mRNA expression by Real-time PCR
TRIZOL solution (SinaClon, Tehran, Iran) was utilized for total RNA isolation from PBMCs. Subsequently, complementary DNA (cDNA) was synthesized using Revert Aid Reverse Transcriptase kit (Thermo Fisher, Waltham, MA). A total volume of 25 μl, containing primers, cDNA, and the SYBR Green Master Mix was prepared for PCR reaction. The optimized conditions for PCR thermocycling were as follows: 10 s at 95 °C, 40 cycles of denaturation: 10 s at 95 °C, annealing and extension level for 30 s at 60 °C, respectively. To confirm the amplification, an electrophoresis analysis on 2% agarose gel were conducted. The sequences of the primers are presented in Table 2 . The β-Actin, as the housekeeping gene, was used to normalize the transcript level of the target genes.
Table 2.
Primer sequence.
| Gene | Primer | Sequence |
|---|---|---|
| IL-1β | Forward | ACGATGCACCTGTACGATCA |
| Reverse | TCTTTCAACACGCAGGACAG | |
| IL-6 | Forward | ACTCACCTCTTCAGAACGAATTG |
| Reverse | CCATCTTTGGAAGGTTCAGGTTG | |
| IL-18 | Forward | GATAGCCAGCCTAGAGGTATGG |
| Reverse | CCTTGATGTTATCAGGAGGATTCA | |
| TNF-α | Forward | CAGAGGGAAGAGTTCCCCAG |
| Reverse | CCTTGGTCTGGTAGGAGACG | |
| β-actin | Forward | AGAGCTACGAGCTGCCTGAC |
| Reverse | AGCACTGTGTTGGCGTACAG |
(Abbreviation: IL-1β: Interleukin-1 β; IL-6: Interleukin-6; IL-18: Interleukin-18; TNF-α: tumor necrosis factor-alpha).
2.4. Measurement of cytokine levels
In order to evaluate the level of IL-1β, IL-6, IL-18, and TNF-α in the supernatant of PBMCs culture and serum, we used Enzyme-linked immunosorbent assay (ELISA) method according to the manufacturer’s instructions. The concentration gradients of the kit standards or positive controls render an estimated sensitivity of < 0.3 pg/ml for IL-6, <0.15 pg/ml for IL-1β, <1 pg/ml for TNF-α and < 0.2 pg/ml for IL-18). First, 100 μl of the initial antibodies were coated in wells of the 96-well plate overnight. Subsequently, the wells were washed with PBS containing 0.005% Tween to remove additional antibodies and were incubated with blocking antibodies for 1 h on shaker. In the next step, 100 μl of samples or standards were added to the wells and incubated for 1 h on a shaker. After washing, first, the wells were incubated with 100 μl of biotinylated antibody for 1 h. Then, 100 μl of Avidin-Biotin-Peroxidase Complex (ABC) were added to wells and incubated for 30 min. After washing, 100 μl of tetra methyl benzidine (TMB) substrate solution was added to the wells. After 20–25 min, finally, Medgenix ELISA reader (BP-800, Biohit, USA) was utilized for measuring the absorbance in 450 nm. The appropriate standard calibration lines were used for calculating the samples concentration using the Softmax software of the reader.
2.5. Statistical analysis
SPSS PC Statistics (version 19.0; SPSS Inc) was used for statistical analysis. Unpaired T test for comparison of differences of immunologic factors between healthy control group and COVID-19 group. The paired T-test was used to compare the results of immunologic factors before and after treatment in either Nano-curcumin or placebo group. The graphs were drawn by the GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA, USA, www.graph pad.com). We reported P values < 0.05 as statistically significant.
3. Results
3.1. mRNA expression level of TNF-α, IL-18, IL-6 and IL-1β in activated PBMCs
TNF-α, IL-18, IL-6 and IL-1β mRNA expression levels were assessed and compared once in COVID-19 patients and healthy control group and once in Nano-curcumin treated and placebo group.
