Abstract
Background
Severe acute respiratory syndrome coronavirus 2, the virus causing COVID-19, is rapidly spreading across sub-Saharan Africa. Hospital-based care for COVID-19 is often needed, particularly among older adults. However, a key barrier to accessing hospital care in sub-Saharan Africa is travel time to the nearest health-care facility. To inform the geographical targeting of additional health-care resources, we aimed to estimate travel time at a 1 km × 1 km resolution to the nearest hospital and to the nearest health-care facility of any type for adults aged 60 years and older in sub-Saharan Africa.
Methods
We assembled a dataset on the geolocation of health-care facilities, separately for hospitals and any type of health-care facility and including both private-sector and public-sector facilities, using data from the OpenStreetMap project and the Kenya Medical Research Institute–Wellcome Trust Programme. Population data at a 1 km × 1 km resolution were obtained from WorldPop. We estimated travel time to the nearest health-care facility for each 1 km × 1 km grid using a cost–distance algorithm.
Findings
9·6% (95% CI 5·2–16·9) of adults aged 60 years or older across sub-Saharan Africa had an estimated travel time to the nearest hospital of 6 h or longer, varying from 0·0% (0·0–3·7) in Burundi and The Gambia to 40·9% (31·8–50·7) in Sudan. For the nearest health-care facility of any type (whether primary, secondary, or tertiary care), 15·9% (95% CI 10·1–24·4) of adults aged 60 years or older across sub-Saharan Africa had an estimated travel time of 2 h or longer, ranging from 0·4% (0·0–4·4) in Burundi to 59·4% (50·1–69·0) in Sudan. Most countries in sub-Saharan Africa contained populated areas in which adults aged 60 years and older had a travel time to the nearest hospital of 12 h or longer and to the nearest health-care facility of any type of 6 h or longer. The median travel time to the nearest hospital for the fifth of adults aged 60 years or older with the longest travel times was 348 min (IQR 240–576; equal to 5·8 h) for the entire population of sub-Saharan Africa, ranging from 41 min (34–54) in Burundi to 1655 min (1065–2440; equal to 27·6 h) in Gabon.
Interpretation
Our high-resolution maps of estimated travel times to both hospitals and health-care facilities of any type can be used by policy makers and non-governmental organisations to help target additional health-care resources, such as makeshift hospitals or transport programmes to existing health-care facilities, to older adults with the least physical access to care. In addition, this analysis shows the locations of population groups most likely to under-report COVID-19 symptoms because of low physical access to health-care facilities. Beyond the COVID-19 response, this study can inform the efforts of countries to improve physical access to care for conditions that are common among older adults in the region, such as chronic non-communicable diseases.
Funding
Bill & Melinda Gates Foundation.
Introduction
Across the world, as of mid September, 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused more than 29 million confirmed infections and the disease it triggers (COVID-19) has led to more than 900 000 reported deaths.1 Although low testing numbers do not allow for a reliable assessment of the extent of the pandemic in sub-Saharan Africa, the region had more than 1 million reported infections and more than 20 000 reported deaths due to SARS-CoV-2 as of mid September, 2020.1 Epidemiological modelling suggests that COVID-19 could lead to between 300 000 and 2·5 million deaths in sub-Saharan Africa, depending on modelling assumptions and the mitigation policies that are adopted.2
There are many barriers to receiving high-quality health care in sub-Saharan Africa, including financial barriers to accessing care, weak supply chains, and understaffing of health-care facilities.3 However, physical distance to the nearest health-care facility and the associated requirements for transport options, cost of transport, and time lost from other income-generating activities consistently figures as one of the most important barriers to accessing both hospital-based care and primary care in the region.4, 5, 6, 7
Research in context.
Evidence before this study
We searched MEDLINE from January, 1966, until May, 2020, for studies with variations of the keywords “physical access”, “distance”, “travel time”, “hospital”, AND “healthcare facility” in the title or abstract. To date, the only studies to systematically map physical access to health-care facilities in sub-Saharan Africa at a high resolution examined access to emergency hospital care (with a focus on women of childbearing age), access to care for children with fever, travel time to the nearest health-care facility for specific populations at risk of viral haemorrhagic fevers, and travel time to the nearest regional-level or district-level hospital.
Added value of this study
The added value of this study is threefold. First, we assembled a new dataset of geotagged health-care facilities, which combines two unique data sources for the geolocation of health-care facilities across sub-Saharan Africa: one-based on crowd-sourced data from OpenStreetMap and one based on information from ministries of health, health management information systems, government statistical agencies, and international organisations. Second, our study is the first to our knowledge to comprehensively map both hospitals and primary health-care facilities, including both public-sector and private-sector facilities, across sub-Saharan Africa. Third, because the COVID-19 pandemic causes a far higher need for hospital services among older than younger population groups, we focused on physical access to health care for adults aged 60 years and older—a population group that is rarely studied in investigations of health-care demand and supply in the region. As such, our maps can inform not only the health system response to COVID-19 but also, more generally, to conditions that are common among older adults in the region, particularly chronic non-communicable diseases and their sequelae.
Implications of all the available evidence
Low physical access to health care in sub-Saharan Africa will probably be a major barrier to receiving care for adults aged 60 years and older with COVID-19. However, there is a wide degree of variation in physical access to health-care facilities for older adults in the region, both between and within countries, which is likely to have an important bearing on the extent to which different population groups within countries are able to access care for COVID-19. Likewise, in areas with a long travel time to the nearest health-care facility of any type (which exist in most countries), symptomatic cases of COVID-19 are probably less likely to be reported to the health-care system.
