Abstract
Background
COVID-19 has affected care home residents internationally, but detailed information on outbreaks is scarce. We aimed to describe the evolution of outbreaks of COVID-19 in all care homes in one large health region in Scotland.
Methods
We did a population analysis of testing, cases, and deaths in care homes in the National Health Service (NHS) Lothian health region of the UK. We obtained data for COVID-19 testing (PCR testing of nasopharyngeal swabs for severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) and deaths (COVID-19-related and non-COVID-19-related), and we analysed data by several variables including type of care home, number of beds, and locality. Outcome measures were timing of outbreaks, number of confirmed cases of COVID-19 in care home residents, care home characteristics associated with the presence of an outbreak, and deaths of residents in both care homes and hospitals. We calculated excess deaths (both COVID-19-related and non-COVID-19-related), which we defined as the sum of deaths over and above the historical average in the same period over the past 5 years.
Findings
Between March 10 and Aug 2, 2020, residents at 189 care homes (5843 beds) were tested for COVID-19 when symptomatic. A COVID-19 outbreak was confirmed at 69 (37%) care homes, of which 66 (96%) were care homes for older people. The size of care homes for older people was strongly associated with a COVID-19 outbreak (odds ratio per 20-bed increase 3·35, 95% CI 1·99–5·63). 907 confirmed cases of SARS-CoV-2 infection were recorded during the study period, and 432 COVID-19-related deaths. 229 (25%) COVID-19-related cases and 99 (24%) COVID-related deaths occurred in five (3%) of 189 care homes, and 441 (49%) cases and 207 (50%) deaths were in 13 (7%) care homes. 411 (95%) COVID-19-related deaths occurred in the 69 care homes with a confirmed COVID-19 outbreak, 19 (4%) deaths were in hospital, and two (<1%) were in one of the 120 care homes without a confirmed COVID-19 outbreak. At the 69 care homes with a confirmed COVID-19 outbreak, 74 excess non-COVID-19-related deaths were reported, whereas ten non-COVID-19-related excess deaths were observed in the 120 care homes without a confirmed COVID-19 outbreak. 32 fewer non-COVID-19-related deaths than expected were reported among care home residents in hospital.
Interpretation
The effect of COVID-19 on care homes has been substantial but concentrated in care homes with known outbreaks. A key implication from our findings is that, if community incidence of COVID-19 increases again, many care home residents will be susceptible. Shielding care home residents from potential sources of SARS-CoV-2 infection, and ensuring rapid action to minimise outbreak size if infection is introduced, will be important for any second wave.
Funding
None.
Introduction
Internationally, institutional care settings for older adults have seen high rates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and COVID-19-related mortality among residents and staff.1 In the UK, care homes provide 24-h nursing care, residential care, or both for older adults who cannot be accommodated at home or in other settings, with increasingly high frailty and needs.2, 3 In the UK and other countries, including the USA, care home funding is typically a mix of self-funded and state-funded, and the care sector has been under increasing financial and capacity strain because of population aging and constraints on public funding.4 Robust national data for the care home population are scarce and data sources are fragmented, meaning our understanding of the needs and outcomes of residents is poor.5, 6
Early epidemiological data identified very high mortality from COVID-19 in some care home settings, for example, affecting 33% of residents in one US facility.7 Atypical presentation of COVID-19 is prevalent, with common presenting symptoms of delirium, postural instability, and diarrhoea in the absence of fever or cough.8, 9 The role of presymptomatic and asymptomatic transmission has become clearer over time. In one US study,10 56% of residents testing positive for COVID-19 were asymptomatic at the time of testing, with most developing symptoms within the next 4 days after testing. In the UK, all residents and staff at four care homes in London with COVID-19 outbreaks were tested twice, 1 week apart.11 40% of residents tested positive, of whom 43% were asymptomatic at the time of testing and 18% had atypical symptoms. 4% of staff tested positive, all of whom were asymptomatic, and viral genome sequencing found evidence of multiple introductions of infection.11
Research in context.
Evidence before this study
We searched PubMed and the medRxiv preprint server on Aug 12, 2020, with the terms (“long-term care” OR “nursing home” OR “care-home” OR “residential care”) AND (“COVID-19” OR “SARS-CoV-2” OR “COVID-19 and SARS-CoV-2”). We restricted our search to publications in English. Existing published work highlights the large effect that COVID-19 has had in care homes and that atypical disease presentation, asymptomatic carriage, and a presymptomatic infectious period are common in both residents and staff. One living systematic review confirmed the international outbreak burden among residents and staff and high but varied international mortality rates. International modelling studies have not accounted for the care home environment and context and have made estimates informed by general community transmission of infection. Only one peer-reviewed study was identified in which US nursing home characteristics associated with outbreaks were assessed, finding associations with larger facility size, urban location, and ethnicity but no association with quality ratings or ownership.
Added value of this study
We used publicly available national data for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing and deaths from COVID-19, which were linked to regulatory public health data, to describe the evolution of outbreaks of COVID-19 in all care homes in one large health region in Scotland. Care homes for older adults had the highest proportion of outbreaks, and the size of these care homes was the key characteristic associated with an outbreak. Many care homes recorded only one case or had short outbreaks, but sustained or repeated outbreaks were also seen. Overall, almost one in six residents had confirmed SARS-CoV-2 infection, but cases were concentrated in a few care homes. Excess deaths (ie, the sum of deaths over and above the historical average in the same period over the past 5 years), both COVID-19-related and non-COVID-19-related, were concentrated in care homes with a confirmed outbreak of COVID-19, not only suggesting that quality and safety of care in the wider care system was not seriously affected but also consistent with COVID-19 having a direct effect on care for other conditions in care homes with an outbreak.