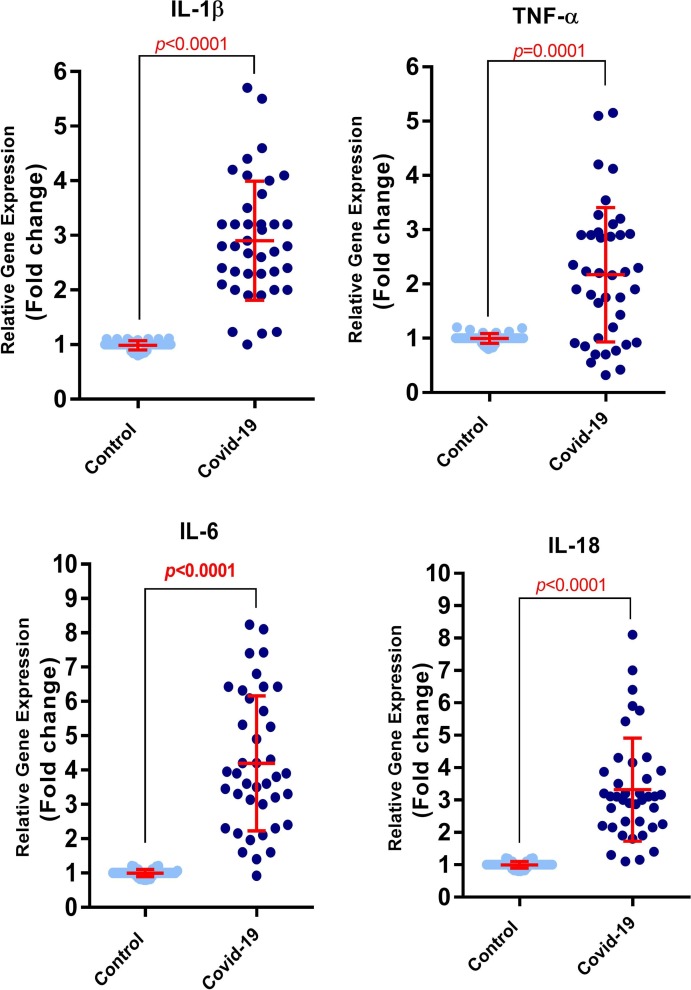
The results of COVID-19 patients and healthy control group indicated that mRNA expression level of all the four inflammatory cytokines were increased in COVID-19 patients compared to the healthy control group (P < 0.0001 for IL-1β, IL-6 and IL-18 and P = 0.0001 for TNF-α; Fig. 1 ).
Fig. 1.
mRNA expression level of TNF-α, IL-18, IL-6, and IL-1β in activated PBMCs isolated from COVID-19 patients and healthy control group. The results showed an upregulation of the mRNA expression of all four inflammatory cytokines in COVID-19 patients compared to healthy control group (P < 0.0001 for IL-1β, IL-6 and IL-18 and P = 0.0001 for TNF-α). (Control group number = 40, COVID-19 group number = 40). Evaluation of mRNA expression level was done using Real-time PCR technique. Unpaired T test was used for statistical analysis and a P < 0.05 was considered as the statistically significant level.
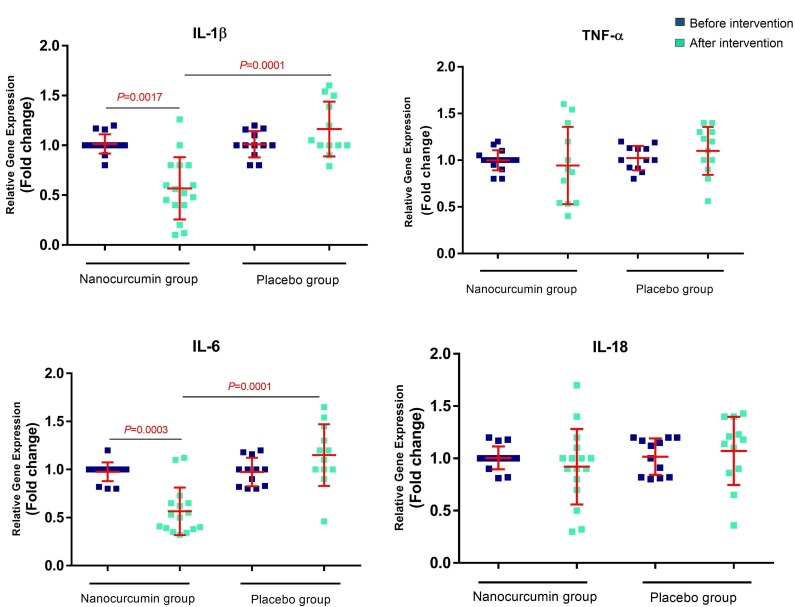
Evaluation of the mRNA expression levels in Nano-curcumin treated and placebo group indicated that the expression level of IL-1β decreased after treatment with Nano-curcumin (P = 0.0017) compared with pre-treatment. Additionally, a significant reduction was observed in the Nano-curcumin group compared to the placebo group (P = 0.0001). Downregulation of the IL-6 mRNA expression was also observed in Nano-curcumin group after treatment (P = 0.0003) and compared with the placebo group (P = 0.0001). There were no statistically significant differences in the expression level of IL-18 and TNF-α before and after intervention in the Nano-curcumin group as well as the placebo groups (Fig. 2 ). All data about mRNA expression level is summarized in Table 3 .
Fig. 2.
mRNA expression level of TNF-α, IL-18, IL-6, and IL-1β in the activated PBMCs isolated from Nano-curcumin treated and placebo group. The expression level of IL-1β and IL-6 decreased after treatment with Nano curcumin compared with the pre-treatment state (P = 0.0017 and 0.0003, respectively) and compared with the placebo group (P = 0.0001 for both). There were no statistically significant differences in the expression level of IL-18 and TNF-α before and after intervention in Nano-curcumin group, and in Nano-curcumin group compared with placebo groups (Nano-curcumin group number = 20, placebo group number = 20). Evaluation of the mRNA expression level was done using Real-time PCR technique. Paired T test was used for statistical analysis and P < 0.05 was considered statistically significant.
