Abstract
Purpose
The components of metabolic syndrome (MS) are risk factors for developing both cardiovascular disease (CVD) and non-alcoholic fatty liver disease (NAFLD). Strain (SI) and strain‑rate imaging (SRI) are able to recognize early changes in cardiac function. Vibration-controlled transient elastography (VCTE) and controlled attenuation parameter (CAP) detect and quantify liver fibrosis and steatosis. We aimed to assess whether there is any correlation between liver fibrosis and steatosis and left ventricular (LV) dysfunction in MS patients.
Patients and Methods
A total of 150 adults with MS were registered in the study. They were compared with a control group of 150 age- and sex-matched adults without MS. After the classic echocardiographic assessment of LV function, two-dimensional speckle echocardiography (2D-STE) was used to evaluate LV peak systolic strain (S) and peak systolic strain rate (SR), while liver steatosis and fibrosis were evaluated by VCTE and CAP.
Results
LV diastolic dysfunction was significantly more frequent among the patients with MS. We found significant differences between the two groups regarding the presence of subtle LV systolic dysfunction, detected by reduced values of S and SR. The risk for LV diastolic dysfunction was 3.6 times higher in MS with severe steatosis and 8 times higher in patients with severe fibrosis, P<0.0001. The risk for LV systolic dysfunction was double in MS with severe steatosis and 1.7 times higher in MS with severe fibrosis, P<0.0001.
Conclusion
In MS patients with normal LV ejection fraction, conventional echocardiography parameters identified diastolic LV dysfunction, while SI and SRI identified subtle impairment of systolic LV dysfunction. The presence of hepatic steatosis and fibrosis increases significantly the risk for cardiac dysfunction in MS patients (P<0.0001).
Keywords: metabolic syndrome, left ventricular function, strain and strain‑rate imaging, liver steatosis and fibrosis, liver elastography
Introduction
Metabolic syndrome (MS) is a condition characterized by the coexistence of multiple cardiovascular risk factors, such as hypertension, abdominal obesity, insulin resistance, hyperglycemia, and dyslipidemia. It usually affects obese and sedentary individuals.1 The incidence of MS increased in the last decades, jeopardizing general population`s health.
It is likely that approximately 25% of the world’s adult population suffers from MS. This condition is associated with an increased risk for diabetes, stroke, and myocardial infarction.1 Several clinical studies indicate that MS is associated with the occurrence of atherosclerotic vascular disease and of heart failure.2 The evidence of myocardial dysfunction in individuals with MS was gathered by studies using conventional and tissue Doppler echocardiography.3 Though conventional echocardiographic parameters for assessment of systolic cardiac function have a poor sensitivity and fail to detect subtle decreases in myocardial contractility. Recently, two-dimensional speckle-tracking echocardiography (2D-STE) has become a more reliable method for the identification of subtle cardiac dysfunction by quantitative evaluation of myocardial deformation.4 One of the key concepts of speckle-tracking is the strain, expressed as percentage (%) and defined as the fractional shorten of a myocardial region. The strain can be assessed in each region of the investigated ventricle (regional strain). The mean of these values gives the peak strain (S), representing the peak ventricular function. This method allows assessing myocardial fibers according to their specific arrangement (longitudinal strain for subendocardial fibers, circumferential and radial strain for subepicardial fibers).5
Non-alcoholic fatty liver disease (NAFLD) represents a frequent metabolic liver disease all over the world, its prevalence ranging from 25% to 45% in Western countries.6 This condition is defined as an accumulation of lipids, mainly triglycerides, in more than 5% of hepatocytes in the absence of excessive alcohol consumption or other secondary causes. NAFLD includes simple steatosis (a benign condition, without any hepatic inflammation and fibrosis), as well as non-alcoholic steatohepatitis (NASH), an aggressive condition that may lead to cirrhosis, hepatocellular carcinoma, death.6
A meta-analysis reported that 35% of NASH patients progress to cirrhosis in about 7 years. But, the main cause of death in NASH patients is represented by CVD, and not by complications of the liver disease.7
NAFLD patients have a significantly higher risk of death due to ischemic heart disease (12% to 16%) when compared to those without NAFLD (1% to 3%).8 This fact indicates a strong correlation between the severity of NAFLD and the risk of cardiovascular disease mortality. This association could be explained by several possible mechanisms. Previous studies showed that NAFLD was related with myocardial insulin resistance,9 as well as with altered LV structure and diastolic function.10
Myocardium uses energy from glucose and fatty acids. This energy uptake is modified in patients with insulin resistance, leading to changes in myocardial structure and to cardiac dysfunction. These facts could explain the increasing prevalence of diastolic heart failure in aging societies, along with increased rates of hypertension, diabetes and obesity.11 Diastolic heart failure has a mortality risk similar to that of systolic heart failure.12
In spite of this, the association between NAFLD, assessed by liver vibration-controlled transient elastography (VCTE) and by controlled attenuation parameter (CAP), and subtle abnormalities in LV function assessed by SI and SRI, was not yet studied.
