Abstract
Objective
The study aimed to investigate the effect of α2A-adrenergic receptor (ADRA2A) on cervical cancer and the potential mechanisms of ADRA2A on phosphatidylinositol 3′-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway in cervical cancer cells.
Methods
In our study, ADRA2A expression was evaluated by analyzing cervical cancer RNA sequencing dataset from the GEPIA. The prognostic values of ADRA2A were evaluated by Kaplan–Meier method using the Cancer Genome Atlas (TCGA) database data. In addition, the expression of ADRA2A in cervical cancer cell lines was detected by qRT-PCR and Western blot. Subsequently, the roles of ADRA2A on cell proliferation, apoptosis, migration, invasion and senescence in HeLa and SiHa cells were evaluated. Moreover, tumorigenesis in nude mice was used to investigate the role of ADRA2A in vivo. We also detected the expression changes of key factors in PI3K/Akt/mTOR pathway after overexpression and silencing of ADRA2A in HeLa and SiHa cells.
Results
ADRA2A expression was significantly downregulated in cervical cancer tissues and cell lines. The high expression of ADRA2A was significantly associated with a better prognosis in cervical cancer patients. ADRA2A overexpression significantly suppressed cell proliferation, migration and invasion, and promoted cell senescence and apoptosis in cervical cancer cells. On the contrary, silencing ADRA2A dramatically facilitated cell proliferation, migration and invasion, and inhibited cell senescence and apoptosis in cervical cancer cells. The expressions of p-PI3K, p-AKT and p-mTOR in cervical cancer cells were notably decreased by ADRA2A overexpression and increased by silencing ADRA2A. In addition, we also confirmed that ADRA2A overexpression could suppress the xenograft tumor growth in vivo.
Conclusion
Our study demonstrated that ADRA2A could suppress cell proliferation, migration and invasion, as well as promote cell senescence and apoptosis through inhibiting PI3K/Akt/mTOR pathway in cervical cancer.
Keywords: cervical cancer, ADRA2A, proliferation, metastasis, senescence, PI3K/Akt/mTOR pathway
Introduction
Cervical cancer is one of the most common malignant tumors in the female reproductive system and ranks the second leading cause of cancer-related death of women.1,2 Despite great achievements in the diagnostic and therapeutic strategies, the mortality of cervical cancer remains high, accounting for about 50%.3,4 Therefore, it is urgent to explore novel prognostic markers and therapeutic targets for the treatment of cervical cancer.
The adrenoceptors consist of α1-adrenoceptors (α1A, α1B, α1D), α2-adrenoceptors (α2A, α2B, α2C) and β-adrenoceptors (β1, β2, β3).5,6 Previous studies have reported that different cancer cell lines can express different adrenoceptor subtypes affecting the biological behavior of tumor cells.7 α2-adrenoceptors are involved in different physiological functions in the human body and have been identified as potential regulators of multiple tumor cell lines.8,9 In recent years, α2A-adrenergic receptor (ADRA2A) is considered to have a positive effect on breast cancer10 and hepatocellular carcinoma.11 However, it is not clear whether ADRA2A has a regulatory effect on cervical cancer.
Some studies have shown that the phosphatidylinositol 3′-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway plays a central role in cell growth, survival, apoptosis, and migration.12–14 Moreover, increasing studies have indicated that PI3K/Akt/mTOR pathway plays an important role in various cancers, including cervical cancer.15,16 Moreover, ADRA2A is reported to be associated with PI3K/AKT/mTOR signaling pathway in many diseases. For example, dexmedetomidine attenuates acute lung injury caused by renal ischemia-reperfusion via ADRA2A/PI3K/Akt pathway.17 Jackson et al18 have suggested that the PI3K pathway contributes importantly to the interaction between α2-adrenoceptors and angiotensin II on renal vascular resistance. However, whether the PI3K/Akt/mTOR pathway is involved in the effect of ADRA2A on cervical cancer remains unclear.
In this study, we explored the role of ADRA2A on cervical cancer progression and its related molecular mechanism. Our data demonstrated that ADRA2A could suppress cell proliferation, migration and invasion, as well as promote cell senescence and apoptosis through inhibiting PI3K/Akt/mTOR pathway in cervical cancer. The results of the present study may provide a prospective therapeutic target for cervical cancer.
Materials and Methods
Bioinformatics Analysis
The GEPIA is an online dataset pooling from the Cancer Genome Atlas (TCGA) projects and the Genotype-Tissue Expression (GTEx) data for survival analysis and gene correlation assessment. ADRA2A expression data based on RNA-Seq were collected from TCGA database for 306 patients with cervical cancer. We performed survival analysis available in R package “survival” using the Kaplan–Meier curve method. To find critical pathways closely related to the initiation and progression of cervical cancer, the gene set enrichment analysis (GSEA) was conducted using Hallmark gene sets.
Cell Culture and Transfection
Human cervical cancer cell lines (HeLa, Caski, C-33A and SiHa) and immortalized epithelial cells of human ectocervix Ect1/E6E7 were supplied by American Type Culture Collection (ATCC, Manassas, USA). All the cells were grown in Dulbecco’s Modified Eagle’s medium (DMEM, Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, USA) and 1% antibiotics (HyClone, USA) at 37°C in an incubator filled with 5% CO2 atmosphere.
The pcDNA3.1-ADRA2A (pcDNA-ADRA2A group), the empty pcDNA3.1 vector (pcDNA3.1-NC group), ADRA2A siRNA (si-ADRA2A group) and ADRA2A siRNA negative control (si-NC group) were synthesized by GenePharm (Shanghai, China) and then, respectively, transfected to HeLa and SiHa cells by using Lipofectamine 3000 (Invitrogen, USA) following to the manufacturer’s protocols. Moreover, HeLa and SiHa cells in pcDNA-ADRA2A and si-ADRA2A groups were cultured in DMEM supplement with 10 μM LY294002 (a selective PI3K inhibitor, #9901, Cell signaling, USA) for 48 h and then named pcDNA-ADRA2A + LY294002 and si-ADRA2A + LY294002 groups cells.