Table 3.
mRNA expression level of inflammatory cytokine.
| Prior to treatment |
Post treatment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 patients (Mean ± SD) | Healthy control (Mean ± SD) | p-value | Nano-curcumin group (Mean ± SD) | Placebo group (Mean ± SD) | p-value | ||||||
| before | after | p-value | before | after | p-value | ||||||
| mRNA expression level of cytokines | IL-1β | 2.9 ± 1.1 | 0.98 ± 0.09 | <0.0001 | 1.01 ± 0.11 | 0.56 ± 0.31 | 0.0017 | 1.01 ± 0.13 | 1.16 ± 0.27 | NS | 0.0001 |
| IL-6 | 0.99 ± 0.09 | 4.19 ± 1.99 | <0.0001 | 0.98 ± 0.09 | 0.58 ± 0.25 | 0.0003 | 0.97 ± 0.14 | 1.15 ± 0.32 | NS | <0.0001 | |
| IL-18 | 3.32 ± 1.57 | 0.99 ± 0.09 | <0.0001 | 1.00 ± 0.10 | 0.93 ± 0.35 | NS | 1.01 ± 0.17 | 1.07 ± 0.35 | NS | NS | |
| TNF-α | 2.17 ± 1.23 | 0.99 ± 0.09 | 0.0001 | 0.99 ± 0.11 | 0.94 ± 0.41 | NS | 1.02 ± 0.13 | 1.09 ± 0.24 | NS | NS | |
(Abbreviation: mRNA: messenger RNA; IL-1β: Interleukin-1 β; IL-6: Interleukin-6; IL-18: Interleukin-18; TNF-α: tumor necrosis factor-alpha; COVID-19: Coronavirus disease 2019; SD: standard deviation; NS: not significant).
3.2. Secretion level of IL-1β, IL-6, TNF-α and IL-18
Cytokines levels were evaluated in serum and supernatant of cultured PBMCs and were compared between COVID-19 patients and healthy control group, as well as between Nano-curcumin‐treated COVID-19 patients and placebo group.
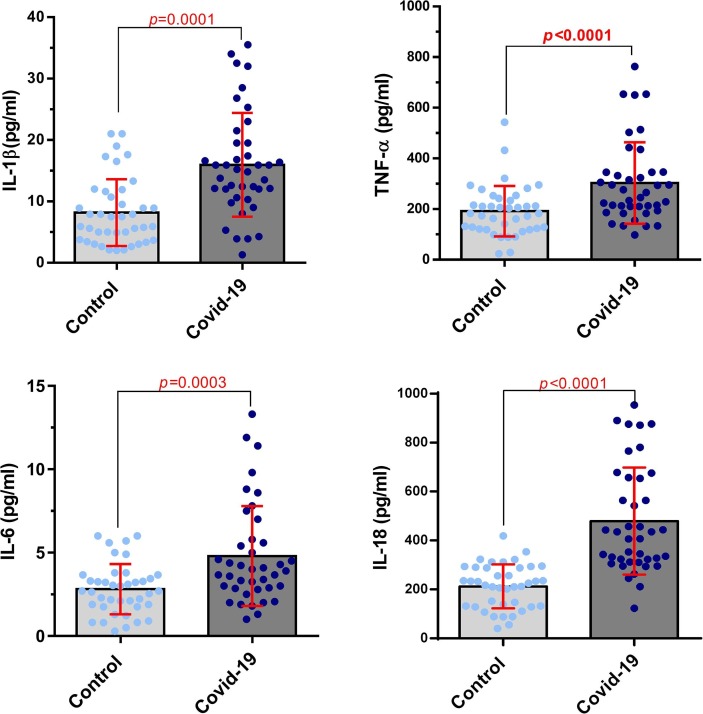
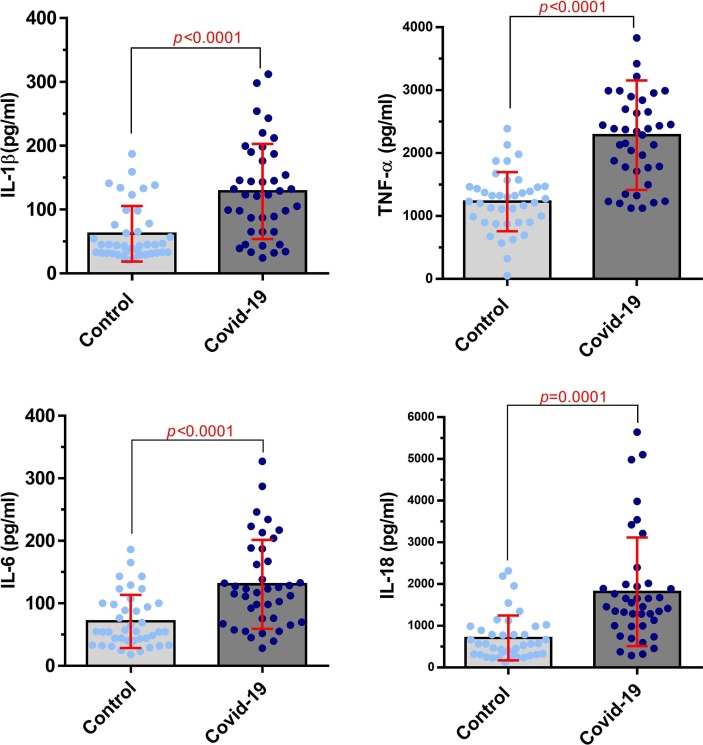
The results of ELISA assessment between COVID-19 patients and healthy control group in serum indicated that the levels of IL-1β, IL-6, TNF-α and IL-18 were significantly higher in the COVID-19 patients compared with the healthy control group (P = 0.0001, 0.0003, P < 0.0001, and P < 0.0001, respectively; Fig. 3 ). As shown in Fig. 4 , almost the same results were obtained in cytokines assessment between COVID-19 patients and healthy control group in the supernatant of cell culture. The results showed that secretion level of inflammatory cytokines were also significantly increased in COVID-19 patients (P < 0.0001 for IL-1β, IL-6, and TNF-α, P = 0.0001 for IL-18).