Implications of all the available evidence
Despite the large effect of COVID-19 on the care home sector, a large susceptible population of residents remains in care homes that avoided an outbreak in the first wave of SARS-CoV-2 infection or that had a small outbreak. Care homes for older people, particularly those of large sizes, are likely to be the most vulnerable to further outbreaks if community transmission rises in the future. Systematic and regular testing and use of whole-genome sequencing is needed to inform understanding of transmission dynamics and support future outbreak detection and management. Future research should consider the built environment and organisation of care as other potentially modifiable factors to support infection control. Improving the quality of national and local data on the care home population is a priority both for COVID-19 and for ensuring this vulnerable population receives better care in the future.
England had the highest cumulative rate of excess deaths (ie, deaths over and above the historical average for the time of year) in Europe up to the end of May, 2020,12 with Scotland third highest, and deaths in care homes were a major contributor to this excess.13 National data are aggregated, which means our understanding of variation between care homes is limited. The aim of our study was to describe the evolution of outbreaks of COVID-19 in all care homes in one health region in Scotland, specifically the timing of outbreaks, number of confirmed cases in residents, care home characteristics associated with the presence of an outbreak, and deaths of residents in both care homes and hospitals.
Methods
Study setting and data sources
Our study included 188 registered care homes and one short-stay and respite facility run by the National Health Service (NHS), which were located within the NHS Lothian health region in the UK, encompassing the city of Edinburgh and surrounding region. NHS Lothian is part of NHS Scotland, which provides universal health-care coverage funded by the UK taxpayer, and so there are no fees for the patient or co-payments for medical care. NHS Scotland health regions have responsibility for delivery of all NHS care, including public health in their respective geographical area. State funding for social care is means-tested and as a result either funded by local authorities or self-funded with some state funding for personal or nursing care. Social care provision for older people is primarily delivered by private providers. Roughly 1 million people live within the geographical area covered by the NHS Lothian health region,14 of whom about 16% are aged 65 years and older and approximately 7% are aged 75 years and older.15 Four Integration Joint Boards, which have the same boundaries as local authority areas in the NHS Lothian region, commission community services. Key milestones in national policy and the local public health response to COVID-19 are summarised in panel 1 .
Panel 1. Initial public health response to COVID-19 in care homes.
February, 2020
In the early phases of the COVID-19 epidemic, NHS Scotland implemented contact tracing to contain community spread of SARS-CoV-2. This time was also used to prepare the NHS for an anticipated influx of seriously ill patients needing hospital care and, frequently, intensive care. In addition to redesigning patient flows to manage increased infection risk, this process meant assertively discharging patients who needed hospital care, with an estimated 900 people in Scotland discharged from hospital to care homes early in the epidemic.
March, 2020
The first positive cases of COVID-19 were diagnosed within the NHS Lothian health region, mainly in travellers returning from Italy and Spain. As positive cases began to be reported and numbers rapidly grew across Scotland, the Scottish Government and Public Health Scotland produced a series of guidance documents, in line with similar publications by Public Health England. The first document related to nursing home and residential care home residents and suspended routine visiting, just as the UK moved from the containment phase to the delay phase of the UK Government's COVID-19 response. Documents were regularly updated and clarifications made about infection control. Concerns arose about the availability and distribution of PPE, with many reports of scarcity for frontline NHS staff, particularly those in the social care sector. Contact tracing was halted nationally on March 13, 2020; subsequently, COVID-19 testing resources were directed towards hospitalised patients and, later, towards NHS staff. Testing of care home residents was available within the NHS Lothian health region from the first week of March but was initially restricted to only the first few cases, to establish the presence of an outbreak, with subsequent residents who showed symptoms of COVID-19 assumed to have the disease. The exception to this policy was care homes with large and prolonged outbreaks, in which wider testing was deployed.
April, 2020
Similar to other parts of the UK, testing in Scotland was extended to care home staff and, as availability of testing improved, the Scottish Government specified that all care home residents who had symptoms of COVID-19 should be tested from April 17, 2020. Importantly, increased responsibility was placed on Directors of Public Health for management of the epidemic in care homes, and NHS health regions were required to provide daily updates to Scottish Government. Public health and health and social care partnerships increasingly provided support to care homes in relation to more systematic testing, infection control, PPE supply, and ensuring safe staffing. Availability of resident testing was higher within the NHS Lothian health region than in many other areas because the regional virology laboratory had capacity, and a public health outreach team was used to swab residents when care homes were unable to do so themselves.
NHS=National Health Service. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. PPE=personal protective equipment.
Local public health data for testing of care home residents for COVID-19 were linked to publicly available data on care home services, collated by the national regulator (the Care Inspectorate) and published in April, 2020. Deaths of residents in care homes were identified from National Records of Scotland (NRS) death registrations and were defined as deaths with an institutional code compatible with a care home, with manual verification by study authors that the address recorded was that of a Care Inspectorate registered care home service. Deaths of care home residents in hospital were identified from NRS death registrations for which the place of death was a hospital, with addresses linked to Care Inspectorate registered care home addresses using postcode and text matching. Linked public health data and data for deaths are complete from March 1 to Aug 2, 2020.
Since our analysis was of public health data at the care home level (with no individual or identifiable patient data), separate research ethics review was not needed and this work was undertaken under generic approval by the Lothian Research Safe Haven and by the NHS Lothian and University of Edinburgh Dataloch partnership agreement. Care Inspectorate data are publicly available and licensed under Open Government, version 3.0.
Procedures
In accordance with NHS Lothian public health team practice during the COVID-19 pandemic, we defined the start of a COVID-19 outbreak in a care home as the date when the first resident had a positive test for SARS-CoV-2, using regional virology laboratory PCR testing of nasopharyngeal swabs. We obtained Care Inspectorate data for five variables: (1) type of care home, which was categorised into care homes for older people, care homes for other adult services (physical or sensory impairment, alcohol and drugs, mental health, respite care, or blood-borne viruses), care homes for people with learning disabilities, and care homes for children and young people; (2) number of beds, with number of registered places used as a proxy in 28 care homes where not available; (3) Risk Assessment Document score, which is a Care Inspectorate score to determine extent and frequency of inspection based on global assessment of care service quality and safety (low, medium, and high risk);16 (4) care home ownership (private, voluntary or not for profit, and local authority); and (5) locality (ie, the Integration Joint Board or local authority in which the care home is located). We used the number of outbreaks of any infectious disease in care homes (primarily norovirus, influenza, and scabies) reported to NHS Lothian Public Health since March, 2014, as a measure of historical infection control practice (categorised into 0, 1–4, and ≥5 outbreaks). We did not investigate availability and quality of personal protective equipment (PPE) because no reliable data were available at the care home level during the study period.