Travel time to the nearest health-care facility and the nearest hospital will probably also play an important role in the ability of health systems in sub-Saharan Africa to respond to SARS-CoV-2, for three main reasons. First, physical access to hospitals will probably affect whether and how timely individuals with COVID-19 are able to seek health care. Although many hospitals in sub-Saharan Africa are not able to provide mechanical ventilation,8, 9 other important components of care for those with severe COVID-19, such as haemodynamic support, supplemental oxygen therapy, and treatment of co-infections (eg, bacterial pneumonia), are more readily available.10, 11, 12 Second, physical access to a health-care facility of any type will probably affect whether and when during the disease course individuals with COVID-19 contact the health-care system. These care-seeking decisions, in turn, have important ramifications for whether the health system is notified of COVID-19 cases and, thus, for monitoring of the pandemic, particularly in settings that are unable to carry out large-scale community-based testing for SARS-CoV-2 infections. Third, physical access to health-care facilities might affect the degree to which individuals with take up effective drugs or a future vaccine against the condition, and possibly how likely they will be able to access a vaccine against SARS-CoV-2.
Having a detailed understanding of where groups of the population are located who are both vulnerable to COVID-19 and have long travel times to the nearest health-care facility can inform where additional health-care resources (eg, makeshift hospitals or programmes to ensure availability of transport to hospitals) are most needed. Furthermore, such knowledge would allow for the identification of geographical areas that are likely to harbour cases of COVID-19 that were not reported to the health system due to low physical access to care, which in turn can inform geographical targeting of testing efforts. More broadly, understanding where older adults reside who have the least physical access to health care can inform efforts of health systems to improve care for conditions that are common in this age group, particularly chronic non-communicable diseases and their sequelae. By assembling a unique dataset from both crowd-sourced data and official records by governments and international organisations, we aimed to create highly detailed maps of estimated travel times for adults aged 60 years and older in sub-Saharan Africa to both the nearest hospital and the closest health-care facility of any type.
Methods
Data sources for geolocation of health-care facilities
We used two data sources for geolocation of health-care facilities. First, we obtained health-care facility data from the OpenStreetMap (OSM) project. Second, we used a geocoded inventory of health-care facilities published by the Kenya Medical Research Institute (KEMRI)–Wellcome Trust Research Programme.13
OSM is a collaborative online platform to map, edit, and share geospatial data globally. Started in 2004, OSM evolved from a crowd-sourced alternative for proprietary map data providers to an important complementary data source used in humanitarian settings14 and a widely used source of information for base maps and for health infrastructure in low-income settings. Querying the OSM database for all objects using the terms “amenity” or “healthcare” as key and either “hospital”, “clinic”, or “doctors” as value, we extracted all health-care facilities mapped in OSM with their geographical coordinates using the ohsome application programming interface. We refer to this dataset as the OSM dataset. We identified 24 571 health-care facilities in the OSM dataset, of which 13 392 were tagged as hospitals.
The KEMRI–Wellcome Trust inventory consists of 98 745 public-sector health-care facilities across all countries of sub-Saharan Africa, except for five small island nations (Cape Verde, Comoros, Mauritius, São Tomé & Príncipe, and Seychelles).13 The primary sources of data for this inventory are master facility lists (MFLs) of national ministries of health and documentation by the UN and non-governmental organisations. Additional sources include websites and data portals by governments of sub-Saharan African countries, health sector reports, and personal communications. We refer to this dataset as the MFL dataset. 52% of health-care facilities in the MFL dataset were manually geocoded by the KEMRI–Wellcome Trust Programme team. For Sudan, Guinea-Bissau, and ten of 18 provinces in Angola, the MFL dataset contains geographical coordinates for hospitals only. The MFL dataset included 92 245 health-care facilities in our study countries, of which 4720 were classified as hospitals. Although the KEMRI–Wellcome Trust Programme team used, among other tools, OSM to assign geocodes to health-care facilities in the MFL dataset that had a missing geocode,13 they did not use OSM to identify health-care facilities that were not already contained in the MFL dataset.
For each of 16 strata resulting from possible permutations of health-care facility type (primary care or hospital), dataset (OSM or MFL), and region, we verified the degree to which the GPS coordinates for a random sample of 20 health-care facilities (320 health-care facilities in total) overlapped with building structures and human settlements in Bing satellite imagery. In addition, we calculated the degree to which the classification of health-care facilities into primary health-care facilities and hospitals overlaps between the OSM and MFL dataset in each country by computing a Jaccard index for radii of 500 m and 1000 m around health-care facilities.
Data source for geolocation of the population
Population counts for adults aged 60 years and older were obtained from the WorldPop project.15 The counts reflect projections for 2020 at a spatial resolution of 1 km2. The WorldPop project built this dataset using a semi-automated dasymetric mapping method that uses a random forest classifier to disaggregate census data at the level of national census tracks to 1 km2 areas.16 Predictors used were geographical properties (eg, topography, climate, and land cover) and the density of human-built features (eg, night-time lights, roads, and buildings).
Estimating travel time to the nearest health-care facility
We merged the two datasets (OSM and MFL) such that estimated travel times were the time to travel to the nearest health-care facility, regardless of the data source in which the facility was listed. We chose this strategy because, in our view, both datasets were more likely to be missing existing health-care facilities than to falsely list a non-existing health-care facility. We estimated travel time to the nearest health-care facility separately for hospitals and health-care facilities of any type. Hospitals were chosen as one entity of interest because most health-care interventions to care for individuals with severe COVID-19 require hospital-based care. Health-care facilities of any type were chosen as an additional entity of interest because physical access to any health-care facility probably affects the degree to which individuals with COVID-19 present to the health-care system and, thus, the extent to which the health-care system is made aware of new COVID-19 cases. In the absence of community-based screening for SARS-CoV-2 infections, and ignoring that more remote areas could experience less SARS-CoV-2 transmission, areas with low physical access to health-care facilities of any type might, thus, have a disproportionately high number of unreported COVID-19 cases.