Fig. 3.
Cytokine levels of IL-1β, IL-6, TNF-α, and IL-18 in COVID-19 patients and healthy control group in serum. The cytokine levels of IL-1β, IL-6, TNF-α, and IL-18 of COVID-19 patients were significantly higher than healthy control group (P = 0.0001, 0.0003, P < 0.0001, and P < 0.0001, respectively) in the serum samples (Control group number = 40, COVID-19 group number = 40). Evaluation of cytokine levels was done by ELISA. Unpaired T test was used for statistical analysis and P < 0.05 was considered statistically significant.
Fig. 4.
Cytokine levels of IL-1β, IL-6, TNF-α, and IL-18 in COVID-19 patients and healthy control group in the supernatant of PBMCs culture. The results showed that the levels of inflammatory cytokine were significantly increased in supernatant of PBMCs culture from COVID-19 patients compared to the control group (P < 0.0001 for IL-1β, IL-6, TNF-α, P = 0.0001 for IL-18; Control group number = 40, COVID-19 group number = 40). Evaluation of cytokine secretion level was done by ELISA. Unpaired T test was used for statistical analysis and a P < 0.05 was considered statistically significant.
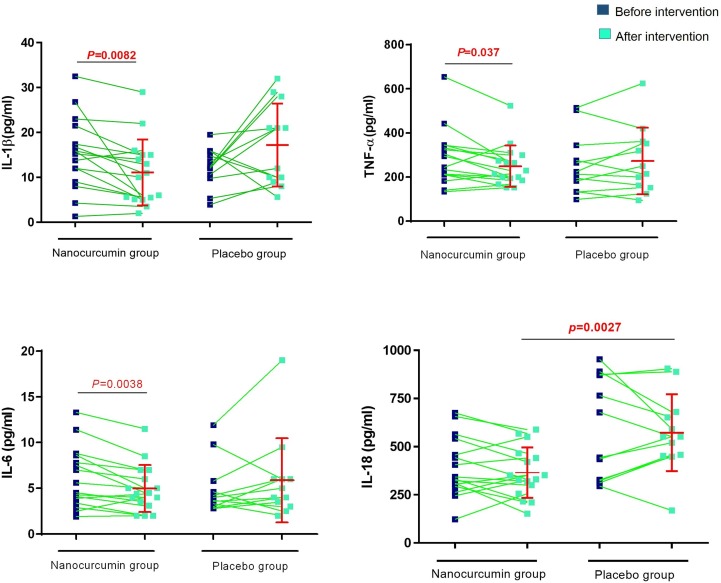
The results of ELISA assessment of cytokine secretion between Nano-curcumin-treated COVID-19 patients and placebo group in serum showed a reduction in the levels of IL-1β, IL-6 and TNF-α after treatment with Nano-curcumin (P = 0.0082, 0.0038, and 0.037, respectively) and in IL-18 when compared to placebo group (P = 0.0027; Fig. 5 ).
Fig. 5.
Cytokine levels of IL-1β, IL-6, TNF-α, and IL-18 in the Nano curcumin‐treated COVID-19 patients and placebo group in serum. Analysis showed a reduction in the secretion level of inflammatory cytokines, including IL-1β, IL-6 and TNF-α (P = 0.0082, 0.0038 and 0.037, respectively). (Nano-curcumin group number = 20, placebo group number = 20). Evaluation of cytokine secretion level was done by ELISA. Paired T test was used for statistical analysis and P < 0.05 was considered statistically significant.
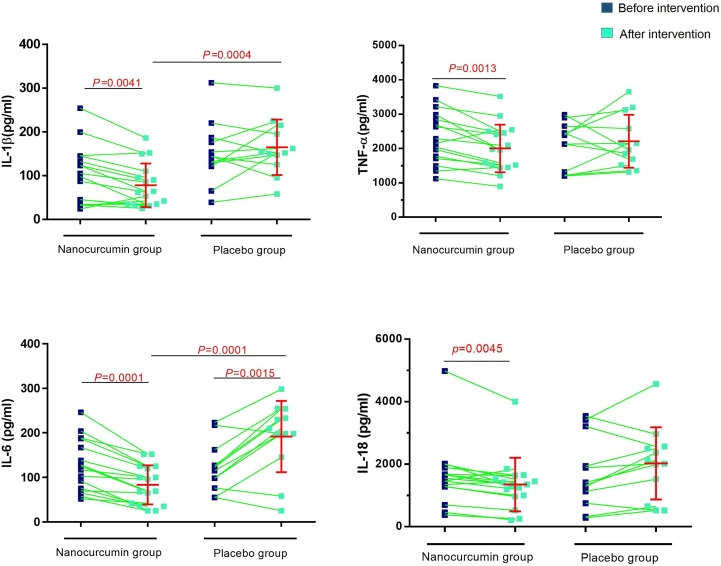
Evaluation of IL-1β, IL-6, TNF-α and IL-18 in supernatant of cultured PBMCs between Nano-curcumin treated COVID-19 patients and placebo group indicated that the level of each cytokine was decreased after intervention (P = 0.0041, 0.0001, 0.0013 and 0.0045, respectively, for IL-1β, IL-6, TNF-α and IL-18) and in comparison to placebo group (P = 0.0004 for IL-1β, P = 0.0001 for IL-6). Also, the secretion level of IL-6 in the placebo group was significantly increased after placebo (P = 0.015; Fig. 6 ). All data about cytokine secretion levels is summarized in Table 4 .