Death was examined in terms of week of death registration, which is legally required to be within 8 days of death, although most deaths are registered within 3 days. Deaths involving COVID-19 (referred to hereafter as COVID-19-related deaths)17 were defined as any death for which a record of confirmed (International Classification of Diseases version 10 [ICD-10] code U07.1) or suspected (ICD-10 code U07.2) COVID-19 was made on the death certificate. All other deaths were defined as non-COVID-19-related.
Statistical analysis
We used logistic regression at care home level to estimate univariate odds ratios (ORs) of the presence of an outbreak by care home characteristics. We then fitted multivariate models, only retaining significantly associated characteristics. Since care home characteristics systematically varied by type of care home, the primary analysis was of care homes for older people and a sensitivity analysis was done of all care homes; for example, care homes for older people were much larger (median 48 beds vs eight for all other types of care homes combined) and were more likely to be in private ownership (68% vs 30%). The number of beds for each type of home was divided by 20 and fitted as a continuous variable; estimated ORs are per 20-bed increase, which we considered clinically meaningful. The evolution of the COVID-19 epidemic at care home and resident level was investigated descriptively, and the estimated dissemination ratio (EDR) in care homes was calculated as the number of cases in a 7-day period divided by the number of cases in the preceding 7 days. EDR requires no assumptions about transmission routes or infectious periods and gives a direct assessment of the slope of the epidemic curve and how that is changing.18 An EDR greater than 1 indicates acceleration of an outbreak and an EDR less than 1 indicates slowing. We defined excess deaths (both COVID-19-related and non-COVID-19-related) as the sum of deaths over and above the historical average in the same period over the past 5 years; we examined excess deaths in all care homes and separately in those with and without an outbreak (but not further stratified because of small numbers). We used IBM SPSS statistics version 24.0 for all analyses.
Role of the funding source
This study received no external funding. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 10 (week 11 of 2020), and Aug 2, 2020 (week 31), residents of 189 care homes were tested for SARS-CoV-2 infection. 109 (58%) care homes provided services for older people, 14 (7%) were for other adult services, 26 (14%) were for people with learning disabilities, and 40 (21%) were for children and young people. These 189 care homes had 5843 beds, of which 5227 (89%) were in care homes for older people.
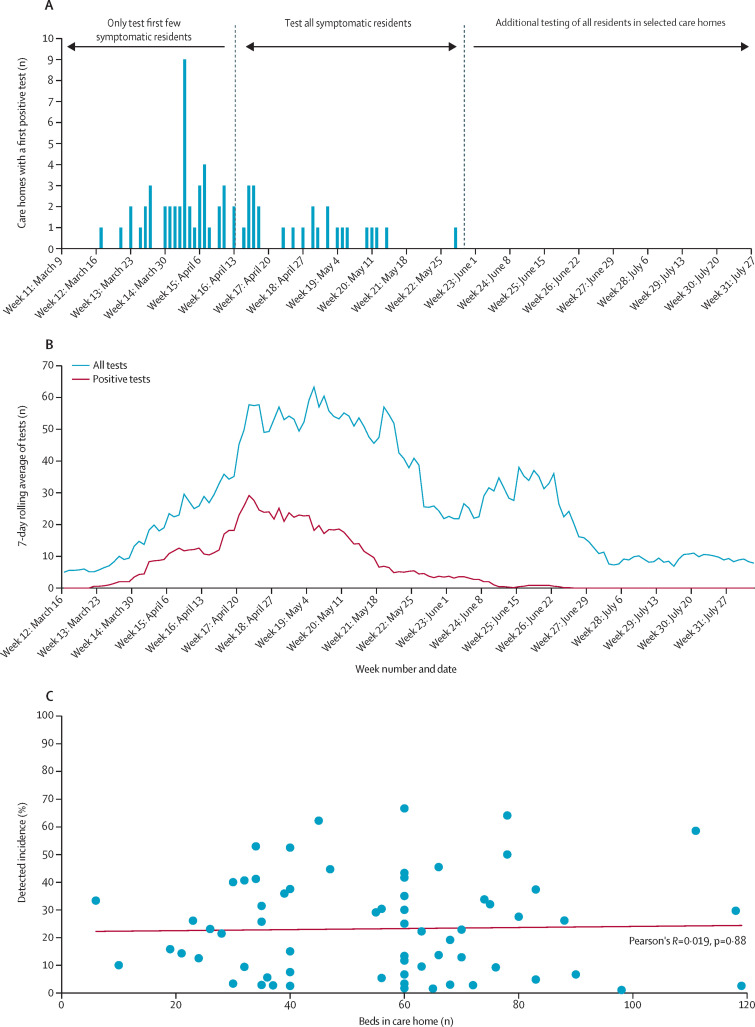
The first test for SARS-CoV-2 in a care home resident was done in the week beginning March 9, 2020 (week 11) and the first positive test was in the week beginning March 16, 2020 (week 12; figure 1A ). 55 outbreaks were recorded in weeks 12–16 (weeks starting March 16 to April 13), with a further 15 outbreaks in weeks 17–22 (weeks starting April 20 to May 25). The final outbreak started on May 14, 2020, and the last positive test in a care home resident was on June 20, 2020.
Figure 1.