Therefore, we wanted to explore whether there is any association between hepatic steatosis and/or fibrosis and cardiac dysfunction in MS patients.
Patients and Methods
Subjects and Methodology
This prospective study was performed between January 2019 and January 2020 in the Department of Cardiology in Timisoara Emergency City Hospital Timisoara and the Department of Gastroenterology and Hepatology in Timisoara Emergency County Hospital. We enrolled adult subjects with MS and compared their demographic, clinical, biological, and echocardiographic characteristics with those of a control group, that included age- and sex-matched adult subjects without MS. All MS patients were evaluated by conventional mono (M) and two-dimensional (2D) echocardiography and by 2D-STE, as well as by VCTE, and by CAP.
Inclusion criteria were: age ≥18 years and a diagnose of MS according to the International Diabetes Federation (IDF) in 2006 criteria.13
Exclusion criteria were: a history or finding of cardiovascular disease; systolic heart failure, defined by (LV ejection fraction ≤50%); ischemic heart disease (exercise testing); hypertrophic cardiomyopathy; moderate or severe valvular heart disease; cardiac pacemakers; known chronic liver diseases due to viral infections, alcohol abuse (>20 g/day in women and >30 g/day in men), or use of drugs that induce steatosis (such as steroids or tamoxifen); end-stage renal disease; pregnancy or lactation; major systemic illness or malignancy.
The diagnosis of MS was based on the 2006 IDF criteria: the presence of central obesity (waist circumference ≥94 in men and ≥80cm in women), plus any two of the following four factors: systolic BP (SBP) ≥130 or diastolic BP (DBP) ≥85 mmHg or treatment of previously diagnosed hypertension; raised fasting plasma glucose (FPG) ≥100 mg/dL or treatment of previously diagnosed type two diabetes; reduced high‑density lipoprotein cholesterol <40/50 mg/dL (men/women) or a treatment specific for this lipid abnormality; increased triglyceride level ≥150 mg/dL or specific treatment for this lipid abnormality.13
Clinical Assessment
All patients underwent a full medical history and a complete clinical examination. Smoking status was defined as current smoking or no smoking. Diabetes was diagnosed in the presence of a FPG ≥126 g/mL or use of insulin or of an oral hypoglycemic agent.13 Resting BP was measured three times in a seated position using an automated oscillometric sphygmomanometer and the average of these measurements was registered. Arterial hypertension was diagnosed according to the 2018 ESC/ESH Guidelines for the management of arterial hypertension as a BP > 140/90 mmHg and/or current antihypertensive therapy.14
Biochemical tests were done on the same day with clinical assessment, echocardiography, liver VCTE and CAP.
Conventional and 2D Speckle-Tracking Echocardiography (STE)
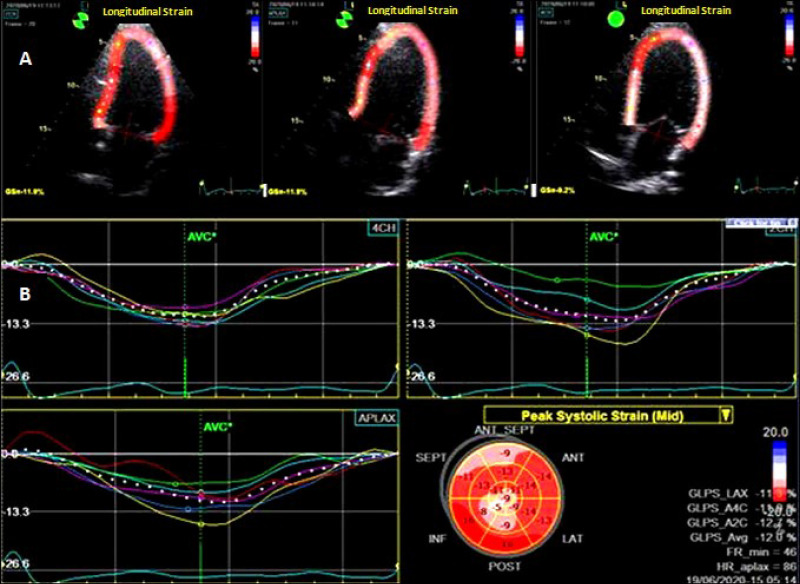
M‑ and 2D-mode echocardiographic evaluations were done using a General Electrics phased array ultrasonoscope (VIVID‑5S dimension, USA) with a 3.5 MHz transducer. The diameters of cardiac chambers and LV ejection fraction (EF) were calculated according to the American Society of Echocardiography guidelines.15 Doppler examination was performed in the 4-chamber apical incidence with the volume specimen placed at the mitral valve tip. We determined the following parameters: the peak E (maximal protodiastolic velocity of the transmitral flow), the peak A (maximal telediastolic velocity), the E/A ratio, and the isovolumic relaxation time (IVRT). LVEF was calculated at 4- and 2-chamber apical incidences by means of the Simpson method. 2D speckle tracking imaging (STI) was assessed for the peak myocardial deformation using a frequency of 70–80 frames/s. The device was adjusted to achieve optimal depth and sectorial width.16 After selecting the frame that allowed the best definition of the endocardium, the endocardium edge was automatically drawn and manually corrected (Figure 1). The software divided automatically the ventricle into 6 equal segments. STI of these 6 segments was analyzed in 4.3 and 2-chambers apical incidences. The peak longitudinal strain (LS) and its rate (LSR) were calculated as the average of the values measured in the 18 analyzed segments. Peak radial (RS) and peak circumferential strain (CS) were calculated from the mean maximum systolic values registered in the 18 LV segments in the short left parasternal axis incidence- at the apex, middle segment, and basal segment of the LV. The circumferential and radial peak strain rates (CSR, RSR) were also calculated. All STI measurements were done during 3 consecutive cardiac cycles, and their arithmetic mean was registered. All echocardiographic assessments were done by the same investigator. The cut-off values for LV diastolic dysfunction were E/A <0.8 and IVRT >100 msec; for LV systolic dysfunction: FEVS<50%, peak LS<-18%, peak CS< −19%, peak RS< 40%.17
Figure 1.