Colony Formation Assay
The clones ability of HeLa and SiHa cells was detected by colony formation assay. Briefly, the transfected were planted into 6-well plates. After 14 days’ incubation, the cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet (Sigma, USA). Finally, the stained colonies were counted under a light microscopy.
5-Ethynyl-20-Deoxyuridine (EdU) Assay
The proliferation ability of HeLa and SiHa cells was measured using a Cell-LightTM EdU assay kit (RiboBio, Guangzhou, China). Simply, the transfected HeLa and SiHa cells were seeded into each well of the 96-well plate and then cultured with 50 μm EdU for 2 h. Afterwards, we added 4′,6-diamidino-2phenylindole (DAPI, Sigma, USA) to each well for staining the cell nuclei of HeLa and SiHa cells. The EdU-positive cells were observed by using a fluorescence microscope.
Flow Cytometry Assay
Annexin-V-fluorescein isothiocyanate (FITC) cell apoptosis assay kit (Biovision, USA) was used to detect apoptotic cells. In brief, the transfected HeLa and SiHa cells were resuspended with binding buffer, incubated with Annexin-V-FITC for 15min and with propidium iodide (PI) for another 15 min. After that, the apoptotic cells were examined with a flow cytometer (BD Biosciences, USA).
Transwell Assay
The invasion and migration abilities of HeLa and SiHa cells were determined by using the transwell boyden chamber (Corning, USA). For invasion assay, each well of the transwell chamber was pre-coated with Matrigel (Millipore, USA). Simply, the transfected HeLa and SiHa cells were harvested and resuspended in serum-free medium. Subsequently, cell suspension was added into the upper chamber, and the medium (500 μL) was added into the lower chamber. After incubation for 24 h, the migratory or invasive cells on the lower surface of the membrane were fixed with 4% paraformaldehyde at room temperature and stained with 0.5% crystal violet at room temperature. The numbers of migratory or invasive cells were counted in 10 randomly selected fields under an inverted microscope.
Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
Senescence β-Galactosidase staining kit (Beyotime, Jiangsu, China) was used to detect the cell senescence of HeLa and SiHa cells. In brief, the transfected HeLa and SiHa cells were fixed with 4% paraformaldehyde for 10 min at room temperature and stained with β-gal staining solution overnight at 37°C. In the end, the senescent cells were observed and counted under a light microscope.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted with the use of TRIZOL reagent (Invitrogen, USA). The synthesis of cDNA was performed with the Revert aid first-strand cDNA synthesis kit (Thermo Fisher, USA). The qRT-PCR was carried out using SYBR Green One-Step RT-PCR Master Mixes (Thermo Fisher) on a Bio-Rad CFX96 Touch qPCR system (Bio-Rad, USA). Primers were supplied by GenePharma (Shanghai, China). The sequences were as follows: ADRA2A (sense): 5′-AGCTCGCTGAACCCTGTTAT-3′ (anti-sense): 5′-CTGACCAGGGTCTGTAAGCA-3′; GAPDH (sense): 5′-GCACCGTCAAGGCTGAGAAC-3′ (anti-sense): 5′-GCCTTCTCCATGGTGGTGAA-3′. The relative gene expression levels were calculated using the 2−ΔΔCt method.
Western Blot Analysis
For protein preparation, cells were homogenized with cell lysis buffer (Cell Signaling, USA), and then the protein concentrations were measured by using the bicinchoninic acid assay (BCA) kit (Thermo, USA). Protein samples were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Next, the membranes were incubated in the primary antibodies (ADRA2A, 1:500, ab85570; Bax, 1:1000, ab77566; Bcl-2, 1:1000, ab32124; MMP-2, 1:1000, ab97779; MMP-9, 1:1000, ab38898; p16, 1:1000, ab220800; p21, 1:1000, ab109199; p53, 1:1000, ab131442, Abcam, UK; phospho-PI3K, 1:300, #9655; PI3K, 1:1000, #4257; phospho-AKT, 1:500, #13,038; AKT, 1:1000, #4685; phospho-mTOR, 1:500, #2971; mTOR, 1:1000, #2983, GAPDH, 1:1000, #2118, Cell signaling, USA) overnight at 4°C. Subsequently, the membranes were incubated with the corresponding horseradish peroxidase-conjugated secondary antibodies (Proteintech, USA) for 2 h at room temperature. The protein bands were visualized with ECL system (Thermo, USA) and then recorded by the LAS 3000 Image Reader (Fujifilm, Tokyo, Japan).
Mouse Xenograft Models
Five-week-old nude female mice weighing 18–20 g were obtained from the Laboratory Animal Centre of Chinese Academy of Sciences. The mice were kept on a 12 h light-dark cycle with access to food and water ad libitum. The mice were divided into two groups (n =6), and then the SiHa cells (1 × 107 cells/mouse) transfected with pcDNA3.1-NC and pcDNA-ADRA2A were injected subcutaneously into the right flank of mice. The tumor volume was determined weekly by the formula: tumor volume = length × width2 × 1/2. Four weeks later, the mice were sacrificed, and then the xenograft tumors were dissected out and weighed. The animal experiments were performed in compliance with the guidelines of the Animal Use Committee of our hospital.
Statistical Analysis
The data were presented as mean ± SEM. All statistical analysis was performed using GraphPad Prism 8.0 software. Statistical analysis was performed using Student’s t-test or one-way ANOVA test. P < 0.05 expresses that the differences were statistically significant.