Evolution of outbreaks
Plots show the number of care homes with a first positive test for SARS-CoV-2 infection, according to current testing policy (A), the 7-day rolling average of all tests and PCR-confirmed cases (B), and detected incidence of COVID-19 by number of care home beds (C). SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
The number of care home residents tested per day rose rapidly, peaking at 50–60 towards the end of April, 2020, when policy changed from testing the first few individuals with symptoms in each care home to testing all people with symptoms. Thereafter, the number of residents tested per day fell until a change in NHS Lothian policy to test all residents in care homes with ongoing outbreaks at the end of May, 2020 (figure 1A). The 7-day moving average of number of residents with a confirmed positive case rose rapidly, peaking at 28 per day on April 23, 2020 (figure 1B), then falling again. Mirroring this trend, the EDR was 7·9 on April 3, 2020, falling to 0·8 on April 16, 2020; a second peak was noted, with an EDR of 2·8 on April 23, 2020, and a steady decline to below 1·0 from April 28, 2020, with values remaining at this level (appendix p 2).
Of 189 care homes in the NHS Lothian region, 69 (37%) had a confirmed COVID-19 outbreak. Among these care homes, 50 (72%) had one or more residents with negative tests before their first confirmed case (table 1 ). 907 cases of SARS-CoV-2 infection were confirmed during the study period. Ten (14%) of 69 care homes only had one case, and 15 (22%) had between two and four cases. 229 (25%) of all cases were in just five (3%) of 189 care homes, and 441 (49%) of all cases were in 13 (7%) care homes, which were widely spread across the NHS Lothian region. The median number of positive cases per care home was seven (IQR 2–17, range 1–65).
Table 1.
Summary of testing data in care homes with COVID-19 outbreaks
| Care homes with recorded COVID-19 outbreak (n=69) | ||
|---|---|---|
| One or more negative tests | 50 (72%) | |
| Positive cases | ||
| 1 | 10 (14%) | |
| 2–4 | 15 (22%) | |
| 5–9 | 12 (17%) | |
| 10–19 | 15 (22%) | |
| 20–29 | 10 (14%) | |
| ≥30 | 7 (10%) | |
| Detected incidence (cases per bed, %) | ||
| 0–9·9 | 23 (33%) | |
| 10–19·9 | 10 (14%) | |
| 20–29·9 | 12 (17%) | |
| 30–39·9 | 10 (14%) | |
| 40–49·9 | 7 (10%) | |
| ≥50 | 7 (10%) | |
Data are n (%).
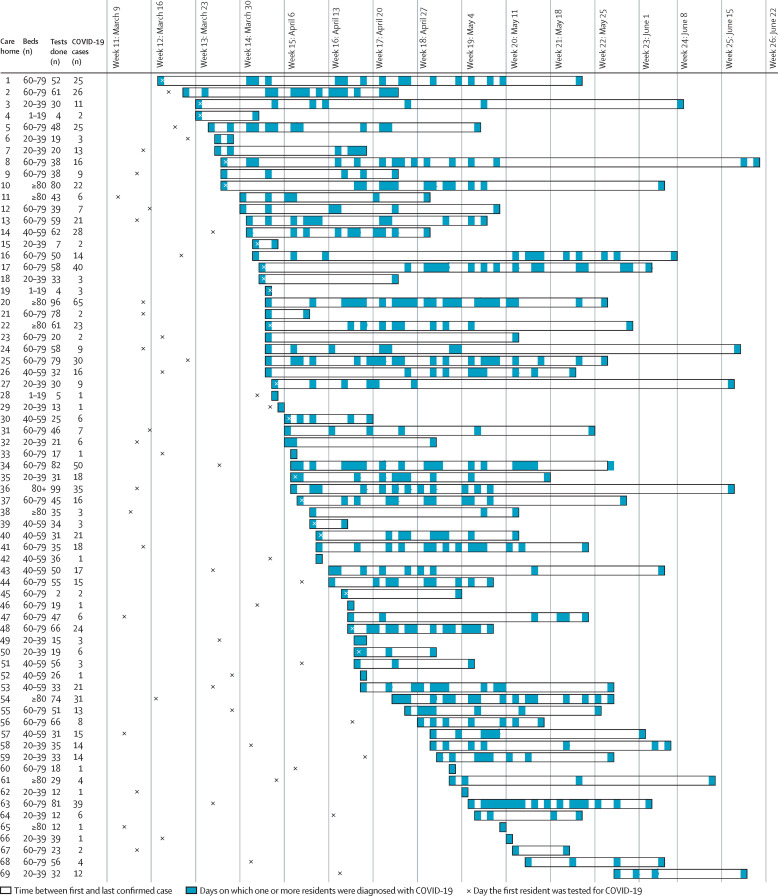
The median detected incidence of COVID-19 (confirmed cases per number of beds) in care homes was 20·7% (IQR 6·4–34·1, range 1·0–66·7), but no relation was noted between the number of beds in the care home and detected incidence (figure 1C). Considerable heterogeneity was seen in patterns of diagnosed infection, with 11 (16%) of 69 care homes with a confirmed outbreak of COVID-19 only having cases diagnosed on 1 day and nine (13%) only having cases diagnosed over 2–14 days (figure 2 ). Although evidence of sustained infection was noted over several weeks in many care homes, in 20 (29%) of 69 care homes there were gaps between diagnosed cases of 14–27 days, and in eight (12%) care homes there were gaps of 28 days or longer, consistent with new infections (figure 2). At the end of follow-up (on Aug 2, 2020) no care homes had had a new case for more than 28 days, consistent with outbreaks ceasing.
Figure 2.
Patterns of outbreak for 69 care homes with an outbreak
66 (96%) of the 69 confirmed COVID-19 outbreaks in any care home were in care homes for older people. Of all 109 care homes for older people, 66 (61%) had an outbreak whereas only three (4%) of the 80 other types of care homes did (table 2 ). Univariate analysis of care homes for older people showed significant associations between a COVID-19 outbreak and number of beds (per 20-bed increase, OR 3·35, 95% CI 1·99–5·63) and a history of previous outbreaks of infectious diseases (five or more vs none, 5·95, 1·94–18·30), but no association was seen with regulatory risk assessment score, ownership, and locality (table 3 ). Once care home number of beds was accounted for, no other characteristic was associated with the presence of an outbreak. Similar results were found in the sensitivity analysis of all care homes (appendix p 4).