Fig. 6.
Cytokine levels of IL-1β, IL-6, TNF-α, and IL-18 in the Nano curcumin‐treated COVID-19 patients and placebo group in supernatant of PBMCs culture. Analysis indicated that the secretion level of each cytokine was increased after intervention (P = 0.0041, 0.0001, 0.0013 and 0.0045, respectively, for IL-1β, IL-6, TNF-α, and IL-18). (Nano-curcumin group number = 20, placebo group number = 20). Evaluation of cytokine secretion level was done by ELISA. Paired T test was used for statistical analysis and P < 0.05 was considered statistically significant.
Table 4.
Secretion level of inflammatory cytokine.
| Prior to treatment | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBMCs | Serum | ||||||||||||||
| COVID-19 patients (Mean ± SD) | Healthy control (Mean ± SD) | p-value | COVID-19 patients (Mean ± SD) | Healthy control (Mean ± SD) | p-value | ||||||||||
| Secretion level of cytokines | IL-1β | 128.4 ± 74.37 | 62.07 ± 43.46 | <0.0001 | 15.95 ± 8.45 | 8.18 ± 5.42 | <0.0001 | ||||||||
| IL-6 | 130.4 ± 71.08 | 71 ± 42.34 | <0.0001 | 4.79 ± 2.99 | 2.81 ± 1.50 | 0.0003 | |||||||||
| IL-18 | 1813 ± 1303 | 709.5 ± 537.3 | <0.0001 | 479.1 ± 218.7 | 212.3 ± 89.55 | <0.0001 | |||||||||
| TNF-α | 2286 ± 869.3 | 1228 ± 470.5 | <0.0001 | 192.1 ± 99.6 | 302.5 ± 160.8 | <0.0001 | |||||||||
| Post treatment | |||||||||||||||
| PBMCs | Serum | ||||||||||||||
| Nano-curcumin group (Mean ± SD) | Placebo group (Mean ± SD) | p- value | Nano-curcumin group (Mean ± SD) | Placebo group (Mean ± SD) | p- value | ||||||||||
| before | after | p-value | before | after | p-value | before | after | p-value | before | after | p-value | ||||
| Secretion level of cytokines | IL-1β | 101.6 ± 65.9 | 78 ± 49.71 | 0.0041 | 151.8 ± 70.3 | 164.8 ± 63.2 | NS | 0.0004 | 15.34 ± 8.0 | 11.11 ± 7.34 | 0.0082 | 12.14 ± 4.41 | 17.22 ± 9.22 | NS | NS |
| IL-6 | 126 ± 58.51 | 83.38 ± 43.91 | <0.0001 | 122.7 ± 54.89 | 191.8 ± 80.15 | 0.0015 | <0.0001 | 6.093 ± 3.28 | 4.96 ± 2.56 | 0.0038 | 4.96 ± 2.90 | 5.87 ± 4.60 | NS | NS | |
| IL-18 | 1611 ± 1025 | 1351 ± 855.7 | 0.0045 | 1710 ± 1137 | 2026 ± 1155 | NS | NS | 392.7 ± 153.7 | 365.4 ± 131.1 | NS | 597.5 ± 264.6 | 572.4 ± 199.7 | NS | 0.0027 | |
| TNF-α | 2347 ± 783.1 | 2004 ± 694.3 | 0.0013 | 2087 ± 675.2 | 2215 ± 769.1 | NS | NS | 288.3 ± 128.3 | 249.2 ± 93.95 | 0.037 | 256.8 ± 135.9 | 273.2 ± 151.3 | NS | NS | |
(Abbreviation: PBMCs: peripheral blood mononuclear cells; IL-1β: Interleukin-1 β; IL-6: Interleukin-6; IL-18: Interleukin-18; TNF-α: tumor necrosis factor-alpha; COVID-19: Coronavirus disease 2019; SD: standard deviation; NS: not significant).
3.3. Clinical manifestation and overall improvement
All the subjects were evaluated in terms of clinical symptoms and primary health issues, such as diabetes, hypertension, chronic disease, cough, fever, headache and dyspnea, there were also some laboratory tests like White blood cell (WBC), lymphocyte, and platelet count and chest radiography in order to evaluate each subject situation. As shown in Table 1, there was no statistically significant difference in the clinical manifestation between Nano-curcumin and placebo group before the treatment, as we randomly selected and divided the groups. However, after treatment with Nano-curcumin, there was statistically significant improvement in almost all the clinical manifestation in Nano-curcumin group, including fever, cough and dyspnea were significantly meliorated (P < 0.0001). There were also some significant differences in clinical symptoms in placebo group, which were because of other supportive medications they received according to treatment protocol. All the obtained information is summarized in Table 5 .