Results
ADRA2A Expression is Low in Cervical Cancer and is Associated with the Progression of Cervical Cancer
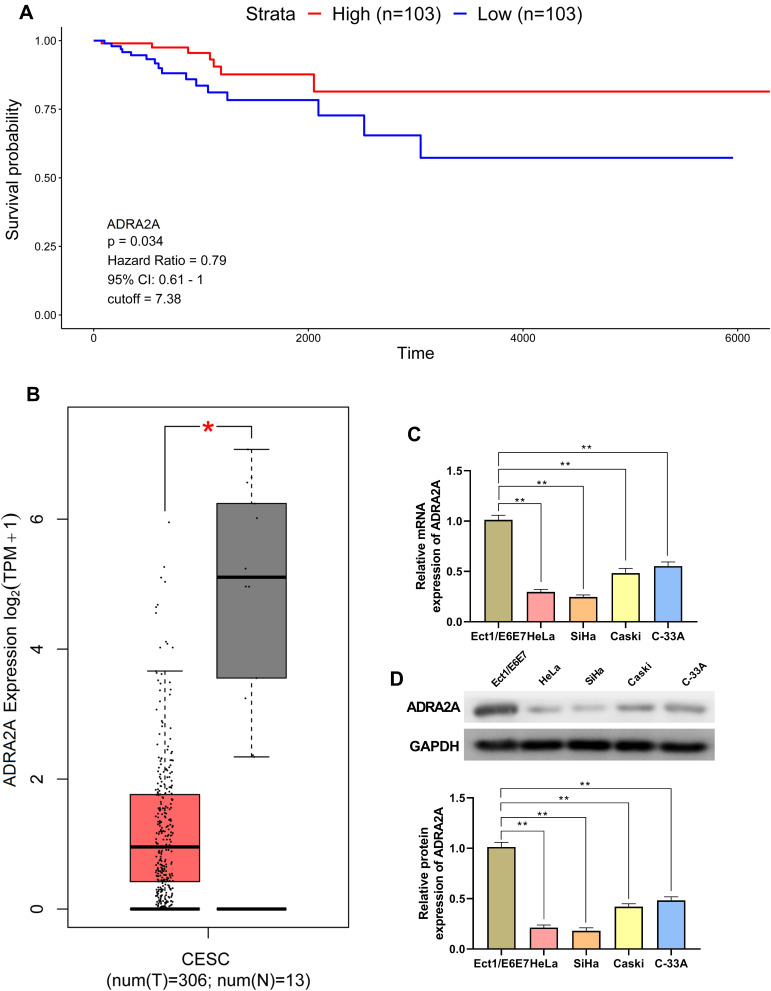
The analysis of TCGA dataset on cervical cancer indicated that patients with high ADRA2A expression had longer survival time than patients with low ADRA2A expression (Figure 1A). Moreover, GEPIA suggested that the expression of ADRA2A was significantly decreased in cervical cancer tissues compared with normal tissue (Figure 1B). Similarly, we also demonstrated that the mRNA and protein expressions of ADRA2A were markedly decreased in Caski cells (P < 0.01) and C-33A cells (P < 0.01), especially in HeLa cells (P < 0.01) and SiHa cells (P < 0.01) relative to Ect1/E6E7 cells (Figure 1C and D). The data indicated that ADRA2A was lowly expressed in cervical cancer and was a good prognostic factor for patients with cervical cancer.
Figure 1.
ADRA2A expression is low in cervical cancer and is associated with the progression of cervical cancer. (A) Kaplan–Meier curve of the cervical cancer clinical outcome for ADRA2A from TCGA. (B) The expression of ADRA2A in cervical cancer tissues from GEPIA. (C) The mRNA expression of ADRA2A in cervical cancer cells was detected by qRT-PCR. (D) The protein expression of ADRA2A in cervical cancer cells was determined by Western blot. *P < 0.05, vs normal tissues group (B); **P < 0.01, vs Ect1/E6E7 cells group (C and D).
ADRA2A Inhibits the Proliferation Ability of HeLa and SiHa Cells
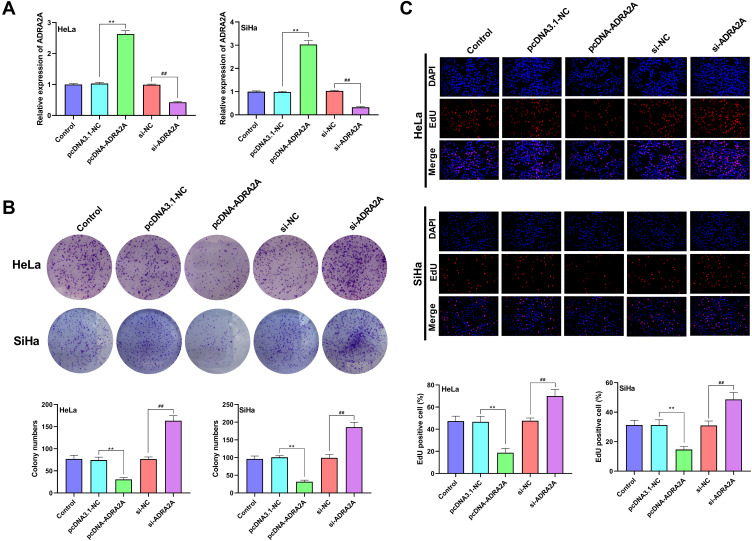
As Figure 2A showed, ADRA2A expression of HeLa and SiHa cells was significantly increased in pcDNA-ADRA2A group compared with pcDNA3.1-NC group (P < 0.01). When compared with si-NC group, ADRA2A expression was significantly decreased in si-ADRA2A group (P < 0.01), suggesting that the transfection was successful. The cell clones and cell proliferation of HeLa and SiHa cells were detected by colony formation assay (Figure 2B) and EdU assay (Figure 2C). The results showed that ADRA2A overexpression significantly reduced clones and proliferation of HeLa and SiHa cells (P < 0.01). On the contrary, silencing ADRA2A markedly increased cell clones and cell proliferation (P < 0.01). All the results indicated that ADRA2A could inhibit the proliferation ability of HeLa and SiHa cells.