We used AccessMod version 5.6.33 to estimate travel time. This program enabled us to create an up-to-date travel model based on the latest available data for land cover and road networks.17 AccessMod uses a raster-based cost–distance algorithm, whereby every raster cell is associated with a cost value that ascertains the time required to travel through this cell. The cost for every cell was modelled using the 2018 Copernicus Global Land Cover product18 and the Shuttle Radar Topography Mission (version 4) digital elevation database as basic impedance surface. Moreover, we used OSM to identify road networks and locations of rivers and open water (which were considered barriers to travel). Aligning with previous studies in sub-Saharan Africa,19 we assigned a travel speed of 100 km/h to motorways and primary roads, 50 km/h to secondary roads, and 30 km/h to tertiary roads. Barren land and built-up areas were assigned a travel speed of 5 km/h and forests a 2 km/h walking speed. The model was created at a spatial resolution of 100 m2. For both OSM and MFL datasets, we calculated the travel time from every cell to the nearest health-care facility of any type and the closest hospital. These results were then aggregated to a 1 km2 resolution to match the resolution of the WorldPop population data. Our analyses assumed that individuals were able to cross national borders to reach the nearest health-care facility, and we did not assign an additional time cost for a border crossing. We did not allow for variations in travel time by time of day or day of the week. As a robustness check, we compared travel time estimates obtained from OpenRouteService (which, similar to our approach implemented in AccessMod, uses road network data from OSM) with those from Google Maps, by selecting at random 40 locations in sub-Saharan Africa and calculating (using each of these two routing services) the travel time from these locations to the nearest health-care facility of any type and the nearest hospital.
Statistical analysis
For every country, we plotted the distribution of travel time separately for hospitals and health-care facilities of any type. In addition, we calculated the median travel time to the nearest hospital for the fifth of adults aged 60 years or older with the longest travel times. We then mapped the estimated travel time at a 1 km × 1 km resolution, both as a continuous variable and when categorising travel time into less than 2 h, 2 h to less than 6 h, 6 h to less than 12 h, and 12 h or longer for the nearest hospital, and less than 1 h, 1 h to less than 2 h, 2 h to less than 6 h, and 6 h or longer for the nearest health-care facility of any type. When summarising our data as binomial proportions, we calculated two-sided 95% CIs using the Wilson score interval.20 The calculation of travel time was done using AccessMod version 5. All other analyses were done in R version 3.6.3.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Across the two datasets (OSM and MFL), the population density of health-care facilities varied. The number of hospitals ranged from 0·067 per 100 000 in Burkina Faso (MFL data) to 11·008 per 100 000 in Central African Republic (OSM data). The number of primary health-care facilities ranged from 0·034 per 100 000 in Eritrea (OSM data) to 28·053 per 100 000 in Gabon (MFL data; table ). The degree to which the classification of healthcare facilities into primary health-care facilities and hospitals overlapped between OSM and MFL datasets in every country is shown in the appendix (pp 132–139). Moreover, we show (separately for every country and each dataset) maps of the location of all health-care facilities contained in the OSM and MFL datasets (appendix pp 107–128). The degree to which these locations (for a stratified random sample of 320 health-care facilities) overlapped with building structures and human settlements in Bing satellite imagery is also shown in the appendix (p 129).
Table.
Population density and number of health-care facilities by country
|
Population (millions) |
Number of health-care facilities |
Number of health-care facilities per 100 000 population |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFL dataset |
OSM dataset |
MFL dataset |
OSM dataset |
|||||||||||
| Total | Aged ≥60 years | Primary care | Hospitals | Total | Primary care | Hospitals | Total | Primary care | Hospitals | Total | Primary care | Hospitals | Total | |
| Central Africa | ||||||||||||||
| Burundi | 13·097 | 0·534 | 619 | 49 | 668 | 22 | 1317 | 1339 | 4·726 | 0·374 | 5·101 | 0·168 | 10·056 | 10·224 |
| Cameroon | 26·265 | 1·673 | 2825 | 181 | 3006 | 478 | 541 | 1019 | 10·756 | 0·689 | 11·445 | 1·820 | 2·060 | 3·880 |
| Central African Republic | 5·360 | 0·143 | 526 | 20 | 546 | 17 | 590 | 607 | 9·814 | 0·373 | 10·187 | 0·317 | 11·008 | 11·326 |
| Chad | 16·435 | 0·747 | 1164 | 79 | 1243 | 90 | 140 | 230 | 7·082 | 0·481 | 7·563 | 0·548 | 0·852 | 1·399 |