Two-dimensional speckle tracking echocardiography of the left ventricle. (A) Longitudinal strain analysis in the apical 2.3, and 4 chamber views (GLPS); (B). Bull’s-eye summary.
Vibration-Controlled Transient Elastography (VCTE) and Controlled Attenuation Parameter (CAP) Measurements
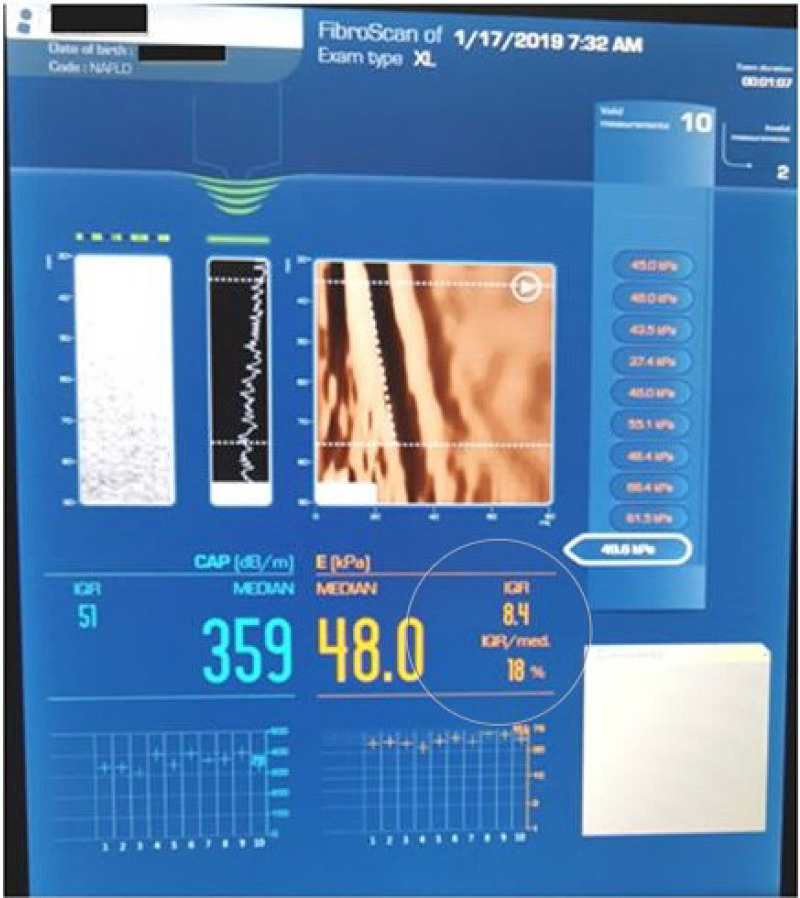
VCTE was performed in the right hepatic lobe, by intercostal approach, after a fasting period longer than 4 hours, using a FibroScan® device (EchoSens, Paris, France). The patient was examined in a supine position with the right arm held in abduction. According to the European recommendations, M (transducer frequency 3.5 MHz) or XL (transducer frequency 2.5 MHz) probes were used.17 In each patient, the examiner meant for 10 valid liver stiffness measurements (LSM) and then their median value was calculated. Reliable measurements were considered those having a median value with an interquartile range interval/median ratio <30%.18 The LSM was expressed in kilopascals (kPa), as shown in Figure 2. To discriminate between the stages of fibrosis, we used the following TE cut-offs: for F≥ 2:8.2 kPa; for F≥3:9.7 kPa; and for F4: 13.6 kPa.19 To discriminate between the stages of steatosis, we used the following CAP cut-offs: S1 (mild) – 274 dB/m, S2 (moderate) – 290 dB/m, S3 (severe) – 302 dB/m.20
Figure 2.
Vibration-controlled transient elastography (VCTE) and controlled attenuation parameter (CAP) from the Fibroscan® device.
Ethics
Informed consent was obtained from all participants in the study. The objective and the strategy of the study were explained in an easy manner for them. The study was conducted in accordance with the Human Rights Declaration of Helsinki and approved by the Ethics Committees of the Timisoara Clinical Emergency City and County Hospitals.