Figure 2.
ADRA2A inhibited the proliferation ability of HeLa and SiHa cells. (A) The expression of ADRA2A in transfected HeLa and SiHa cells was detected by qRT-PCR. (B) The colony numbers of HeLa and SiHa cells were analyzed by colony formation assay. (C) The proliferation of HeLa and SiHa cells was measured by EdU assay. **P < 0.01, vs pcDNA3.1-NC group; ##P < 0.01, vs si-NC group.
ADRA2A Promotes the Apoptosis Ability of HeLa and SiHa Cells
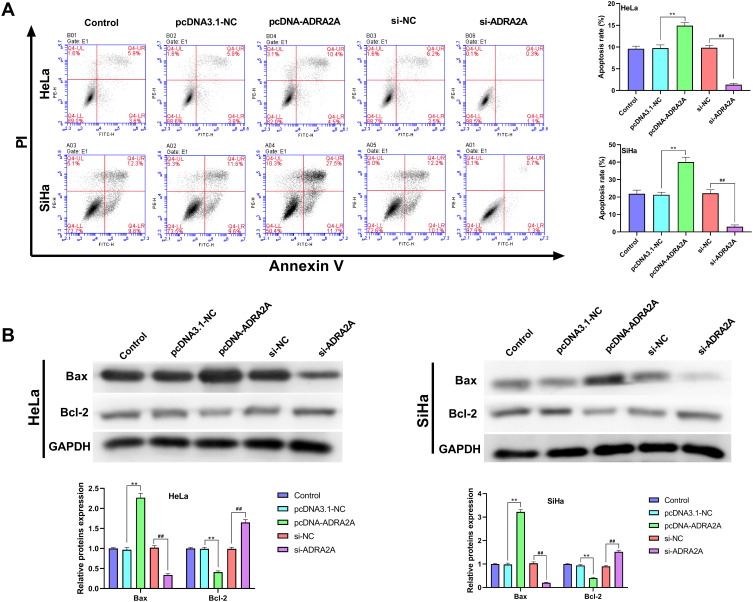
The results of flow cytometry showed that the apoptosis of HeLa and SiHa cells was significantly increased by ADRA2A overexpression (P < 0.01) and decreased by silencing ADRA2A (P < 0.01) (Figure 3A). In addition, the results of Western blot showed that ADRA2A overexpression significantly promoted the expression of Bax in HeLa and SiHa cells (P < 0.01), and inhibited the expression of Bcl-2 (P < 0.01) (Figure 3B). On the contrary, silencing ADRA2A markedly suppressed Bax expression (P < 0.01) and facilitated Bcl-2 expression in HeLa and SiHa cells (P < 0.01) (Figure 3B). These results suggested that ADRA2A could promote the apoptosis ability of HeLa and SiHa cells.
Figure 3.
ADRA2A promoted the apoptosis ability of HeLa and SiHa cells. (A) The apoptosis of HeLa and SiHa cells was tested by flow cytometry. (B) The expressions of Bax and Bcl-2 in HeLa and SiHa cells were determined by Western blot. **P < 0.01, vs pcDNA3.1-NC group; ##P < 0.01, vs si-NC group.
ADRA2A Inhibits the Ability of Migration and Invasion in HeLa and SiHa Cells
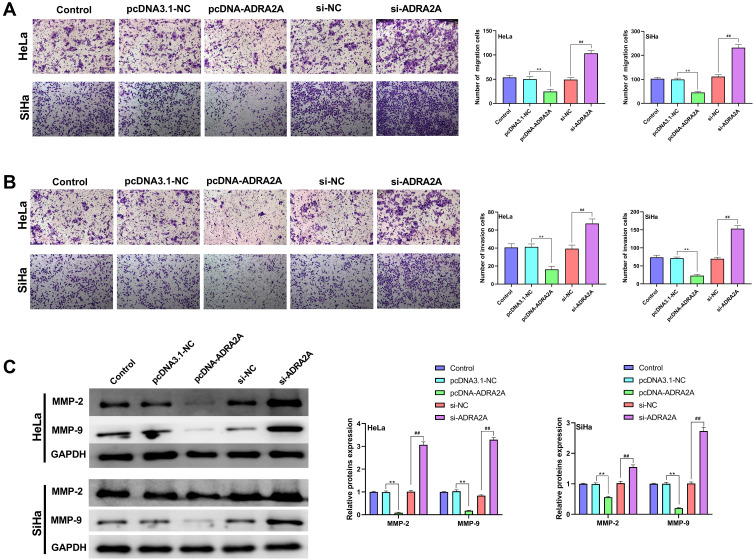
The results of transwell assay revealed that ADRA2A overexpression dramatically decreased the migration and invasion in HeLa and SiHa cells (P < 0.01), while silencing ADRA2A notably increased cell migration and invasion (P < 0.01) (Figure 4A and B). In addition, to further investigate the effect of ADRA2A on the migration and invasion, Western blot was performed. Figure 4C results showed that the expressions of MMP-2 and MMP-9 in HeLa and SiHa cells were significantly inhibited by ADRA2A overexpression (P < 0.01) and promoted by silencing ADRA2A (P < 0.01). Altogether, these results demonstrated that ADRA2A could inhibit the ability of migration and invasion in HeLa and SiHa cells.
Figure 4.
ADRA2A inhibited the abilities of migration and invasion in HeLa and SiHa cells. (A) The migration of HeLa and SiHa cells was measured by transwell assay. (B) The invasion of HeLa and SiHa cells was measured by transwell assay. (C) The expressions of MMP-2 and MMP-9 in HeLa and SiHa cells were determined by Western blot. **P < 0.01, vs pcDNA3.1-NC group; ##P < 0.01, vs si-NC group.