| DR Congo | 89·636 | 3·908 | 14096 | 432 | 14528 | 1383 | 724 | 2107 | 15·726 | 0·482 | 16·208 | 1·543 | 0·808 | 2·351 |
| Equatorial Guinea | 0·925 | 0·044 | 28 | 14 | 42 | 2 | 5 | 7 | 3·027 | 1·514 | 4·541 | 0·216 | 0·541 | 0·757 |
| Gabon | 1·829 | 0·124 | 513 | 17 | 530 | 153 | 56 | 209 | 28·053 | 0·930 | 28·983 | 8·367 | 3·062 | 11·429 |
| Congo (Brazzaville) | 5·244 | 0·176 | 308 | 27 | 335 | 81 | 87 | 168 | 5·873 | 0·515 | 6·388 | 1·545 | 1·659 | 3·204 |
| East Africa | ||||||||||||||
| Djibouti | 0·671 | 0·023 | 50 | 13 | 63 | 6 | 26 | 32 | 7·449 | 1·937 | 9·386 | 0·894 | 3·873 | 4·767 |
| Eritrea | 5·955 | 0·259 | 252 | 20 | 272 | 2 | 19 | 21 | 4·232 | 0·336 | 4·568 | 0·034 | 0·319 | 0·353 |
| Ethiopia | 111·731 | 4·780 | 5014 | 164 | 5178 | 184 | 272 | 456 | 4·488 | 0·147 | 4·634 | 0·165 | 0·243 | 0·408 |
| Kenya | 51·513 | 2·202 | 5608 | 394 | 6002 | 279 | 811 | 1090 | 10·887 | 0·765 | 11·651 | 0·542 | 1·574 | 2·116 |
| Rwanda | 13·299 | 0·512 | 538 | 48 | 586 | 55 | 82 | 137 | 4·046 | 0·361 | 4·406 | 0·414 | 0·617 | 1·030 |
| Somalia | 12·459 | 0·574 | 760 | 73 | 833 | 7 | 40 | 47 | 6·100 | 0·586 | 6·686 | 0·056 | 0·321 | 0·377 |
| South Sudan | 14·112 | 0·545 | 1684 | 41 | 1725 | 36 | 68 | 104 | 11·933 | 0·291 | 12·224 | 0·255 | 0·482 | 0·737 |
| Sudan | 45·292 | 2·374 | 5 | 259 | 264 | 88 | 300 | 388 | 0·011 | 0·572 | 0·583 | 0·194 | 0·662 | 0·857 |
| Tanzania | 61·897 | 2·762 | 6159 | 222 | 6381 | 1015 | 977 | 1992 | 9·950 | 0·359 | 10·309 | 1·640 | 1·578 | 3·218 |
| Uganda | 45·982 | 2·043 | 3582 | 121 | 3703 | 1728 | 546 | 2274 | 7·790 | 0·263 | 8·053 | 3·758 | 1·187 | 4·945 |
| Southern Africa | ||||||||||||||
| Angola | 29·150 | 1·043 | 1289 | 150 | 1439 | 76 | 162 | 238 | 4·422 | 0·515 | 4·936 | 0·261 | 0·556 | 0·816 |
| Botswana | 2·443 | 0·130 | 560 | 28 | 588 | 80 | 77 | 157 | 22·924 | 1·146 | 24·071 | 3·275 | 3·152 | 6·427 |
| Eswatini | 1·362 | 0·060 | 124 | 6 | 130 | 6 | 25 | 31 | 9·107 | 0·441 | 9·548 | 0·441 | 1·836 | 2·277 |
| Lesotho | 2·232 | 0·187 | 92 | 14 | 106 | 20 | 44 | 64 | 4·121 | 0·627 | 4·748 | 0·896 | 1·971 | 2·867 |
| Madagascar | 27·555 | 0·969 | 2497 | 117 | 2614 | 59 | 218 | 277 | 9·062 | 0·425 | 9·486 | 0·214 | 0·791 | 1·005 |
| Malawi | 20·052 | 0·843 | 574 | 83 | 657 | 36 | 194 | 230 | 2·863 | 0·414 | 3·276 | 0·180 | 0·967 | 1·147 |
| Mozambique | 31·732 | 1·452 | 1499 | 61 | 1560 | 740 | 148 | 888 | 4·724 | 0·192 | 4·916 | 2·332 | 0·466 | 2·798 |
| Namibia | 2·734 | 0·164 | 322 | 37 | 359 | 48 | 83 | 131 | 11·780 | 1·354 | 13·133 | 1·756 | 3·036 | 4·792 |
| South Africa | 56·423 | 4·614 | 3951 | 329 | 4280 | 252 | 644 | 896 | 7·002 | 0·583 | 7·586 | 0·447 | 1·141 | 1·588 |
| Zambia | 18·784 | 0·713 | 1163 | 89 | 1252 | 61 | 129 | 190 | 6·192 | 0·474 | 6·665 | 0·325 | 0·687 | 1·012 |
| Zimbabwe | 17·363 | 0·927 | 1031 | 170 | 1201 | 98 | 148 | 246 | 5·938 | 0·979 | 6·917 | 0·564 | 0·852 | 1·417 |
| West Africa | ||||||||||||||
| Benin | 12·418 | 0·717 | 771 | 48 | 819 | 227 | 214 | 441 | 6·209 | 0·387 | 6·595 | 1·828 | 1·723 | 3·551 |
| Burkina Faso | 20·829 | 1·061 | 1711 | 14 | 1725 | 292 | 175 | 467 | 8·214 | 0·067 | 8·282 | 1·402 | 0·840 | 2·242 |
| Ghana | 30·256 | 1·582 | 1679 | 178 | 1857 | 256 | 333 | 589 | 5·549 | 0·588 | 6·138 | 0·846 | 1·101 | 1·947 |
| Guinea | 14·260 | 1·024 | 1482 | 36 | 1518 | 240 | 97 | 337 | 10·393 | 0·252 | 10·645 | 1·683 | 0·680 | 2·363 |
| Guinea-Bissau | 2·027 | 0·098 | 0 | 8 | 8 | 11 | 18 | 29 | 0·000 | 0·395 | 0·395 | 0·543 | 0·888 | 1·431 |
| Ivory Coast | 25·170 | 0·911 | 1638 | 95 | 1733 | 793 | 260 | 1053 | 6·508 | 0·377 | 6·885 | 3·151 | 1·033 | 4·184 |
| Liberia | 4·953 | 0·243 | 668 | 33 | 701 | 126 | 51 | 177 | 13·488 | 0·666 | 14·154 | 2·544 | 1·030 | 3·574 |
| Mali | 20·542 | 1·154 | 1446 | 18 | 1464 | 678 | 140 | 818 | 7·039 | 0·088 | 7·127 | 3·301 | 0·682 | 3·982 |
| Mauritania | 4·509 | 0·282 | 626 | 19 | 645 | 30 | 70 | 100 | 13·883 | 0·421 | 14·304 | 0·665 | 1·552 | 2·218 |
| Niger | 24·140 | 1·280 | 2794 | 41 | 2835 | 154 | 113 | 267 | 11·574 | 0·170 | 11·744 | 0·638 | 0·468 | 1·106 |
| Nigeria | 205·773 | 10·227 | 18714 | 887 | 19601 | 557 | 2888 | 3445 | 9·094 | 0·431 | 9·526 | 0·271 | 1·403 | 1·674 |
| Senegal | 17·384 | 0·943 | 1198 | 27 | 1225 | 306 | 184 | 490 | 6·891 | 0·155 | 7·047 | 1·760 | 1·058 | 2·819 |
| Sierra Leone | 6·951 | 0·433 | 1060 | 28 | 1088 | 148 | 145 | 293 | 15·249 | 0·403 | 15·652 | 2·129 | 2·086 | 4·215 |
| The Gambia | 2·186 | 0·107 | 91 | 5 | 96 | 11 | 58 | 69 | 4·162 | 0·229 | 4·391 | 0·503 | 2·653 | 3·156 |
| Togo | 8·296 | 0·534 | 149 | 37 | 186 | 92 | 172 | 264 | 1·796 | 0·446 | 2·242 | 1·109 | 2·073 | 3·182 |
| Sub-Saharan Africa | ||||||||||||||
| All countries | 1131·227 | 55·123 | 90 860 | 4732 | 95 592 | 11 023 | 13 391 | 24 414 | 8·032 | 0·417 | 8·449 | 0·988 | 1·183 | 2·277 |
MFL=master facility list. OSM=OpenStreetMap.