Statistical Analysis
Statistical analysis was performed using the MedCalc statistical software version 12.7.7 (MedCalc Software, Ostend, Belgium). Continued data were presented as mean ± 1 standard deviation (SD). Qualitative variables were expressed as numbers and percentages. Differences between groups were compared by the paired t-test. Linear and logistic regression were used for univariate and multivariate analysis of factors that may influence the echocardiographic LV function variables. The association between two variables was assessed using Pearson`s correlation coefficient (r). The 95% confidence intervals were calculated for each predictive test. A P- value < 0.05 was considered significant for all statistical tests.
Results
A total of 208 MS patients were screened. Thirty (16%) were removed from the study because of inappropriate quality of the echocardiographic image, while 28 (15%) were excluded because of failure at VCTE and CAP.
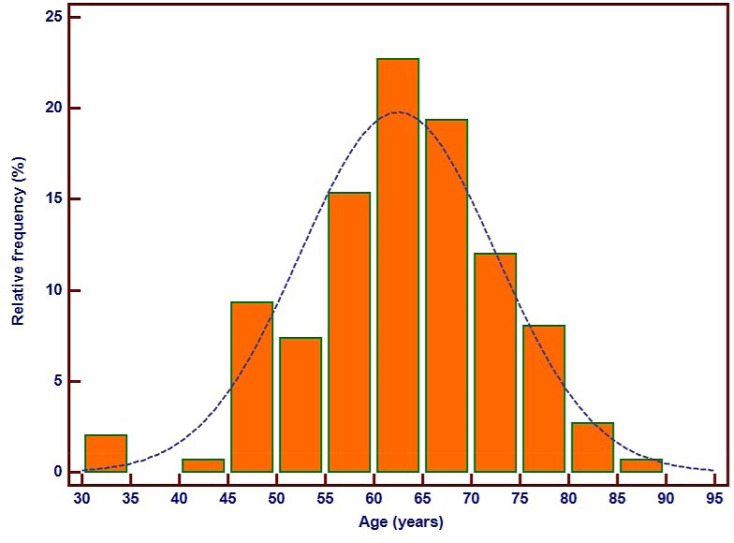
One hundred and fifty MS subjects were registered in the study. One hundred and fifty age- and sex-matched subjects without MS were registered in the control group. The baseline demographic, clinical, and biochemical characteristics are shown in Table 1. The mean age of the patients was 62.4±10 years (limits: 31–85), 54% (82) were males. The frequency distribution of the MS patients` age is shown in Figure 3. No significant differences were observed between the MS subjects and the controls regarding smoking status, heart rate, low– density lipoprotein (LDL)-cholesterol, and serum transaminase levels. MS patients had significantly more frequent diabetes mellitus and systemic hypertension, had higher weights, body mass indexes, and waist circumferences, higher values of fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), and of triglycerides, but significantly lower values of total and high– density lipoprotein (HDL) cholesterol.
Table 1.
Clinical and Biochemical Characteristics of MS and Control Groups
| With MS (n=150) | Controls (n=150) | P value | |
|---|---|---|---|
| Systemic hypertension (n, %) | 120 (80%) | 68 (45%) | <0.0001 |
| Diabetes mellitus | 134 (89%) | 36 (24%) | <0.0001 |
| Smoking (current, %) | 15 (10%) | 18 (12%) | 0.58 |
| Systolic BP (mmHg) | 141.6±18 | 131.27±12 | <0.0001 |
| Diastolic BP (mmHg) | 84.6±11 | 73.23 ± 6.97 | <0.0001 |
| Heart rate (beats/min) | 75.6 ± 11.4 | 73.11 ± 10.8 | 0.05 |
| BMI (kg/m2) | 32.7± 5.2 | 29.7±3.8 | <0.0001 |
| Weight (kg) | 91±7 | 77±9 | <0.0001 |
| Waist circumference (cm) | 112±13 | 97.00 ± 4 | <0.0001 |
| Total cholesterol | 174±39 | 197±44 | <0.0001 |
| HDL (mgl/dL) | 45.2± 12.7 | 48.3±13 | 0.03 |
| LDL (mgl/dL) | 109.4±33 | 110.5±32 | 0.76 |
| Triglyceride (mgl/dL) | 159.1± 89.5 | 134.4±80.4 | 0.01 |
| FPG (mgl/dL) | 130± 42 | 109±12 | <0.0001 |
| HbA1c | 7.1±0.9 | 5.2±0.8 | <0.0001 |
| ASAT | 24±9 | 23±5 | 0.23 |
| ALAT | 37±7 | 36±5 | 0.15 |
Notes: Data are expressed as mean ± SD or number (percentage). Statistically significant values are presented in bold (P<0.05).
Abbreviations: MS, metabolic syndrome; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; ASAT, aspartate amino transferase; ALAT, alanine amino transferase.
Figure 3.
Frequency distribution of age in metabolic syndrome patients.