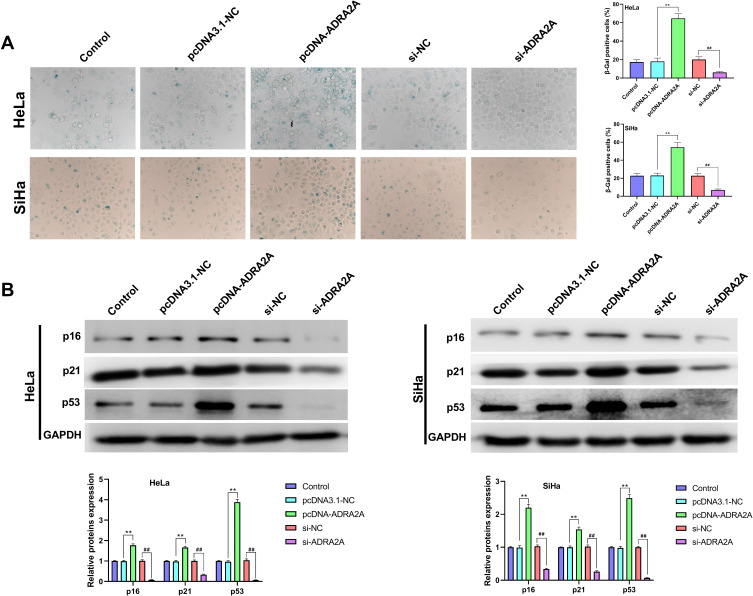
ADRA2A Promotes the Senescence of HeLa and SiHa Cells
As shown in Figure 5A, ADRA2A overexpression significantly promoted the senescence of HeLa and SiHa cells (P < 0.01), and silencing ADRA2A notably inhibited cell senescence (P < 0.01). Additionally, the results of Western blot also confirmed that the expressions of p16, p21 and p53 in HeLa and SiHa cells were dramatically elevated by ADRA2A overexpression (P < 0.01) and reduced by silencing ADRA2A (P < 0.01) (Figure 5B). All the results indicated that ADRA2A could promote the senescence of HeLa and SiHa cells.
Figure 5.
ADRA2A promoted the senescence of HeLa and SiHa cells. (A) The senescence of HeLa and SiHa cells were tested by SA-β-gal staining. (B) The expressions of p16, p21 and p53 in HeLa and SiHa cells were measured by Western blot. **P < 0.01, vs pcDNA3.1-NC group; ##P < 0.01, vs si-NC group.
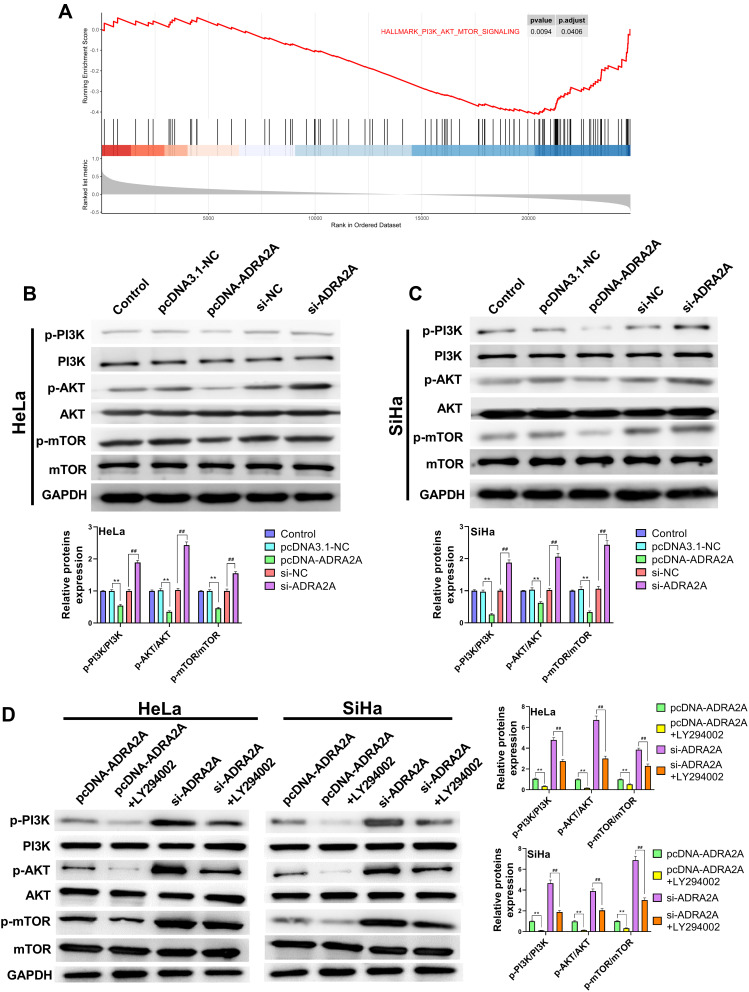
ADRA2A Blocks PI3K/AKT/mTOR Pathway in HeLa and SiHa Cells
The GSEA showed that the expression of ADRA2A was associated with the “PI3K/AKT/mTOR signaling pathway” (Figure 6A). To further confirm this hypothesis, we detected the related proteins of PI3K/AKT/mTOR pathway by Western blot. And the results revealed that the expressions of p-PI3K, p-AKT and p-mTOR in HeLa and SiHa cells were significantly reduced by ADRA2A overexpression (P < 0.01) and dramatically elevated by silencing ADRA2A (P < 0.01) (Figure 6B and C), suggesting that ADRA2A could regulate PI3K/AKT/mTOR signaling pathway. Then, to further verify this hypothesis, LY294002 was performed in the subsequent experiment. As Figure 6D showed, the treatment of LY294002 could significantly enhance the inhibitory effect of ADRA2A overexpression on the expressions of p-PI3K, p-AKT and p-mTOR in HeLa and SiHa cells (P < 0.01). On the contrary, the treatment of LY294002 markedly reversed the promoting effect of ADRA2A silencing on the expressions of p-PI3K, p-AKT and p-mTOR (P < 0.01) (Figure 6D). All these results demonstrated that ADRA2A could block PI3K/AKT/mTOR signaling pathway in HeLa and SiHa cells.