Across sub-Saharan Africa, the proportion of adults aged 60 years and older with an estimated travel time of greater than 6 h to the nearest hospital was 9·6% (95% CI 5·2–16·9), ranging from 0·0% (0·0–3·7) in Burundi and The Gambia to 40·9% (31·8–50·7) in Sudan (appendix p 9). For health-care facilities of any type and using a travel time cutoff of 2 h, the corresponding proportions were 15·9% (95% CI 10·1–24·4) across sub-Saharan Africa, ranging from 0·4% (0·0–4·4) in Burundi to 59·4% (50·1–69·0) in Sudan (appendix p 10).
The distribution of travel time to the nearest hospital for adults aged 60 years and older varied greatly across countries (figure 1 ), ranging from a distribution in which most of the population was within 60 min travel time (eg, in Burundi) to distributions in which the population was almost equally spread across the range of travel times (eg, 0 min to 4 h, in Ethiopia). The median travel time to the nearest hospital for the fifth of adults aged 60 years or older with the longest travel times was 348 min (IQR 240–576; equal to 5·8 h) for the entire population of sub-Saharan Africa, ranging from 41 min (34–54) in Burundi to 1655 min (1065–2440; equal to 27·6 h) in Gabon. By contrast, for the nearest health-care facility of any type, the distribution was skewed towards very short travel times (figure 2 ), with the proportion of adults aged 60 years and older who reside within 30 min of the nearest facility being at least 25% in 43 of the 44 study countries. Travel time distributions are shown separately for the MFL and OSM datasets (appendix pp 11–14).
Figure 1.
Distribution of travel time to the nearest hospital for adults aged 60 years and older, by country in sub-Saharan Africa
Countries are shown in ascending order by the proportion of adults aged 60 years and older in their population who reside in a 1 km × 1 km area that has an estimated travel time of 6 h or longer to the nearest hospital.
Figure 2.
Distribution of travel time to the nearest health-care facility of any type for adults aged 60 years and older, by country in sub-Saharan Africa
Countries are shown in ascending order by the proportion of adults aged 60 years and older in their population who reside in a 1 km × 1 km area that has an estimated travel time of 2 h or longer to the nearest health-care facility.
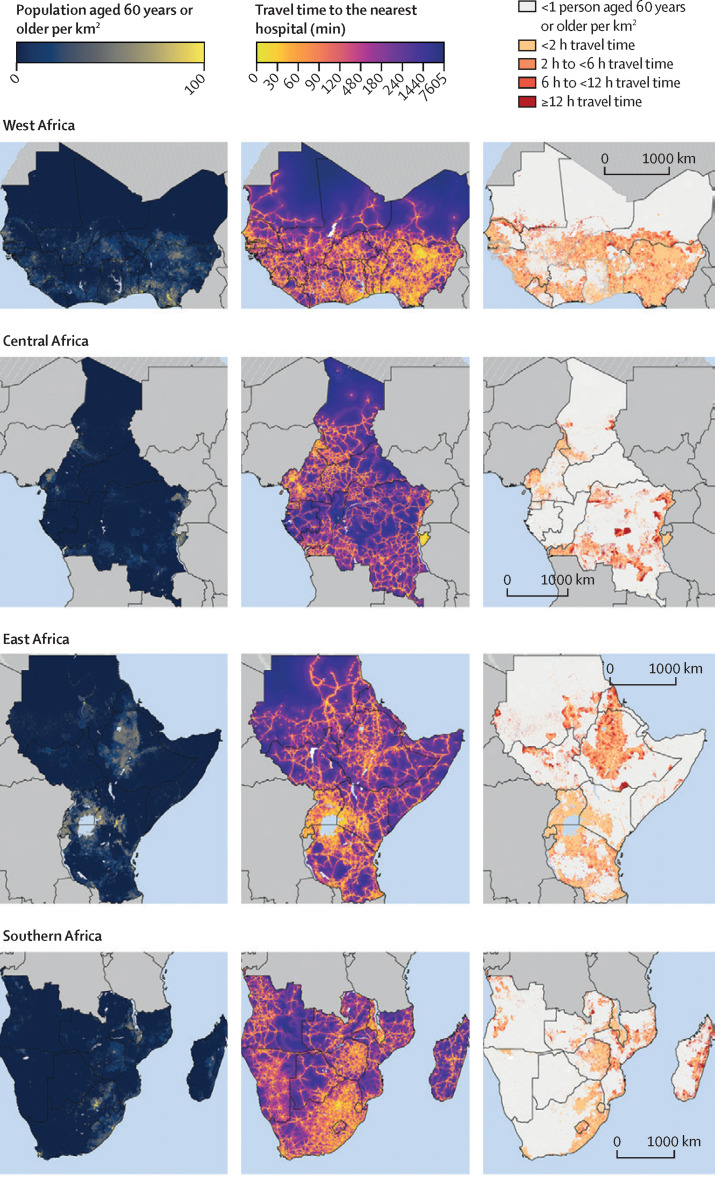
Figure 3 consists of three columns of maps; the first column shows the population density of adults aged 60 years and older and the second shows the estimated travel time among these adults to the nearest hospital at a 1 km × 1 km resolution. The third column of maps focuses on populated areas (which we defined as areas with at least one adult aged 60 years and older per km2) and categorises travel time into less than 2 h, 2 h to less than 6 h, 6 h to less than 12 h, and 12 h or more. This column shows that almost all countries in sub-Saharan Africa contain populated areas that have an estimated travel time to the nearest hospital of 12 h or longer (indicated as areas in dark red). Countries with many of these populated 1 km2 areas with poor physical access to hospital care included DR Congo, Ethiopia, Madagascar, Mauritania, Mozambique, South Sudan, and Sudan. Detailed maps created separately for each country are shown in the appendix (pp 15–58). Regional maps were also created using only the MFL dataset (appendix p 59) and only the OSM dataset (appendix p 60).