Table 2.
Care home and testing characteristics
| Beds | No resident testing done | One or more residents tested, but no positive tests | One or more residents with a positive test (outbreak) | ||
|---|---|---|---|---|---|
| All care homes (n=189) | 23 (1–119) | 65 (34%) | 55 (29%) | 69 (37%) | |
| Older people (n=109) | 42 (10–119) | 3 (3%) | 40 (37%) | 66 (61%) | |
| Other adult (n=14)* | 8 (4–60) | 9 (64%) | 4 (29%) | 1 (7%) | |
| Learning disabilities (n=26) | 5 (4–11) | 16 (62%) | 9 (35%) | 1 (4%) | |
| Children and young people (n=40) | 5 (1–45) | 37 (93%) | 2 (5%) | 1 (3%) | |
Data are median (range) or n (%).
For physical or sensory impairment, alcohol and drugs, mental health, short-break and respite care (including a National Health Service-run service), and blood-borne viruses.
Table 3.
Unadjusted logistic regression analysis of characteristics of care homes for older people associated with an outbreak
| Care homes for older people (n=109) | Univariate odds ratio (95% CI) | ||
|---|---|---|---|
| Number of beds (per 20 bed increase) | .. | 3·35 (1·99–5·63)* | |
| Regulatory risk assessment score† | |||
| Low (2767 beds) | 38/64 (59%) | 1 (ref) | |
| Medium (1153 beds) | 12/24 (50%) | 0·68 (0·27–1·76) | |
| High (1307 beds) | 16/21 (76%) | 2·19 (0·71–6·72) | |
| Ownership | |||
| Private (3835 beds) | 42/74 (57%) | 1 (ref) | |
| Voluntary or not for profit (597 beds) | 10/16 (63%) | 1·27 (0·70–6·53) | |
| Local authority (795 beds) | 14/19 (74%) | 2·13 (0·42–3·86) | |
| Outbreaks of other infectious diseases in past 6 years | |||
| None (1212 beds) | 14/34 (41%) | 1 (ref) | |
| 1–4 (2030 beds) | 27/44 (61%) | 2·27 (0·91–5·66) | |
| ≥5 (2030 beds) | 25/31 (81%) | 5·95 (1·94–18·30) | |
| Locality | |||
| Locality 1 (3169 beds) | 41/65 (63%) | 1 (ref) | |
| Locality 2 (654 beds) | 9/17 (56%) | 0·66 (0·22–1·93) | |
| Locality 3 (554 beds) | 7/11 (64%) | 1·02 (0·27–3·86) | |
| Locality 4 (850 beds) | 9/16 (56%) | 0·75 (0·25–2·28) | |
Data are n/N (%) unless otherwise specified.
Once care home number of beds was accounted for, no other characteristic was associated with the presence of an outbreak.
Care Inspectorate risk assessment score determined on independent inspection.
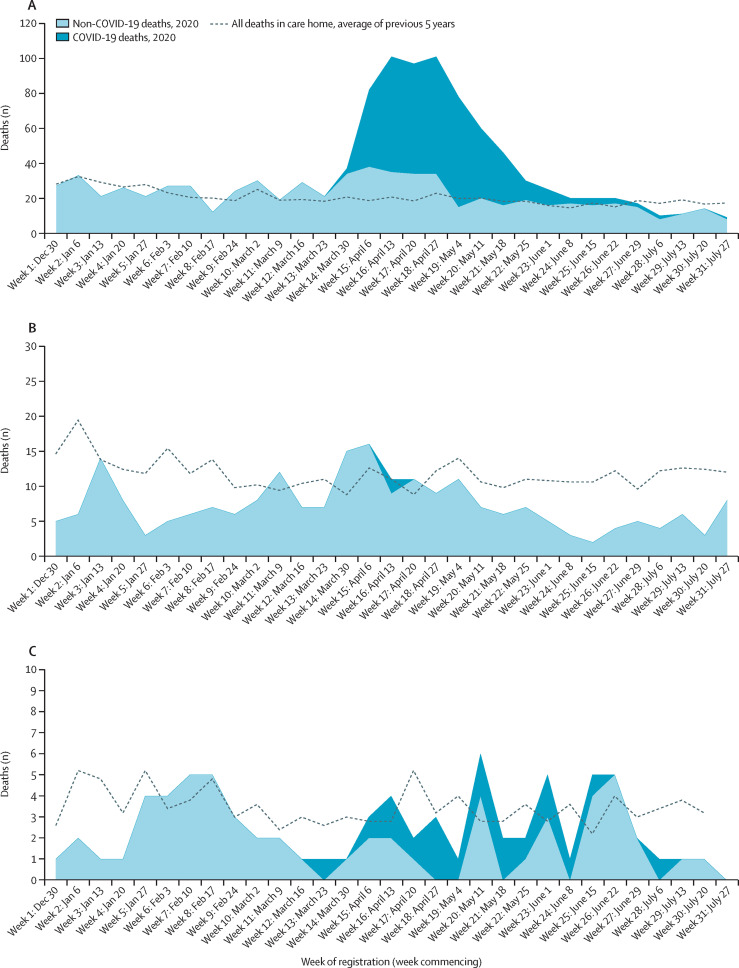
432 care home residents died and had COVID-19 recorded on their death certificates. Of these COVID-19-related deaths, 413 (96%) occurred in care homes and 19 (4%) occurred in hospital. 99 (24%) COVID-19-related deaths in care homes occurred in five (3%) care homes and 207 (50%) were in 13 (7%) care homes. The number of COVID-19-related deaths rose rapidly during the study period, peaking at 64–70 per week in weeks 16–19 (weeks beginning April 13 to May 4, 2020; appendix p 3). Of 413 COVID-19-related deaths occurring in care homes, 411 (99%) were in the 69 care homes with a confirmed outbreak. In these 69 care homes, 480 excess deaths were recorded in weeks 13–26 (weeks beginning March 23 to June 22, 2020), representing 65% of all deaths in these care homes in that time, of which 406 (85%) deaths were COVID-19-related and 74 (15%) were non-COVID-19-related excess, after which mortality was below historical averages. The peak ratio of observed to expected deaths was 5·3 in week 17 (week beginning April 20, 2020). From week 27 (week beginning June 29, 2020), total deaths consistently fell below expected levels, although five COVID-19-related deaths were recorded in this period (figure 3A ). By comparison, two COVID-19-related deaths were reported in care homes without a confirmed outbreak; on the death certificates, one death was confirmed as COVID-19-related and one was suspected as COVID-19-related, but both were untested and not known to Public Health Scotland. An additional ten non-COVID-19-related excess deaths were recorded in these 120 care homes in weeks 14–17 (weeks beginning March 30 to April 20, 2020); fewer deaths occurred throughout the remainder of follow-up than expected compared with historical averages (figure 3B). 27 deaths of care home residents in hospital were non-COVID-19-related, which is 32 fewer than expected compared with historical averages (figure 3C).