Figure 6.
ADRA2A blocked PI3K/AKT/mTOR pathway in HeLa and SiHa cells. (A) GSEA suggested that ADRA2A expression was correlated with PI3K/AKT/mTOR pathway. (B) The expressions of p-PI3K, PI3K, p-AKT, AKT, p-mTOR and mTOR in HeLa cells were measured by Western blot. (C) The expressions of p-PI3K, PI3K, p-AKT, AKT, p-mTOR and mTOR in C-33A cells were measured by Western blot. (D) After treatment of LY294002, the expressions of p-PI3K, PI3K, p-AKT, AKT, p-mTOR and mTOR in HeLa and C-33A cells were measured by Western blot. **P < 0.01, vs pcDNA3.1-NC group; ##P < 0.01, vs si-NC group (B and C); **P < 0.01, vs pcDNA-ADRA2A group, ##P < 0.01, vs si-ADRA2A group (D).
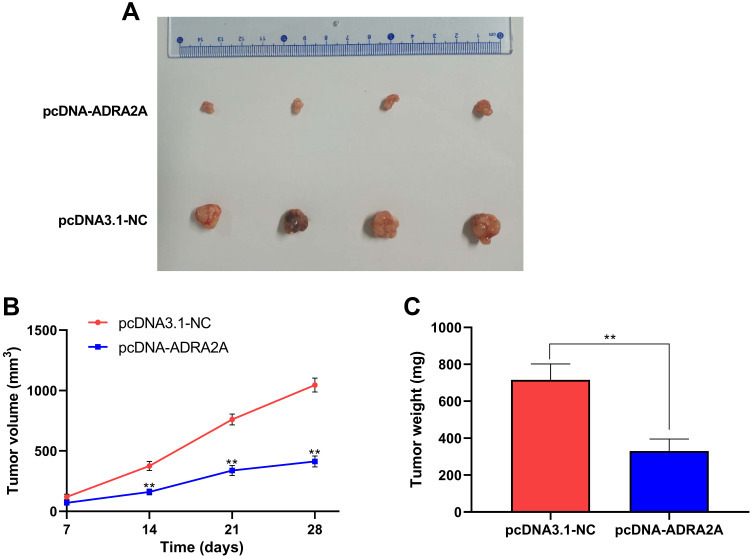
ADRA2A Inhibits Xenograft Tumor Growth
To confirm the function of ADRA2A on cervical cancer cell growth in vivo, the xenograft assay was performed (Figure 7A). As Figure 7B showed, tumor volume was significantly reduced in pcDNA-ADRA2A group at 14 days (P < 0.01), 21 days (P < 0.01) and 28 days (P < 0.01) post-injection when compared with pcDNA3.1-NC group. Moreover, tumor weight was also notably decreased at 28 days post-injection in pcDNA-ADRA2A group relative to pcDNA3.1-NC group (P < 0.01) (Figure 7C). Altogether, these results demonstrated that ADRA2A overexpression could inhibit tumor growth in vivo.
Figure 7.
ADRA2A inhibited xenograft tumor growth. (A) Representative images of xenograft tumors. (B) The xenograft tumor volume in pcDNA3.1-NC and pcDNA-ADRA2A groups. (C) The xenograft tumor weight in pcDNA3.1-NC and pcDNA-ADRA2A groups. **P < 0.01, vs pcDNA3.1-NC group.
Discussion
Despite the morbidity and mortality of cervical cancer have declined over the past thirty years, the 5-year survival rate of advanced patients is still low.19,20 Thus, there is an urgent need to find a new molecular approach to better understand this disease and identify a novel therapeutic target. In our study, we demonstrated that ADRA2A could suppress cell proliferation, migration and invasion, and also promote cell senescence and apoptosis through inhibiting PI3K/Akt/mTOR pathway in cervical cancer.
More and more studies have indicated that ADRA2A can play an important role in central nervous system, cardiovascular system, platelet aggregation, blood pressure, insulin secretion and lipolysis.10 In addition to these functions, ADRA2A is thought to be involved in the progression of multiple cancers.10,11 Previous studies have confirmed that ADRA2A is abnormally expressed in human colon carcinoma cell line HT29.21 A study by Yoo et al22 has suggested that KG-135 could repress the progression of prostate cancer through inhibiting cyclin and regulating the expression of ADRA2A and TNFRSF25. In our study, we confirmed that ADRA2A expression was downregulated in cervical cancer cells. Moreover, the analysis of GEPIA indicated that ADRA2A expression was significantly decreased in cervical cancer tissues and the TCGA analysis showed that the high expression of ADRA2A was significantly associated with a better prognosis in cervical cancer patients, suggesting that ADRA2A might be a tumor-suppressed gene in cervical cancer. To confirm this hypothesis, we detected the effect of ADRA2A on biological behaviors of cervical cancer cells and found that ADRA2A significantly suppressed cell proliferation, migration and invasion, as well as promoted cell senescence and apoptosis in HeLa and SiHa cells. Then, we further investigate the mechanism of ADRA2A on cervical cancer cell apoptosis, migration, invasion and senescence. Increasing evidence show that cancer cells may acquire resistance to apoptosis via the upregulation of antiapoptotic proteins such as Bcl-2 or via the downregulation of proapoptotic proteins such as Bax.23 Our data demonstrated that ADRA2A significantly increased Bax expression and decreased Bcl-2 expression in cervical cancer cells, which further confirmed that ADRA2A could facilitate cell apoptosis in cervical cancer. MMP-2 and MMP-9 degrade extracellular matrix as a prerequisite for cellular invasion and have been reported to play an important role in cancer metastasis.24–26 In this study, we found that ADRA2A could inhibit the expressions of MMP-2 and MMP-9, thus repressing the migration and invasion of cervical cancer cells. At present, cell senescence is considered as the third mechanism of the intracellular cancer prevention and serves as a potent barrier to malignant transformation.27,28 More and more researches have demonstrated that cellular senescence is associated with the activation of p53-p21KIP1/CIP1 and p16INK4A tumor suppressor pathways.29 Our results revealed that ADRA2A notably increased the expressions of p16, p21 and p53, further suggesting that ADRA2A could promote cervical cancer cell senescence. In addition, we also confirmed that ADRA2A could suppress the xenograft tumor growth in vivo.