Table 5.
Subjects clinical and laboratory information (before and after treatment).
| Nano-curcumin group | P value | Placebo group (n = 12) | P value | |||
|---|---|---|---|---|---|---|
| Before(n = 20) | After(n = 16) | After(n = 12) | Before(n = 20) | |||
| Fever | ||||||
| <37.3 °C | 1 (5%) | 10(62.5%) | <0.0001 | 4(33.33%) | 0(0%) | 0.003 |
| 37.3–38.0 °C | 9 (45%) | 6 (37.5%) | 7 (58.33%) | 8 (40%) | ||
| 38.1–39.0 °C | 7 (37%) | 0 (0%) | 1 (8.33%) | 7 (35%) | ||
| >39.0 °C | 4 (20%) | 0 (0%) | 0 (0%) | 5 (25%) | ||
| Cough | 12 (60%) | 2(12.5%) | <0.0001 | 6 (50%) | 11 (55%) | NS |
| Headache | 1 (5%) | 0(0%) | – | 0(0%) | 2 (10%) | – |
| Dyspnea | 5 (25%) | 1(6.25%) | <0.0001 | 1(8.33%) | 4 (20%) | 0.01 |
| White blood cell count, × 109/L | <0.0001 | NS | ||||
| <4 | 5 (25%) | 2 (12.5%) | 2 (16.66%) | 5 (25%) | ||
| 4–10 | 9 (45%) | 3 (18.75%) | 4 (33.33%) | 8 (40%) | ||
| >10 | 6 (30%) | 2(12.25%) | 6 (50%) | 7 (35%) | ||
| Lymphocyte count, × 109/L | <0.0001 | NS | ||||
| <1·0 | 13 (65%) | 4 (25%%) | 6 (50%) | 11 (55%) | ||
| ≥1·0 | 7 (35%) | 12 (75%%) | 6 (50%) | 9 (45%) | ||
| Platelet count, × 109/L | <0.0001 | 0.04 | ||||
| <100 | 11 (55%) | 6 (37.5%) | 5 (41.66%) | 11 (55%) | ||
| ≥100 | 9 (45%) | 10 (62.5%) | 7(58.33%) | 8 (40%) | ||
| Creatinine, μmol/L | 0.002 | NS | ||||
| ≤133 | 18 (90%) | 13 (81.25%) | 9 (75%) | 17 (85%) | ||
| >133 | 2 (10%) | 3 (18.75%) | 3 (25%) | 3 (15%) | ||
| Lactate dehydrogenase, U/L | <0.0001 | NS | ||||
| ≤245 | 13 (65%) | 15 (93.75%) | 7 (58.33%) | 13 (65%) | ||
| >245 | 7 (35%) | 1 (6.25%) | 5 (41.66%) | 7 (35%) | ||
| Bilateral involvement of chest radiographs | 19 (95%) | 10(62.5%) | <0.0001 | 9 (75%) | 19 (95%) | 0.002 |
Ultimately, the fatality rate of Nano-curcumin group was 20% (4 out of 20) and the placebo group was 40% (8 out of 20). Sixteen recovered patients out of 20 cases of Nano-curcumin group were discharged from the hospital with overall improvement. However, in placebo group, 12 out of 20 subjects were recovered and discharged from the hospital.
4. Discussion
A group of 40 patients with laboratory confirmed COVID-19 infection were included in our study. Patients had serious, sometimes fatal pneumonia and some cases with acute disease developed ARDS, who needed ICU admission and oxygen therapy. Early evidences had demonstrated that the pro-inflammatory cytokines, such as IL-1β, IL-6 and IL-12, are dramatically increased in serum of these patients. Indeed, the over-activation of immune system and cytokine storm are the key suspects of the disease that are related with broad lung damage and mortality in COVID-19 patients. According to the studies by Okabayashi and colleagues, elevated level of IL-6 secretion and subsequently dysregulation of IL-6 signaling may be the main reason of severe inflammation during SARS [26]. The study of Huang et al. also indicated that the lung injury in the COVID-19 patients was due to wide inflammatory cytokine secretion, including IL-1β, IFN-γ, IP10, and MCP1, which activated T-helper-1 (Th1) cell responses. They also observed that the disease severity and ICU admission requirement were related to higher amount of inflammatory cytokines in plasma [10]. Elevated secretion of IL-6 was more highlighted in the period of COVID-19 and it was suggested that the increased amount of IL-6 is related to worsening the symptoms and progressing the disease to critical stage, according to the studies of Ruan et al., Wang et al., and Henry et al. [27], [28], [29].
We also observed that the secretion and mRNA expression level of pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α, and IL-18 were dramatically higher in COVID-19 patients in comparison with healthy controls. We evaluated the amount of cytokine secretion in both serum and supernatant of cultured PBMCs, and the levels of cytokines were significantly higher in both comparing to the healthy controls. Therefore, our observations highlighted the requirement for a beneficial anti-inflammatory agent in order to modulate the high amount of these harmful cytokines.