Figure 3.
Deaths of care home residents in care homes and in hospital
Plots show deaths of residents in care homes with a confirmed COVID-19 outbreak (A), in care homes without an outbreak (B), and in hospitals (C). Data obtained from NRS death registrations.13 Data are by week of registration (which is later than date of death). NRS data are provisional and could be subject to change, particularly later data if registration was delayed. NRS=National Records of Scotland.
Discussion
The findings of our population analysis show that, in the NHS Lothian health region of the UK, just over a third of care homes (69 of 189 [37%]) had a confirmed COVID-19 outbreak, but with wide variation in the size, duration, and pattern of outbreaks. The number of beds was strongly associated with the presence of an outbreak. No other care home characteristics were associated with outbreaks once number of beds was accounted for. However, care home size was not associated with outbreak size measured as the percentage of residents subsequently confirmed to have COVID-19. Many care homes had only one reported case of COVID-19 or a short outbreak, but in some care homes sustained or repeated outbreaks were reported. 413 COVID-19-related deaths were recorded in care homes. In the 120 care homes without a confirmed COVID-19 outbreak, only two COVID-19-related deaths were reported; neither person had been tested for SARS-CoV-2. Ten non-COVID-19-related excess deaths were recorded in these 120 care homes early in the epidemic period (March 30 to April 20, 2020). 19 care home residents died while in hospital with COVID-19, which was recorded on the death certificate. 32 fewer non-COVID-19-related deaths occurred in hospital during the epidemic, which is consistent with some shift of deaths that would have occurred anyway from hospital to care home.
The strengths of our analysis are that we included all care homes in one geographical area using linked data and investigated deaths in both care homes and hospitals. Availability of resident testing in this region was higher and started earlier than in many other areas because the regional virology laboratory had capacity and a public health outreach team supported rapid testing as part of the outbreak response programme. The limitations of our analysis include under-ascertainment of cases due to false-negative tests and that testing policy varied over time. Case numbers are, therefore, likely to be underestimated, particularly early in the epidemic.11 However, the absence of a large mortality effect in care homes without a confirmed COVID-19 outbreak mean it is unlikely that any large outbreaks were missed. The decision to include COVID-19 on death certificates, and whether to code as confirmed or suspected COVID-19, probably also varied over time in relation to test availability. We, therefore, report the UK-wide definition of COVID-19-related deaths without subgroup analysis, but interpretation should be cautious.17 Finally, we included 189 care homes in our analysis and, thus, it is relatively underpowered to examine associations with care home characteristics.
Data from the Care Quality Commission show that 36% of care homes in England had reported an outbreak of COVID-19 by May 17, 2020,19 compared with 37% of care homes in the NHS Lothian health region in this study. In Scottish care homes, a larger proportion of COVID-19 deaths are reported to have occurred than in English care homes (47% vs 28%),13, 20 although these proportions are both within the range reported internationally (from 24% in Hungary to 82% in Canada).1 This difference could reflect differing hospital admission practices in England and Scotland since approximately 12% of COVID-19-related deaths of care home residents in England are in hospital21 versus only 4% in our study. A London point-prevalence study of four care homes with about 400 residents found 26% mortality, higher than that noted in our study, which probably reflects selection of care homes with large outbreaks.11 Similar to our study findings, US data showed a significant association between confirmed COVID-19 outbreaks in older people's nursing homes and home size and no association with regulatory quality ratings.22 By contrast with our findings, no association between presence of outbreaks and care home size was noted in a study of nursing homes for older people in Ontario, Canada, but, similar to our findings, the concentration of confirmed cases in care homes was considerable (10% of care homes had 86% of confirmed cases).23 In the same study, crowding (ie, more shared rooms, bathrooms, or both) was not associated with a confirmed COVID-19 outbreak but it was associated with larger outbreak size and higher mortality rates,23 similar to findings of the UK Vivaldi study,24 in which COVID-19 incidence increased with higher occupancy and lower staffing ratios. A study in the US State of Georgia reported associations between worse infection control practice and larger outbreak size.25 Overall, there are consistent associations between care home size and the presence of an outbreak, whereas other characteristics could contribute more to outbreak size once an outbreak starts.
The effect of COVID-19 on care homes has been very large; in our study, cases and deaths were concentrated not only in care homes with a confirmed outbreak but also within this group, since a quarter of cases and deaths were in just five care homes and half were in 13 care homes. However, most care homes for older people have not had a confirmed outbreak of COVID-19, or have rapidly controlled their outbreak, meaning that large numbers of residents will remain susceptible to SARS-CoV-2 infection. Any future increase in community transmission is, therefore, likely to drive further care home outbreaks without stricter measures to control ingress.