Previous researches have shown that PI3K/Akt/mTOR pathway affects a variety of biological processes, such as cell survival, proliferation, invasion, autophagy, apoptosis, senescence and cell cycle, and is closely associated with many types of malignant tumors.12,30–32 Moreover, the activated PI3K/AKT/mTOR pathway frequently occurs in metastatic or recurrent cervical cancer, suggesting that it may be a potential therapeutic target in cervical cancer treatment.33,34 For example, ARHGAP17 could inhibit tumor progression through upregulating P21 and P27 expression and inhibiting PI3K/AKT pathway in cervical cancer.35 BMX/Etk could promote cell proliferation of cervical cancer cells via the regulation of PI3K/AKT/mTOR signal transduction pathway.36 Che et al37 have suggested that TRIP4 can facilitate tumor growth and metastasis and modulate radiosensitivity of cervical cancer via activating PI3K/AKT pathway. Li et al38 have indicated that fibulin-3 could promote cervical cancer cell growth and metastasis by activating PI3K/AKT/mTOR pathway. In addition, the GSEA demonstrated that the inhibitory role of ADRA2A in cervical cancer was closely related to the PI3K/AKT/mTOR pathway. To further confirm this hypothesis, we evaluated the function of ADRA2A on the PI3K/Akt/mTOR pathway-related proteins p-PI3K, PI3K, p-AKT, AKT, p-mTOR and mTOR, and found that ADRA2A could inhibit the activation of PI3K/Akt/mTOR pathway in cervical cancer cells, which was consistent with previous researches.
As a conclusion, in this research, we found that ADRA2A was lowly expressed in both cervical cancer tissues and cells. In addition, we also demonstrated that ADRA2A could suppress cell proliferation, migration and invasion, and also promote cell senescence and apoptosis through inhibiting PI3K/Akt/mTOR pathway in cervical cancer. These findings suggested that ADRA2A may be used as a potential therapeutic strategy for cervical cancer in future.
Funding Statement
There is no funding to report.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
Weina Wang and Xin Guo are co-first authors. The authors declare that they have no conflicts of interest for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Feng C, Dong J, Chang W, Cui M, Xu T. The progress of methylation regulation in gene expression of cervical cancer. Int J Genomics. 2018;2018:8260652. doi: 10.1155/2018/8260652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KB, Shim SH, Lee JM. Comparison between adjuvant chemotherapy and adjuvant radiotherapy/chemoradiotherapy after radical surgery in patients with cervical cancer: a meta-analysis. J Gynecol Oncol. 2018;29(4):e62. doi: 10.3802/jgo.2018.29.e62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vázquez-Sánchez AY, Hinojosa LM, Parraguirre-Martínez S, et al. Expression of K(ATP) channels in human cervical cancer: potential tools for diagnosis and therapy. Oncol Lett. 2018;15(5):6302–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hein L, Kobilka BK. Adrenergic receptors from molecular structure to in vivo function. Trends Cardiovasc Med. 1997;7(5):137–145. doi: 10.1016/S1050-1738(97)00034-0 [DOI] [PubMed] [Google Scholar]
- 6.Civantos Calzada B, Aleixandre de Artiñano A. Alpha-adrenoceptor subtypes. Pharmacol Res. 2001;44(3):195–208. [DOI] [PubMed] [Google Scholar]
- 7.Shen SG, Zhang D, Hu HT, Li JH, Wang Z, Ma QY. Effects of alpha-adrenoreceptor antagonists on apoptosis and proliferation of pancreatic cancer cells in vitro. World J Gastroenterol. 2008;14(15):2358–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruzzone A, Piñero CP, Castillo LF, et al. Alpha2-adrenoceptor action on cell proliferation and mammary tumour growth in mice. Br J Pharmacol. 2008;155(4):494–504. doi: 10.1038/bjp.2008.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruzzone A, Piñero CP, Rojas P, et al. α(2)-Adrenoceptors enhance cell proliferation and mammary tumor growth acting through both the stroma and the tumor cells. Curr Cancer Drug Targets. 2011;11(6):763–774. doi: 10.2174/156800911796191051 [DOI] [PubMed] [Google Scholar]
- 10.Kaabi B, Belaaloui G, Benbrahim W, et al. ADRA2A germline gene polymorphism is associated to the severity, but not to the risk, of breast cancer. Pathol Oncol Res. 2016;22(2):357–365. doi: 10.1007/s12253-015-0010-0 [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, French B, Tillman B, French S. Different roles of FAT10, FOXO1, and ADRA2A in hepatocellular carcinoma tumorigenesis in patients with alcoholic steatohepatitis (ASH) vs non-alcoholic steatohepatitis (NASH). Exp Mol Pathol. 2018;105(1):144–149. doi: 10.1016/j.yexmp.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han C, Xing G, Zhang M, et al. Wogonoside inhibits cell growth and induces mitochondrial-mediated autophagy-related apoptosis in human colon cancer cells through the PI3K/AKT/mTOR/p70S6K signaling pathway. Oncol Lett. 2018;15(4):4463–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara F, Lessi F, Vitagliano O, Birkenghi E, Rossi G. Current Therapeutic Results and Treatment Options for Older Patients with Relapsed Acute Myeloid Leukemia. Cancers. 2019;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke M, Mo L, Li W, Zhang X, Li F, Yu H. Ubiquitin ligase SMURF1 functions as a prognostic marker and promotes growth and metastasis of clear cell renal cell carcinoma. FEBS Open Bio. 2017;7(4):577–586. doi: 10.1002/2211-5463.12204 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Zhang W, Zhou Q, Wei Y, et al. The exosome-mediated PI3k/Akt/mTOR signaling pathway in cervical cancer. Int J Clin Exp Pathol. 2019;12(7):2474–2484. [PMC free article] [PubMed] [Google Scholar]
