COVID-19, caused by the highly contagious RNA virus SARS-CoV-2, has infected over 8 million and killed over four hundred thousand worldwide as of June, 2020. In response, the research community around the world is racing to develop treatments. The major thrust of the efforts appears to be three-pronged: antivirals, vaccines, and mitigators of tissue damage and cytokine storm. Here I propose the hypothesis that glycine, a non-essential amino acid, should be evaluated as a beneficial mitigator of tissue damage and cytokine storm in COVID-19 patients.
An increasing body of literature has documented that COVID-19 can attack multiple cell types throughout the body(1). These include cells in the lung, heart and blood vessels, liver, kidneys, intestines, and brain. Tissue damage in one or more of these organs are associated with the severity and mortality of COVID-19 patients. Although the exact cause of how COVID-19 kills remains unknown, there are some important clues. The first is the association of cytokine storms with mortality. Cytokine storms have been associated with acute respiratory distress syndrome (ARDS), a major cause of mortality(2). Elevated levels of pro-inflammatory cytokines, such as IL-2, IL-7, G-CSF, IP10, MCP1, MIP1A, and TNF-α, were correlated with COVID-19 severity(3). Similarly, GM-CSF and IL6 were observed at significantly higher levels in COVID-19 patients needing ICU care compared to those that do not(4). Another potential cause of death was increased blood clotting, vessel constriction, and cardiac damage in COVID-19 patients. Possible reasons include SARS-CoV2 induced death of endothelial cells and constriction of blood vessels and ischemia; or secondary cardiac damage after lung damage, which leads to hypoxia; or damage caused by cytokine storm. In addition to lung, heart, and blood vessels, significant tissue damage has also been observed in the kidney, GI tract, liver, and the brain. Taken together, a picture emerges that tissue damage in the lung and other organs causes the release of an abundance of pathogen-associated molecular patterns (PAMPS) and damage-associated molecular patterns (DAMPS). The PAMPS and DAMPS subsequently lead to the onset of cytokine release syndrome (CRS), ARDS, and secondary hemophagocytic lymphohistiocytosis (sHLH)(5), which can cause multiple organ failure and COVID-19 mortality.
Given the prominent association of CRS with COVID-19 mortality, an important emerging treatment strategy that is being widely evaluated is the use of anticytokine or immunomodulatory drugs. One example is tocilizumab, a clinically approved anti-IL6 receptor antibody, showed some promise in reducing mortality in COVID-19 patients(4). Another anti-IL6R antibody being evaluated is sarilumab. In addition, inhibitors of the JAK1 and JAK2 kinases, such as baricitinib and ruxolitinib, which act downstream of IL6, are also being evaluated for treating severely ill COVID19 patients and have shown some promise (6, 7). In addition to the above pharmaceutical-based approach, another recently proposed unorthodox treatment of COVID-19 associated ARDS and CRS is low dose irradiation of the lung(8–12). The radiation-based approach was proposed based on historical observations of successful control of virus or bacteria-induced pneumonia(13). Interestingly, early results from an a small clinical trial showed promising results in 4 of 5 radiotherapy treated COVID-19 patients(14). However, given the early stages of the trials, it is too early to conclude whether any of the above strategies will be effective against COVID-19 induced CRS. Additional approaches/ideas are clearly needed.
I propose here that glycine, a non-essential amino acid, may be an effective mitigator for both the tissue damage and cytokine storm in COVID-19 patients. Previous studies have demonstrated both the cytoprotective and anti-inflammatory properties of glycine in human patients and animal models. Dietary intake of glycine significantly reduced endotoxin/ischemia-induced liver and lung tissue damage and extended overall survival in rats(15). Oral glycine prevented rat hemorrhagic shock and liver injury(16). Glycine also blunted endotoxin-induced superoxide and proinflammatory cytokine TNF-α production in alveolar macrophages.(17) Glycine has been further shown to have anti-inflammatory and protective roles in experimental models of acute pancreatitis(18), gastric ulcer(19), and arthritis(20). Furthermore, glycine attenuated TNF-α and IL-6 production in obese mice(21), a very relevant finding since obesity has been linked with increased COVID-19 hospitalizations(22). In human cystic fibrosis patients, oral glycine at 0.5g/kg/d reduced inflammatory cytokines TNF-α, IL-6, and G-CSF and improve clinical status(23). Importantly, no major untoward sides effects were observed in human patients who had taken high doses of 0.5-1.0 g/kg for 8 weeks(24, 25) or 5 years(26).