Figure 3.
Maps showing population density and travel time to the nearest hospital for adults aged 60 years or older, by sub-Saharan African region
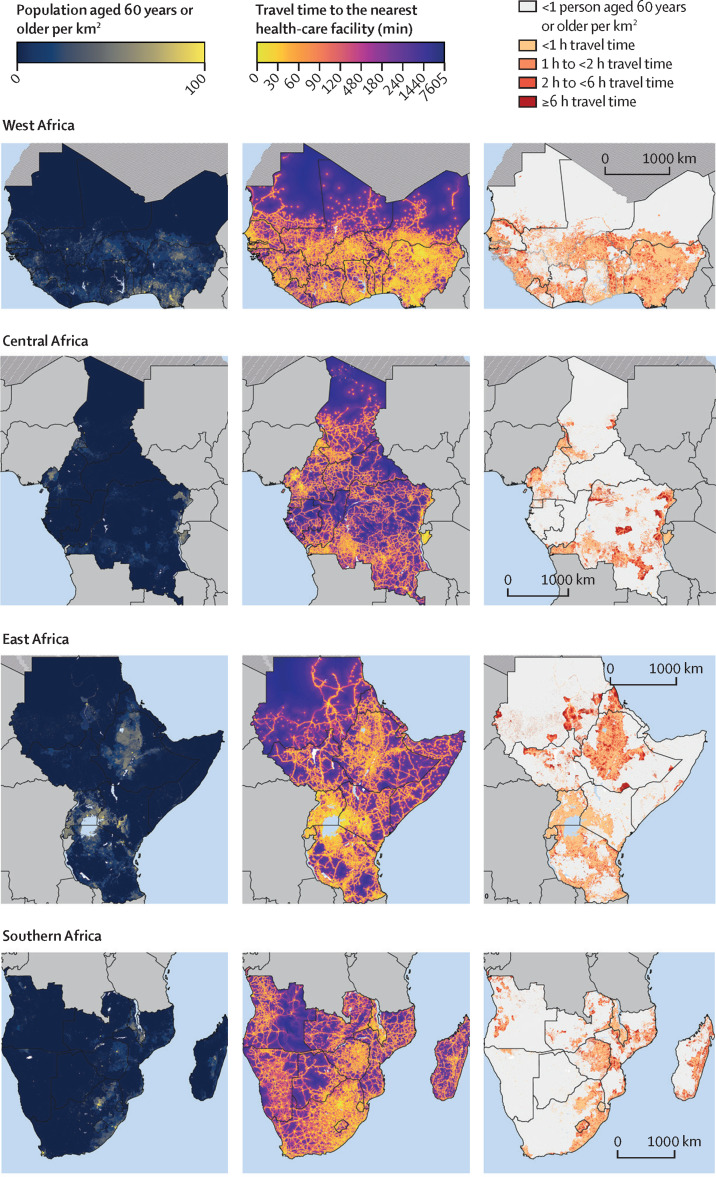
Figure 4 also consists of three columns of maps; the first column shows the population density of adults aged 60 years and older and the second shows the estimated travel time among these adults to the nearest health-care facility of any type at a 1 km × 1 km resolution. The third column of maps focuses on populated areas (again, defined as areas with at least one adult aged 60 years and older per km2) and categorises travel time into less than 1 h, 1 h to less than 2 h, 2 h to less than 6 h, and 6 h or more. Countries with a high number of populated 1 km2 areas with poor physical access to a health-care facility included Angola, DR Congo, Ethiopia, Madagascar, Mozambique, South Sudan, and Sudan. Maps created separately for each country are shown in the appendix (pp 61–104). Regional maps were also created using only the MFL dataset (appendix p 105) and only the OSM dataset (appendix p 106).
Figure 4.
Maps showing population density and travel time to the nearest health-care facility for adults aged 60 years or older, by sub-Saharan African region
Discussion
The findings of our study show that approximately 10% of adults aged 60 years and older across sub-Saharan Africa have an estimated travel time to the nearest hospital of 6 h or longer. Thus, physical access to health care will probably play a major role in whether older adults in this world region will be able to seek care for COVID-19. By precisely identifying where older adults are residing who have an especially high estimated travel time to the nearest hospital, our high-resolution maps can inform policy makers about where interventions to increase physical access to hospital care are needed most urgently. Such interventions could include transport programmes to existing hospitals and establishment of makeshift hospitals. Moreover, our maps of estimated travel time to the nearest health-care facility of any type could help guide policy makers about which populations are least likely to present to the health-care system when they suffer from COVID-19 symptoms because of low physical access to health care. This information, in turn, could be helpful for interpretation of monitoring data for new cases of COVID-19 from different areas within countries and for targeting of testing efforts to those populations that have the greatest need for such tests.
The usefulness and policy relevance of this analysis goes beyond informing countries' responses to the SARS-CoV-2 pandemic. Physical access (ie, the time required to travel to a health-care facility, available transport options, and costs for transport) is one of the main barriers to accessing health care in sub-Saharan Africa.4, 5, 6, 7 Yet, currently very little detailed evidence is available on how physical access to health care varies across sub-Saharan Africa, particularly within countries. Such evidence, however, is crucial to guide policy makers in identifying those areas that have the greatest need for community outreach programmes, establishment of new health-care facilities, and improved transport infrastructure. Our study helps fill this important evidence gap for older adults in the region and is, thus, of high relevance for informing countries' efforts to improve care for conditions that affect older adults, particularly chronic non-communicable diseases. Specifically, that study builds on the findings of existing studies17, 19, 21, 22, 23 that have mapped physical access to health care in sub-Saharan Africa at a subnational level within countries. Ouma and colleagues investigated access to emergency hospital care in sub-Saharan Africa.17 This study differs from ours in that it focused on women of childbearing age (aged 15–49 years) rather than older adults, did not include primary health-care facilities or any private-sector health-care facilities, did not use OSM data, used a cutoff for travel time of 2 h or less or greater than 2 h (based on a target set by the Global Surgery 2030 Lancet Commission)24 rather than analysing the whole distribution, analysed data from 2015, and did not provide detailed country-by-country maps. Other relevant studies have focused on the effect of physical access to a health-care facility on the probability of seeking care for a febrile episode in children,19 estimating travel time to health-care facilities among populations at risk of viral haemorrhagic fevers,21 and examining physical access to major district and regional hospitals.22 In addition, while not focusing directly on physical access to care, South and colleagues23 have mapped health-care facility locations in sub-Saharan Africa using a combination of OSM and MFL data as well as direct information from national ministries of health.