As suggested in most studies, curcumin is among the strongest anti-inflammatory agents [30]. The results of our study also showed that the mortality rate of Nano-curcumin group was 20% (4 out of 20), while it was 40% (8 out of 20) in the placebo group, suggesting the advantageous therapeutic (anti-inflammatory) effects of Nano-curcumin on the COVID-19 patients. Therefore, Nano-curcumin was able to reduce mortality rate probably through reducing cytokine storm. It seems that high levels of inflammatory cytokines are considered as negative prognosis and are directly related to fatality in COVID-19 patients.
Curcumin is a bright yellow chemical material in plants that has been confirmed to have multiple features, including anticancer, anti-inflammatory, anti-invasive, anti-angiogenic, immunomodulatory, and antioxidant activities in several diseases [31]. The prompt metabolism, poor oral bioavailability, weak aqueous solubility, and systemic deletion of curcumin have been pointed as notable drawbacks for the clinical application of curcumin [32]. In order to overcome these challenges, biodegradable polymer nanoparticles (Nano-curcumin), which were also used in our trial, have been used for improving the solubility, stability, and half-life, as well as promoting the free radical scavenging activity of curcumin [33].
Curcumin, as a prominent immune modulatory factor, suppresses the expression of multiple pro-inflammatory cytokines, such as IL-6, IL-10, TNF-α, IL-12, and chemokines. According to several studies, curcumin is able to decrease the mRNA expression and cytokine secretion level of IL-1, IL-6 and TNF-α in inflammatory disease, as discussed in the study of Yetkin et al. [34], Schneider et al. [35] and Mouzaoui et al. [36]. A meta-analysis by Derosa et al. also revealed that curcumin was able to decrease the amount of circulating IL-6 in patients with systemic inflammation [37], while the same effect of curcumin on TNF-α was observed in meta-analysis of Sahebkar et al. [38]. Indeed, curcumin acts as an anti-inflammatory and immunomodulatory agent and is capable of decreasing the proliferation and differentiation of inflammatory cells and related cytokines. Regarding these points, in the current study, a dose of 160 mg of Nano-curcumin daily was used for 14 days in 20 patients with active COVID-19, and then the mRNA expression and secretion of cytokines were measured in these patients before and after treatment.
In conclusion, here we tried to determine the potential therapeutic effects of curcumin on the modulation of inflammatory state in the COVID-19 patients. The results showed that the mRNA expression of IL-1β and IL-6 were dramatically decreased after receiving Nano-curcumin. Nonetheless, Nano-curcumin was not able to diminish the mRNA of IL-18 and TNF-α. Also, we observed a significant decrease in secretion of IL-1β and IL-6 in supernatant of cultured PBMCs, even though there was no significant decrease in the level of these cytokines in serum of patients. IL-18 level was significantly reduced just in patient’s serum compared with the control group, but no statistically significant difference was observed in secretion of IL-18 and TNF-α in supernatant and TNF-α level in serum. It seems that Nano-curcumin is able to efficiently modulate the mRNA and secretion of IL-1β and IL-6, rather than IL-18 and TNF-α.
All in all, this study suggests that Nano-curcumin may be used as an innovative therapeutic agent for COVID-19 patients by regulating inflammatory response. Further investigations are required to evaluate the accurate pathways controlled by Nano-curcumin in COVID-19 patients to cover up the limitation of this trial.
5. Author statement
All authors have been involved in drafting the manuscript. The author M. A and H. V desined project and critically reviewed the manuscript content.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgment
This study was supported by Tuberculosis and Lung Disease Research Center of Tabriz University of Medical Sciences, Tabriz, Iran (Grant Number: 65203).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107088.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis, advances in virus research. Elsevier. 2011:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19), Statpearls [internet] StatPearls Publishing. 2020 [PubMed] [Google Scholar]
- 5.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit. Care. 2020;24:1–5. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A. Taghizadieh, H. Mikaeili, M. Ahmadi, H. Valizadeh, Acute kidney injury in pregnant women following SARS-CoV-2 infection: A case report from Iran, Respiratory medicine case reports (2020) 101090. [DOI] [PMC free article] [PubMed]
- 8.M. Ghaebi, A. Osali, H. Valizadeh, L. Roshangar, M. Ahmadi, Vaccine development and therapeutic design for 2019‐nCoV/SARS‐CoV‐2: Challenges and chances, Journal of cellular physiology (2020). [DOI] [PMC free article] [PubMed]
- 9.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2):1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 10.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchetti C., Swartzwelter B., Koenders M.I., Azam T., Tengesdal I.W., Powers N., de Graaf D.M., Dinarello C.A., Joosten L.A. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res. Therapy. 2018;20(1):169. doi: 10.1186/s13075-018-1664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Z.S. Ulhaq, G.V. Soraya, Interleukin-6 as a potential biomarker of COVID-19 progression, Médecine et Maladies Infectieuses (2020). [DOI] [PMC free article] [PubMed]