Mechanistically, it has been suggested that the anti-inflammatory properties of glycine in macrophages and other leukocytes are dependent on the expression of a glycine-gated chloride channel in these cells(27, 28). Glycine caused hyperpolarization of macrophages, which blunted endotoxin-induced calcium influxes and membrane depolarization that were necessary for free radicals and TNF-α induction(29). However, the precise mechanisms through which glycine attenuates inflammatory cytokines are still not well understood. One likely mechanism is its potent ability to inhibit pyroptosis(30), a pro-inflammatory form of cell death that often accompanies microbial infections, including those from SARS-CoV-2(31), as part of the organism’s innate immune response. Fig. 1 shows a schematic of potential mechanisms.
Figure 1. A hypothetical mechanism of how glycine may reduce SARS-CoV-2 mediated tissue damage and cytokine storm.
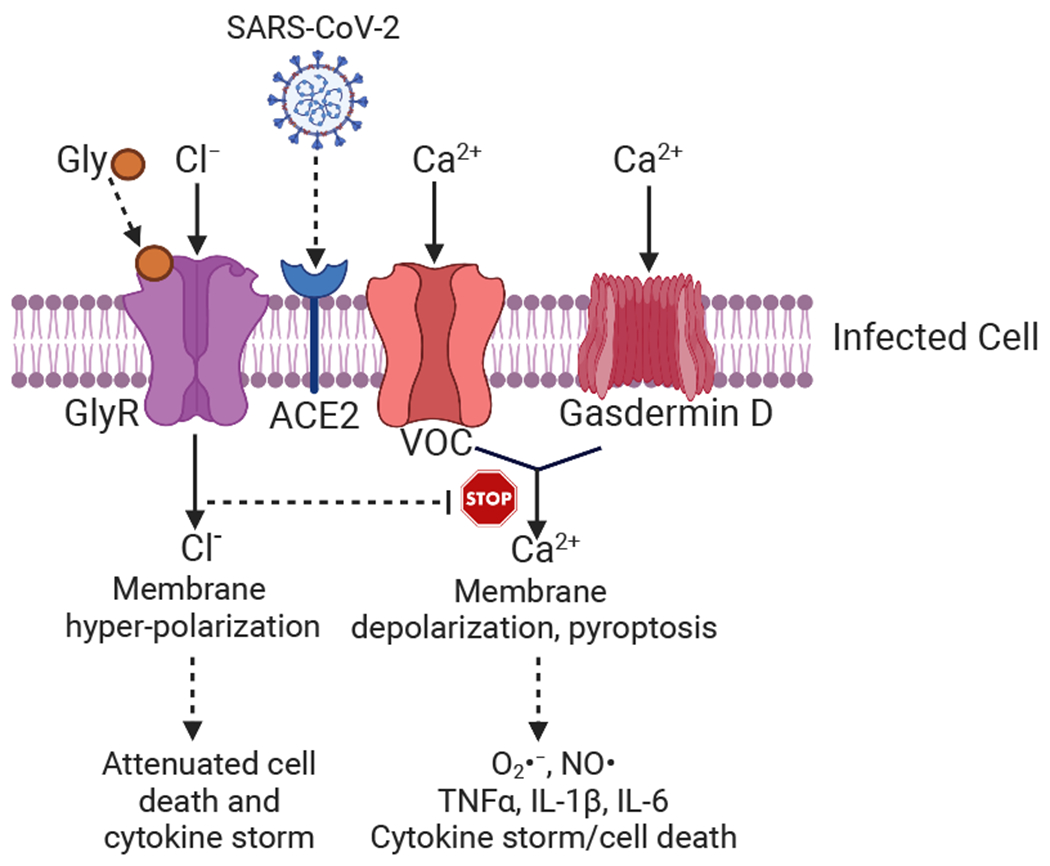
SARS-CoV-2 can infect target cells through the ACE2 protein. The infection may cause cell death through pyroptosis, which requires calcium influxes from voltage operated calcium channel (VOC) or gasdermin D pores and ensuing cellular membrane depolarization, free radical generation, and secretion of pro-inflammatory cytokines. Glycine, by binding its receptor GlyR, induces a chloride influx that causes cellular membrane hyperpolarization that protects the cells from pyroptosis and proinflammatory cytokine secretion.