Excess deaths not attributed to COVID-19 largely occurred in care homes with a confirmed outbreak. At least some of these deaths would have happened anyway in hospital since we observed lower than expected numbers of non-COVID-19-related deaths of care home residents in hospital during the epidemic period. This finding suggests that non COVID-19-related excess deaths in care home residents in this health region were not strongly related to wider changes to the system of care (eg, inappropriate withdrawal of primary care). Whether some deaths not attributed to COVID-19 were due to COVID-19 (eg, false-negative tests or undiagnosed COVID-19 in residents presenting with atypical symptoms) or were an indirect effect (eg, related to changes in care for uninfected residents when staff were overwhelmed or short-staffed) needs further detailed investigation.
Our analysis highlights that univariate associations with care home characteristics can be misleading, because many characteristics are strongly associated with care home size, which unsurprisingly dominates associations with the presence of a COVID-19 outbreak.22 Although care home size cannot be easily altered, discrete, self-contained units could be created within care homes comprising smaller numbers of staff and residents. Such efforts will be complicated by the built environment of the individual care home and will be difficult to sustain without rapid outside support during any large COVID-19 outbreak, when staff absence through illness risk can compromise safe care. Additional measures to respond to new outbreaks of COVID-19 will be needed, including maintaining good provision of PPE, better support for infection control, active surveillance of residents and staff to ensure early detection of outbreaks and ongoing transmission, support for staff self-isolation, and staffing support for care homes with outbreaks (panel 2 ). However, COVID-19 has high infectivity, which is reflected in high rates of nosocomial infection in both health and social care settings.26 Infection control is intrinsically difficult in care home settings because a priority is to maintain social and cognitive function through interaction. Shielding care homes and its residents from further outbreaks is essential, but poses difficulties—for example, in relation to the effect of preventing or minimising family and friends visiting on the quality of life of residents.27
Panel 2. Additional measures taken to contain outbreaks of COVID-19 in care homes.
Extended staff and resident testing
In May, 2020, nasopharyngeal swabbing and PCR testing for SARS-CoV-2 was introduced for all staff (permanent and agency) and residents in care homes (and other closed settings such as prisons) with new or sustained outbreaks. A key aim was to identify staff with no or low-level symptoms. For example, staff who tested positive for SARS-CoV-2 frequently did not show symptoms meeting the formal case definition for COVID-19 (ie, fever, cough, or loss of sense of smell or taste) but about half of staff showed a range of other low-level symptoms of the disease.
Contact tracing and reinforcing household isolation
Staff with positive tests for COVID-19 underwent contact tracing, and advice about self-isolation and household isolation was reinforced (as many staff in care homes live with other care workers).
Support for care workers with COVID-19 to self-isolate
Care workers are a low-paid, marginalised workforce who typically have worse employment conditions than do NHS staff, in relation to hourly wage and paid sick leave, which makes self-isolation and household isolation challenging. The NHS Lothian health region and Public Health Scotland worked together to develop advice and behavioural interventions. In some care homes with ongoing outbreaks, minimal or no sick pay was a clear barrier to staff self-isolating. The NHS Lothian health region supported rapid access to alternatives to employment-related sick pay via health and social care partnerships and the Public Health Act (Scotland) 2008, although one barrier was ensuring that sick pay was promptly approved and paid.
Support for staffing
From the middle of May, 2020, care homes with staffing difficulties in the face of an outbreak could request support from health and social care partnerships via access to the NHS Lothian health region staff bank. Bank staff are tested 48 h before going into any care home.
NHS=National Health Service. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Several outstanding issues need to be addressed. First, SARS-CoV-2 transmission dynamics in this context are not well understood, including how infection gets into care homes and the extent to which outbreaks are sustained by ongoing transmission within care homes or by introduction of new infections. More systematic testing of both residents and staff, with whole-genome sequencing to trace transmission chains, will be of great value and help inform public health response. Second, in our analysis we investigated high-level care home characteristics (eg, number of beds) and ignored spatial and other clustering effects, including when care homes have shared ownership. Research is needed to understand the relative importance of the built environment, the nature and intensity of staff–resident and resident–resident interactions (which will vary by resident group), variations in use of agency staff and investment in staff training, access to and effective use of PPE, and infection control procedures. Some of these data can be obtained by survey but some might need direct observation of care, which is challenging during an epidemic. Third, research is needed to better understand the causes of mortality, including investigating variation in the attribution of death to be COVID-19-related, and the circumstances and likely causes of other deaths. Finally, COVID-19 has highlighted how difficult it is to reliably identify who lives in care homes from routine data.5 Thus, our regression analysis is at care home level and we can only estimate incidence using number of beds as the denominator, which has hampered our understanding of the effect of COVID-19. More broadly, we need care home residence to be accurately recorded to support systematic understanding of the needs and patterns of care of a highly vulnerable but often overlooked population and care sector. Similar issues apply to people receiving social care in their own homes, who are also vulnerable and largely invisible in routine data.
COVID-19 outbreaks have, up to now, been concentrated within a few care homes, with repeated or sustained outbreaks that have affected a large number of residents. Many care homes in the NHS Lothian health region have not yet had an outbreak. There is, therefore, considerable risk of further outbreaks with many deaths in care homes if community incidence of COVID-19 increases again. Allowing families and friends to visit residents again is important for quality of life but needs to be balanced against the need to shield residents in areas where community incidence is high or increasing. Early detection of outbreaks through regular testing, reliable PPE supply, support for robust infection control, and measures to ensure safe staffing are all likely to be needed to contain the size of established outbreaks.
For Care Inspectorate data see https://www.careinspectorate.com/index.php/publications-statistics/93-public/datastore
Data sharing
Data are available on request from the corresponding author, subject to NHS Scotland disclosure controls to prevent identification of individuals.
Acknowledgments
Acknowledgments
We thank Audrey Pringle, Jenni Strachan, Lindsey Murphy, Louise Wellington, and Peter Harrison (NHS Lothian Health Protection Team) for data collection and comments on the paper; Alison Milne, Dan Clutterbuck, and members of the enhanced outreach testing team; and Alison McCallum (Director of Public Health, Health Data Research UK) for support for policy and public dissemination.