- 16.Lu R, Yang Z, Xu G, Yu S. miR-338 modulates proliferation and autophagy by PI3K/AKT/mTOR signaling pathway in cervical cancer. Biomed Pharmacother. 2018;105:633–644. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Chen Q, He X, et al. Dexmedetomidine attenuates lung apoptosis induced by renal ischemia-reperfusion injury through α(2)AR/PI3K/Akt pathway. J Transl Med. 2018;16(1):78. doi: 10.1186/s12967-018-1455-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson EK, Gao L, Zhu C. Mechanism of the vascular angiotensin II/alpha2-adrenoceptor interaction. J Pharmacol Exp Ther. 2005;314(3):1109–1116. [DOI] [PubMed] [Google Scholar]
- 19.Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ. 2001;164(7):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- 20.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–265. doi: 10.1136/jcp.55.4.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devedjian JC, Schaak S, Gamet L, Denis-Pouxviel C, Paris H. Regulation of alpha 2A-adrenergic receptor expression in the human colon carcinoma cell line HT29: SCFA-induced enterocytic differentiation results in an inhibition of alpha 2C10 gene transcription. Proc Assoc Am Physicians. 1996;108(4):334–344. [PubMed] [Google Scholar]
- 22.Yoo JH, Kwon HC, Kim YJ, Park JH, Yang HO. KG-135, enriched with selected ginsenosides, inhibits the proliferation of human prostate cancer cells in culture and inhibits xenograft growth in athymic mice. Cancer Lett. 2010;289(1):99–110. [DOI] [PubMed] [Google Scholar]
- 23.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Bloomston M, Zervos EE, Rosemurgy AS 2nd. Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002;9(7):668–674. doi: 10.1007/BF02574483 [DOI] [PubMed] [Google Scholar]
- 25.Xu Q, Li P, Chen X, et al. miR-221/222 induces pancreatic cancer progression through the regulation of matrix metalloproteinases. Oncotarget. 2015;6(16):14153–14164. doi: 10.18632/oncotarget.3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb AH, Gao BT, Goldsmith ZK, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer. 2017;17(1):434. doi: 10.1186/s12885-017-3418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000–1011. doi: 10.1016/j.cell.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornsby PJ. Senescence as an anticancer mechanism. J Clin Oncol. 2007;25(14):1852–1857. doi: 10.1200/JCO.2006.10.3101 [DOI] [PubMed] [Google Scholar]
- 29.Adams PD. Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell. 2009;36(1):2–14. doi: 10.1016/j.molcel.2009.09.021 [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Fisher RC, Signs S, et al. Inhibition of PI3K/Akt/mTOR signaling in PI3KR2-overexpressing colon cancer stem cells reduces tumor growth due to apoptosis. Oncotarget. 2017;8(31):50476–50488. doi: 10.18632/oncotarget.9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy D, Ghosh P, Kumavath R. Strophanthidin Attenuates MAPK, PI3K/AKT/mTOR, and Wnt/β-catenin signaling pathways in human cancers. Front Oncol. 2019;9:1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murugan AK. Special issue: PI3K/Akt signaling in human cancer. Semin Cancer Biol. 2019;59:1–2. doi: 10.1016/j.semcancer.2019.10.022 [DOI] [PubMed] [Google Scholar]
- 33.Li YJ, Wang Y, Wang YY. MicroRNA-99b suppresses human cervical cancer cell activity by inhibiting the PI3K/AKT/mTOR signaling pathway. J Cell Physiol. 2019;234(6):9577–9591. doi: 10.1002/jcp.27645 [DOI] [PubMed] [Google Scholar]
- 34.Tian Q, Wang L, Sun X, Zeng F, Pan Q, Xue M. Scopoletin exerts anticancer effects on human cervical cancer cell lines by triggering apoptosis, cell cycle arrest, inhibition of cell invasion and PI3K/AKT signalling pathway. J BUON. 2019;24(3):997–1002. [PubMed] [Google Scholar]
- 35.Guo Q, Xiong Y, Song Y, Hua K, Gao S. ARHGAP17 suppresses tumor progression and up-regulates P21 and P27 expression via inhibiting PI3K/AKT signaling pathway in cervical cancer. Gene. 2019;692:9–16. doi: 10.1016/j.gene.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Cui N, Zheng PS, Yang WT. BMX/Etk promotes cell proliferation and tumorigenicity of cervical cancer cells through PI3K/AKT/mTOR and STAT3 pathways. Oncotarget. 2017;8(30):49238–49252. doi: 10.18632/oncotarget.17493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Che Y, Li Y, Zheng F, et al. TRIP4 promotes tumor growth and metastasis and regulates radiosensitivity of cervical cancer by activating MAPK, PI3K/AKT, and hTERT signaling. Cancer Lett. 2019;452:1–13. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Qi C, Liu X, Li C, Chen J, Shi M. Fibulin-3 knockdown inhibits cervical cancer cell growth and metastasis in vitro and in vivo. Sci Rep. 2018;8(1):10594. doi: 10.1038/s41598-018-28906-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]