- 13.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. The Lancet. 2020;395(10234):1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolati S., Ahmadi M., Aghebti-Maleki L., Nikmaram A., Marofi F., Rikhtegar R., Ayromlou H., Yousefi M. Nanocurcumin is a potential novel therapy for multiple sclerosis by influencing inflammatory mediators. Pharmacol. Rep. 2018;70(6):1158–1167. doi: 10.1016/j.pharep.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Yavarpour-Bali H., Ghasemi-Kasman M., Pirzadeh M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019;14:4449. doi: 10.2147/IJN.S208332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1/A):363–398. [PubMed] [Google Scholar]
- 19.Hewlings S.J., Kalman D.S. Curcumin: a review of its’ effects on human health. Foods. 2017;6(10):92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolati S., Ahmadi M., Rikhtegar R., Babaloo Z., Ayromlou H., Aghebati-Maleki L., Nouri M., Yousefi M. Changes in Th17 cells function after nanocurcumin use to treat multiple sclerosis. Int. Immunopharmacol. 2018;61:74–81. doi: 10.1016/j.intimp.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Bose S., Panda A.K., Mukherjee S., Sa G. Curcumin and tumor immune-editing: resurrecting the immune system. Cell division. 2015;10(1):6. doi: 10.1186/s13008-015-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afolayan F.I., Erinwusi B., Oyeyemi O.T. Immunomodulatory activity of curcumin-entrapped poly d, l-lactic-co-glycolic acid nanoparticles in mice. Integrative Medicine Res. 2018;7(2):168–175. doi: 10.1016/j.imr.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trivedi M.K., Mondal S.C., Gangwar M., Jana S. Immunomodulatory potential of nanocurcumin-based formulation. Inflammopharmacology. 2017;25(6):609–619. doi: 10.1007/s10787-017-0395-3. [DOI] [PubMed] [Google Scholar]
- 24.Hatamipour M., Sahebkar A., Alavizadeh S.H., Dorri M., Jaafari M.R. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iranian J. Basic Medical Sci. 2019;22(3):282. doi: 10.22038/ijbms.2019.32873.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corkum C.P., Ings D.P., Burgess C., Karwowska S., Kroll W., Michalak T.I. Immune cell subsets and their gene expression profiles from human PBMC isolated by Vacutainer Cell Preparation Tube (CPT™) and standard density gradient. BMC immunology. 2015;16(1):1–18. doi: 10.1186/s12865-015-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.T. Okabayashi, H. Kariwa, S.i. Yokota, S. Iki, T. Indoh, N. Yokosawa, I. Takashima, H. Tsutsumi, N. Fujii, Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections, Journal of medical virology 78(4) (2006) 417-424. [DOI] [PMC free article] [PubMed]
- 27.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive care medicine. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Z. Wang, B. Yang, Q. Li, L. Wen, R. Zhang, Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China, Clinical infectious diseases (2020). [DOI] [PMC free article] [PubMed]
- 29.Henry B.M., De Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chem. Laboratory Medicine (CCLM) 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 30.Zhao G., Liu Y., Yi X., Wang Y., Qiao S., Li Z., Ni J., Song Z. Curcumin inhibiting Th17 cell differentiation by regulating the metabotropic glutamate receptor-4 expression on dendritic cells. Int. Immunopharmacol. 2017;46:80–86. doi: 10.1016/j.intimp.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Catanzaro M., Corsini E., Rosini M., Racchi M., Lanni C. Immunomodulators inspired by nature: a review on curcumin and echinacea. Molecules. 2018;23(11):2778. doi: 10.3390/molecules23112778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dei Cas M., Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential. Nutrients. 2019;11(9):2147. doi: 10.3390/nu11092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gera M., Sharma N., Ghosh M., Huynh D.L., Lee S.J., Min T., Kwon T., Jeong D.K. Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget. 2017;8(39):66680. doi: 10.18632/oncotarget.19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Z.Y. Ay, B. Bakır, Ş.B. Bozkurt, S.A. Kayis, S.S. Hakki, Positive effect of curcumin on experimental peridontitis via suppression of IL-1-beta and IL-6 expression level, International Journal for Vitamin and Nutrition Research (2020). [DOI] [PubMed]
- 35.Schneider A., Hossain I., VanderMolen J., Nicol K. Comparison of remicade to curcumin for the treatment of Crohn’s disease: A systematic review. Complementary Therapies Medicine. 2017;33:32–38. doi: 10.1016/j.ctim.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Mouzaoui S., Rahim I., Djerdjouri B. Aminoguanidine and curcumin attenuated tumor necrosis factor (TNF)-α-induced oxidative stress, colitis and hepatotoxicity in mice. Int. Immunopharmacol. 2012;12(1):302–311. doi: 10.1016/j.intimp.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Derosa G., Maffioli P., Simental-Mendia L.E., Bo S., Sahebkar A. Effect of curcumin on circulating interleukin-6 concentrations: a systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016;111:394–404. doi: 10.1016/j.phrs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Sahebkar A., Cicero A.F., Simental-Mendía L.E., Aggarwal B.B., Gupta S.C. Curcumin downregulates human tumor necrosis factor-α levels: A systematic review and meta-analysis ofrandomized controlled trials. Pharmacol. Res. 2016;107:234–242. doi: 10.1016/j.phrs.2016.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.