Compared to most other repurposed drugs that are being evaluated for COVID-19 treatment, glycine is very affordable and widely available as a nutritional supplement. Many of the drugs under evaluation are biologics. Therefore, even if their clinical trials produce good results, it will take time to ramp up their production to meet the steep demand generated by the rapidly rising patient population worldwide. Furthermore, the cost of such medicines may limit their use in less developed countries. In comparison, glycine, if proven to be beneficial in clinical trials, can be rapidly deployed to COVID-19 patients around the world.
Because of its excellent safety record, promising anti-inflammatory properties, and wide availability and affordability, I propose that clinical trials with glycine as a mitigator of cytokine storms should be conducted as soon as possible in COVID-19 patients. It may be administered either orally in patients with moderate symptoms or intravenously in patients with severe symptoms at doses of 0.5-1.0 g/kg/d. In early stages of the disease, glycine may suppress or blunt the onset of virus-induced cytokine storm. In late stages of the disease, its cytoprotective effect may protect lung tissues from severe damage and ARDS, the leading cause of the mortality. Under these circumstances, it may be used in conjunction with other treatments, such as dexamethasone, which was shown to reduce cell death in advanced stage patients(32); or radiotherapy, as reported recently(14); or anti-viral agents, such as remdesivir(33). It is likely that glycine may enhance the therapeutic effects of those treatments.
References
- 1.Wadman M, Couzin-Frankel J, Kaiser J, Matacic C, A rampage through the body. Science 2020; 368, 356–60. [DOI] [PubMed] [Google Scholar]
- 2.Ruan Q, Yang K, Wang W, Jiang L, Song J, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. , Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. , Pathogenic T cells and inflammatory monocytes incite inflammatory strom in severe COVID-19 patients. National Science Review 2020, nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. , COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395, 1033–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D, Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. , Ruxolitinib in treatmen tof severe coronovirus disease 2019 (COVID-19): A multicenter, single-blind, randomized contrlled trial. Journal of Allergy and Clinical Immunology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JJ, Mitigating coronavirus-induced acute respiratory distress syndrome by radiotherapy. iScience 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby C, Mackenzie M, Is low dose radiation therapy a potential treatment for COVID-19 pneumonia ? Radiotherapy and Oncology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhawan G, Kapoor R, Dhawan R, Singh R, Monga B, Giordano J, et al. , Low dose radiation therapy as a potential life saving treatment for COVID-19-induced acute respiratory distress syndrome (ARDS). Radiother Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodel F, Arenas M, Ott OJ, Fournier C, Georgakilas AG, Tapio S, et al. , Low-dose radiation therapy for COVID-19 pneumopathy: what is the evidence? Strahlenther Onkol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corry PM A Radiation Mitigator as a Potential Treatment for COVID-19. Radiat Res 2020. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese EJ, Dhawan G, How radiotherapy was historically used to treat pneumonia: could it be useful today? Yale J Biol Med 2013; 86, 555–70. [PMC free article] [PubMed] [Google Scholar]