Another key contribution of our study is the collation of a new dataset of geotagged health-care facilities in sub-Saharan Africa. By making this dataset available in the public domain and including the location of other age groups (not merely adults aged 60 years and older), we enable researchers and policy makers to run their own analyses for various demographic groups and add to (or alter) the list of geotagged health-care facilities in a country. Currently, no authoritative source exists for the location of all health-care facilities in sub-Saharan Africa. We have combined data from the only two existing sources of data for geolocation of health-care facilities in the region (OSM and MFL). We chose this approach because it is highly likely that neither dataset is complete, as shown by the fact that, in some countries, the MFL dataset listed a higher number of health-care facilities than did the OSM dataset, whereas the opposite was the case in other countries. Because the OSM project relies on volunteers to map and tag health-care facilities, OSM data by itself could underestimate the density of health-care facilities in an area. Moreover, because the categorisation of a health-care facility as a primary care facility or a hospital relies on the judgment or knowledge of the person tagging the facility, the OSM dataset is likely to have inaccuracies in categorisation. For instance, OSM listed far more hospitals than primary-care facilities in the Central Africa Republic, which seems unlikely to be correct. The fact that OSM contained a higher number of health-care facilities in many countries than did the MFL dataset, particularly hospitals, is encouraging in that OSM seems to be a useful source of information for geolocation of health-care facilities. Importantly, OSM data are likely to improve over time as coverage of smartphones increases in sub-Saharan Africa and more volunteers map out their local areas. We will update our dataset on a regular basis. Similarly, the afrihealthsites package25 aims to make spatial data on health-care facilities in sub-Saharan Africa more accessible to data analysts around the world. Moving forward, it will be important to continuously monitor the validity of the data entered into the OSM and MFL datasets, a task that would ideally be accomplished by ministries of health of sub-Saharan African countries.
Our study has several limitations. First, although by combining MFL and OSM datasets we have possibly provided the most comprehensive source of data to date for the geolocation of health-care facilities, it is still likely that we have missed a substantial proportion of health-care facilities. The level of omissions will vary between countries, because both participation in the OSM project and the degree to which documentation used for the MFL dataset was available and complete differ across countries.26 Second, we do not have any data for either the readiness of health-care facilities to provide care or the quality of care provided at health-care facilities. Similarly, we did not have information on the functioning of referral systems from primary to secondary and tertiary care, which affects access to effective health care for COVID-19 and other conditions requiring specialised care. These factors are also likely to vary across and within countries. Third, a limitation of our analysis for the COVID-19 response is that governments might decide that not all hospitals in a country should be providing care for COVID-19. Fourth, our analysis does not consider that vulnerability to COVID-19 is probably affected by factors beyond age that vary across and within countries, including HIV, tuberculosis, and malnutrition. We decided against including these factors in our analysis because it is still largely unknown which conditions increase the risk for experiencing a severe disease course, and to what degree, in sub-Saharan Africa. Fifth, we did not investigate duplication of health-care facilities between MFL and OSM datasets. Our findings are, thus, estimates for travel time to the nearest health-care facility, regardless of whether the facility is contained in the MFL or OSM dataset. This strategy does not introduce any bias so long as the same health-care facility has the same or very similar geographical coordinates in both datasets. It is, however, possible that the geographical coordinates for the same health-care facility differed between the two datasets, in which case our analysis would consider these to be two different health-care facilities and, thus, underestimate the true travel time. Sixth, our travel time numbers are approximations that, for example, do not take into account the frequency of transport services and assign an estimated (rather than measured) travel speed to different types of roads. Similarly, we assumed that individuals were able to cross national borders and incurred no additional time cost from doing so. In border regions where these assumptions do not hold true, our estimated travel times would, thus, underestimate the true travel time. Finally, our analysis focuses on only one aspect of access to health care and does not, for instance, consider financial barriers to accessing care.
Most countries in sub-Saharan Africa contain populated areas in which older adults have little to no physical access to a hospital and (albeit to a lesser extent) health-care facilities of any type. If COVID-19 becomes a generalised pandemic that infects large swathes of populations in the region, then it will be older adults living in these areas who are in especially high need for either improved transport options to existing hospitals or provision of makeshift hospital care. Beyond their usefulness for the COVID-19 response, our maps could also inform health systems planning for other conditions that commonly affect older adults, such as expansion of care for chronic non-communicable diseases.
For more on OpenStreetMap see https://www.openstreetmap.org
For more on use of OSM in humanitarian settings see https://www.missingmaps.org/
For more on use of OSM to map health infrastructure see https://healthsites.io/
For more on the process of mapping health-care facilities see https://www.hotosm.org/
For the Shuttle Radar Topography Mission database see http://srtm.csi.cgiar.org
For data and maps see https://doi.org/10.11588/data/RGM2AW
Data sharing
All data and high-resolution versions of all maps in this manuscript are available online.
Acknowledgments
Acknowledgments
This study was funded by the Bill & Melinda Gates Foundation (agreement no INV-016002). PG was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (award no KL2TR003143). POO is funded under the IDeAL's Project (DELTAS Africa Initiative [DEL-15-003]). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences Alliance for Accelerating Excellence in Science in Africa and is supported by the New Partnership for Africa's Development Planning and Coordinating Agency with funding from the Wellcome Trust (no 107769/Z/10/Z) and the UK Government. POO is also supported by funds provided under R W Snow's Wellcome Trust Principal Fellowship (nos 103602 and 212176). EAO is supported as Wellcome Trust Intermediate Fellow (no 201866). POO and EAO acknowledge the support of the Wellcome Trust to the Kenya Major Overseas Programme (no 203077). MR and SL were supported by the Klaus Tschira Stiftung. The views expressed in this publication are those of the authors. We acknowledge the contribution of scientists at the KEMRI–Wellcome Trust Programme, who assembled the MFL dataset since 2010, including Robert Snow, Peter Macharia, and Joseph Maina.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps.