Contributors
BG, CE, FG, NH, JES, LW, and JKB had the idea for the study. KET was responsible for PCR testing and quality assurance. GB, NH, SS, MT, DG, DMcC, and RO contributed to data collection and management. BG, NH, SS, MT, JKB, and BG did primary data analysis, with LW, FG, JES, CE, DG, DMcC, and RO contributing to interpretation. All authors contributed to drafting and revision and approved the submitted paper.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Salcher-Konrad M, Jhass A, Naci H, Tan M, El-Tawil Y, Comas-Herrera A. COVID-19 related mortality and spread of disease in long-term care: a living systematic review of emerging evidence. medRxiv. 2020 doi: 10.1101/2020.06.09.20125237. published online Aug 1. (preprint) [DOI] [Google Scholar]
- 2.Barker RO, Hanratty B, Kingston A, Ramsay S, Matthews FE. Changes in health and functioning of care home residents over two decades: what can we learn from population based studies? medRxiv. 2020 doi: 10.1101/2020.08.05.20168740. published online Aug 6. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton J, Lynch E, Love S, Rintoul J, Starr J, Shenkin S. Who lives in Scotland's care homes? Descriptive analysis using routinely collected social care data 2012–16. J R Coll Physicians Edinb. 2019;49:12–22. doi: 10.4997/JRCPE.2019.103. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski DC, Mor V. Nursing home care in crisis in the wake of COVID-19. JAMA. 2020;324:23–24. doi: 10.1001/jama.2020.8524. [DOI] [PubMed] [Google Scholar]
- 5.Burton JK, Guthrie B. Identifying who lives in a care home: a challenge to be conquered. Age Ageing. 2018;47:322–323. doi: 10.1093/ageing/afx200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanratty B, Burton J, Goodman C, Gordon A, Spilsbury K. Covid-19 and lack of linked datasets for care homes. BMJ. 2020;369 doi: 10.1136/bmj.m2463. [DOI] [PubMed] [Google Scholar]
- 7.McMichael TM, Currie DW, Clark S. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dora A, Winnett A, Jatt L. Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for veterans—Los Angeles, California. MMWR Morb Mortal Wkly Rep. 2020;69:651–655. doi: 10.15585/mmwr.mm6921e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lithander FE, Neumann S, Tenison E. COVID-19 in older people: a rapid clinical review. Age Ageing. 2020;49:501–515. doi: 10.1093/ageing/afaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arons MM, Hatfield KM, Reddy SC. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham NSN, Junghans C, Downes R. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020;81:411–419. doi: 10.1016/j.jinf.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Office for National Statistics Comparisons of all-cause mortality between European countries and regions: January to June 2020. July 30, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/comparisonsofallcausemortalitybetweeneuropeancountriesandregions/januarytojune2020
- 13.National Records of Scotland Deaths involving coronavirus (COVID-19) in Scotland. https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/general-publications/weekly-and-monthly-data-on-births-and-deaths/deaths-involving-coronavirus-covid-19-in-scotland
- 14.National Records of Scotland Mid-year population estimates. April 30, 2020. https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/population/population-estimates/mid-year-population-estimates
- 15.National Records of Scotland Mid-2018 population estimates Scotland. April 25, 2019. https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/population/population-estimates/mid-year-population-estimates/mid-2018
- 16.The Care Inspectorate . The Care Inspectorate; Dundee, UK: 2020. Risk assessment: information for care service providers (OPS-0312-123) [Google Scholar]
- 17.Office for National Statistics Deaths involving COVID-19, England and Wales: deaths occurring in June 2020. July 17, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsinvolvingcovid19englandandwales/deathsoccurringinjune2020
- 18.Morris R, Sanson R, Stern M, Stevenson M, Wilesmith J. Decision-support tools for foot and mouth disease control. Rev Sci Tech. 2002;21:557–567. doi: 10.20506/rst.21.3.1363. [DOI] [PubMed] [Google Scholar]
- 19.Care Quality Commission . Care Quality Commission; London, UK: 2020. COVID-19 insight. [Google Scholar]
- 20.Office for National Statistics Deaths registered weekly in England and Wales, provisional: week ending 12 June 2020. June 23, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredweeklyinenglandandwalesprovisional/weekending12june2020
- 21.Office for National Statistics Number of deaths in care homes notified to the Care Quality Commission, England. Sept 8, 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/numberofdeathsincarehomesnotifiedtothecarequalitycommissionengland
- 22.Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of US nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68:1653–1656. doi: 10.1111/jgs.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown KA, Jones A, Daneman N. Association between nursing home crowding and COVID-19 infection and mortality in Ontario, Canada. medRxiv. 2020 doi: 10.1101/2020.06.23.20137729. published online June 23. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutey-Magni PF, Williams H, Jhass A. Covid-19 infection and attributable mortality in UK long term care facilities: cohort study using active surveillance and electronic records (March–June 2020) medRxiv. 2020 doi: 10.1101/2020.07.14.20152629. published online July 15. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telford CT, Bystrom C, Fox T. Assessment of infection prevention and control protocols, procedures, and implementation in response to the COVID-19 pandemic in twenty-three long-term care facilities in Fulton County, Georgia. medRxiv. 2020 doi: 10.1101/2020.08.13.20174466. published online Aug 15. (preprint) [DOI] [Google Scholar]
- 26.Zhou Q, Gao Y, Wang X. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8:629. doi: 10.21037/atm-20-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Down A, Dhillon A, Stretch G. COVID-19 in care homes: atypical presentations and high mortality rates mean outbreak management needs to include health and social care—early identification of atypical clinical signs, and complete segregation of cases, not cohorting, is essential. Preprints. 2020 doi: 10.20944/preprints202006.0060.v1. published online June 7. (preprint) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request from the corresponding author, subject to NHS Scotland disclosure controls to prevent identification of individuals.