- 14.Hess CB, Buchwald ZS, Stokes W, Swithcenko JM, Nasati TH, Weinberg BD, et al. , Low-dose whole-lung radiation for COVID-19 pneumonia: planned day-7 interim analysis of a registered clinical trial. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikejima K, Iimuro Y, Forman DT, Thurman RG, A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol 1996; 271, G97–103. [DOI] [PubMed] [Google Scholar]
- 16.Mauriz JL, Matilla B, Culebras JM, Gonzalez P, Gonzalez-Gallego J, Dietary glycine inhibits activation of nuclear factor kappa B and prevents liver injury in hemorrhagic shock in the rat. Free Radic Biol Med 2001; 31, 1236–44. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler MD, Thurman RG, Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am J Physiol 1999; 277, L952–9. [DOI] [PubMed] [Google Scholar]
- 18.Ceyhan GO, Timm AK, Bergmann F, Gunther A, Aghdassi AA, Demir IE, et al. , Prophylactic glycine administration attenuates pancreatic damage and inflammation in experimental acute pancreatitis. Pancreatology 2011; 11, 57–67. [DOI] [PubMed] [Google Scholar]
- 19.Tariq M, Al Moutaery AR, Studies on the antisecretory, gastric anti-ulcer and cytoprotective properties of glycine. Res Commun Mol Pathol Pharmacol 1997; 97, 185–98. [PubMed] [Google Scholar]
- 20.Li X, Bradford BU, Wheeler MD, Stimpson SA, Pink HM, Brodie TA, et al. , Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: role for glycine-gated chloride channel. Infect Immun 2001; 69, 5883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alarcon-Aguilar FJ, Almanza-Perez J, Blancas G, Angeles S, Garcia-Macedo R, Roman R, et al. , Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice. Eur J Pharmacol 2008; 599, 152–8. [DOI] [PubMed] [Google Scholar]
- 22.Lighter J, Philips M, Hochman S, Sterling S, Johnson D, Francois F, et al. , Obesity in patients younger than 60 years is a risk facotr for COVID-19 hospital admission. Clinical Infectious Diseases 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vargas MH, Del-Razo-Rodriguez R, Lopez-Garcia A, Lezana-Fernandez JL, Chavez J, Furuya MEY, et al. , Effect of oral glycine on the clinical, spirometric and inflammatory status in subjects with cystic fibrosis: a pilot randomized trial. BMC Pulm Med 2017; 17, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC, Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am J Psychiatry 2000; 157, 826–8. [DOI] [PubMed] [Google Scholar]
- 25.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M, Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry 1999; 56, 29–36. [DOI] [PubMed] [Google Scholar]
- 26.Cleveland WL, DeLaPaz RL, Fawwaz RA, Challop RS, High-dose glycine treatment of refractory obsessive-compulsive disorder and body dysmorphic disorder in a 5-year period. Neural Plast 2009; 2009, 768398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Froh M, Thurman RG, Wheeler MD, Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol 2002; 283, G856–63. [DOI] [PubMed] [Google Scholar]
- 28.Colquhoun D, Sivilotti LG, Function and structure in glycine receptors and some of their relatives. Trends Neurosci 2004; 27, 337–44. [DOI] [PubMed] [Google Scholar]
- 29.Ikejima K, Qu W, Stachlewitz RF, Thurman RG, Kupffer cells contain a glycine-gated chloride channel. Am J Physiol 1997; 272, G1581–6. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg JM, Bienholz A, Venkatachalam MA, The role of glycine in regulated cell death. Cell Mol Life Sci 2016; 73, 2285–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP, The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20, 363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kupferschmidt KA cheap steroid is the first drug shown to reduce death in COVID-19 patients. Science 2020. [Google Scholar]
- 33.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. , Remdesivir for the Treatment of Covid-19 - Preliminary Report. N Engl J Med 2020. [DOI] [PubMed] [Google Scholar]