Contributors
PG wrote the first draft of the manuscript. MR and PG did the data analysis. PG, MR, SL, and AZ had the idea for the study. All authors provided input on iterations of the manuscript and approved the final version.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.European Centre for Disease Prevention and Control Daily number of new reported cases of COVID-19 by country worldwide. Sept 14, 2020. https://www.ecdc.europa.eu/en/publications-data/download-todays-data-geographic-distribution-covid-19-cases-worldwide
- 2.Walker P, Whittaker C, Watson O, et al. The global impact of COVID-19 and strategies for mitigation and suppression. March 26, 2020. https://spiral.imperial.ac.uk:8443/handle/10044/1/77735 [DOI] [PMC free article] [PubMed]
- 3.Kruk ME, Gage AD, Arsenault C, et al. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6:e1196–e1252. doi: 10.1016/S2214-109X(18)30386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimes CE, Bowman KG, Dodgion CM, Lavy CBD. Systematic review of barriers to surgical care in low-income and middle-income countries. World J Surg. 2011;35:941–950. doi: 10.1007/s00268-011-1010-1. [DOI] [PubMed] [Google Scholar]
- 5.Kironji AG, Hodkinson P, de Ramirez SS, et al. Identifying barriers for out of hospital emergency care in low and low-middle income countries: a systematic review. BMC Health Serv Res. 2018;18:291. doi: 10.1186/s12913-018-3091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyei-Nimakoh M, Carolan-Olah M, McCann TV. Access barriers to obstetric care at health facilities in sub-Saharan Africa-a systematic review. Syst Rev. 2017;6:110. doi: 10.1186/s13643-017-0503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav. 2014;18:1199–1223. doi: 10.1007/s10461-014-0729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baelani I, Jochberger S, Laimer T, et al. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit Care. 2011;15:R10. doi: 10.1186/cc9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma X, Vervoort D. Critical care capacity during the COVID-19 pandemic: global availability of intensive care beds. J Crit Care. 2020 doi: 10.1016/j.jcrc.2020.04.012. published online Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajewski J, Pittalis C, Lavy C, et al. Anesthesia capacity of district-level hospitals in Malawi, Tanzania, and Zambia: a mixed-methods study. Anesth Analg. 2020;130:845–853. doi: 10.1213/ANE.0000000000004363. [DOI] [PubMed] [Google Scholar]
- 11.Kruk ME, Leslie HH, Verguet S, Mbaruku GM, Adanu RMK, Langer A. Quality of basic maternal care functions in health facilities of five African countries: an analysis of national health system surveys. Lancet Glob Health. 2016;4:e845–e855. doi: 10.1016/S2214-109X(16)30180-2. [DOI] [PubMed] [Google Scholar]
- 12.Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387:168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 13.Maina J, Ouma PO, Macharia PM, et al. A spatial database of health facilities managed by the public health sector in sub Saharan Africa. Sci Data. 2019;6:134. doi: 10.1038/s41597-019-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nic Lochlainn LM, Gayton I, Theocharopoulos G, et al. Improving mapping for Ebola response through mobilising a local community with self-owned smartphones: Tonkolili District, Sierra Leone, January 2015. PLoS One. 2018;13 doi: 10.1371/journal.pone.0189959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tatem AJ. WorldPop, open data for spatial demography. Sci Data. 2017;4 doi: 10.1038/sdata.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens FR, Gaughan AE, Linard C, Tatem AJ. Disaggregating census data for population mapping using random forests with remotely-sensed and ancillary data. PLoS One. 2015;10 doi: 10.1371/journal.pone.0107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouma PO, Maina J, Thuranira PN, et al. Access to emergency hospital care provided by the public sector in sub-Saharan Africa in 2015: a geocoded inventory and spatial analysis. Lancet Glob Health. 2018;6:e342–e350. doi: 10.1016/S2214-109X(17)30488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchhorn M, Smets B, Bertels L, et al. Copernicus Global Land Service: Land Cover 100m: epoch 2018: Africa demo (deprecated) Dec 31, 2019. https://zenodo.org/record/3518087#.X1eCpZ5KhPY
- 19.Alegana VA, Maina J, Ouma PO, et al. National and sub-national variation in patterns of febrile case management in sub-Saharan Africa. Nat Commun. 2018;9 doi: 10.1038/s41467-018-07536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- 21.Hulland EN, Wiens KE, Shirude S, et al. Travel time to health facilities in areas of outbreak potential: maps for guiding local preparedness and response. BMC Med. 2019;17:232. doi: 10.1186/s12916-019-1459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juran S, Broer PN, Klug SJ, et al. Geospatial mapping of access to timely essential surgery in sub-Saharan Africa. BMJ Glob Health. 2018;3 doi: 10.1136/bmjgh-2018-000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.South A, Dicko A, Herringer M, et al. A rapid and reproducible picture of open access health facility data in Africa to support the COVID-19 response [version 1; peer review: 1 not approved] Wellcome Open Res. 2020;5:157. doi: 10.12688/wellcomeopenres.16075.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meara JG, Leather AJM, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386:569–624. doi: 10.1016/S0140-6736(15)60160-X. [DOI] [PubMed] [Google Scholar]
- 25.van der Walt A, South A. Exploring open African health facility data (version 1.1) Zenodo. 2020 doi: 10.5281/zenodo.3871224. published online June 1. [DOI] [Google Scholar]
- 26.Quattrone G, Capra L, De Meo P. Proceedings of the 18th ACM Conference on Computer Supported Cooperative Work & Social Computing; Vancouver, BC, Canada. March 14–18, 2015. There's no such thing as the perfect map: quantifying bias in spatial crowd-sourcing datasets; pp. 1021–1032. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and high-resolution versions of all maps in this manuscript are available online.