Echocardiography data are presented in Table 2. We found no statistically significant differences between MS and controls regarding the conventional variables of LV structure and systolic function. One hundred and thirty-five (90%) MS patients had reduced E/A ratio and prolonged IVRT, these parameters indicating LV diastolic dysfunction due to abnormal relaxation. SI and SRI detected subtle LV systolic dysfunction in 47 (31%) MS patients, reflected by reduced LS and LSR. Compared with controls, MS patients had significantly impaired LS (P < 0.0001), and LSR (P < 0.0001). No significant differences were observed among MS and control patients regarding CS, CSR, RS and RSR.
Table 2.
Left Ventricular Echocardiographic Data in MS and Control Groups
| MS (n=150) | Controls (n=150) | P value | |
|---|---|---|---|
| Conventional echocardiography | |||
| End DD (mm) | 49.00 ± 3.20 | 48.69 ± 2.94 | 0.38 |
| End SD (mm) | 30.34 ± 2.50 | 29.85 ± 2.68 | 0.10 |
| EF (%) | 51.7±0.6 | 51.8±0.2 | 0.05 |
| FS (%) | 37.93 ± 2.91 | 38.00 ± 3.50 | 0.85 |
| E (m/s) | 0.69 ± 0.15 | 0.88 ± 0.12 | <0.0001 |
| A (m/s) | 0.89 ± 0.17 | 0.61 ± 0.10 | <0.0001 |
| E/A ratio | 0.81 ± 0.21 | 1.47 ± 0.23 | <0.0001 |
| IVRT (msec) | 110.1±18 | 105.3±21 | 0.02 |
| 2D Speckle tracking echocardiography | |||
| GLS (%) | 19.9 ± 2.3 | 21.3 ± 1.9 | <0.0001 |
| GLSR (1/sec) | 1.58 ± 0.18 | 1.62 ± 0.1 | 0.01 |
| GCS (%) | 22.7 ± 2.1 | 23.0± 2.2 | 0.22 |
| GCSR (1/sec) | 1.59 ± 0.4 | 1.62 ± 0.4 | 0.51 |
| GRS (%) | 47.5 ± 5.5 | 47.7 ± 5.0 | 0.74 |
| GRSR (1/sec) | 2.3 ± 0.5 | 2.4 ± 0.4 | 0.05 |
Notes: Data are expressed as mean ± SD or number (percentage). Statistically significant values are presented in bold (P<0.05).
Abbreviations: MS, metabolic syndrome; End SD, end systolic diameter; End DD, end diastolic diameter; EF, ejection fraction; FS, fractional shortening; E, protodiastolic filling wave; A, end diastolic filling wave; IVRT, isovolumic relaxation time; 2D, two dimensional; GLS, global longitudinal strain; GLSR, global longitudinal strain rate; GCS, global circumferential strain; GCSR, global circumferential strain rate; GRS, global radial strain; GRSR, global radial strain rate.
In the study group of 150 SM subjects, the distribution of steatosis severity assessed by CAP was the following: 14% (21) patients had no steatosis–S0, 7% (11) had S1, 7% (11) had S2, and 71% (107) patients S3. The proportion of S0 was significantly lower, while the proportion of S3 was significantly higher than in the controls (P<0.0001), Table 3.
Table 3.
Evaluation of Liver Fibrosis and Steatosis
| MS (n=150) | Controls (n=150) | P value | |
|---|---|---|---|
| CAP, dB/m | 335.2± 51.2 | 255.56 ± 60.8 | <0.0001 |
| Steatosis stage | |||
| S0 | 21 (14%) | 95 (63%) | <0.0001 |
| S1 | 11 (7%) | 15 (10%) | 0.35 |
| S2 | 11 (7%) | 3 (2%) | 0.03 |
| S3 | 107 (71%) | 37 (25%) | <0.0001 |
| LSM, kPa | 7.24 ± 3.25 | 6.52± 2.85 | 0.04 |
| Fibrosis stage | |||
| F0–1 | 87 (58%) | 118 (79%) | 0.0001 |
| F2 | 28 (19%) | 11 (7%) | 0.002 |
| F3 | 20 (13%) | 14 (9%) | 0.26 |
| F4 | 15 (10%) | 8 (5%) | 0.10 |
Notes: Data are expressed as mean ± SD or number (percentage). Statistically significant values are shown in bold (P<0.05).
Abbreviations: CAP, controlled attenuation parameter; LSM, liver stiffness measurements; S, steatosis; F, fibrosis.
Regarding the severity of liver fibrosis, according to VCTE measurements, 58% (87) of the MS subjects had no or mild fibrosis–F0 and F1, 19% (28) had F2, 13% (20) F3, and 10% (15 subjects) F4. Among the controls, significantly more subjects (79%) had no or mild fibrosis (P<0.001), and significantly less (7%) presented F2 (P=0.002).
In univariate regression analysis, the variables associated with reduced LS in MS patients were diabetes mellitus, waist circumference, age, and liver stiffness measurement, while factors associated with reduced LSR were diabetes, waist circumference and liver stiffness measurement.
In multivariate analysis, the factors independently associated with reduced LS were diabetes (P<0.005) and LSM (P<0.0001). Reduced LSR was also independently associated in multivariate analysis with diabetes (P< 0.02) and with LSM (P<0.001), as seen in Table 4.
Table 4.
Factors Associated with Subtle LV Systolic Dysfunction in MS Patients
| GLS | ||||||
|---|---|---|---|---|---|---|
| Variables | Univariate Analysis | Multivariate Analysis | ||||
| β | SE | P | β | SE | P | |
| Waist circumference | −0.997 | 0.440 | 0.02 | – | – | – |
| Diabetes mellitus | −1.451 | 0.375 | 0.0002 | −1.026 | 0.353 | 0.004 |
| Age (years) | −0.047 | 0.019 | 0.01 | −0.030 | 0.017 | 0.08 |
| LSM (kPa) | −0.593 | 0.100 | <0.0001 | −0.293 | 0.052 | <0.0001 |
| GLSR | ||||||
| Variables | Univariate analysis | Multivariate analysis | ||||
| β | SE | P | β | SE | P | |
| Waist circumference | −0.003 | 0.001 | 0.008 | −0.001 | 0.001 | 0.08 |
| Diabetes mellitus | −0.098 | 0.030 | 0.001 | −0.074 | 0.029 | 0.01 |
| LSM (kPa) | −0.018 | 0.004 | <0.0001 | −0.0158 | 0.004 | 0.0006 |
Notes: Data are expressed as mean ± SD or number (percentage). Statistically significant values are presented in bold (P<0.05).
Abbreviations: MS, metabolic syndrome; GLS, global longitudinal strain; GLSR, global longitudinal strain rate; LSM, liver stiffness measurement; ß, beta coefficient from regression analysis; SE, standard error.
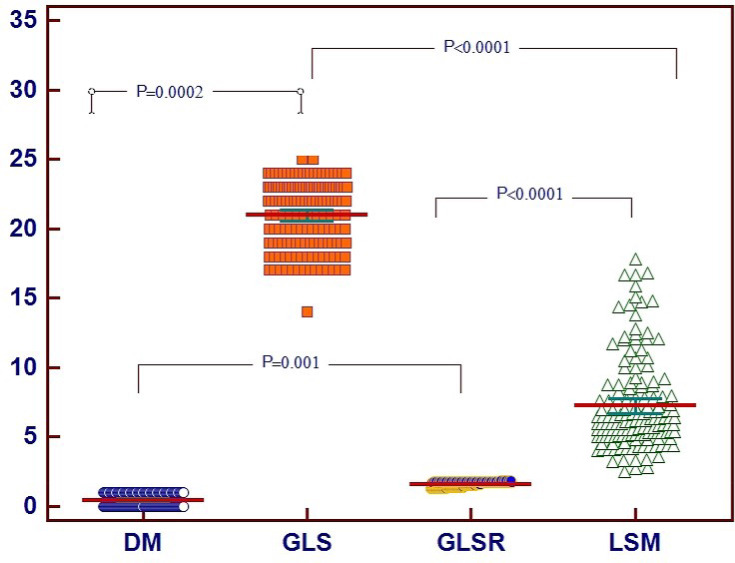
The correlations between the independent variables associated with subtle LV systolic function in MS patients are shown in Figure 4.
Figure 4.
Correlations between the independent variables associated with subtle LV systolic function in MS patients.
Abbreviations: MS, metabolic syndrome; DM, diabetes mellitus; LSM, liver stiffnessmeasurement; GLS, peak longitudinal strain; GLSR, peak longitudinal strain rate.
LVEF was similar in the two groups (P=0.05), but the 2D-STE identified a significant difference in LS values between the case and control groups. 2D-STE was able to assess a subtle LV systolic dysfunction in 47 MS patients (46%) and in 12 controls (8%), P<0.0001. The risk for LV systolic dysfunction was 3 times higher in the hypertensive (OR=8.7; 95% CI: 5.1 to 14.8, P<0.0001) and 5.5 times higher in diabetic patients with MS (OR=18.3; 95% CI: 9.8 to 34.2, P<0.0001).
The risk for LV diastolic dysfunction was 3.6 times higher in MS with severe steatosis (OR=3.6; 95% CI: 1.9 to 6.8, P<0.0001) and 8 times higher in patients with severe fibrosis (OR=14.8; 95% CI: 8.7 to 25.1, P<0.0001). The risk for LV systolic dysfunction was double in MS with severe steatosis (OR=3.6; 95% CI: 1.9 to 6.8, P<0.0001) and 1.7 times higher in MS with severe fibrosis (OR=4.1; 95% CI: 2.1 to 7.7, P<0.0001).
Discussion
MS is described as a group of cardiovascular risk factors, such as abdominal obesity, dyslipidemia, glucose intolerance, insulin resistance, and hypertension.21,22 This condition is related with increased risk for diabetes, stroke, myocardial infarction, and heart failure (HF).23 HF is a clinical syndrome induced by any cardiac structural or functional injury that reduces the capacity of the ventricles to fill with or eject blood.12 The HF syndrome has been compared to an iceberg. The visible segment includes the symptomatic HF patients, most of them being diagnosed in the primary healthcare sites. The invisible segment “below the waterline” includes the asymptomatic patients, with subtle left ventricular dysfunction.24,25 Early identification of subtle LV dysfunction in MS, as well as the understanding of the role of each of the MS components in affecting myocardial structure and function could help to establish and predict the risk of CVD in MS. Early determination of LV systolic dysfunction is enabled by 2D STE. This technique, that does not involve additional costs compared to conventional echocardiography, identifies a group of high-risk patients in whom the symptoms of heart failure are not yet present, In these patients (life style changes targeting obesity, alcohol abuse, sedentary habits, high salt intake), and the management of hypertension may prevent and delay the progression to symptomatic heart failure. But, 2D STE-guided therapy of heart failure is less studied. As the cardioprotective effects of angiotensin-converting enzyme inhibitors and of beta-blockers are demonstrated, these drugs should be chosen for the therapy of arterial hypertension in MS patients.
Most MS patients also develop NAFLD. Some authors reported a correlation between the incidence of NAFLD and a high amount of body fat and.26 Unexpectedly, the most frequent cause of death in patients with NAFLD is CVD and not a complication of the liver disease. Individuals with NASH have a much higher risk of death due to ischemic heart disease (12% to 16%) compared to those with NAFLD (1% to 3%). This indicates that the risk of CVD mortality increases along with the severity of NAFLD.27,28
Early detection of both heart and liver disease in MS is of great importance, as appropriate life-style modifications and medical interventions could avoid or postpone the development of heart failure and of NASH cirrhosis. These actions may reduce not only morbidity and mortality, but also the costs for health insurance.26
In our study, MS was associated with reduced cardiac function and with liver steatosis and fibrosis. This is important, as all the participants in the study had no symptoms and no history of heart failure with LVEF<50%, atherosclerotic cardiovascular disease, or liver disease.
To our information, this is the first work that evaluated the association between subclinical LV dysfunction, assessed by 2D speckle echocardiography, and liver fibrosis and steatosis, assessed by transient VCTE and CAP, in adult subjects with MS.
In the present study, 150 consecutive patients with MS were enrolled in the study, additive to 150 apparently healthy age- and sex-matched controls. The patients` age ranged from 31 to 85 years. Fifty-four percent were males. Eighty-nine percent of the MS patients had diabetes mellitus, based on the level of HbA1c, and 80% were hypertensive. Body mass index (BMI) among the MS patients ranged from 24 to 42 kg/m2, while waist circumference ranged from 85 to 140 cm.
Conventional 2D Doppler echocardiography identified LV diastolic dysfunction in 135 MS patients (90%) and in 30 (20%) controls, P<0.0001. The risk for LV diastolic dysfunction was 1.6 times higher in MS patients with hypertension (OR=2.25, 95% CI: 1.1 to 4.3, P=0.01). In the Strong Heart Study, MS was also found to be linked with LV systolic and diastolic dysfunction.27
Although LVEF was similar in the two groups (P=0.05), 2D-STE identified a subtle LV systolic dysfunction in 47 MS patients (46%) and in 12 controls (8%), P<0.0001. The risk for LV systolic dysfunction was 3 times higher in the hypertensive (P<0.0001) and 5.5 times higher in diabetic patients with MS (P<0.0001).
In multivariate analysis, diabetes mellitus and liver stiffness measurement were independently associated with both diastolic and systolic LV dysfunction (P<0.0001). The risk for LV diastolic dysfunction was 3.6 times higher in MS with severe steatosis (P<0.0001) and 8 times higher in patients with severe fibrosis (P<0.0001). The risk for LV systolic dysfunction was double in MS with severe steatosis (P<0.0001) and 1.7 times higher in MS with severe fibrosis (P<0.0001).
Even if some studies suggested that subjects with NAFLD were at risk for LV structural injuries and diastolic dysfunction,28–30 the association with hepatic steatosis and fibrosis was not proven due to insufficient use of ultrasonography or computed tomography. In our study the participants were carefully investigated, using conventional and 2D-STE for cardiac evaluation, and liver VCTE and CAP to identify and measure liver fibrosis and steatosis.
The present study also demonstrated for the first time that subtle LV systolic dysfunction, detected by SI and SRI, was significantly related to hepatic fibrosis and steatosis.
Limitations
With the presently existing equipment, SR measurement has a quite poor signal/noise ratio and is load sensitive. Also, S measurements are done alongside a single ultrasound scan line. The assessment of liver steatosis and fibrosis was done non-invasively in all patients, without performing a liver biopsy, which is the gold standard method.
Conclusion
Our study indicates that MS patients have a high prevalence of LV diastolic and systolic dysfunction. While diastolic heart dysfunction can be detected by conventional echocardiographic measurements, the assessment of subtle systolic dysfunction needs speckle tracking echocardiography. This finding recommends 2D STE as a routine echocardiographic examination in MS patients, as revealing and early treatment of cardiac disorders are vital issues for better outcomes in these subjects. Cardiac dysfunction in MS patients was significantly and independently associated with severe hepatic fibrosis and steatosis, detected by transient VCTE and CAP. Early assessing of both heart and liver disease in MS individuals is important, in order to initiate lifestyle changes and medical therapy, aimed to correct all cardiovascular risk factors, including abdominal obesity.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T, Katz R, Jenny NS, et al. Metabolic syndrome, inflammation, and incident heart failure in the elderly: the cardiovascular health study. Circ Heart Fail. 2008;1(4):242–248. doi: 10.1161/CIRCHEARTFAILURE.108.785485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong H, P H, Tan HW, et al. Impaired left ventricular systolic and diastolic function in patients with metabolic syndrome as assessed by strain and strain rate imaging. Diabetes Res Clin Pract. 2009;83(3):300–307. doi: 10.1016/j.diabres.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 4.Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28(2):183–193. doi: 10.1016/j.echo.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 5.Hwang JW, Kang SJ, Lim HS, et al. Impact of arterial stiffness on regional myocardial function assessed by speckle tracking echocardiography in patients with hypertension. J Cardiovasc Ultrasound. 2012;20(2):90–96. doi: 10.4250/jcu.2012.20.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi Z, Koenig A, Abdelatif D, et al. Peak epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 7.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49(4):608–612. doi: 10.1016/j.jhep.2008.06.018 [DOI] [PubMed] [Google Scholar]
- 8.Azzam H, Malnick S. Non-alcoholic fatty liver disease - the heart of the matter. World J Hepatol. 2015;7:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lautamaki R, Borra R, Iozzo P, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291(2):E282–E290. doi: 10.1152/ajpendo.00604.2005 [DOI] [PubMed] [Google Scholar]
- 10.Fotbolcu H, Yakar T, Duman D, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17:457–463. [PubMed] [Google Scholar]
- 11.Kim G, Jo K, Kim KJ, et al. Visceral adiposity is associated with altered myocardial glucose uptake measured by (18) FDG-PET in 346 subjects with normal glucose tolerance, prediabetes, and type 2 diabetes. Cardiovasc Diabetol. 2015;14(1):148. doi: 10.1186/s12933-015-0310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pionikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129–2200. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – A new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 14.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):1–98. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–271. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 16.Pellerin D, Sharma R, Elliott P, et al. Tissue doppler, strain, and strain rate imaging for the assessment of left and right systolic ventricular function. Heart. 2003;89(Suppl III):9–17. doi: 10.1136/heart.89.suppl_3.iii9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cottrell C, Kirkpatrick JN. Echocardiographic strain imaging and its use in the clinical setting. Expert Rev Cardiovasc Ther. 2010;8(1):93–102. doi: 10.1586/erc.09.165 [DOI] [PubMed] [Google Scholar]
- 18.EASL-ALEH. Clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. doi: 10.1016/j.jhep.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 19.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–1730. doi: 10.1053/j.gastro.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 20.Dietrich A, Bamber C, Berzigotti J, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Eur J Ultrasound. 2017;38:e16–e47. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 22.Balkau B, Valensi P, Eschwège E, Slama G. A review of the metabolic syndrome. Diabetes Metab. 2007;33(6):405–413. doi: 10.1016/j.diabet.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 23.Von Bibra H, St John Sutton M. Diastolic dysfunction in diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia. 2010;53(6):1033–1045. doi: 10.1007/s00125-010-1682-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo JM, Park TH, Lee DY, et al. Subclinical myocardial dysfunction in metabolic syndrome patients without hypertension. J Cardiovasc Ultrasound. 2011;19(3):134–139. doi: 10.4250/jcu.2011.19.3.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mavrea A, Gyalai KI, Citu I, et al. Long term prognosis and modes of death in heart failure patients with reduced versus preserved left ventricular left ventricular systolic function. Eur Sci J. 2013;3:246–253. [Google Scholar]
- 26.Sporea I, Mare R, Popescu A, et al. Screening for liver fibrosis and steatosis in a large cohort of patients with type 2 diabetes using vibration controlled transient elastography and controlled attenuation parameter in a single-center real-life experience. J Clin Med. 2020;9(4):1032. doi: 10.3390/jcm9041032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinali M, de Simone G, Roman MJ, et al. Cardiac markers of pre-clinical disease in adolescents with the metabolic syndrome: the strong heart study. J Am Coll Cardiol. 2008;52(11):932–938. doi: 10.1016/j.jacc.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(03):221–235. doi: 10.1055/s-0035-1562943 [DOI] [PubMed] [Google Scholar]
- 29.Ruberg FL, Chen Z, Hua N, et al. The relationship of ectopic lipid accumulation to cardiac and vascular function in obesity and metabolic syndrome. Obesity (Silver Spring). 2010;18(6):1116–1121. doi: 10.1038/oby.2009.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: A 1H-magnetic resonance spectroscopy study. Circulation. 2007;116(10):1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614 [DOI] [PubMed] [Google Scholar]